Abstract
Indocyanine Green Angiography (or ICGA) is a technique performed by ophthalmologists to diagnose abnormalities of the choroidal and retinal vasculature of various eye diseases such as age-related macular degeneration (AMD). ICGA is especially useful to image the posterior choroidal vasculature of the eye due to its capability of penetrating through the pigmented layer with its infrared spectrum. ICGA time course can be divided into early, middle, and late phases. The three phases provide valuable information on the pathology of eye problems. Although time-course ICGA by intravenous (IV) injection is widely used in the clinic for the diagnosis and management of choroid problems, ICGA by intraperitoneal injection (IP) is commonly used in animal research. Here we demonstrated the technique to obtain high-resolution ICGA time-course images in mice by tail-vein injection and confocal scanning laser ophthalmoscopy. We used this technique to image the choroidal lesions in a mouse model of age-related macular degeneration. Although it is much easier to introduce ICG to the mouse vasculature by IP, our data indicate that it is difficult to obtain reproducible ICGA time course images by IP-ICGA. In contrast, ICGA via tail vein injection provides high quality ICGA time-course images comparable to human studies. In addition, we showed that ICGA performed on albino mice gives clearer pictures of choroidal vessels than that performed on pigmented mice. We suggest that time-course IV-ICGA should become a standard practice in AMD research based on animal models.
Keywords: Medicine, Issue 84, Indocyanine Green Angiography, ICGA, choroid vasculature, age-related macular degeneration, AMD, Polypoidal Choroidal Vasculopathy, PCV, confocal scanning laser ophthalmoscope, IV-ICGA, time-course ICGA, tail-vein injection
Introduction
Indocyanine green angiography (ICGA) is a diagnostic test to image problems related to blood vessels in the eye. The absorption spectrum of ICG ranges from 790-805 nm while the emission spectrum ranges from 770-880 nm with the peak emission at 835 nm 1. This is different from the other popular dye, sodium fluorescein, whose spectrum falls in the visible range. The infrared spectrum enables ICG to penetrate through retinal pigment epithelium (RPE), serosanguineous fluid, and lipid exudates, all of which can easily block visualization by sodium-fluorescein based fluorescein angiography (FA). ICG is 98% protein-bound in the vasculature resulting in less extravasation, allowing enhanced imaging of choroidal vessels and choroidal lesions1,2. ICGA is almost the only choice to visualize choroidal vasculature, which is posterior to RPE. Figure 1 shows the comparison of ICGA and FA in imaging vasculature in mouse eyes. FA can be used to image the retinal vasculature well but not the choroidal vasculature. In contrast, ICGA can be used to image both retinal and choroidal vasculature. ICGA is performed with high-resolution digital imaging systems or scanning laser ophthalmoscopes (SLO) together with infrared-sensitive video cameras, which we will use in this study.
In the clinic, ICGA has been recommended in diagnosing a number of chorioretinal disorders involving the choroidal vasculature including Polypoidal Choroidal Vasculopathy (PCV), Retinal Angiomatous Proliferation (RAP), angioid streaks, vitelliform macular dystrophy, central serous chorioretinopathy, choroidal hemangioma, hemorrhaging retinal arteriolar macroaneurysms, choroidal tumors, and certain forms of posterior uveitis1,3. The combination of ICGA with FA and Optical Coherence Tomography (OCT) provide powerful tools for the clinicians in the diagnosis and management of exudative age-related macular degeneration (AMD)4-10. ICGA is especially useful for diagnosing conditions involving the choroid. In fact, ICGA is considered the gold standard for diagnosing PCV, a variant of exudative AMD11-13. PCV is characterized by a network of branching vessels with terminal polypoidal dilations in the choroidal vasculature11-13. PCV is frequently associated with recurrent serosanguineous detachments of the RPE and retina with leakage and bleeding from the polypoidal components11,14,15. We recently reported the generation of the first PCV animal model by transgenically expressing human HTRA1, a multi-functional serine protease, in mouse retinal pigment epithelium (RPE)16. We showed that increased HTRA1 induced characteristic features of PCV, e.g. polypoidal lesions.
