Abstract
Rationale
Early, accurate detection of degenerative neurological disorders such as Alzheimer's Disease (AD) is essential for therapies designed to slow disease progression. Performance of a touch-screen mediated visuo-spatial paired-associates learning (vsPAL) task predicts neurocognitive decline in elderly populations presenting with mild cognitive impairment and distinguishes AD patients from elderly depressed individuals. Translation of this cognitive task to a nonhuman model may therefore provide an improved tool for study of the etiology and treatment of dementia.
Objective
The goal of the current study was to contrast cholinergic and glutamatergic contributions to performance of this AD-sensitive task by challenging rhesus monkeys performing vsPAL with muscarinic antagonist and non-competitive NMDA antagonist drugs.
Methods
Monkeys (7) were trained to perform vsPAL and then serially challenged with acute doses of scopolamine (3, 10, 17 µg/kg, i.m.) and ketamine (0.3, 1.0, 1.78 mg/kg, i.m.).
Results
Scopolamine produced a dose × difficulty related impairment of both recognition memory and incremental acquisition aspects of task performance. In contrast, ketamine administration resulted in a dose-dependent impairment of recognition memory but not incremental acquisition.
Conclusions
Monkeys' performance of a task sensitive to AD in humans was impaired by two classic pharmacological models of cognitive impairment therefore supporting the use of this nonhuman model to explore mechanisms of AD-associated cognitive decline. The differential pattern of impairment observed is consistent with a hypothesis that muscarinic mechanisms are required for linking external events with an existing internal representation, whereas NMDA mechanisms are required for the formation/strengthening of such an internal representation.
Keywords: Learning, Memory, Scopolamine, Ketamine, CANTAB, Alzheimer's Disease
Introduction
Early and accurate detection of neurodegenerative disorders such as Alzheimer's Disease, Parkinson's Disease and Huntington's Disease is crucial for maintaining maximal quality of life with the currently available treatments. The ability to detect such conditions in the very earliest stages would also be important for new disease-modifying treatments, e.g., (Blesch et al. 1998; Kinscherf et al. 2000). In addition, a more precise understanding of cognitive disturbance in the earliest stages of disease progression would help to refine nonhuman models of the disorder, thereby speeding development of new therapeutic strategies.
The most commonly used diagnostic tool for identifying individuals with Alzheimer's Disease (AD) antemortem is a battery of neuropsychological tests (McKhann et al. 1984; Welsh et al. 1992) although conclusive diagnosis currently requires postmortem examination of brain tissue. Neuropsychological testing is likely to remain the standard in the absence of a reliable biomarker, given the relative inexpense of such tests compared with alternative diagnostics such as current imaging techniques (Massoud et al. 2000; McMahon et al. 2000). Consequently, refinement of the sensitivity and the specificity of the cognitive/behavioral tests used to evaluate cognitive function remains a continuing goal. Improving the specificity of such tests is important in differentiating AD individuals from other populations who may perform poorly on neuropsychological tests. For example elderly subjects with major depression perform poorly on tests of memory that AD subjects perform poorly (Lichtenberg et al. 1995; Zakzanis et al. 1998). It would therefore be crucial to distinguish depressed individuals from AD individuals when proposing to use any risky or invasive treatment. Refinement of the sensitivity of neuropsychological tests would allow possible disease-modifying treatments, e.g., (Kinscherf et al. 2000), to be employed at the earliest stages of neuronal loss, thereby minimizing damage. On a practical level any reduction in the number of test-battery items required to detect AD would lead to a reduced cost of clinical evaluation and decreased burden on the evaluated patients. Finally, an improved neuropsychological test may be useful to evaluate the efficacy of novel medications in clinical trials. These reasons and others have motivated a number of attempts to determine which behavioral tests may be most specific for, and sensitive to, cognitive impairments early in AD, e.g., (Flicker et al. 1991; Fowler et al. 1997; Masur et al. 1994; Perry and Hodges 2000). Nonhuman models of AD can take advantage of such efforts by adopting non-verbal, automated, behavioral tests which are homologous to those shown to be most sensitive and specific in the evaluation of AD patients. The present study was conducted to use one such nonverbal test, with demonstrated sensitivity and specificity for AD in humans, to explore cognitive function in nonhuman primates.
