Abstract
Human Immunodeficiency Virus (HIV) initiates infection by fusing its envelope membrane with the cell membrane through a process which is triggered through interactions with the cellular receptor and coreceptor. While the mechanism of HIV fusion has been extensively studied, the point of its entry into cells remains controversial. HIV has long been thought to fuse directly with the cell plasma membrane. However, several lines of evidence suggest that endocytic entry of HIV can lead to infection and, moreover, that endocytosis could be the predominant HIV entry pathway into different cell types. This review discusses recent findings pertinent to HIV entry routes and novel approaches to pinpoint the sites of virus entry.
Introduction
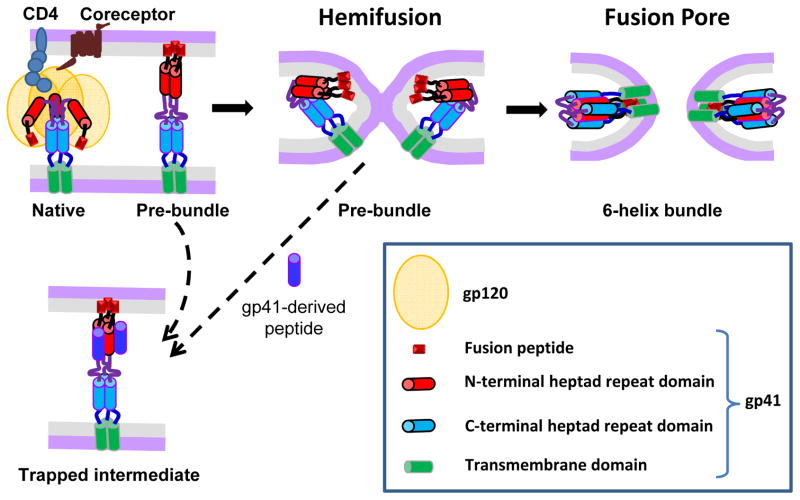
Fusion between the HIV envelope membrane and the host cell membrane is a key step in viral entry that leads to the nucleocapsid release into the cytoplasm. The fusion process is triggered through sequential interactions between the HIV envelope glycoprotein (Env) and cellular receptor (CD4) and coreceptors, CCR5 or CXCR4 (Fig. 1). Following the formation of ternary Env-receptor-coreceptor complexes, the transmembrane gp41 subunit of Env refolds into the thermodynamically stable 6-helix bundle (6HB) structure (reviewed in [1]). Intermediate conformations of gp41 formed en route to 6HB (referred to as pre-bundles or pre-hairpins, Fig. 1) transiently expose conserved functionally important gp41 epitopes. These epitopes are targeted by inhibitory peptides and neutralizing antibodies which block fusion by inhibiting the 6HB formation [1].
Figure 1.
HIV Env-mediated membrane fusion and its inhibition. Key steps of HIV fusion following the engagement of CD4 and coreceptor by the gp120 subunit of Env glycoprotein are illustrated. The gp41 subunit refolds from its native conformation through a series of pre-bundle structures that expose conserved heptad repeat domains (shown as red and blue cylinders) into the post-fusion 6-helix bundle structure. Peptides derived from the C-terminal heptad repeat domain of gp41 (dark blue) bind to the complementary N-terminal domains (red) and prevent the formation of helical bundles. The dashed arrow between a hemifused state and the peptide-trapped intermediate is intended to show that this intermediate, and even nascent fusion pores, can be reversed in the presence of the peptides.
While the general principles of HIV Env-mediated fusion are reasonably well understood, the virus entry pathway(s) resulting in productive infection remain controversial [1–4]. The identification of productive entry pathways is confounded by the fact that most HIV particles appear to be degraded by a cell while only a minor fraction establishes infection. Another issue is that, unlike many other enveloped viruses, HIV Env-mediated membrane fusion does not usually require low pH (e.g., [5,6]). This shows that HIV fusion is not restricted to acidic intracellular compartments and can, therefore, occur at the cell surface or in endosomes. This review attempts to reconcile discrepant reports regarding the HIV entry route, focusing primarily on cell-free virus, which is somewhat more amenable to studies of the entry point than a cell-to-cell transmission route. Here, the term HIV entry will be used to refer to a sequence of events leading to viral fusion (and, potentially, to infection). This is in contrast to bulk virus endocytosis, which includes non-productive and productive pathways and is therefore referred to as internalization or uptake.
Traditional approaches to elucidating the point of HIV entry
Experimental approaches aimed at defining the site of HIV entry often lacked the ability to directly relate the readout (e.g., fusion or infection) to the virus entry pathway. For example, biochemical techniques, such as cellular fractionation, detect the cytosolic delivery of the viral nucleoproteins [7,8], but do not reveal the preceding pathways. Functional approaches to delineate the virus entry pathway(s) usually rely on disruption of endocytosis or vesicular trafficking and measuring its effect on viral fusion/infection. These interventions include: (i) raising endosomal pH [8–10] and (ii) blocking distinct endocytic pathways with specific inhibitors (when available) or by knocking down the expression or function of key proteins involved in virus uptake [11–17]. Several electron microscopy studies have captured HIV fusion events both at the plasma membrane (PM) and in endosomes of macrophages, CD4+ T cells and trophoblasts [6,15,18–20]. The experimental approaches outlined above also produced evidence for the existence of both entry routes, but, for the reasons discussed in the next section, endocytic entry has been viewed as a non-productive pathway.