Here we demonstrated the use of time-course ICGA by tail vein injection in AMD research using our HTRA1 mouse model. Our data suggest that IV-ICGA is superior to IP (or subcutaneous (SC))-ICGA that are currently used in the field17,18 for characterizing lesions in the choroid.
Statement on Animal Research
Animal experiments were conducted according to protocols approved by Institutional Animal Care and Use Committee (IACUC), and were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Protocol
1. Preparation of Instruments
The procedure is performed in a procedure room in an animal facility.
Wear facemasks, hair bonnets, surgical gowns, sterile foot-covers, and gloves before beginning the experiment.
Heat water in a beaker to ~40 °C on a hotplate.
Place a sterile blue pad on top of a heating pad that will be used later to maintain the mouse's body temperature during imaging. Turn on the heating pad.
- Prepare the Imaging System:
- Remove the dust cover and turn on the laser.
- Take out the 55° lens and mount it onto the machine.
- Open the imaging software from the computer and input the information of the mouse for imaging under a new patient's sheet (e.g. genotype, age, etc.). Under "device type", choose Infrared (IR) mode.
Note: It has been reported that the use of an external double aspheric lens can improve the image quality17-20 although we have no problem in obtaining high quality images by IV-ICGA without using external lenses (see representative results, Figures 1-4).
2. Tail Vein Injection of ICGA
Dilate mouse eyes with 1% Tropicamide ophthalmic solution and wait 5 min.
Weigh the mouse to determine the amount of anesthesia (Ketamine/Xylazine/Acepromazine 65-100/10-20/1-3 mg/kg) needed.
Retrieve a sterile 1-ml syringe along with a 32 G needle. Inject the mouse intraperitoneally with the anesthetics (13 mg/ml Ketamine, 2.6 mg/ml Xylazine, 0.3 mg/ml Acepromazine in sterile PBS). Wait until the mouse is fully anesthetized (~5 min).
Place the mouse tail into 40 °C warm water to cause vasodilatation of the vein.
Retrieve a 1 ml syringe with a 32 G needle. Withdraw the desired amount of ICG, typically 50 μl of 1 mg/ml ICG, which is sterile filtered with a 0.2 µM syringe filter into a sterile tube, for a 25 g mouse (2 mg/kg). Be careful not to introduce any air into the syringe.
Wipe the tail with an alcohol swab to sterilize the area to be injected.
Hold the tail with one hand so that the lateral tail vein is upward. With the bevel of the needle facing upward, inject the needle ~2 mm into the vein at a minimal angle. Be careful not to perforate the vein. Draw back on the syringe slightly and look for traces of blood flow into the needle hub, which indicates that the needle was successfully inserted into the vein.
Slowly inject ICG into the vein. There should be minimal resistance when injecting. Remove the needle and apply an alcohol swab directly to the injection site for ~5-10 sec to stop any bleeding. The mouse is then ready for imaging. In order to catch the early phase (0-4 min after injection), it is essential to image the mouse quickly.
Note: Mouse eyes can easily get dry and can develop cataract under anesthesia. It is important to keep the eye moist by applying sterile PBS during the procedure. Wipe off excess PBS with a sterile cotton swab before ICGA recording. Other labs have used a contact lens to avoid dehydration of the cornea17-20.
3. ICG Angiography
Start to take images 30-40 sec after ICG injection, which permits the capture of the early phase of choroidal filling until retinal and choroidal circulations are at maximum brightness (0-4 min). The retinal vasculature is best visualized at focus ~35-45 diopters and choroidal vasculature is visualized at 10-15 diopters. Note: During the first examination of an animal model, it is recommended to capture images from all angles (nasal, temporal, dorsal and ventral) to identify all possible abnormalities in the vasculature. During the early phase, both medium and large choroidal arteries and veins are well visualized. In the animal model used in this protocol, choroidal lesions (e.g. polypoidal dilations) may start to appear 1 min into the early phase.