A series of neuropsychological studies has provided evidence that a touch-screen mediated, computerized, visuo-spatial paired-associates learning task (vsPAL) may offer improved sensitivity and specificity for the detection of AD. The task involves learning to associate visual stimuli with distinct spatial locations on a trial-by-trial basis, performance of which has been demonstrated to decline with age in large-sample factor analytic studies (Rabbitt and Lowe 2000; Robbins et al. 1994). Sahakian and colleagues have further demonstrated impaired performance of vsPAL in AD patients relative to age- and premorbid IQ-matched controls, and recognition-memory impaired Parkinson’s patients (Sahakian et al. 1990; Sahakian et al. 1988; Sahgal et al. 1991). Recent prospective investigations based on these findings have provided additional confirmation that the vsPAL task is particularly sensitive to, and specific for, symptoms of neurocognitive decline associated with AD. One such study has shown that a six-month decline in vsPAL performance in patients presenting with mild cognitive symptoms predicts later progression to AD (Fowler et al. 1995; 1997). The Sahakian group has also demonstrated that vsPAL scores of 'questionably demented' individuals at presentation predict a decline in the mini-mental state exam score over the subsequent eight months (Swainson et al. 2001). The nonverbal nature of this putative AD-sensitive task supports its use in nonhuman subjects to investigate specific brain mechanisms which contribute to vsPAL performance and therefore may be altered early in AD.
The objective of the present study was to contrast the roles of muscarinic cholinergic and N-methyl-D-aspartate (NMDA) glutamatergic receptors in the performance of the vsPAL task in rhesus monkeys. The muscarinic antagonist scopolamine has been shown to impair memory and other performance measures in humans, monkeys and rodents in support of the so-called "cholinergic hypothesis", i.e., that memory failure in AD is causally related to the early and significant loss of cholinergic markers in the brain (see (Bartus 2000; Iversen 1997; Ridley and Baker 1991) for review). Similarly, NMDA glutamatergic receptors have been demonstrated to play a crucial role in long-term potentiation, a putative cellular mechanism subserving memory (for review see Huang and Stevens 1998; Nicoll and Malenka 1999), and numerous studies have demonstrated that NMDA antagonists interfere with memory performance in several species (e.g., Buffalo et al. 1994; Ghoneim et al. 1985; Morris et al. 1986). More specifically, NMDA antagonists have been demonstrated to impair learning (or encoding) but not memory retrieval at subanaesthetic doses in human subjects (Hetem et al. 2000; Oye et al. 1992) and several studies have shown that the non-competitive NMDA antagonists dizocilpine (Boyce et al. 1991; Buffalo et al. 1994; Harder et al. 1998; Ogura and Aigner 1993) and phencyclidine (Frederick et al. 1995; Rupniak et al. 1992; Thompson et al. 1986) impair learning and memory in monkeys. Furthermore, recognition memory is significantly impaired in monkeys challenged with a combination of dizocilpine and scopolamine at doses lower than would produce deficits when given individually (Matsuoka and Aigner 1996), thereby demonstrating a behavioral interaction and, potentially, a mechanistic interaction.
While the majority of pharmacological studies in monkeys have illustrated similarities between the effect of muscarinic and NMDA blockade on learning or memory processes, few have specifically contrasted muscarinic versus NMDA modulation to explore possible differences which may be important for the detection and/or treatment of cognitive failure early in AD. As suggested in a recent review (Bartus 2000), any specific pharmacological model of dementia may not encompass all aspects of age-related cognitive failure and it may reasonably be expected that an "ideal" model of a complex neurodegenerative disease like AD would require concomitant manipulation of several transmitter systems. By extension, efforts to contrast multiple pharmacological models, as with the present study, may help to further delineate the specific contributions of various neurotransmitter system components to particular aspects of cognitive disruption. Such contrasts may advance a more specific pharmacological model of memory failure and potentially a better understanding of the relationship between brain alterations and memory failure in normal aging and AD.
