Abstract
In general, presence of the metabolic syndrome is associated with significant cardiovascular mortality and represents a growing public health concern in the United States. Patients with a schizophrenia have a three times greater risk of death compared to the general population, with cardiovascular disease being the most common cause of this mortality. Use of the atypical antipsychotics (AAPs) to treat schizophrenia contributes significantly to this cardiovascular disease risk. While currently several different clinical guidelines exist to monitor for the metabolic consequences of AAP use, implementation is lacking. Due to the under monitoring of side effects and the lack of alternative treatment choices in schizophrenia, research has focused on the identification of various biomarkers and pharmacogenomic targets to focus on those at greatest risk for metabolic syndrome, thus aiming to increase the efficacy and minimize the side effects of the AAPs. This has led to several different lines of research. This manuscript focuses on summarizing the differing metabolic syndrome criteria, monitoring guidelines for AAPs and the role of folic acid as it relates to metabolic syndrome within the schizophrenia population. It will concentrate not only on the pharmacogenomics of folic acid metabolism, but also its epigenetic interaction with the environment. From this work, genetic variation within both the methylenetetrahydrofolate reductase (MTHFR) and catechol-o-methyl transferase (COMT) genes has been associated with increased metabolic syndrome risk in schizophrenia patients treated with AAPs. Furthermore, the combination of folate pharmacogenetics and epigenetics has uncovered relationships between methylation, schizophrenia disease, treatment type and metabolic syndrome. Despite the several areas of biomarker research for schizophrenia related metabolic syndrome, translation to the clinical setting is still lacking and further studies are needed to bridge this gap. Future folate supplementation research may prove to be an easy and effective clinical tool for the prevention and/or treatment of metabolic syndrome associated with AAP treatment, but clearly more work needs to be done in this area.
Keywords: Schizophrenia, Metabolic Syndrome, Folic Acid, Homocysteine, MTHFR, Epigenetics
1. Introduction
In the general population, the presence of the metabolic syndrome is associated with significant cardiovascular mortality and represents a growing public health concern in the United States (1,2). While the term, metabolic syndrome, has been coined within the past 20 years (previously called Syndrome X), the constellation of symptoms that make up metabolic syndrome (such as central adiposity and elevations in cholesterol, blood glucose, and blood pressure) have historically been recognized as risk factors for cardiovascular disease (3–5). Metabolic syndrome is seen in about 25% of men and women (6). For those meeting metabolic syndrome criteria, the population-attributable risk estimates for cardiovascular disease, coronary heart disease, and diabetes mellitus are 34%, 29%, and 62% for men and 16%, 8% and 47% for women (7). Thus, presence of the metabolic syndrome criteria is associated with increased risk for significant cardiovascular morbidity and mortality and has undoubtedly become a national health crisis as the rates of this illness continue to rise. Unfortunately the risks for metabolic syndrome in those with a serious mental illness such as schizophrenia or bipolar disorders are more than double that seen in the general population which has also resulted in a significant proportion of the morbidity and mortality seen within these populations (8–10). Although the exact cause for this increased risk for the metabolic syndrome in serious mental illness is unknown, the high prevalence of atypical antipsychotic use has been suggested as being a major contributor (8,11). Much work has been done examining the pharmacogenomics of atypical antipsychotic metabolic consequences; however consensus regarding these risks currently does not remain. One promising line of work has focused on folic acid and its pharmacogenetically regulated metabolism through the methylenetetrahydrofolate reductase (MTHFR) enzyme. Thus the purpose of this review is to focus on the increased risk for metabolic syndrome within the schizophrenia population. It will give a brief background examining the different criteria used for a diagnosis of metabolic syndrome followed by a summary regarding monitoring for metabolic syndrome within the schizophrenia population. Lastly the role of biomarkers for the detection of metabolic syndrome within schizophrenia will be touched on with a summarization of the available literature regarding folate pharmacogenomics and epigenomics in antipsychotic-associated metabolic syndrome within this population.
2. Criteria for Diagnosis of Metabolic Syndrome
Before discussing the increased incidence of the metabolic syndrome within the schizophrenia population, a thorough understanding of the different criteria currently available is necessary. Although there is significant overlap between these differing criteria, there is no one set of criteria that are consistency used by all, and as such this lack of consistency makes comparing the rates of metabolic syndrome across populations difficult. Additionally differing patient populations (i.e. treatment naïve and non-treatment naïve) are often included when examining the true incidence of metabolic syndrome due to atypical antipsychotic use. Thus, current estimates range from 13.4%, as seen within the Comparison of Atypicals for First Episode (CAFE) trial (12) which included younger drug naive subjects, to 40%–52% as reported in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) trial, as well as other recent larger database studies which did not include treatment naïve subjects (9,11,13).
In looking at the different criteria available for the diagnosis of metabolic syndrome, those defined by the National Cholesterol Education Program (NCEP) in 2001, and the International Diabetes Federation (IDF) in 2006 (14,15) appear to be the most commonly cited and referenced when examining the overall risk in schizophrenia. In examining the NCEP criteria it can be seen that a diagnosis of metabolic syndrome can be given when patients meet at least 3 of the following criteria: abdominal obesity (waist circumference ≥ 40 inches in males or 35 inches in females), elevated triglycerides (≥ 150 mg/dL), low HDL (< 40 mg/dL in men or < 50 mg/dL in women), elevated blood pressure (≥ 130 / 85 or on antihypertensive medication), or elevated fasting glucose (≥ 100 mg/dL or on medication for diabetes) (16). This popular NCEP definition, which was subsequently updated with a lower impaired fasting glucose threshold by the American Heart Association in 2005, was published in the third Adult Treatment Protocol (ATP III-A). Since 2001, various definitions have typically been updates to the original ATP III definition (17) and so these competing definitions have significant overlap with key components such as glucose dysregulation, and central adiposity. It is only the NCEP ATP III-A guideline that do not require any core elements be present for a diagnosis of metabolic syndrome. In contrast to this, both the International Diabetes Foundation and the European Group for the Study of Insulin Resistance require either a BMI >30 kg/m2 or elevated insulin levels, respectively as part of their core definition. Thus for all of these guidelines, at the heart each are the same core risk factors, it is just how they are used in defining a diagnosis of metabolic syndrome that potentially allows for some of the variation seen in the incidence of metabolic syndrome within the seriously mentally ill populations. To overcome this, some groups have worked together to create a more symbiotic approach to developing metabolic syndrome criteria (with added ethic and race specificity) such as the consensus definition suggested by Alberti and colleagues (18). Thus while it is easy to understand that presence of the metabolic syndrome confers with it an increased risk for cardiovascular disease, understanding the specific criteria that need to be met in order to obtain this diagnosis is often not so simple which may contribute to the confusion often associated with metabolic syndrome and its diagnosis (19). The anticipated release of the ATP-IV guidelines however, could result in another update to the definition and criteria of the metabolic syndrome and possibly include ethnic and race specificity which may further complicate this issue.