Set the image focus on the vasculature. Control for brightness and focus using the control module and focus knob, respectively. These values are adjustable digitally and are easily kept constant. Keep the distance from the mouse eye to the camera lens constant to ensure image quality is reproducible using the technique that follows. Note: Since the device can only image a portion of the posterior eye, we try to keep the focus, brightness, and the distance between the camera lens and the mouse eye constant as we image the entire posterior eye from different angles. The key to this is to align the circle-shaped luminescence emitted by the ICG through the eye with the field of view of the camera. This is accomplished by making left-to-right, up-and-down, and in-and-out adjustments of the camera position until the entire image has no dark areas. When the luminescence and the field of view of the camera are lined up, the distance from the eye to the lens will be reproducible for the next set of images as well as at an optimal distance for quality imaging.
Once the vasculature is in focus, capture the image frames by pressing the round black button on the acquisition module. The round black button can also be used to reduce or enhance the signal of ICG for best image quality.
Determine the optimal view angle and focusing depth to image choroidal lesions. It is important to keep the position of the eye, the focusing depth, and other device settings fixed for the entire time-course ICGA. The images are saved by pressing acquire button on the touch screen panel of the acquisition module.
Acquire images in the middle phase at 6-15 min after injection. Note: Both the choroidal and retinal vessels become less distinct. Choroidal vasculature appears as diffuse fluorescence. Choroidal lesions exhibiting hyperfluorescence emerge in contrast to the fading surrounding normal background fluorescence.
Acquire images in the late phase at 17-25 min after injection. Note: Hyperfluorescence fades. Both choroidal and retinal vessels are no longer visible. The optic nerve head becomes black. Hyperfluorescent choroidal lesions have maximal contrast with the fading background.
After finishing the acquisition of images, apply a clear lubricant eye gel to mouse eyes and leave the mouse on a heating pad for recovery.
Return mice to their cages and holding area. Export images as Tiff or JPEG files for further analysis.
Note: The timing of each phase is not absolute. We found that the timing of each phase might change depending on the amount of ICG injected. More ICG tends to prolong each phase. The best way to define a phase is according to the key features of each phase listed above.
Representative Results
We performed ICGA time course in HTRA1 transgenic mice and control WT littermates, both of which are on the CD1 background. The albino CD1 background was selected to facilitate indocyanine green angiography (ICGA) imaging (see DISCUSSION). Some aneurism like dilations began to appear in the early phase in the HTRA1 mouse (Figure 2, a red arrow indicates the dilation at the tip of a vessel and a red circle indicates a cluster type polypoidal lesion). Choroidal vessels are clearly visible in both WT and HTRA1 mice during this early fill-in stage of the ICG dye. In the middle phase, the hyperfluorescent lesions in the early phase became clearer and more lesions started to appear while the choroidal vessels started to fade in the HTRA1 mouse (yellow circles indicate the appearance of more lesions). In the late phase, choroidal lesions of the HTRA1 mouse became more "distinct" as all the vessels faded away into the background. The optic nerve head was dark in both WT and HTRA1 mice (green arrows). The key features of the three phases are similar to the ICGA time course in human AMD patients (early phase, 0-3 min; middle phase, 5-15 min; late phase, 18-22 min)1.
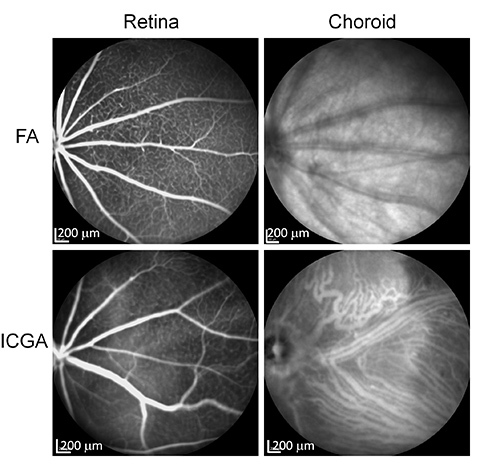 Figure 1. Comparison of FA and ICGA in imaging mouse retinal and choroidal vasculature. WT CD1 mice were imaged by IV-FA and IV-ICGA using a Multi-Modality Imaging System. Retinal vessels can be seen in both FA and ICGA. Choroidal vessels can only be seen in ICGA. Click here to view larger image.