The present study therefore compares muscarinic and NMDA contributions to monkeys’ performance of a task that is specifically sensitive to AD. It is hypothesized that monkeys will perform the task in a manner similar to humans, thus performance is expected to decline as trial difficulty is increased. Based on previous observations in nonhuman primates performing tests of pattern or spatial memory, challenge with ketamine and scopolamine are each predicted to impair performance in a dose-related as well as task difficulty-related manner. Finally, since previous studies have reported qualitatively similar impairments of memory performance following muscarinic versus NMDA blockade, as well as additive effects of simultaneous blockade, it is predicted that the effects of scopolamine and ketamine challenge of vsPAL performance will be similar.
Materials and methods
Animals
Eight male rhesus monkeys (Macaca mulatta) served as subjects. The monkeys were approximately 5 years of age and weighed 6.0–8.5 kg at the beginning of the study. Animals were individually housed and fed in the home cage after completion of the daily testing session. The animals' normal diet (Lab Diet 5045, PMI Nutrition International) was supplemented with fruit or vegetables four days per week and water was available ad libitum in the home cage at all times. Principles of laboratory animal care (Guide for the Care and use of Laboratory Animals, National Academy Press, 1996) were followed, and all protocols were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute. The monkeys were concurrently being trained on components of a behavioral test battery at the initiation of the present study (see below) and had not received any experimental drug treatments prior to the current study. All animals were, however, immobilized with ketamine in doses of 5–10 mg/kg (i.m.) no less than semiannually for the purposes of routine care and health monitoring prior to the present study.
Apparatus
For behavioral testing, animals were transferred to the testing room in transport cages modified by the removal of several bar sections from the front to allow the animal to easily reach out of the cage. The transport cage was placed in front of a computer monitor fitted with a touch-sensitive screen on which visual stimuli were presented. Animals were previously trained to reach out of the cage to touch the location on the screen at which stimuli are presented to obtain a food pellet reward. Stimulus presentation and response detection were controlled by a micro-computer equipped with a version of the CAmbridge Neuropsychological Test Automated Battery (CANTAB; CeNeS Pharmaceuticals, PLC) designed for use with non-human primates. Following correct responses, a dispenser delivered 190 mg flavored pellets (P.J. Noyes Co., Lancaster, NH) to a bin mounted on the front of the cage. A white-noise generator remained on, and the subject was left alone in the testing room during each behavioral session.
Visuo-Spatial Paired Associates Learning (vsPAL) Task
The vsPAL task is a memory task that requires learning to associate given stimuli with particular spatial locations on a trial by trial basis, as schematized in Figure 1. This task was trained as part of a neuropsychological test battery designed to concurrently access multiple cognitive domains in nonhuman primates. Five of the tasks are part of the nonhuman primate CANTAB and require monkeys to respond by touching the touch-sensitive computer screen and are reinforced with the delivery of food pellet reinforcers. In addition to vsPAL, tests of short-term recognition memory (delayed matching to sample; DMS), short-term spatial memory (self-ordered spatial search; SOSS), reaction time (RT) and reinforcer efficacy (progressive ratio; PR) are included. The sixth task requires the animal to extract raisins from holes in a transparent plastic board (bimanual motor skill; BMS). Animals are generally trained 5 days per week. Three of the six tasks are performed during each daily test session as is outlined in Table 1 (also see Weed et al. 1999).
Figure 1. Schematic of the visuo-spatial paired-associates learning task trials.
One to four colored visual stimuli are sequentially presented in distinct spatial locations in the sample phase of each trial. Following an observing response, each stimulus is then re-displayed in multiple locations in the choice phase of the trial. The order of stimuli in the choice phase is randomized relative to the sample order for each trial. Accurate trial performance requires the correct selection of the sample location for each stimulus within a trial. Animals are allowed up to 6 attempts to complete each trial correctly (see Methods).
TABLE 1.