Fusion with the plasma membrane
Direct HIV fusion with the PM has long been regarded as the only productive pathway. The following observations support this notion: (1) HIV Env is capable of mediating cell-cell fusion at neutral pH (e.g., [5]), and HIV particles can fuse two adjacent cells, a phenomenon referred to as “fusion from without” [21]; (2) mutations in the cytoplasmic domains of CD4 or coreceptors that impair constitutive and ligand-mediated endocytosis of these proteins do not inhibit HIV infection [22–24]; (3) although HIV particles pseudotyped with the Vesicular Stomatitis Virus (VSV) G protein enter via endocytosis and are highly infectious, the fact that infection by these pseudoviruses no longer requires Nef and is less sensitive to mutations that alter the capsid stability [25–27] suggests distinct entry routes for HIV and VSV G pseudotypes; and (4) HIV, but not VSV G pseudoviruses, can infect resting CD4+ T cells [28,29] (but see the next section for further discussion).
HIV Env-mediated cell signaling and actin remodeling have been implicated in multiple steps of entry, from CD4/coreceptor clustering [30–34] to reverse transcription [35,36]. Since actin remodeling should help the released HIV core to penetrate the cortical actin layer [26,36] (Fig. 3), the requirement for signaling and actin dynamics has been interpreted as evidence for HIV fusion with the PM [13,34,37]. The regulation of actin dynamics through CXCR4 signaling is particularly important for HIV infection of resting CD4+ T cells [37,38]. In this context, the inability of VSV G pseudotypes to infect resting T cells [28,29] has been taken as evidence against endocytic entry leading to productive infection [29]. However, signaling and actin dynamics could also be required for receptor-mediated virus endocytosis. In fact, the inability of VSV G pseudoviruses to infect resting T cells appears to stem from the lack of pseudovirus internalization in the absence of CD4 and/or coreceptor signaling [28,39].
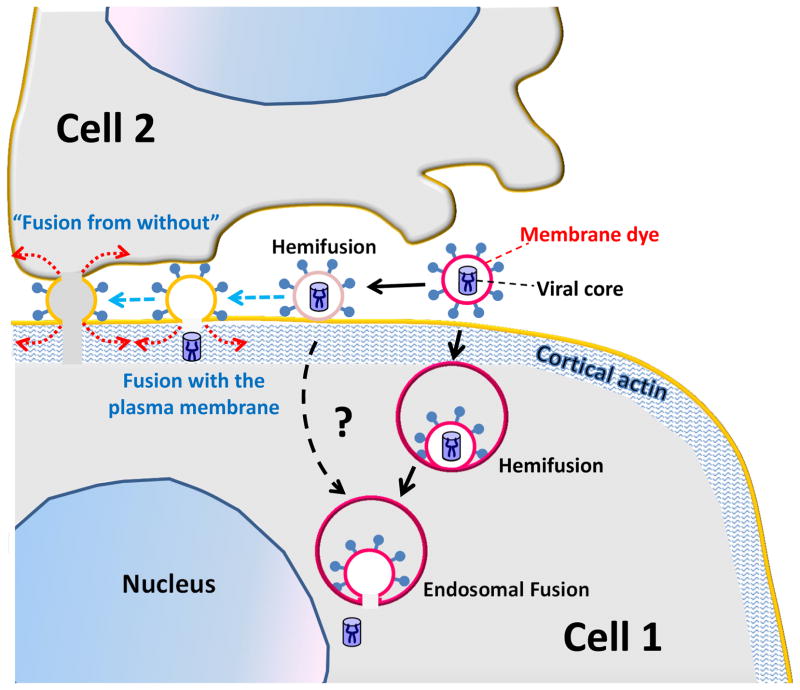
Figure 3.
A model for regulation of the HIV entry sites. HIV fusion with endosomes may be augmented by cellular factors (not shown) that help enlarge the pore. Whereas fusion with the plasma membrane does not normally progress beyond hemifusion, conditions that allow for lateral force generation (red dotted arrows) by cells, in this case, virus being bound to two adjacent cells, are conducive to force generation and can thus allow virus-cell or cell-cell fusion (“fusion from without”). The events that were experimentally observed by single virus imaging [46] are shown by solid black arrows and those hypothesized to occur between two cells are shown by dashed blue arrows.
Similar to cell-free viruses, the point of HIV entry upon cell-to-cell transmission is controversial. While several studies concluded that, following the formation of a virological synapse with an antigen presenting cell, HIV fuses with the PM of the uninfected cell [32,40,41], others found that this transmission mode relies on virus endocytosis [42–45].
Evidence for HIV entry through endocytosis
Accumulating evidence supports productive HIV entry through endocytosis in different cell types. First, raising endosomal pH enhances HIV fusion and infection, presumably due to the reduced virus degradation in intracellular compartments [8–10]. Second, inhibition of clathrin- and dynamin-dependent endocytosis or macropinocytosis (depending on the cell type) suppresses HIV uptake, fusion and infection [11,16,46,47].
Two complementary non-invasive strategies have been recently developed to define the point of HIV entry. The first approach compares the kinetics of HIV escape from a membrane-impermeant inhibitory peptide that blocks fusion of surface-accessible virions to the kinetic of escape from low temperature that halts all fusion events (Fig. 2). The gp41-derived peptide used in these experiments inhibits HIV fusion by binding to the complementary N-terminal heptad repeat domains on gp41 pre-hairpins and interfering with the formation of 6HBs (Fig. 1). The two possible escape routes from the inhibitory peptide are: (i) fusion with the PM and formation of peptide-resistant 6HBs [48] and (ii) endocytosis of HIV-CD4-coreceptor complexes prior to addition of the 6HB inhibitor followed by fusion with endosomes (Fig. 2A). It is unlikely that HIV acquires resistance to the inhibitory peptide prior to fusing with the PM, since the requisite number of 6HBs is formed after the pore opening [48].