Figure 1. Comparison of FA and ICGA in imaging mouse retinal and choroidal vasculature. WT CD1 mice were imaged by IV-FA and IV-ICGA using a Multi-Modality Imaging System. Retinal vessels can be seen in both FA and ICGA. Choroidal vessels can only be seen in ICGA. Click here to view larger image.
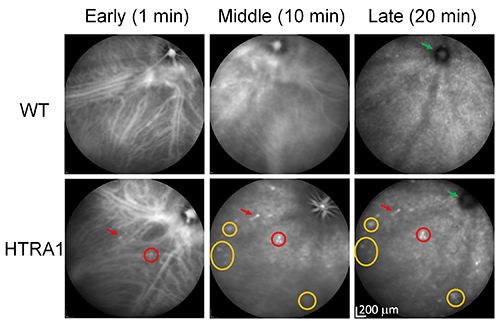 Figure 2. ICGA time course of HTRA1 transgenic mice by IV injection. A WT control and a HTRA1 transgenic mouse were imaged by ICGA (with tail vein injection). A red arrow indicates the dilation at the tip of a vessel (single polyp) and a red circle indicates a cluster type polypoidal lesion, which appeared in the early phase. Yellow circles indicate several lesions that appeared in the middle phase. Green arrows point to the dark optic nerve head in both WT and HTRA1 mice. Note that polypoidal lesions appear in the early phase of ICGA and become more distinct in the middle phase as in human studies21-24. Discrete dot lesions appear in the middle phase and become clear in the late phases (e.g. the biggest yellow circle indicating three dot lesions). Click here to view larger image.
Figure 2. ICGA time course of HTRA1 transgenic mice by IV injection. A WT control and a HTRA1 transgenic mouse were imaged by ICGA (with tail vein injection). A red arrow indicates the dilation at the tip of a vessel (single polyp) and a red circle indicates a cluster type polypoidal lesion, which appeared in the early phase. Yellow circles indicate several lesions that appeared in the middle phase. Green arrows point to the dark optic nerve head in both WT and HTRA1 mice. Note that polypoidal lesions appear in the early phase of ICGA and become more distinct in the middle phase as in human studies21-24. Discrete dot lesions appear in the middle phase and become clear in the late phases (e.g. the biggest yellow circle indicating three dot lesions). Click here to view larger image.
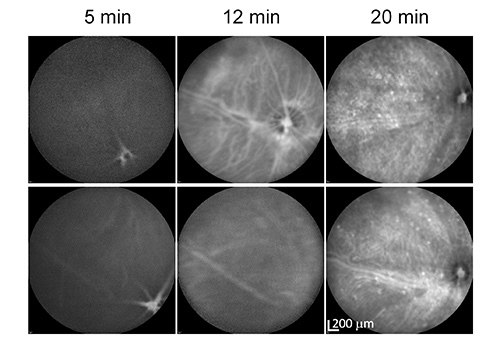 Figure 3.ICGA time course of HTRA1 transgenic mice by IP injection. HTRA1 transgenic mice were imaged 5, 12, and 20 min after IP injection of ICG. The images of the two panel rows were taken from two different HTRA1 transgenic mice. Note that the choroidal vasculature is mostly invisible even 5 min after injection (12 min for the mouse in the lower panels). Click here to view larger image.