Schedule for Each Drug Challenge Study
| Monday | Tuesday | Wednesday | Thursday | Friday | |
|---|---|---|---|---|---|
| Week 1 | DMS/RT/BMS | vsPAL/RT/BMS | PR/SOSS/BMS | DMS/RT/BMS | PR/vsPAL/BMS |
| Baseline | Drug Dose 1 | Baseline | Vehicle | Drug Dose 2 | |
| Week 2 | PR/SOSS/BMS | DMS/RT/BMS | vsPAL/RT/BMS | PR/SOSS/BMS | DMS/RT/BMS |
| Baseline | Drug Dose 1 | Baseline | Vehicle | Drug Dose 2 | |
| Week 3 | PR/vsPAL/BMS | PR/SOSS/BMS | DMS/RT/BMS | vsPAL/RT/BMS | PR/SOSS/BMS |
| Baseline | Drug Dose 1 | Baseline | Vehicle | Drug Dose 2 | |
| Week 4 | DMS/RT/BMS | vsPAL/RT/BMS | PR/SOSS/BMS | DMS/RT/BMS | PR/vsPAL/BMS |
| Baseline | Drug Dose 3 | Baseline | Vehicle | Drug Dose 3 | |
| Week 5 | PR/SOSS/BMS | DMS/RT/BMS | PR/vsPAL/BMS | PR/SOSS/BMS | DMS/RT/BMS |
| Baseline | Drug Dose 3 | Baseline | Vehicle | Drug Dose 3 | |
| Week 6 | vsPAL/RT/BMS | PR/SOSS/BMS | DMS/RT/BMS | PR/vsPAL/BMS | PR/SOSS/BMS |
| Baseline | Drug Dose 3 | Baseline | Vehicle | Drug Dose 3 | |
| Week 7 | DMS/RT/BMS | vsPAL/RT/BMS | PR/SOSS/BMS | DMS/RT/BMS | PR/vsPAL/BMS |
| Baseline | Drug Dose 2 | Baseline | Vehicle | Drug Dose 1 |
The schedule required to double-determine the effects of 3 doses of one drug on vsPAL performance is presented in the table. The current study was conducted in the context of evaluating drug effects on all battery tasks as depicted. Animals completed one of three memory tasks (vsPAL, DMS, SOSS), and either PR or RT on alternating days. The BMS task was completed at the end of each session. Thus the behavioral schedule repeated every 3 weeks. Drug challenges were performed Tue and Fri in an ascending-descending order for each memory task combination. DMS, Delayed Match to Sample; RT, Reaction Time; BMS, Bimanual Motor Skill; vsPAL, visuo-spatial Paired Associates Learning; SOSS, Self-Ordered Spatial Search
The stimuli for the vsPAL task consist of 18 distinct colored shapes or patterns. To begin a trial, one sample stimulus is presented in one of four possible target locations (center of the left, right, top or bottom edge of the screen) and the animal must make an observing touch to its location within 30 sec. For the more difficult trials, second, third and/or fourth sample stimuli are presented (a 0.5 sec screen blank follows each observing response) in unique locations prior to the choice phase of the trial. After each sample stimulus has been presented, and following a 1 sec screen blank, one of the sample stimuli (pseudo-randomly selected) is simultaneously presented in 2–4 target locations (choice presentation).The animal is then required to touch the target location in which that stimulus was originally presented to obtain a reinforcer pellet. The next choice is then presented following a 0.5 sec screen blank. Touches directed to the stimulus in an incorrect location (error) or a failure to touch within 30 sec (omission) instantiate an additional 4 sec screen blank prior to presentation of the subsequent choice. A successful trial completion requires the accurate selection of each of the 1–4 stimulus-location associations presented in the sample phase. If a subject fails to successfully complete a given trial on the first attempt, the same set of stimulus-location associations are presented again, in a new sample- and choice-order. Animals are allowed up to 5 additional attempts to successfully complete the set of stimulus-location associations in a given trial. If the subject does not succeed after 6 total attempts the trial is terminated and a next trial is initiated, (i.e., a new set of stimulus-location associations is presented). Task performance is measured by several measures including initial-attempt trial completion success (the proportion of trials successfully completed on the first attempt), overall trial completion success (the proportion of trials successfully completed after 1–6 attempts), mean attempts per overall completed trial, (the average number of attempts made on initially-failed but ultimately successful trials), percent task completed (the proportion of trials on which both sample and choice responses were made), total number of correct choices (the sum of accurate individual choice responses, across both correct and failed attempts to complete a trial), total number of incorrect choices (the sum of inaccurate choice responses), sample latency (the average time from sample onset to the screen touch) and choice latency (the average time from choice stimuli onset to the screen touch, for all correct choice responses). All monkeys were trained with gradual increases in the session difficulty until they were performing sessions under terminal conditions consisting of 35 trials blocked as follows: 5 × 1-stimulus trials, 10 × 2-, 3- and 4-stimuli trials (see Figure 1). (N.b. This version of vsPAL designed for use with nonhuman primates differs slightly from the version used in human studies (e.g., Fowler et al. 1997; Swainson et al. 2001). In the human version of the task, the sample stimuli are presented in 6–8 possible locations delimited by white square outlines on the screen. In the choice phase, the relevant stimulus is presented in the center of the display while white squares are presented at each possible target location. The subject must touch the white square where the given stimulus was previously presented for a successful trial completion.)