3. Metabolic syndrome Within Schizophrenia
Presence of a mental illness has long been associated with increased overall mortality (20–22). Cardiovascular disease undoubtedly also contributes to excess morbidity and mortality in individuals with a serious mental illness. In patients with schizophrenia, it is estimated that approximately 34% of deaths among male patients and 31% of deaths among female patients are attributed to cardiovascular disease which is only surpassed by suicide (21,23). In general, schizophrenia is an often debilitating mental illness that affects approximately 1% of the population (24) usually manifesting not only through positive and negative symptoms, but significant cognitive dysfunction as well (25). The overall goal of treatment for schizophrenia is remission of symptoms, and for most individuals with schizophrenia, antipsychotic medications play an important role in this process. While our pharmacotherapeutic treatment choices for schizophrenia have expanded over the last few decades, pharmacologically these medications traditionally achieve their effect through blockade of the dopamine 2 receptor (26). More recently, the atypical antipsychotics (AAPs) (olanzapine, clozapine, risperidone, paliperidone, iloperidone, quetiapine, asenapine, lurasidone, aripirazole, and ziprasidone) have become the first line treatment for schizophrenia due to their differing serotonin antagonism, primarily at 5HT2A and 5HT2C, their possible association with negative symptom improvement, as well as attenuation of extrapyramidal side effects (27).
Although AAPs are effective for the treatment of schizophrenia, their use has now become common place in other mental illnesses and younger age groups as well. Most AAPs carry significant risks such as diabetes, weight gain and dyslipidemia which, as previously discussed, make up the constellation of cardiovascular risk factors outlined in the metabolic syndrome criteria (28–31). Patients taking AAPs frequently manifest early symptoms of metabolic syndrome followed by the actual development of more serious cardiac complications. The end result is up to 30 years of life lost (23,32) for those with schizophrenia. Furthermore, recent studies have suggested the standardized mortality ratio (SMR) for cardiac disease may be increasing in schizophrenia patients relative to the general population following the introduction of AAPs (33,34). These findings are particularly concerning given the known association between these medications and CVD risk factors, adding biological plausibility to the epidemiological findings. A comparison of the Clinical Antipsychotic Trial of Intervention Effectiveness (CATIE) participants with schizophrenia to matched controls from the National Health and Nutrition Examination Survey (NHANES III) on ten-year risk of coronary heart disease based on the Framingham Heart Study formula demonstrated an elevation in risk for coronary heart disease of 34% in males and 50% in females with schizophrenia (35). Similar elevations in cardiac risk with schizophrenia have been demonstrated in other studies (36,37) and are much higher than that reported in the Framingham Heart Study Offspring Study.
Until recently, significant weight gain with AAP use was the primary research focus involving AAP metabolic complications, and in fact, CATIE showed that over 30% of subjects gained greater than 7% of their baseline body weight with at least 18 months of AAP treatment (38). This trial also confirmed that overall men and women with schizophrenia were 85% and 137% more likely to develop metabolic syndrome, respectfully, than NHANES matched controls (9) and that “Clinical attention must be given to monitoring for this syndrome and minimizing the risks associated with antipsychotic treatment”. This work has been replicated by other groups showing that schizophrenia patients treated with AAPs have a two to four fold greater risk for metabolic syndrome compared to the general population (39). The precise explanation for this increased risk linked to AAPs remains unknown; however the body of literature regarding the risks seen with AAP use has grown substantially throughout the last decade (40). In addition, recent guidelines based on this work have been developed in an effort to help mitigate the cardiovascular risks seen with AAP use in persons with a serious mental illness.
4. Monitoring Recommendations for AAP use in Schizophrenia
Although much of this literature contributes the risk for metabolic syndrome in the seriously mentally ill population to atypical antipsychotic use, not all clinicians agree that this is simply a medication associated side effect. Regardless of the etiology, there is consensus regarding the importance of routine monitoring for metabolic syndrome within the mental health population and several specific guidelines have been published for those receiving an atypical antipsychotic. The first of these guidelines was authored within the United States and was published on behalf of the American Diabetes Association (ADA) and the American Psychiatric Association (APA) in 2004 (41). Key to these guidelines is the routine monitoring of weight, waist circumference, blood pressure, fasting plasma glucose level, and fasting lipid profile. Table 1 is a summary of the APA/ADA monitoring guideline recommendations. In addition to these practice guidelines, several others have been published such as those supported by the United Kingdom’s National Institute for Health and Clinical Excellence (NICE) and the Quality and Outcomes Framework (QOF) (42,43) as well as publications published in the Canadian Journal of Psychiatry (44,45). Finally, much work has looked at the quality of proposed guidelines for monitoring metabolic syndrome within schizophrenia which is beyond the scope of this review however several reviews are available (46,47). While there might be slight differences in the exact monitoring these various guidelines recommend; they all highlight the importance of continued monitoring and preventative care for those with a serious mental illness especially in those populations receiving AAPs.
Table 1.