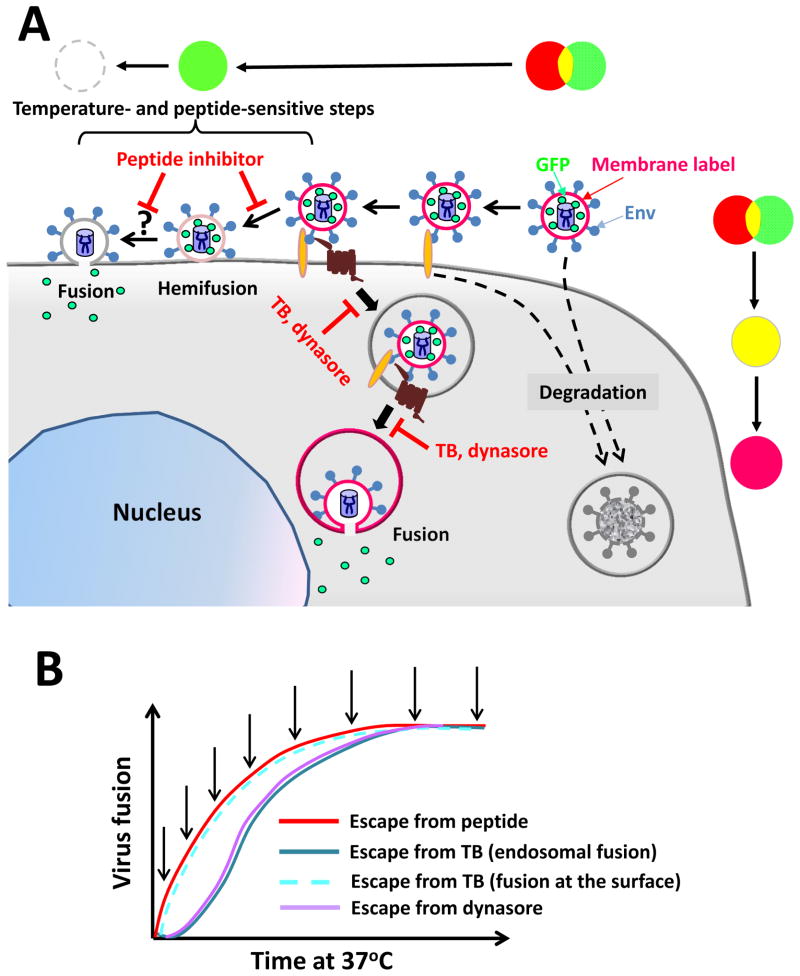
Figure 2.
Alternative HIV entry routes into a cell and experimental strategies to define the point of entry. (A) Sequential steps of hemifusion and fusion and redistribution of the viral membrane and content markers are illustrated for the HIV entry at the cell surface and through endocytosis. Virions that do not engage a requisite number of CD4 and coreceptors are degraded (dashed arrows). The pseudocolor transformation schemes corresponding to these pathways are shown above and on the right side of the cartoon. (B) Kinetic measurements of HIV fusion by adding a peptide inhibitor of gp41 6-helix bundles, dynamin inhibitor (dynasore) or by lowering the temperature (temperature block, TB) at indicated time points (arrows). If fusion occurs at the cell surface, the kinetics of escape from all three inhibitors should be similar (illustrated for the TB experiments by a dashed line).
Kinetic measurements revealed that HIV escaped from the inhibitory peptide earlier than from the temperature block in several cell lines of epithelial and lymphoid origin [46,47] (Fig. 2B). The delayed resistance to low temperature compared to the membrane-impermeable 6HB inhibitor implies that a large fraction of virions enter an endocytic pathway and fuse with endosomes [46]. However, this approach does not rule out the possibility that a fraction of viruses fuses directly with the PM, especially in activated primary CD4+ T cells for which the above kinetic difference is marginal [47].
The second experimental approach employs time-resolved imaging of single virus fusion with live cells. Here, pseudoviruses bearing HIV Env are co-labeled with a fluorescent membrane dye and a GFP-based viral content marker that is released from particles into the cytoplasm following the virus fusion (Fig. 2A and [46]). The loss of viral content thus marks virus-cell fusion regardless of whether this event occurs at the cell surface or in endosomes (Fig. 2A). The viral membrane marker also vanishes upon HIV fusion with the PM. Thus, the disappearance of the membrane marker without loss of the viral content signifies hemifusion at the cell surface. In contrast, the retention of a membrane marker following the viral content release into the cytoplasm demonstrates the virus fusion with a small sub-cellular compartment. Labeled pseudoviruses consistently fused with endosomes in HeLa-derived and T-derived cells, as evidenced by the loss of viral content and retention of the membrane marker [46]. In contrast, almost without exception, the release of a membrane marker into the PM did not culminate in release of the viral content. Moreover, attempts to redirect the HIV fusion to the PM by slowing down the virus uptake or by accelerating the fusion process through synchronizing the post-coreceptor binding steps were not successful [47].
Collectively, these findings indicate that HIV fusion with the PM may be disfavored for reasons that are presently not understood. It is possible that restriction factors, such as tetraspanins and syntenin-1 [49–52] can interfere with HIV-PM fusion. Alternatively, endosome-associated factors may be required for efficient HIV fusion. A potential candidate for such factor is dynamin [11,12,46]. Measurements of the time course of HIV escape from inhibition by dynasore, a small molecule dynamin inhibitor, implicate dynamin in augmenting HIV-endosome fusion long after the virus uptake (Fig. 2) [46]. Dynamin has also been shown to play a role in cell-to-cell transmission of HIV and Env-mediated cell-cell fusion [45,53].