Drug Challenge
The drug challenge studies were initiated in monkeys following training on the terminal-contingency version of the task for at least two months during which the animals completed one of the memory tasks (DMS, SOSS or vsPAL) on sequential days in a continuing rotation. Either the PR or the RT task was completed in combination with each memory task and the BMS task was trained each day at the end of the session. Therefore, the following combinations of battery tests were completed on sequential days: PR/SOSS/BMS one day, DMS/RT/BMS on the following day and either PR/vsPAL/BMS or vsPAL/RT/BMS on the third day (see Table 1). During the drug challenge studies, non-injection baseline sessions were administered twice (Mon, Wed), drug sessions were administered twice (Tue, Fri) and a vehicle (physiological saline) session was administered once (Thur) per week as outlined in Table 1. Therefore within each 3 week period the vsPAL task was evaluated twice under baseline conditions, twice under drug challenge and once following vehicle administration. (To ensure stable responding prior to each drug study, vehicle was injected three days per week (Tue, Thur, Fri) for a minimum of two weeks or until performance following injections was equivalent to non-injection performance.) Doses of ketamine (0.3, 1.0, 1.78 mg/kg, i.m.; 10 min prior to session) were evaluated twice for each SOSS, DMS or PAL task combination in an ascending-descending order per test combination, as is outlined in Table 1 (each of the two “vsPAL” combinations, i.e., PR/vsPAL/BMS and vsPAL/RT/BMS, was evaluated once at each dose). Doses of scopolamine (3, 10, 17 µg/kg, i.m.; 20 min prior to session) were evaluated twice for the PAL sessions only, again in an ascending-descending order; we have previously described the effect of scopolamine on the other test battery tasks in a different cohort of monkeys (Taffe et al. 1999). All drug doses were administered in a standard injection volume of 0.1 ml per kg of bodyweight. Four animals received ketamine first followed by scopolamine and the other four received scopolamine first and ketamine second. In both cases a minimum two-week washout period, in which no drugs were administered, was instituted between testing of the two compounds.
Data Analysis
Trial attempts on which a monkey omitted responding were not included in the calculation of trial completion success, therefore incorrect trial attempts reflect inaccurate choices rather than a failure to respond. The trial completion success scores for each drug challenge study were evaluated by 3-way repeated measures ANOVA which included factors for task difficulty (1–4 stimuli trials), drug condition (baseline, vehicle and 3 drug doses) and trial-completion success measure (initial-attempt success, overall success). The remaining task measures (attempts per overall correct, percent task completed, sample latency, choice latency, number of correct responses, number of incorrect responses) were analyzed by repeated measures ANOVA which included the single factor of drug condition. Post hoc exploration of any significant main effect or interaction in the 3-way ANOVA was conducted using the Tukey-Kramer procedure whereas the Dunnett procedure was used to explore significant effects in the 1-way ANOVA (vehicle as the control condition). All analyses were conducted using GB-STAT v7.0 for Windows (Dynamic Microsystems, Inc., Silver Spring MD), the criterion for significance in all tests was p < 0.05 and the criterion values were corrected for all possible comparisons in the post hoc tests.