Summary of American Diabetes Association and American Psychiatric Association Monitoring Guidelines for the implementation of Atypical Antipsychotics (41)
| Monitoring Parameters | Baseline | 4 weeks | 8 weeks | 12 weeks | Annually | Every 5 years |
|---|---|---|---|---|---|---|
| Personal/Family History | X | X | ||||
| Weight (BMI) | X | X | X | X | X (Obtain Quarterly) | X |
| Waist Circumference | X | X | ||||
| Blood Pressure | X | X | X | |||
| Fasting Plasma Glucose | X | X | X | |||
| Fasting Lipid Profile | X | X | X |
Unfortunately the reality is that, despite clear recommendations, these guidelines are not being followed (48–51). Adherence to these guidelines has recently become a priority research area for many in an effort to document some of the health disparities related to those with a serious mental illness. In a recent meta-analysis on this topic, Mitchell and colleagues found 48 studies on the topic of metabolic monitoring in mental health (47). As part of their meta-analysis they found that across these studies, routine baseline monitoring was very low and that only blood pressure and triglyceride monitoring were occurring in more than half of patients receiving an atypical antipsychotic. More specifically weight was only monitored in 48% of patients, followed by glucose in 44%, cholesterol in 42% and lipids and glycosylated hemoglobin (HbA1c) in less than 25% of patients. Thus, while we have many different monitoring guidelines to choose from which can be used to guide the treatment of those with a serious mental illness, these recommendations are not being consistently followed. Additionally these authors examined the literature regarding monitoring changes after specific educational interventions were made for clinicians regarding these guidelines. They found that monitoring in areas like blood pressure, weight gain, glucose and lipids did increase, but that overall the rates of monitoring were still low related to glucose (56%) and lipids (29%)(47). The low use of these guidelines in clinical practice is of concern and indicates that this patient group does not receive adequate testing or monitoring for metabolic complications. Furthermore, clinicians must use this monitoring to guide treatment of identified metabolic abnormalities with appropriate medications in order to prevent future cardiovascular consequences from the metabolic syndrome.
While monitoring for metabolic complications of AAP use continues to be an emphasized issue, the research community has continued to work towards understanding the mechanisms behind these medication side effects resulting in the identification of different biomarkers which have been proposed for their potential use in the clinic (52–56). Potentially having a biomarker for the metabolic side effects seen with AAP use would be highly desirable, as it would allow the clinician to easily measure a patients risk for metabolic syndrome before any medication is administered. This would aid in the effort to personalize mental illness pharmacotherapy and optimize treatment. While the research on various biomarkers related to the risk for weight gain and metabolic syndrome seen with AAPs is fairly expansive (56–58), no definitive recommendations have currently been translated into clinical practice and thus work within this area must continue.
5. Folic Acid Pharmacogenetics and Epigenetics
Our continued understanding of the pharmacogenomics of antipsychotic-associated metabolic syndrome has highlighted the importance of environment and nutrition in the body’s ability to regulate the genome (59) though dietary folic acid intake and its pharmacogenetically regulated metabolism. Briefly, folate is a water soluble B-vitamin involved in the synthesis, repair, and methylation of DNA (60) whose effective utilization is dependent on adequate daily intake as well as genetically altered metabolism (60). Within the AldoMet cycle, the methylenetetrahydrofolate reductase (MTHFR) enzyme metabolizes folate to methyltetrahydrofolate (5-methyl THF) which then converts homocysteine to methionine and adenosyl methionine by methionine synthetase (MTR) (Figure 1). Reduced MTHFR activity results in hyperhomocysteinemia, which is associated with cardiovascular disease. The AldoMet cycle’s final product is the universal methyl donor for several biological methylation reactions. It is these methyl groups which form the basis for epigenetic modulation of DNA processes, which is beyond the scope of this review (61).
Figure 1.
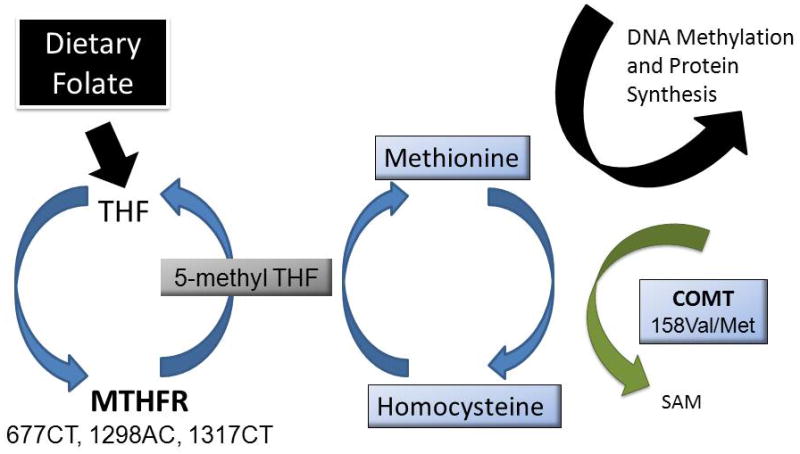
The Aldo Met cycle converts homocysteine to methionine and is facilitated by folate and methylenetetrahydrolfate reductase (MTHFR). Catechol-o-methyltransferase (COMT) converts methionine to S-adenyl methionine (SAM), also producing homocysteine. Genetic variants within these enzymes affect their efficiency within this cycle.
MTHFR relies on dietary folate as well as genetic variants in determining its efficiency (62). When inadequate amounts of 5-methyl THF are available for MTR, homocysteine increases and s-adenosyl methionine formation is reduced, resulting in DNA hypomethylation (63,64). Thus, folic acid plays an important role in maintaining genomic stability as well as homocysteine levels (65). Genetic variation within this enzyme has also been shown to affect its efficiency. For MTHFR, the 677C/T variant, resulting in an alanine to valine substitution is the most prominent and produces a thermo-labile variant with reduced activity (66). The T allele is relatively common, with homozygosity occurring in up to 20% of North American and European populations (60). Individuals with a TT genotype have a 70% reduction in MTHFR activity, compared to the CC genotype group, while heterozygotes have a 35% reduction (67). Of the AldoMet cycle enzymes, the MTHFR 677TT variant is the best characterized and is most consistently associated with hyperhomocysteinemia, cardiovascular disease, metabolic syndrome and methylation status. This relationship is exaggerated by low dietary intake and reduced total body stores of folic acid (68). Research currently points to MTHFR in the development of metabolic syndrome in mental health patients taking AAPs as summarized and discussed below (28,31).