Figure 3.ICGA time course of HTRA1 transgenic mice by IP injection. HTRA1 transgenic mice were imaged 5, 12, and 20 min after IP injection of ICG. The images of the two panel rows were taken from two different HTRA1 transgenic mice. Note that the choroidal vasculature is mostly invisible even 5 min after injection (12 min for the mouse in the lower panels). Click here to view larger image.
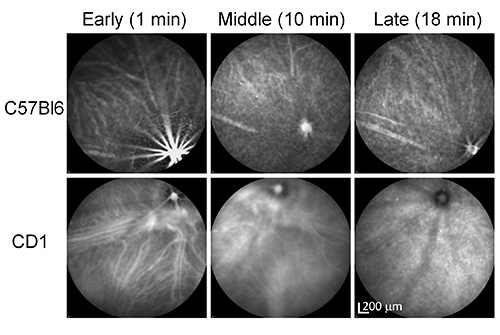 Figure 4. ICGA time course of a pigmented (C57Bl6) and an albino (CD1) mouse by IV injection. Note the difference in the clarity of choroidal vasculature between the pigmented and the albino mice. Click here to view larger image.
Figure 4. ICGA time course of a pigmented (C57Bl6) and an albino (CD1) mouse by IV injection. Note the difference in the clarity of choroidal vasculature between the pigmented and the albino mice. Click here to view larger image.
Discussion
In this study, we demonstrated the use of ICGA to image choroidal lesions in HTRA1 transgenic mice. The characteristics of the early, middle, and late phases of ICGA in our mouse model match the time course well in human studies1. This is important to make better comparisons between human pathology and animal phenotypes, which are invaluable for research on pathophysiological mechanisms and treatment strategies of conditions related to the choroid such as AMD.
We first performed ICGA in mice by IP injection and found that the time course was highly variable from mouse to mouse, probably due to the variable absorption of the ICG dye from the body cavity in the abdomen (Figure 3). This makes it difficult to compare with human studies performed by IV injection. In addition, the angiographic features of different phases of IV-ICGA are very useful for characterizing different types of choroidal lesions in animal models. Most people choose to avoid IV injection (e.g. in FA) for mice due to the technical challenge of performing tail vein injection (mouse tail veins are tiny). However, the effort is well spent considering the reproducible nature of this technique and the amount of information obtained. Once we mastered the technique of tail vein injection, the other steps are rather similar to IP-ICGA. It is worthwhile to mention that one has to get everything prepared beforehand (e.g. the Imaging System) in order to capture the very short early phase (0-4 min). We compared IP-ICGA vs. IV-ICGA for studying various HTRA1 transgenic mice. We have done ~100 mice for each method. The conclusion is that IV-ICGA is superior to IP-ICGA for characterizing lesions in the choroid. Time-course IV-ICGA has become our standard practice to examine AMD mouse models. For the same reason, we suggest that researchers should consider performing IV-FA for animal research.
Other than the injection route, we noticed that pigment color also influences the quality of ICGA. Previous studies also reported this "pigmentation effect"18,25. However, no information is available on the influence of the coat color on the different phases of ICGA. We compared ICGA between pigmented mice (C57Bl6) and albino mice (CD1) by time course IV-ICGA. Big choroidal vessels appear fuzzy and less clear while the small vessels are difficult to see in C57Bl6 mice, which is in sharp contrast to the much sharper images of both big and small choroidal vessels in CD1 mice (Figure 4). The biggest difference was observed in the early phase although the middle phase is also affected. There is no big difference in the late phase ICGA due to the fading of the ICG signal in the choroidal vessels. Apparently, ICG fluorescence can be partially blocked by the RPE and melanocytes in the choroid in pigmented mice. As a suggestion, one may want to consider breeding their AMD models into the CD1 background to obtain high resolution ICGA.
Although FA is more widely used on AMD animal models, ICGA is essential in detecting abnormalities in the choroidal vasculature. The ability to observe mouse choroidal vasculature at high resolution in real time can greatly help researchers in characterizing AMD mouse models and in correlating with histopathological data. The combination of ICGA, FA and OCT will be extremely useful in characterizing the phenotype of AMD models as in the diagnosis of AMD in human patients. Since the mouse is currently the most widely used animal model for AMD research26-29, time-course IV-ICGA can play a wider role in the research community.