Results
One of the animals designated for the drug challenges failed to complete at least 50% of the task following the first administration of the lowest two doses of both scopolamine and ketamine and was consequently removed from each drug study. Therefore data from a total of 7 animals contributed to each analysis. The main vsPAL measures (initial-attempt and overall trial completion success) are illustrated in Figure 2 and the ancillary vsPAL measures are illustrated in Figure 3 for both drug challenges. Under baseline and vehicle conditions for each study the monkeys’ performance declined with trial difficulty (Figure 2), which is consistent with human studies which have shown that the number of attempts required to complete a given vsPAL trial (Coull et al. 1995), as well as the proportion reaching criterion (Beats et al. 1996) and the number of errors committed (Robbins et al. 1992), in patient populations depends on the number of stimulus-location associations per trial. Similarly, monkeys were able to learn with repeated exposure since overall trial success was higher than initial-attempt trial success under baseline and vehicle conditions. Monkeys’ performance of the task is therefore qualitatively similar to human performance and the use of this model to investigate relationships between pharmacology and brain function is supported. There was no effect of the intramuscular injection protocol for either drug study since post hoc exploration of significant main effects (for all performance measures) failed to confirm any differences between vehicle and baseline conditions (see Figures 2, 3). Likewise, there were no observed differences between monkeys that received ketamine first and those that received scopolamine first, consequently the drug order was not included in the statistical analyses.
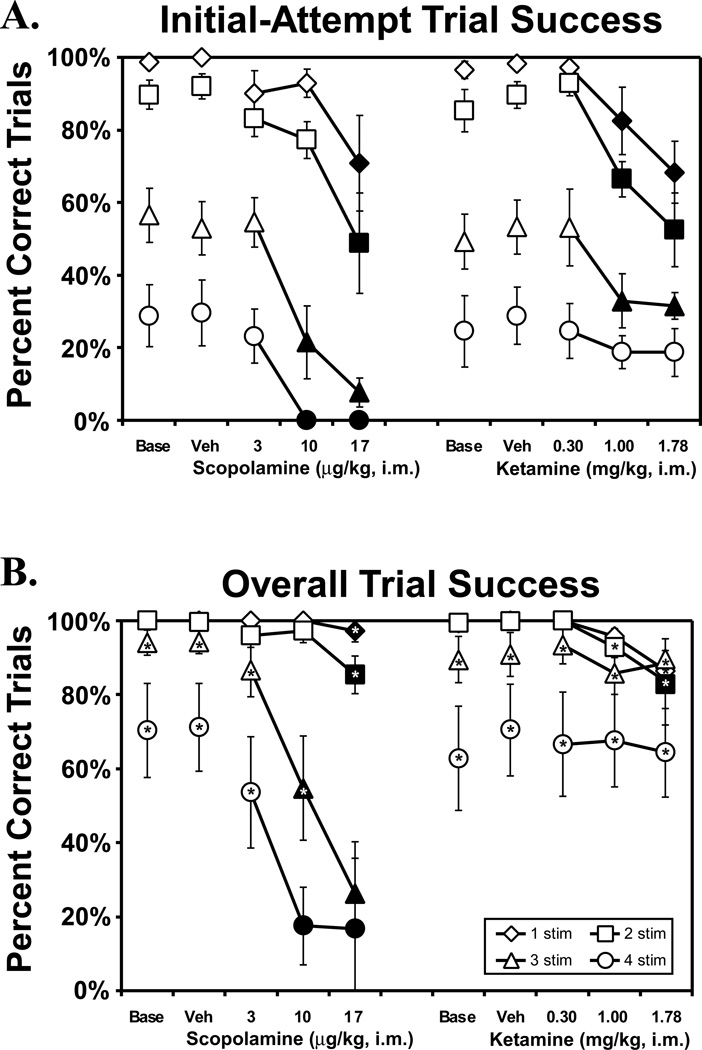
Figure 2. Effect of scopolamine and ketamine on initial-attempt and trial completion success.
The mean (N = 7; ± SEM) proportion of successfully completed trials are presented by trial type (i.e., difficulty) for monkeys challenged acutely with doses of scopolamine and ketamine. Closed symbols indicate significant differences from vehicle performance within a trial type and * indicates a significant difference between initial and overall trial completion success for a given trial type in each drug treatment condition. A complete description of the statistical analysis is provided in the Methods. Base indicates noninjection baseline sessions; Veh indicates vehicle injection sessions.
Figure 3. Effect of ketamine and scopolamine on ancillary vsPAL measures.
Group means (N = 7; ± SEM), collapsed across trial-type, are presented for the total number of correct and incorrect responses made (Panels A), average latency of sample responses and correct choices (Panel B) and the proportion of the task completed (PTC) and the number of attempts required to complete an initially-failed trial (Panel C) following acute challenge with doses of scopolamine and ketamine. Significant differences from vehicle performance are indicated by *. A complete description of the statistical analysis is provided in the Methods. B indicates noninjection baseline sessions; V indicates vehicle injection sessions.