Pertinent to the metabolism of homocysteine is the enzyme catechol-O-methyl transferase (COMT). Despite the lack of clarity concerning COMT’s role in the pathogenesis of schizophrenia, it has been shown that the 158Met variant produces a more thermolabile protein resulting in reduced activity compared to the 158Val variant. Those with the Val/Val genotype have 30–50% greater activity than those with the Met/Met genotype (69). Thus, in relation to homocysteine metabolism, individuals with the COMT 158Val allele would have higher COMT activity leading to increased homocysteine concentrations, which may be exaggerated in individuals who also have MTHFR variants associated with hyperhomocysteinemia (70). The risks seen with the AldoMet variants are often exaggerated in situations of low folate exposure (71) and therefore dietary assessments as well as genetic measurements are dually important to understanding homocysteine, cardiovascular disease and the risk of metabolic syndrome within those receiving AAPs.
6. Role of Folic Acid in Atypical Antipsychotic Metabolic Syndrome
Multiple studies have demonstrated relationships between MTHFR gene variants and schizophrenia pathogenesis, but more recently this work has begun to focus on the role of aberrant folate metabolism as it relates to metabolic syndrome risk in the schizophrenia population using AAPs. To identify available literature associated with this topic, a pubmed search was conducted using combinations of the following words: schizophrenia, folate, metabolic syndrome, antipsychotic, pharmacogenetic, epigenetic, MTHFR, COMT and MTR. A total of 22 studies were found, 15 were excluded either because they were not conducted in humans, were conducted without reference to antipsychotic use, were reviews or did not relate to metabolic syndrome. Furthermore, references of included articles were searched for further literature sources. Table 2 is a summary of those studies on this topic that are discussed below.
Table 2.
Summary of studies for folic acid pharmacogenomics and metabolic syndrome with atypical antipsychotic use in schizophrenia.
| Author | Year | Subjects | Genotype | Outcomes | Results |
|---|---|---|---|---|---|
| Ellingrod VL and colleagues | 2008 | 58 patients with schizophrenia taking an antipsychotic for at least 12 months | MTHFR 677C>T MTHFR 1298A>C | Metabolic Syndrome HOMA-IR | MTHFR T allele resulted in a 3.6 times more likelihood of developing AAP associated metabolic syndrome (p = 0.02). Also, the TT genotype patients were at greater risk for insulin resistance and increasing waist circumference (p = 0.0006). |
| Van Winkel and colleagues | 2010 | 518 patients with schizophrenia | MTHFR 677C>T MTHFR 1298A>C | Association between genotype and metabolic syndrome | MTHFR 1298 C/C genotype had a 2.4 times risk of developing metabolic syndrome (p = 0.009) |
| Van Winkel and colleagues | 2010 | 155 patients with schizophrenia or schizoaffective disorder newly started on a atypical antipsychotic. Patients with diabetes or metabolic syndrome at baseline were excluded | MTHFR 677C>T MTHFR 1298A>C | Genotype × time interactions for metabolic variables (weight, waist circumference, fasting glucose, 120 minute OGTT level and lipids | No significant effect for 677C>T variant. Significant genotype × time interaction for 1298A>C and weight (p=0.006), waist (p=0.050), fasting glucose (p=0.024) and 120 minute OGTT levels (p=0.018), with a dose-response pattern with increasing C-allele loading. |
| Ellingrod VL and colleagues | 2012 | 237 patients with schizophrenia and bipolar patients taking an antipsychotic for at least 6 months | MTHFR 677C>T MTHFR 1298A>C COMT 158Val>Met | Metabolic Syndrome | Metabolic syndrome was related to age, smoking and the MTHFR 677T and COMT 158Val alleles (χ2=34.4, p<0.0001). |
| Lott SA and colleagues | 2012 | 85 schzophrenia patients taking an atypical antipsychotic | COMT 158Val>Met and COMT-s promoter methylation | Genotype, promoter methylation and metabolic syndrome | Associations found between COMT 158MetMet genotype, COMT-S promoter methylation and metabolic syndrome |
| Burghardt KJ and colleagues | 2012 | 133 patients with schizophrenia stable on an antipsychotic | MTHFR 677C>T and LINE-1 methylation | Genotype and global methylation measure | LINE-1 methylation lower in females carrying the MTHFR 677 TT genotype when controlling for serum folate |
| Melas PA and colleagues | 2012 | 171 schizophrenia patients and 171 controls | LUMA methylation, COMT-s methylation | Disease onset, treatment type and global methylation measure | LUMA methylation related to schizophrenia onset and antipsychotic type |
Abbreviations: HOMA-IR - Homeostatic Model Assessment Insulin Resistance, MTHFR –Methylenetetrahyrofolate reductase, COMT – Catechol-o-Methyl Transferase, LINE-1 long interspersed nucleotide element-1, LUMA - Luminometric assay.
The first report of this relationship included 58 subjects with schizophrenia who were receiving AAPs. It was reported that patients with schizophrenia who carried a MTHFR 677T allele had a 3.6 times greater risk for meeting metabolic syndrome criteria while taking an AAP (p = 0.02) (28). Additionally, the data showed that after controlling for waist circumference, those with the MTHFR 677T allele were also at increased risk for developing higher levels of insulin resistance (28). At this time, this report was the first to examine the relationship between MTHFR variants and metabolic syndrome risk within this population. This study was then followed up by Van Winkel and colleagues (72). While this group also found a relationship between MTHFR and metabolic syndrome within schizophrenia, the authors reported that the MTHFR 1298A>C allele instead of the 677C>T allele was related to a significant increase in risk of metabolic syndrome (p = 0.02). Overall these authors found that patients with the 1298C/C genotype had a 2.4 times increase risk of metabolic syndrome (p = 0.009) which was similar to our previously reported odds ratio of 2.54 for the 677 T variant (28,72). Van Winkel and colleagues also conducted a prospective, naturalistic 3 month follow-up study to evaluate the association between MTHFR 677C>T and 1298A>C variants and metabolic parameters after initiation of an AAP. In this study they found genotype × time associations between the 1298A>C variant and measures of glucose, weight and waist circumference. Although this study did not measure the occurrence of metabolic syndrome over time, it supports their earlier results where schizophrenia patients with the 1298 C allele have genetic loading for metabolic side effects from AAPs (73).