A model for HIV entry and fusion
Current data appear to support the view that the HIV entry route depends on the cell type and their activation status (reviewed in [3]). The site of HIV fusion can also depend on the mode of transmission (free virus vs. cell-to-cell transfer). In addition, there is evidence that different Env glycoproteins can direct the virus entry through distinct routes. Entry of some CD4-independent HIV strains appears to require low pH and endocytic machinery [10,54,55]. Also, the block for the HIV-2 MCR infection in non-permissive cells can be rescued by pseudotyping this virus with HIV-1 Env, by inhibiting clathrin-/dynamin-dependent uptake or by disrupting actin filaments [56,57].
To reconcile discrepant results regarding the HIV entry routes, it has been proposed that the point of entry is determined by the kinetic competition between fusion and endocytosis [3]. Thus, cells exhibiting high endocytic activity would support endosomal fusion, whereas those that slowly internalize the virus would favor fusion with the PM. Two lines of evidence argue against this model. First, a comparison of the kinetics of lipid mixing at the cell surface and content release from endosome [46] shows that ~80% of hemifusion events at the PM occurred within the first 10 min, before the onset of endosomal fusion. In other words, the initiation of HIV fusion on the cell surface of HeLa-derived cells is relatively quick, but particles hemifused at the cell surface fail to progress to full fusion. Second, fusion with the PM could not be achieved by slowing down or blocking HIV endocytosis: inhibition of dynamin impaired HIV uptake and resulted in exaggerated lipid mixing at the cell surface without allowing for detectable viral content release [47]. Therefore, at least in HeLa-derived cells the entry route appears to be independent by the rates of HIV endocytosis and fusion. These rates, however, can determine the relative efficiency of fusion/infection, since viruses that fail to engage a requisite number of CD4 and coreceptors on the cell surface prior to endocytosis should be degraded in lysosomes (Fig. 2A). Kinetic experiments showed that the rate of CD4 and coreceptor engagement is defined by their surface density and affinity to Env, whereas bulk virus uptake is virtually independent of the receptor expression [58]. This accounts for the lower infectivity in cells with fewer CD4 or coreceptors.
Based on the above considerations, we propose an alternative hypothesis that HIV Env glycoproteins are only capable of creating a local hemifusion or a small pore, whereas the most energy-costly pore enlargement process [59] is driven by the host cell. Indeed, Abl kinase and Wave2 signaling-mediated actin rearrangement has been implicated in promoting the conversion of HIV hemifusion to full fusion [60]. A key distinction between entry and fusion of cell-free HIV and “fusion from without” or cell-cell fusion induced by Env is that, in the latter cases, the Env-bearing membrane cannot be readily internalized by the target cell: Env is either expressed on an effector cell or on a virus wedged between adjacent cells. We therefore propose that cell-cell fusion and “fusion from without” are driven by lateral force resulting from Env-mediated signaling and actin remodeling [61], whereas the ease with which cells internalize free HIV would minimize the potential for force generation on the cell surface. This model is supported by the observation that coverslip-immobilized HIV particles were able to fuse with the PM of overlaid target cells [62]. In addition, HIV-cell fusion and infection of susceptible cell lines are not inhibited by actin depolymerization [26,46,63,64], whereas cell-to-cell transmission [32,63,65] and Env-mediated cell-cell fusion [30,61] are sensitive to actin inhibitors. Additional host factors that can help dilate the HIV fusion pore are membrane bending proteins, including dynamin ([46] and see the previous section), which could stabilize the edge of a fusion pore, as have also been suggested in [2].
Conclusions
Endocytic HIV entry could offer advantages relative to fusion with the PM by ensuring: (i) virus delivery to the perinuclear space; (ii) protection from cytosolic restriction factors; and (iii) by shortening the cell surface exposure of gp41 intermediates targeted by 6HB inhibitors and neutralizing antibodies. The latter notion is supported by the correlation between the lifetimes of gp41 pre-bundles on the cell surface and the HIV sensitivity to gp41-derived inhibitory peptides [58,66,67]. However, in spite of evidence supporting the ability of HIV to enter from endosomes, the role of endocytosis in productive infection of different cell types remains to be defined.
To elucidate the HIV entry pathways, it would be helpful to reach consensus on interpreting the experimental results. Below are a few suggestions that might reconcile discrepant findings. First, the observation that HIV fuses with the PM or endosomes by electron microscopy, fluorescence microscopy or other means does not rule out the alternative pathway nor does it demonstrate productive entry. Second, the appearance of HIV p24 in the cytosolic fraction as a result of fusion does not identify the point of HIV entry. Third, HIV fusion inhibitors should not prevent virus uptake, since bulk endocytosis occurs irrespective of Env or CD4 (e.g., [8]). (Note, however, that cell-to-cell transfer requires Env-CD4 interactions to form/maintain the virological synapse [43,45]). Thus, the fact that these inhibitors block infection (as they should), but not the virus uptake or cell-to-cell transmission [7,43] could not be used as an argument for endocytic entry. Fourth, the requirement for cellular signaling and actin dynamics does not necessarily mean that HIV fuses at the cell surface. Fifth, virus co-localization with intracellular compartments in fixed cells does not constitute a proof of productive entry pathway.