Disclosures
YF is an inventor of two pending patents that are relevant to the AMD mouse model used in this work. SK, ZB, and ADJ have nothing to disclose.
Acknowledgments
This work was supported by NIH grant 1R01EY022901, the Career Development Award from Research to Prevent Blindness (RPB), C.M.Reeves & M.A. Reeves Foundation, E. Matilda Ziegler Foundation for the Blind, Knights Templar Eye Foundation, and an unrestricted grant to the Department of Ophthalmology at the University of Utah from RPB. We thank Balamurali Ambati for technical assistance on the Spectralis Multi-Modality Imaging System and Tao Zhang for discussions and comments on the manuscript.
References
- Duane TD, Tasman W, Jaeger EA. Chapter 4a, Indocyanine Green Angiography. Duane's clinical ophthalmology on CD-ROM. Philadelphia: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- Alfaro DV. Age-related macular degeneration : a comprehensive textbook. Lippincott Williams & Wilkins; 2006. [Google Scholar]
- Yannuzzi LA. Indocyanine green angiography: a perspective on use in the clinical setting. Am. J. Ophthalmol. 2011;151:745–751. doi: 10.1016/j.ajo.2011.01.043. [DOI] [PubMed] [Google Scholar]
- Destro M, Puliafito CA. Indocyanine green videoangiography of choroidal neovascularization. Ophthalmology. 1989;96:846–853. doi: 10.1016/s0161-6420(89)32826-0. [DOI] [PubMed] [Google Scholar]
- Scheider A, Schroedel C. High resolution indocyanine green angiography with a scanning laser ophthalmoscope. Am. J. Ophthalmol. 1989;108:458–459. doi: 10.1016/s0002-9394(14)73325-2. [DOI] [PubMed] [Google Scholar]
- Guyer DR, et al. Digital indocyanine-green angiography in chorioretinal disorders. Ophthalmology. 1992;99:287–291. doi: 10.1016/s0161-6420(92)31981-5. [DOI] [PubMed] [Google Scholar]
- Yannuzzi LA, Slakter JS, Sorenson JA, Guyer DR, Orlock DA. Digital indocyanine green videoangiography and choroidal neovascularization. Retina. 1992;12:191–223. [PubMed] [Google Scholar]
- Regillo CD, Benson WE, Maguire JI, Annesley WH. Indocyanine green angiography and occult choroidal neovascularization. Ophthalmology. 1994;101:280–288. doi: 10.1016/s0161-6420(94)31350-9. [DOI] [PubMed] [Google Scholar]
- Scheider A, Kaboth A, Neuhauser L. Detection of subretinal neovascular membranes with indocyanine green and an infrared scanning laser ophthalmoscope. Am. J. Ophthalmol. 1992;113:45–51. doi: 10.1016/s0002-9394(14)75752-6. [DOI] [PubMed] [Google Scholar]
- Kuck H, Inhoffen W, Schneider U, Kreissig I. Diagnosis of occult subretinal neovascularization in age-related macular degeneration by infrared scanning laser videoangiography. Retina. 1993;13:36–39. doi: 10.1097/00006982-199313010-00009. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Engelbert M, Iida T, Freund KB, Yannuzzi LA. Polypoidal choroidal vasculopathy: a review. Surv. Ophthalmol. 2010;55:501–515. doi: 10.1016/j.survophthal.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Ciardella AP, Donsoff IM, Yannuzzi LA. Polypoidal choroidal vasculopathy. Ophthalmol. Clin. N. Am. 2002;15:537–554. doi: 10.1016/s0896-1549(02)00053-6. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Yannuzzi LA, Slakter JS, Sorenson J, Orlach DA. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina. 1995;15:100–110. doi: 10.1097/00006982-199515020-00003. [DOI] [PubMed] [Google Scholar]