More recently, our group has gone on to confirm our initial findings in a separate group of 237 subjects with bipolar disorder or schizophrenia who were screened for metabolic syndrome and genotyped for both MTHFR and COMT variants. In addition, subjects underwent a fairly comprehensive assessment for dietary and lifestyle factors (i.e. physical exercise, medication use, 24 hour food recount, and smoking assessment) as well as folate exposure. Overall, 41% of our subjects met metabolic syndrome criteria (n=98). There were no significant differences in age, gender, AAP exposure, or BMI between genotype groups. We found that occurrence of the metabolic syndrome was related to age, smoking and MTHFR 677T and COMT 158Val alleles (p<0.0001). Those with these two risk alleles (MTHFR 677T and COMT 158 Val alleles) met metabolic syndrome criteria at a much earlier age than those without these alleles (46 vs. 52 years) (31).
Additionally, studies have looked at the epigenetics of AAP-associated metabolic syndrome due to the link between folate pharmacogenetics and methylation as described above. Epigenetics is a rapidly growing field in psychiatry due to the known influence of environment on mental illness and yet, it requires cautious interpretation due to the complexities of epigenetic mechanisms and as study designs within schizophrenia continue to be defined (74). One such study investigated the role of the soluble COMT (COMT-s) methylation promoter status and metabolic syndrome in the peripheral blood samples from schizophrenia patients largely on atypical antipsychotics (75). This study found that COMT genotype was an indicator of COMT methylation status of the two CpG sites investigated (p=0.0044 for site 1 and p=0.027 for site 2). Furthermore, those homozygous for the met/met COMT genotype showed a positive correlation between CpG site methylation and metabolic syndrome (site 1: p=0.001 and site 2: p=0.001). In addition to this investigation, a different study using peripheral blood samples found that females carrying the MTHFR 677TT genotype had the lowest measure of global methylation, measured using the long interspersed nucleotide element-1 (LINE-1), which may help to explain the gender metabolic syndrome differences seen in schizophrenia (76). Finally, investigators have reported that global DNA methylation (using the Luminometric assay) differed based on schizophrenia onset status as well as treatment type (with AAPs users having lower levels of global of methylation) (77). Although this study did not look at metabolic indices it does begin to show that methylation status can be affected not only by antipsychotic treatment but by antipsychotic class.
Therefore, these studies provide evidence of a link between different enzymes related to folic acid metabolism and an increased risk of metabolic syndrome for patients with schizophrenia when taking an AAP. The results could possibly provide the evidence for pharmacogenetic testing of patients before starting an antipsychotic medication in an effort to reduce this risk or in addition to direct dietary and lifestyle interventions for those at greatest risk. Given that pharmacogenetic assays for MTHFR and its variants are commercially available and often done within other medical specialties, the era of personalized medicine for schizophrenia may not be so far off.
7. Summary and Conclusions
It is now well known that use of the atypical antipsychotics is perhaps the most effective treatment we currently have for schizophrenia and other serious mental illnesses. Unfortunately due to their associated risk for metabolic syndrome, use of these medications may be placing these individuals at greater risk for several comorbidities, resulting in an accumulation of life years lost due to cardiovascular disease. There currently is a lack of consensus regarding the specific criteria which should be used when diagnosing metabolic syndrome within the serious mentally ill population, although some agreement does exist regarding which criteria are important and potentially place individuals at greater risk for the development of cardiovascular disease. While monitoring guidelines for the use of atypical antipsychotics and metabolic syndrome risk have been developed and widely circulated, the reality is that they are often not followed for many reasons. Although many biomarkers have been proposed for the possible prevention of metabolic syndrome seen with atypical antipsychotic use, this research has not been successfully translated into the clinic. The role of folic acid in the development of these metabolic complications is currently being investigated and current data suggests that for those with the MTHFR 677T and COMT 158Val allele, metabolic syndrome risk may be elevated or occurring at a younger age. Furthermore, evidence is beginning to show schizophrenia subjects reside in a global hypomethylation and possibly less stable genetic state. While the natural next step in this currently research is folate supplementation, ongoing work in this area is not yet available. Studies using folate supplementation in schizophrenia patients using AAPs and carrying these increased risk pharmacogenomics and epigenomic targets are needed in order to begin to translate this research into practice. Thus, at this time, educating patients and their caregivers about the importance of a balanced healthy diet with exercise as well as proper pharmacotherapeutic management of metabolic abnormalities is crucial to combating the staggering cardiovascular mortality seen within this group. For those whose diets do not include the minimum recommended daily allowance of folate, a supplement may be appropriate until such a time when conclusive data can be presented regarding the role of folic acid in the diagnosis and detection of metabolic syndrome within schizophrenia.
Acknowledgments
The following sources were utilized for this publication: The following funding sources were utilized for this publication: NIMH (R01 MH082784) and NIMH (K08 MH64158).