It appears that the major source of conflicting reports regarding HIV entry sites is the difference in assays, which are often indirect and somewhat invasive. It is therefore critical to design and implement strategies to define the HIV entry route(s) into biologically relevant target cells. Although studies of HIV entry into primary CD4+ T cells and macrophages are challenging, it is essential to define the site(s) of fusion with these cells which differ from artificial target cells in many aspects. Toward this goal, the development of non-invasive virus labeling and single virus imaging techniques, such as real-time super-resolution fluorescence microscopy, along with well-characterized drugs and other targeted interventions, should reveal the exact sites of virus fusion. Post-fusion assays must also be developed in order to determine whether the released HIV cores establish productive infection.
Highlights.
I discuss controversial findings regarding the HIV entry sites into host cells
There is evidence both for HIV fusion with the plasma membrane and endosomes
New evidence highlight the role of endocytosis in productive HIV entry
A force generation model of HIV fusion is proposed to reconcile the current results
Acknowledgments
I thank the members of my laboratory for their hard work and helpful discussions. I would also like to apologize to the authors whose work is not cited here due to the limited space. The work on HIV entry in my laboratory has been supported by the NIGMS R01 GM054787 grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilen CB, Tilton JC, Doms RW. Molecular mechanisms of HIV entry. Adv Exp Med Biol. 2012;726:223–242. doi: 10.1007/978-1-4614-0980-9_10. [DOI] [PubMed] [Google Scholar]
- 2.Blumenthal R, Durell S, Viard M. HIV viral entry and envelope glycoprotein mediated fusion. J Biol Chem. 2012 doi: 10.1074/jbc.R112.406272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Permanyer M, Ballana E, Este JA. Endocytosis of HIV. anything goes. Trends Microbiol. 2010;18:543–551. doi: 10.1016/j.tim.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Melikyan GB. Membrane fusion mediated by human immunodeficiency virus envelope glycoprotein. Curr Top Membr. 2011;68:81–106. doi: 10.1016/B978-0-12-385891-7.00004-0. [DOI] [PubMed] [Google Scholar]
- 5.McClure MO, Marsh M, Weiss RA. Human immunodeficiency virus infection of CD4-bearing cells occurs by a pH-independent mechanism. Embo J. 1988;7:513–518. doi: 10.1002/j.1460-2075.1988.tb02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stein BS, Gowda SD, Lifson JD, Penhallow RC, Bensch KG, Engleman EG. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987;49:659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- 7.Janas AM, Dong C, Wang JH, Wu L. Productive infection of human immunodeficiency virus type 1 in dendritic cells requires fusion-mediated viral entry. Virology. 2008;375:442–451. doi: 10.1016/j.virol.2008.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marechal V, Clavel F, Heard JM, Schwartz O. Cytosolic Gag p24 as an index of productive entry of human immunodeficiency virus type 1. J Virol. 1998;72:2208–2212. doi: 10.1128/jvi.72.3.2208-2212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fredericksen BL, Wei BL, Yao J, Luo T, Garcia JV. Inhibition of endosomal/lysosomal degradation increases the infectivity of human immunodeficiency virus. J Virol. 2002;76:11440–11446. doi: 10.1128/JVI.76.22.11440-11446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fackler OT, Peterlin BM. Endocytic entry of HIV-1. Curr Biol. 2000;10:1005–1008. doi: 10.1016/s0960-9822(00)00654-0. [DOI] [PubMed] [Google Scholar]
- **11.Daecke J, Fackler OT, Dittmar MT, Krausslich HG. Involvement of clathrin-mediated endocytosis in human immunodeficiency virus type 1 entry. J Virol. 2005;79:1581–1594. doi: 10.1128/JVI.79.3.1581-1594.2005. This study was the first to demonstrate the link between clathrin- and dynamin-dependent endocytosis and HIV fusion/infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *12.Carter GC, Bernstone L, Baskaran D, James W. HIV-1 infects macrophages by exploiting an endocytic route dependent on dynamin, Rac1 and Pak1. Virology. 2011;409:234–250. doi: 10.1016/j.virol.2010.10.018. Using pharmacological interventions, the authors found that HIV enters macrophages through non-classical macropinocytosis. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Exposito L, Barroso-Gonzalez J, Puigdomenech I, Machado JD, Blanco J, Valenzuela-Fernandez A. HIV-1 requires Arf6-mediated membrane dynamics to efficiently enter and infect T lymphocytes. Mol Biol Cell. 2011;22:1148–1166. doi: 10.1091/mbc.E10-08-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *14.Gobeil LA, Lodge R, Tremblay MJ. Macropinocytosis-like HIV-1 internalization in macrophages is CCR5 dependent and leads to efficient but delayed degradation in endosomal compartments. J Virol. 2013;87:735–745. doi: 10.1128/JVI.01802-12. The authors show that macrophages inernalize HIV in CCR5-dependent manner and propose that this is pathway can lead to fusion and infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marechal V, Prevost MC, Petit C, Perret E, Heard JM, Schwartz O. Human immunodeficiency virus type 1 entry into macrophages mediated by macropinocytosis. J Virol. 2001;75:11166–11177. doi: 10.1128/JVI.75.22.11166-11177.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *16.von Kleist L, Stahlschmidt W, Bulut H, Gromova K, Puchkov D, Robertson MJ, MacGregor KA, Tomlin N, Pechstein A, Chau N, et al. Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell. 2011;146:471–484. doi: 10.1016/j.cell.2011.06.025. This article reports the discovery of a specific inhibitor of clathrin-mediated endocytosis, which also inhibited HIV infection. [DOI] [PubMed] [Google Scholar]