- Coppens G, Spielberg L, Leys A. Polypoidal choroidal vasculopathy, diagnosis and management. Bull. Soc. belge d'Ophtalmol. 2011. pp. 39–44. [PubMed]
- Tsujikawa A, et al. Pigment epithelial detachment in polypoidal choroidal vasculopathy. Am. J. Ophthalmol. 2007;143:102–111. doi: 10.1016/j.ajo.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Jones A, et al. Increased expression of multifunctional serine protease, HTRA1, in retinal pigment epithelium induces polypoidal choroidal vasculopathy in mice. Proc. Natl. Acad. Sci. U.S.A. 2011;108:14578–14583. doi: 10.1073/pnas.1102853108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alex AF, Heiduschka P, Eter N. Retinal fundus imaging in mouse models of retinal diseases. Methods Mol. Biol. 2013;935:41–67. doi: 10.1007/978-1-62703-080-9_3. [DOI] [PubMed] [Google Scholar]
- Seeliger MW, et al. In vivo confocal imaging of the retina in animal models using scanning laser ophthalmoscopy. Vision Res. 2005;45:3512–3519. doi: 10.1016/j.visres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Fischer MD, Zhour A, Kernstock CJ. Phenotyping of mouse models with OCT. Methods Mol. Biol. 2013;935:79–85. doi: 10.1007/978-1-62703-080-9_5. [DOI] [PubMed] [Google Scholar]
- Jian Y, Zawadzki RJ, Sarunic MV. Adaptive optics optical coherence tomography for in vivo mouse retinal imaging. J. Biomed. Opt. 2013;18 doi: 10.1117/1.JBO.18.5.056007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardella AP, Donsoff IM, Huang SJ, Costa DL, Yannuzzi LA. Polypoidal choroidal vasculopathy. Surv. Ophthalmol. 2004;49:25–37. doi: 10.1016/j.survophthal.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Sasahara M, et al. Polypoidal choroidal vasculopathy with choroidal vascular hyperpermeability. Am. J. Ophthalmol. 2006;142:601–607. doi: 10.1016/j.ajo.2006.05.051. [DOI] [PubMed] [Google Scholar]
- Silva RM, et al. Polypoidal choroidal vasculopathy and photodynamic therapy with verteporfin. Graefes Arch. Clin. Exp. Ophthalmol. 2005;243:973–979. doi: 10.1007/s00417-005-1139-4. [DOI] [PubMed] [Google Scholar]
- Yannuzzi LA, et al. Polypoidal choroidal vasculopathy masquerading as central serous chorioretinopathy. Ophthalmology. 2000;107:767–777. doi: 10.1016/s0161-6420(99)00173-6. [DOI] [PubMed] [Google Scholar]
- Janssen A, et al. Abnormal vessel formation in the choroid of mice lacking tissue inhibitor of metalloprotease-3. Invest. Ophthalmol. Vis. Sci. 2008;49:2812–2822. doi: 10.1167/iovs.07-1444. [DOI] [PubMed] [Google Scholar]
- Ding X, Patel M, Chan CC. Molecular pathology of age-related macular degeneration. Prog. Retin. Eye Res. 2009;28:1–18. doi: 10.1016/j.preteyeres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossniklaus HE, Kang SJ, Berglin L. Animal models of choroidal and retinal neovascularization. Prog. Retin. Eye Res. 2010;29:500–519. doi: 10.1016/j.preteyeres.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennesi ME, Neuringer M, Courtney RJ. Animal models of age related macular degeneration. Mol. Aspects Med. 2012;33:487–509. doi: 10.1016/j.mam.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizabeth Rakoczy P, Yu MJ, Nusinowitz S, Chang B, Heckenlively JR. Mouse models of age-related macular degeneration. Exp. Eye Res. 2006;82:741–752. doi: 10.1016/j.exer.2005.10.012. [DOI] [PubMed] [Google Scholar]