References
- 1.Maggi S, Noale M, Gallina P, Bianchi D, Marzari C, Limongi F, et al. Metabolic syndrome, diabetes, and cardiovascular disease in an elderly Caucasian cohort: the Italian Longitudinal Study on Aging. J Gerontol A Biol Sci Med Sci. 2006 May;61(5):505–510. doi: 10.1093/gerona/61.5.505. [DOI] [PubMed] [Google Scholar]
- 2.Onat A, Hergenc G, Turkmen S, Yazici M, Sari I, Can G. Discordance between insulin resistance and metabolic syndrome: features and associated cardiovascular risk in adults with normal glucose regulation. Metabolism. 2006 Apr;55(4):445–452. doi: 10.1016/j.metabol.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Levitan EB, Song Y, Ford ES, Liu S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med. 2004 Oct 25;164(19):2147–2155. doi: 10.1001/archinte.164.19.2147. [DOI] [PubMed] [Google Scholar]
- 4.Eckel RH. Obesity and heart disease: a statement for healthcare professionals from the Nutrition Committee, American Heart Association. Circulation. 1997 Nov 4;96(9):3248–3250. doi: 10.1161/01.cir.96.9.3248. [DOI] [PubMed] [Google Scholar]
- 5.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002 Dec 14;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 6.Wilson PW, Nam BH, Pencina M, D’Agostino RBS, Benjamin EJ, O’Donnell CJ. C-reactive protein and risk of cardiovascular disease in men and women from the Framingham Heart Study. Arch Intern Med. 2005 Nov 28;165(21):2473–2478. doi: 10.1001/archinte.165.21.2473. [DOI] [PubMed] [Google Scholar]
- 7.Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005 Nov 15;112(20):3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 8.Patel JK, Buckley PF, Woolson S, Hamer RM, McEvoy JP, Perkins DO, et al. Metabolic profiles of second-generation antipsychotics in early psychosis: findings from the CAFE study. Schizophr Res. 2009 Jun;111(1–3):9–16. doi: 10.1016/j.schres.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 9.McEvoy JP, Meyer JM, Goff DC, Nasrallah HA, Davis SM, Sullivan L, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005 Dec 1;80(1):19–32. doi: 10.1016/j.schres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Meyer JM, Nasrallah HA, McEvoy JP, Goff DC, Davis SM, Chakos M, et al. The Clinical Antipsychotic Trials Of Intervention Effectiveness (CATIE) Schizophrenia Trial: clinical comparison of subgroups with and without the metabolic syndrome. Schizophr Res. 2005 Dec 1;80(1):9–18. doi: 10.1016/j.schres.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Correll C, Frederickson A, Kane J, Manu P. Equally increased risk for metabolic syndrome in patients with bipolar disorder and schizophrenia treated with second-generation antipsychotics. Bipolar Disord. 2008;10(7):788–797. doi: 10.1111/j.1399-5618.2008.00625.x. [DOI] [PubMed] [Google Scholar]
- 12.Patel JK, Buckley PF, Woolson S, Hamer RM, McEvoy JP, Perkins DO, et al. Metabolic profiles of second-generation antipsychotics in early psychosis: Findings from the CAFE study. Schizophr Res. 2009;111(1–3):9–16. doi: 10.1016/j.schres.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 13.Correll C, Druss B, Lombardo I, O’Gorman C, Harnett J, Sanders K, et al. Findings of a U.S. national cardiometabolic screening program among 10,084 psychiatric outpatients. Psychiatric Services. 2010;61(9):892–898. doi: 10.1176/ps.2010.61.9.892. [DOI] [PubMed] [Google Scholar]
- 14.Choi SH, Ahn CW, Cha BS, Chung YS, Lee KW, Lee HC, et al. The prevalence of the metabolic syndrome in Korean adults: comparison of WHO and NCEP criteria. Yonsei Med J. 2005 Apr 30;46(2):198–205. doi: 10.3349/ymj.2005.46.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998 Jul;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 16.Davidson MH. A symposium: National Cholesterol Education Program Adult Treatment Panel III: Impact and implementation of the new guidelines. Introduction. Am J Cardiol. 2002 Mar 7;89(5A):1C–2C. [PubMed] [Google Scholar]
- 17.Grundy S, Cleeman J, Daniels S, Donato K, Eckel R, Franklin B, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: Executive Summary. Critical pathways in cardiology. 2005;4(4):198–203. doi: 10.1097/00132577-200512000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the Metabolic Syndrome. Circulation. 2009 Oct 20;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 19.Prasad H, Ryan DA, Celzo MF, Stapleton D. Metabolic syndrome: definition and therapeutic implications. Postgrad Med. 2012 Jan;124(1):21–30. doi: 10.3810/pgm.2012.01.2514. [DOI] [PubMed] [Google Scholar]
- 20.Harris EC, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry. 1998 Jul;173:11–53. doi: 10.1192/bjp.173.1.11. [DOI] [PubMed] [Google Scholar]
- 21.Brown S. Excess mortality of schizophrenia. A meta-analysis. The British Journal of Psychiatry. 1997 Dec 01;171(6):502–508. doi: 10.1192/bjp.171.6.502. [DOI] [PubMed] [Google Scholar]
- 22.Joukamaa M, Heliovaara M, Knekt P, Aromaa A, Raitasalo R, Lehtinen V. Mental disorders and cause-specific mortality. Br J Psychiatry. 2001 Dec;179:498–502. doi: 10.1192/bjp.179.6.498. [DOI] [PubMed] [Google Scholar]
- 23.Colton C, Manderscheid R. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Preventing chronic disease. 2006;3(2):A42–A42. [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss A, Movahed R, Dym H. Schizophrenia: current therapy and review. J Oral Maxillofac Surg. 2011 Jan;69(1):192–198. doi: 10.1016/j.joms.2010.06.178. [DOI] [PubMed] [Google Scholar]