- 17.Vidricaire G, Tremblay MJ. A Clathrin, Caveolae, and Dynamin-independent Endocytic Pathway Requiring Free Membrane Cholesterol Drives HIV-1 Internalization and Infection in Polarized Trophoblastic Cells. J Mol Biol. 2007 doi: 10.1016/j.jmb.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Goto T, Harada S, Yamamoto N, Nakai M. Entry of human immunodeficiency virus (HIV) into MT-2, human T cell leukemia virus carrier cell line. Arch Virol. 1988;102:29–38. doi: 10.1007/BF01315560. [DOI] [PubMed] [Google Scholar]
- 19.Grewe C, Beck A, Gelderblom HR. HIV: early virus-cell interactions. J Acquir Immune Defic Syndr. 1990;3:965–974. [PubMed] [Google Scholar]
- 20.Pauza CD, Price TM. Human immunodeficiency virus infection of T cells and monocytes proceeds via receptor-mediated endocytosis. J Cell Biol. 1988;107:959–968. doi: 10.1083/jcb.107.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clavel F, Charneau P. Fusion from without directed by human immunodeficiency virus particles. J Virol. 1994;68:1179–1185. doi: 10.1128/jvi.68.2.1179-1185.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maddon PJ, McDougal JS, Clapham PR, Dalgleish AG, Jamal S, Weiss RA, Axel R. HIV infection does not require endocytosis of its receptor, CD4. Cell. 1988;54:865–874. doi: 10.1016/s0092-8674(88)91241-x. [DOI] [PubMed] [Google Scholar]
- 23.Pelchen-Matthews A, Clapham P, Marsh M. Role of CD4 endocytosis in human immunodeficiency virus infection. J Virol. 1995;69:8164–8168. doi: 10.1128/jvi.69.12.8164-8168.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bedinger P, Moriarty A, von Borstel RC, 2nd, Donovan NJ, Steimer KS, Littman DR. Internalization of the human immunodeficiency virus does not require the cytoplasmic domain of CD4. Nature. 1988;334:162–165. doi: 10.1038/334162a0. [DOI] [PubMed] [Google Scholar]
- 25.Aiken C. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J Virol. 1997;71:5871–5877. doi: 10.1128/jvi.71.8.5871-5877.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell EM, Nunez R, Hope TJ. Disruption of the actin cytoskeleton can complement the ability of Nef to enhance human immunodeficiency virus type 1 infectivity. J Virol. 2004;78:5745–5755. doi: 10.1128/JVI.78.11.5745-5755.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brun S, Solignat M, Gay B, Bernard E, Chaloin L, Fenard D, Devaux C, Chazal N, Briant L. VSV-G pseudotyping rescues HIV-1 CA mutations that impair core assembly or stability. Retrovirology. 2008;5:57. doi: 10.1186/1742-4690-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agosto LM, Yu JJ, Liszewski MK, Baytop C, Korokhov N, Humeau LM, O’Doherty U. The CXCR4-tropic human immunodeficiency virus envelope promotes more-efficient gene delivery to resting CD4+ T cells than the vesicular stomatitis virus glycoprotein G envelope. J Virol. 2009;83:8153–8162. doi: 10.1128/JVI.00220-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *29.Yu D, Wang W, Yoder A, Spear M, Wu Y. The HIV envelope but not VSV glycoprotein is capable of mediating HIV latent infection of resting CD4 T cells. PLoS Pathog. 2009;5:e1000633. doi: 10.1371/journal.ppat.1000633. This study provides evidence for the existence of a post-fusion block for infection of resting CD4+ T cells by VSV G pseudotypes. The authors concluded that an endicytic route does not lead to productive entry into these cells and that therefore latent HIV infection is established by fusion with the plasma membrane (see reference 39 for a different conclusion) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallo SA, Puri A, Blumenthal R. HIV-1 gp41 six-helix bundle formation occurs rapidly after the engagement of gp120 by CXCR4 in the HIV-1 Env-mediated fusion process. Biochemistry. 2001;40:12231–12236. doi: 10.1021/bi0155596. [DOI] [PubMed] [Google Scholar]
- 31.Iyengar S, Hildreth JE, Schwartz DH. Actin-dependent receptor colocalization required for human immunodeficiency virus entry into host cells. J Virol. 1998;72:5251–5255. doi: 10.1128/jvi.72.6.5251-5255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jolly C, Kashefi K, Hollinshead M, Sattentau QJ. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J Exp Med. 2004;199:283–293. doi: 10.1084/jem.20030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barrero-Villar M, Cabrero JR, Gordon-Alonso M, Barroso-Gonzalez J, Alvarez-Losada S, Munoz-Fernandez MA, Sanchez-Madrid F, Valenzuela-Fernandez A. Moesin is required for HIV-1-induced CD4-CXCR4 interaction, F-actin redistribution, membrane fusion and viral infection in lymphocytes. J Cell Sci. 2009;122:103–113. doi: 10.1242/jcs.035873. [DOI] [PubMed] [Google Scholar]
- 34.Jimenez-Baranda S, Gomez-Mouton C, Rojas A, Martinez-Prats L, Mira E, Ana Lacalle R, Valencia A, Dimitrov DS, Viola A, Delgado R, et al. Filamin-A regulates actin-dependent clustering of HIV receptors. Nat Cell Biol. 2007;9:838–846. doi: 10.1038/ncb1610. [DOI] [PubMed] [Google Scholar]
- 35.Bukrinskaya A, Brichacek B, Mann A, Stevenson M. Establishment of a functional human immunodeficiency virus type 1 (HIV-1) reverse transcription complex involves the cytoskeleton. J Exp Med. 1998;188:2113–2125. doi: 10.1084/jem.188.11.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spear M, Guo J, Wu Y. The trinity of the cortical actin in the initiation of HIV-1 infection. Retrovirology. 2012;9:45. doi: 10.1186/1742-4690-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **37.Yoder A, Yu D, Dong L, Iyer SR, Xu X, Kelly J, Liu J, Wang W, Vorster PJ, Agulto L, et al. HIV envelope-CXCR4 signaling activates cofilin to overcome cortical actin restriction in resting CD4 T cells. Cell. 2008;134:782–792. doi: 10.1016/j.cell.2008.06.036. The authors show the key role of cofilin-mediated actin remodeling in infection of resting CD4+ T cells. This finding is consistent with the model that cortical actin restricts the penetration of HIV core into the cytosol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Y, Yoder A. Chemokine coreceptor signaling in HIV-1 infection and pathogenesis. PLoS Pathog. 2009;5:e1000520. doi: 10.1371/journal.ppat.1000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **39.Pace MJ, Agosto L, O’Doherty U. R5 HIV Env and VSV-G Cooperate to Mediate Fusion to Naive CD4+T Cells. J Virol. 2011 doi: 10.1128/JVI.01851-10. This study demonstrates that the failure of VSV G pseudotypes to infect resting CD4+ T cells is due to the inability of these cells to internalize particles that do not activate proper signaling pathways. Thus, the difference in infectivity of HIV and VSV pseudoviruses stems from the block upstream of VSV G-mediated fusion (lack of endocytosis) and not from a post-fusion block, as has been suggested in the reference 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin N, Welsch S, Jolly C, Briggs JA, Vaux D, Sattentau QJ. Virological synapse-mediated spread of human immunodeficiency virus type 1 between T cells is sensitive to entry inhibition. J Virol. 2010;84:3516–3527. doi: 10.1128/JVI.02651-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Felts RL, Narayan K, Estes JD, Shi D, Trubey CM, Fu J, Hartnell LM, Ruthel GT, Schneider DK, Nagashima K, et al. 3D visualization of HIV transfer at the virological synapse between dendritic cells and T cells. Proc Natl Acad Sci U S A. 2010;107:13336–13341. doi: 10.1073/pnas.1003040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clotet-Codina I, Bosch B, Senserrich J, Fernandez-Figueras MT, Pena R, Ballana E, Bofill M, Clotet B, Este JA. HIV endocytosis after dendritic cell to T cell viral transfer leads to productive virus infection. Antiviral Res. 2009;83:94–98. doi: 10.1016/j.antiviral.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 43.Sloan RD, Kuhl BD, Mesplede T, Munch J, Donahue DA, Wainberg MA. Productive entry of HIV-1 during cell-to-cell transmission via dynamin-dependent endocytosis. J Virol. 2013 doi: 10.1128/JVI.00815-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *44.Dale BM, McNerney GP, Thompson DL, Hubner W, de Los Reyes K, Chuang FY, Huser T, Chen BK. Cell-to-cell transfer of HIV-1 via virological synapses leads to endosomal virion maturation that activates viral membrane fusion. Cell Host Microbe. 2011;10:551–562. doi: 10.1016/j.chom.2011.10.015. Delayed maturation of HIV particles transmitted via the virological synapse in endosomes of a recipient uninfected cell is proposed to trigger the fusion reaction and be responsible for endocytic entry of this virus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bosch B, Grigorov B, Senserrich J, Clotet B, Darlix JL, Muriaux D, Este JA. A clathrin-dynamin-dependent endocytic pathway for the uptake of HIV-1 by direct T cell-T cell transmission. Antiviral Res. 2008 doi: 10.1016/j.antiviral.2008.06.004. [DOI] [PubMed] [Google Scholar]
- **46.Miyauchi K, Kim Y, Latinovic O, Morozov V, Melikyan GB. HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell. 2009;137:433–444. doi: 10.1016/j.cell.2009.02.046. This is the first study to implement novel mimimally invasive techniques specifically designed to delineate the HIV entry sites. Both kinetic measurements of virus escape from fusion inhibtors and imaging single particle fusion implied that HIV fused with endosomes, but not with the plasma membrane. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *47.de la Vega M, Marin M, Kondo N, Miyauchi K, Kim Y, Epand RF, Epand RM, Melikyan GB. Inhibition of HIV-1 endocytosis allows lipid mixing at the plasma membrane, but not complete fusion. Retrovirology. 2011;8:99. doi: 10.1186/1742-4690-8-99. This study attempted to redirect the HIV fusion to the plasma membrane by blocking the virus uptake. The fact that this intervention exaggerated the HIV hemifusion at the cell surface, but did not promote full fusion implies that the plasma membrane is not conducive for HIV fusion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Markosyan RM, Cohen FS, Melikyan GB. HIV-1 envelope proteins complete their folding into six-helix bundles immediately after fusion pore formation. Mol Biol Cell. 2003;14:926–938. doi: 10.1091/mbc.E02-09-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gordon-Alonso M, Yanez-Mo M, Barreiro O, Alvarez S, Munoz-Fernandez MA, Valenzuela-Fernandez A, Sanchez-Madrid F. Tetraspanins CD9 and CD81 modulate HIV-1-induced membrane fusion. J Immunol. 2006;177:5129–5137. doi: 10.4049/jimmunol.177.8.5129. [DOI] [PubMed] [Google Scholar]