- 25.Chan KK, Xu JQ, Liu KC, Hui CL, Wong GH, Chen EY. Executive function in first-episode schizophrenia: A three-year prospective study of the Hayling Sentence Completion Test. Schizophr Res. 2012 Jan 18; doi: 10.1016/j.schres.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Roerig JL, Steffen KJ, Mitchell JE. Atypical antipsychotic-induced weight gain: insights into mechanisms of action. CNS Drugs. 2011 Dec 1;25(12):1035–1059. doi: 10.2165/11596300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005 Sep 22;353(12):1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 28.Ellingrod VL, Miller DD, Taylor SF, Moline J, Holman T, Kerr J. Metabolic syndrome and insulin resistance in schizophrenia patients receiving antipsychotics genotyped for the methylenetetrahydrofolate reductase (MTHFR) 677C/T and 1298A/C variants. Schizophr Res. 2008 Jan;98(1–3):47–54. doi: 10.1016/j.schres.2007.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gautam S, Meena PS. Drug-emergent metabolic syndrome in patients with schizophrenia receiving atypical (second-generation) antipsychotics. Indian J Psychiatry. 2011 Apr;53(2):128–133. doi: 10.4103/0019-5545.82537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009 Jan 15;360(3):225–235. doi: 10.1056/NEJMoa0806994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellingrod VL, Taylor SF, Dalack G, Grove TB, Bly MJ, Brook RD, et al. Risk factors associated with metabolic syndrome in bipolar and schizophrenia subjects treated with antipsychotics: the role of folate pharmacogenetics. J Clin Psychopharmacol. 2012 Apr;32(2):261–265. doi: 10.1097/JCP.0b013e3182485888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laursen TM, Munk-Olsen T, Vestergaard M. Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr Opin Psychiatry. 2012 Mar;25(2):83–88. doi: 10.1097/YCO.0b013e32835035ca. [DOI] [PubMed] [Google Scholar]
- 33.Hansen V, Jacobsen BK, Arnesen E. Cause-specific mortality in psychiatric patients after deinstitutionalisation. Br J Psychiatry. 2001 Nov;179:438–443. doi: 10.1192/bjp.179.5.438. [DOI] [PubMed] [Google Scholar]
- 34.Osby U, Correia N, Brandt L, Ekbom A, Sparen P. Time trends in schizophrenia mortality in Stockholm county, Sweden: cohort study. BMJ. 2000 Aug 19–26;321(7259):483–484. doi: 10.1136/bmj.321.7259.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goff DC, Sullivan LM, McEvoy JP, Meyer JM, Nasrallah HA, Daumit GL, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res. 2005 Dec 1;80(1):45–53. doi: 10.1016/j.schres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Cohn T, Prud’homme D, Streiner D, Kameh H, Remington G. Characterizing coronary heart disease risk in chronic schizophrenia: high prevalence of the metabolic syndrome. Can J Psychiatry. 2004 Nov;49(11):753–760. doi: 10.1177/070674370404901106. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M. Prevalence of Metabolic Syndrome and Metabolic Abnormalities in Schizophrenia and Related Disorders--A Systematic Review and Meta-Analysis. Schizophr Bull. 2011 Dec 29; doi: 10.1093/schbul/sbr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nasrallah HA. Metabolic findings from the CATIE trial and their relation to tolerability. CNS Spectr. 2006 Jul;11(7 Suppl 7):32–39. doi: 10.1017/s1092852900026663. [DOI] [PubMed] [Google Scholar]
- 39.Saari KM, Lindeman SM, Viilo KM, Isohanni MK, Jarvelin MR, Lauren LH, et al. A 4-fold risk of metabolic syndrome in patients with schizophrenia: the Northern Finland 1966 Birth Cohort study. J Clin Psychiatry. 2005 May;66(5):559–563. doi: 10.4088/jcp.v66n0503. [DOI] [PubMed] [Google Scholar]
- 40.De Hert M, Detraux J, van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011 Oct 18;8(2):114–126. doi: 10.1038/nrendo.2011.156. [DOI] [PubMed] [Google Scholar]
- 41.American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004 Feb;27(2):596–601. doi: 10.2337/diacare.27.2.596. [DOI] [PubMed] [Google Scholar]
- 42.Kozumplik O, Uzun S. Recommendations from treatment guidelines for schizophrenia regarding monitoring of side effects of antipsychotics: brief review. Psychiatr Danub. 2009 Mar;21(1):95–98. [PubMed] [Google Scholar]
- 43.Dixon A, Khachatryan A. A review of the public health impact of the Quality and Outcomes Framework. Qual Prim Care. 2010;18(2):133–138. [PubMed] [Google Scholar]
- 44.Poulin MJ, Cortese L, Williams R, Wine N, McIntyre RS. Atypical antipsychotics in psychiatric practice: practical implications for clinical monitoring. Can J Psychiatry. 2005 Aug;50(9):555–562. doi: 10.1177/070674370505000909. [DOI] [PubMed] [Google Scholar]
- 45.Cohn TA, Sernyak MJ. Metabolic monitoring for patients treated with antipsychotic medications. Can J Psychiatry. 2006 Jul;51(8):492–501. doi: 10.1177/070674370605100804. [DOI] [PubMed] [Google Scholar]
- 46.De Hert M, Vancampfort D, Correll CU, Mercken V, Peuskens J, Sweers K, et al. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. Br J Psychiatry. 2011 Aug;199(2):99–105. doi: 10.1192/bjp.bp.110.084665. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell AJ, Delaffon V, Vancampfort D, Correll CU, De Hert M. Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychol Med. 2012 Jan;42(1):125–147. doi: 10.1017/S003329171100105X. [DOI] [PubMed] [Google Scholar]
- 48.Marder S, Essock S, Miller A, Buchanan R, Casey D, Davis J, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. 2004;161(8):1334–1349. doi: 10.1176/appi.ajp.161.8.1334. [DOI] [PubMed] [Google Scholar]
- 49.Morrato E, Druss B, Hartung D, Valuck R, Allen R, Campagna E, et al. Metabolic testing rates in 3 state Medicaid programs after FDA warnings and ADA/APA recommendations for second-generation antipsychotic drugs. Arch Gen Psychiatry. 2010;67(1):17–24. doi: 10.1001/archgenpsychiatry.2009.179. [DOI] [PubMed] [Google Scholar]
- 50.Morrato E, Newcomer J, Kamat S, Baser O, Harnett J, Cuffel B. Metabolic screening after the American Diabetes Association’s consensus statement on antipsychotic drugs and diabetes. Diabetes Care. 2009;32(6):1037–1042. doi: 10.2337/dc08-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weissman E, Jackson C, Schooler N, Goetz R, Essock S. Monitoring metabolic side effects when initiating treatment with second-generation antipsychotic medication. Clin Schizophr Relat Psychoses. 2012;5(4):201–207. doi: 10.3371/CSRP.5.4.4. [DOI] [PubMed] [Google Scholar]