- 50.Gordon-Alonso M, Rocha-Perugini V, Alvarez S, Moreno-Gonzalo O, Ursa A, Lopez-Martin S, Izquierdo-Useros N, Martinez-Picado J, Munoz-Fernandez MA, Yanez-Mo M, et al. The PDZ-adaptor protein syntenin-1 regulates HIV-1 entry. Mol Biol Cell. 2012;23:2253–2263. doi: 10.1091/mbc.E11-12-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krementsov DN, Weng J, Lambele M, Roy NH, Thali M. Tetraspanins regulate cell-to-cell transmission of HIV-1. Retrovirology. 2009;6:64. doi: 10.1186/1742-4690-6-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weng J, Krementsov DN, Khurana S, Roy NH, Thali M. Formation of syncytia is repressed by tetraspanins in human immunodeficiency virus type 1-producing cells. J Virol. 2009;83:7467–7474. doi: 10.1128/JVI.00163-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lai W, Huang L, Ho P, Montefiori D, Chen CH. The role of dynamin in HIV type 1 Env-mediated cell-cell fusion. AIDS Res Hum Retroviruses. 2011;27:1013–1017. doi: 10.1089/aid.2010.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshii H, Kamiyama H, Goto K, Oishi K, Katunuma N, Tanaka Y, Hayashi H, Matsuyama T, Sato H, Yamamoto N, et al. CD4-independent human immunodeficiency virus infection involves participation of endocytosis and cathepsin B. PLoS ONE. 2011;6:e19352. doi: 10.1371/journal.pone.0019352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maurin T, Fenard D, Lambeau G, Doglio A. An envelope-determined endocytic route of viral entry allows HIV-1 to escape from secreted phospholipase A2 entry blockade. J Mol Biol. 2007;367:702–714. doi: 10.1016/j.jmb.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 56.Liu L, Oliveira NM, Cheney KM, Pade C, Dreja H, Bergin AM, Borgdorff V, Beach DH, Bishop CL, Dittmar MT, et al. A whole genome screen for HIV restriction factors. Retrovirology. 2011;8:94. doi: 10.1186/1742-4690-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harrison IP, McKnight A. Cellular entry via an actin and clathrin-dependent route is required for Lv2 restriction of HIV-2. Virology. 2011;415:47–55. doi: 10.1016/j.virol.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 58.Miyauchi K, Kozlov MM, Melikyan GB. Early steps of HIV-1 fusion define the sensitivity to inhibitory peptides that block 6-helix bundle formation. PLoS Pathog. 2009;5:e1000585. doi: 10.1371/journal.ppat.1000585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chernomordik LV, Kozlov MM. Protein-lipid interplay in fusion and fission of biological membranes. Annu Rev Biochem. 2003;72:175–207. doi: 10.1146/annurev.biochem.72.121801.161504. [DOI] [PubMed] [Google Scholar]
- **60.Harmon B, Campbell N, Ratner L. Role of Abl kinase and the Wave2 signaling complex in HIV-1 entry at a post-hemifusion step. PLoS Pathog. 2010;6:e1000956. doi: 10.1371/journal.ppat.1000956. This study demonstrates the reqirement for cell signaling for efficient HIV fusion and infection. A key finding is that, in the absence of signaling and downstream actin remodeling, HIV fusion is arrested at a hemifusion stage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pontow SE, Heyden NV, Wei S, Ratner L. Actin cytoskeletal reorganizations and coreceptor-mediated activation of rac during human immunodeficiency virus-induced cell fusion. J Virol. 2004;78:7138–7147. doi: 10.1128/JVI.78.13.7138-7147.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Markosyan RM, Cohen FS, Melikyan GB. Time-resolved imaging of HIV-1 Env-mediated lipid and content mixing between a single virion and cell membrane. Mol Biol Cell. 2005;16:5502–5513. doi: 10.1091/mbc.E05-06-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *63.Hubner W, McNerney GP, Chen P, Dale BM, Gordon RE, Chuang FY, Li XD, Asmuth DM, Huser T, Chen BK. Quantitative 3D video microscopy of HIV transfer across T cell virological synapses. Science. 2009;323:1743–1747. doi: 10.1126/science.1167525. Using quantitative fluorescence microscopy, the authors provided first evidence for the role of endocytosis in cell-to-cell transmission of HIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yonezawa A, Cavrois M, Greene WC. Studies of ebola virus glycoprotein-mediated entry and fusion by using pseudotyped human immunodeficiency virus type 1 virions: involvement of cytoskeletal proteins and enhancement by tumor necrosis factor alpha. J Virol. 2005;79:918–926. doi: 10.1128/JVI.79.2.918-926.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blanco J, Bosch B, Fernandez-Figueras MT, Barretina J, Clotet B, Este JA. High level of coreceptor-independent HIV transfer induced by contacts between primary CD4 T cells. J Biol Chem. 2004;279:51305–51314. doi: 10.1074/jbc.M408547200. [DOI] [PubMed] [Google Scholar]
- 66.Steger HK, Root MJ. Kinetic dependence to HIV-1 entry inhibition. J Biol Chem. 2006;281:25813–25821. doi: 10.1074/jbc.M601457200. [DOI] [PubMed] [Google Scholar]
- 67.Demirkhanyan L, Marin M, Lu W, Melikyan GB. Sub-Inhibitory Concentrations of Human alpha-defensin Potentiate Neutralizing Antibodies against HIV-1 gp41 Pre-Hairpin Intermediates in the Presence of Serum. PLoS Pathog. 2013;9:e1003431. doi: 10.1371/journal.ppat.1003431. [DOI] [PMC free article] [PubMed] [Google Scholar]