- 52.Lett TA, Wallace TJ, Chowdhury NI, Tiwari AK, Kennedy JL, Muller DJ. Pharmacogenetics of antipsychotic-induced weight gain: review and clinical implications. Mol Psychiatry. 2012 Mar;17(3):242–266. doi: 10.1038/mp.2011.109. [DOI] [PubMed] [Google Scholar]
- 53.Jin H, Meyer JM, Mudaliar S, Jeste DV. Impact of atypical antipsychotic therapy on leptin, ghrelin, and adiponectin. Schizophr Res. 2008 Mar;100(1–3):70–85. doi: 10.1016/j.schres.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin H, Meyer J, Mudaliar S, Henry R, Khandrika S, Glorioso DK, et al. Use of clinical markers to identify metabolic syndrome in antipsychotic-treated patients. J Clin Psychiatry. 2010 Oct;71(10):1273–1278. doi: 10.4088/JCP.09m05414yel. [DOI] [PubMed] [Google Scholar]
- 55.Roerig J, Steffen K, Mitchell J. Atypical antipsychotic-induced weight gain: insights into mechanisms of action. 2011 Dec 1;25(12):1035–1059. doi: 10.2165/11596300-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 56.Zhang JP, Malhotra AK. Pharmacogenetics and antipsychotics: therapeutic efficacy and side effects prediction. Expert Opin Drug Metab Toxicol. 2011 Jan;7(1):9–37. doi: 10.1517/17425255.2011.532787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perlis RH. Translating biomarkers to clinical practice. Mol Psychiatry. 2011 Nov;16(11):1076–1087. doi: 10.1038/mp.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mrazek DA, Lerman C. Facilitating clinical implementation of pharmacogenomics. JAMA. 2011 Jul 20;306(3):304–305. doi: 10.1001/jama.2011.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Friso S, Choi SW. Gene-nutrient interactions in one-carbon metabolism. Curr Drug. doi: 10.2174/1389200052997339. [DOI] [PubMed] [Google Scholar]
- 60.Friso S, Choi SW, Girelli D, Mason JB, Dolnikowski GG, Bagley PJ, et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acad Sci U S A. 2002 Apr 16;99(8):5606–5611. doi: 10.1073/pnas.062066299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zeisel S. Genetic polymorphisms in methyl-group metabolism and epigenetics: lessons from humans and mouse models. Brain Res. 2008;1237:5–11. doi: 10.1016/j.brainres.2008.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gueant JL, Gueant-Rodriguez RM, Anello G, Bosco P, Brunaud L. Genetic determinants of folate and vitamin B12 metabolism: a common pathway in neural tube defect and Down syndrome? 2003 Nov;41(11):1473–1477. doi: 10.1515/CCLM.2003.226. [DOI] [PubMed] [Google Scholar]
- 63.Choi SW, Mason JB. Folate status: effects on pathways of colorectal carcinogenesis. J Nutr. 2002 Aug;132(8 Suppl):2413S–2418S. doi: 10.1093/jn/132.8.2413S. [DOI] [PubMed] [Google Scholar]
- 64.Choi SW, Kim YI, Weitzel JN, Mason JB. Folate depletion impairs DNA excision repair in the colon of the rat. Gut. 1998 Jul;43(1):93–99. doi: 10.1136/gut.43.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scott JM. Folate and vitamin B12. Proc Nutr Soc. 1999;58(02):441. doi: 10.1017/s0029665199000580. [DOI] [PubMed] [Google Scholar]
- 66.Goyette P, Christensen B, Rosenblatt DS, Rozen R. Severe and mild mutations in cis for the methylenetetrahydrofolate reductase (MTHFR) gene, and description of five novel mutations in MTHFR. Am J Hum Genet. 1996 Dec;59(6):1268–1275. [PMC free article] [PubMed] [Google Scholar]
- 67.Sharp L, Little J. Polymorphisms in Genes Involved in Folate Metabolism and Colorectal Neoplasia: A HuGE Review. American Journal of Epidemiology. 2004 Mar 01;159(5):423–443. doi: 10.1093/aje/kwh066. [DOI] [PubMed] [Google Scholar]
- 68.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995 May;10(1):111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 69.Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004 Nov;75(5):807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tunbridge EM, Harrison PJ, Warden DR, Johnston C, Refsum H, Smith AD. Polymorphisms in the catechol-O-methyltransferase (COMT) gene influence plasma total homocysteine levels. Am J Med Genet B Neuropsychiatr Genet. 2008 Sep 5;147B(6):996–999. doi: 10.1002/ajmg.b.30700. [DOI] [PubMed] [Google Scholar]
- 71.Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG, et al. MTHFR 677C-->T polymorphism and risk of coronary heart disease: a meta-analysis. JAMA. 2002 Oct 23–30;288(16):2023–2031. doi: 10.1001/jama.288.16.2023. [DOI] [PubMed] [Google Scholar]
- 72.van Winkel R, Rutten BP, Peerbooms O, Peuskens J, van Os J, De Hert M. MTHFR and risk of metabolic syndrome in patients with schizophrenia. Schizophr Res. 2010 Jun 12;121(1–3):193–198. doi: 10.1016/j.schres.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 73.van Winkel R, Moons T, Peerbooms O, Rutten B, Peuskens J, Claes S, et al. MTHFR genotype and differential evolution of metabolic parameters after initiation of a second generation antipsychotic: an observational study. Int Clin Psychopharmacol. 2010 Jun 2;25(5):270–276. doi: 10.1097/YIC.0b013e32833bc60d. [DOI] [PubMed] [Google Scholar]
- 74.Ekstrom TJ, Lavebratt C, Schalling M. The importance of epigenomic studies in schizophrenia. Epigenomics. 2012 Aug;4(4):359–362. doi: 10.2217/epi.12.33. [DOI] [PubMed] [Google Scholar]
- 75.Lott SA, Burghardt PR, Burghardt KJ, Bly MJ, Grove TB, Ellingrod VL. The Influence of Metabolic Syndrome, Physical Activity, and Genotype on Catechol-O-Methyl Transferase Promoter-Region Methylation in Schizophrenia. The Pharmacogenomics Journal. 2012 Mar 6; doi: 10.1038/tpj.2012.6. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burghardt KJ, Pilsner JR, Bly MJ, Ellingrod VL. DNA methylation in schizophrenia subjects: gender and MTHFR 677C/T genotype differences. Epigenomics. 2012 Jun;4(3):261–268. doi: 10.2217/epi.12.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Melas PA, Rogdaki M, Osby U, Schalling M, Lavebratt C, Ekstrom TJ. Epigenetic aberrations in leukocytes of patients with schizophrenia: association of global DNA methylation with antipsychotic drug treatment and disease onset. FASEB J. 2012 Jun;26(6):2712–2718. doi: 10.1096/fj.11-202069. [DOI] [PubMed] [Google Scholar]