Scopolamine
Acute challenge with scopolamine reduced performance of the vsPAL task in a memory-load specific manner as is illustrated in Figure 2. Overall, the drug impaired both initial-attempt and overall trial completion success (main effect of drug condition; F4,24 = 28.37, p < .05) and furthermore, performance depended on the trial type which demonstrated that increasing the number of stimulus-location associations required for a trial increased the difficulty or memory-load of the task (main effect of trial type, F3,18 = 98.76, p < .05). The scopolamine-associated performance decrement depended on trial difficulty, as confirmed by a significant interaction between drug condition and trial type (F12,72 = 3.69, p < .05). Post hoc analysis of this interaction confirmed that scopolamine significantly reduced both initial-attempt and overall trial completion success relative to vehicle for the 3-stim and 4-stim trials at the 10 and 17 µg doses and for 1-stim and 2-stim trials at the 17 µg dose (Figure 2). A further analysis of the percent of choices (as opposed to trials) that were correct on the first attempt at each trial produced an outcome similar to the initial-attempt trial completion measure (data not shown). Thus, performance on the most difficult trials was most sensitive to the disrupting effects of scopolamine whether measured as initial-attempt trial completion accuracy, initial-attempt choice accuracy or overall trial completion accuracy.
Scopolamine also interfered with the incremental-acquisition or learning aspects of vsPAL. Monkeys were able to learn with repeated attempts at a given trial since overall trial completion success was significantly higher than initial-attempt success (main effect of completion measure; F1,6 = 543.51, p < .05), however, this effect depended on both trial difficulty and drug condition (significant interactions between completion measure and trial type, F3,18 = 8.93, p < .05; and between all three factors; F12,72 = 6.02, p < .05). The fact that the overall completion success was not improved over initial-attempt success only for 4-stim trials at the 10 and 17 µg doses and for 3-stim trials at the 17 µg dose indicates that scopolamine was able to block learning in a dose- and difficulty-dependent manner.
The ancillary measures of vsPAL performance also illustrate a detrimental effect of scopolamine on the monkeys' ability to complete the task (Figure 3). Consistent with the decreases in initial-attempt and overall trial success illustrated in Figure 2, scopolamine significantly reduced the total number of correct responses made (F4,24 = 38.06, p < .05; Figure 3A). Somewhat less consistent with the success measures is the fact that scopolamine significantly reduced the overall number of incorrect choices made (F4,24 = 7.81, p < .05; Figure 3A) however this outcome can be explained as a lowered responsivity and/or task persistance since the percent task completed was also significantly decreased by scopolamine (F4,24 = 9.29, p < .05; Figure 3B). In this context of decreased overall responsivity, the fact that scopolamine did not significantly alter (F4,24 = 0.99, p = .43) the number of attempts required to learn those trials which were initially-failed but ultimately completed successfully (~2 attempts, see Figure 3B) suggests that animals stopped responding after failing a couple of attempts at a given trial. Finally, scopolamine slowed responding as evidenced by the significantly increased latencies of sample responses (F4,24 = 12.58, p < .05; Figure 3C) as well as accurate choice responses (F4,24 = 4.31, p < .05; Figure 3C). The fact that response latencies to sample presentations were more affected by scopolamine than choice latencies indicates that animals were less able to remain “on-task“ or to sustain attention between trials. This distractability from task demands may explain the decreased task persistance.
Ketamine
Acute challenge with ketamine also interfered with trial completion success (main effect of drug condition; (F4,24 = 14.23; p < .05) as is illustrated in Figure 2. What is also apparent in the figure is that ketamine challenge impaired initial-attempt trial completion success while not greatly affecting overall trial completion success (interaction between completion measure and drug condition; F4,24 = 10.59; p < .05). As with the scopolamine study, further analysis of the percent of correct choices on the initial attempt produced an outcome similar to the analysis of the initial-attempt trial completion measure (data not shown). Post hoc exploration confirmed that the 1.0 and 1.78 mg/kg doses of ketamine significantly impaired initial-attempt success relative to vehicle performance for the 1-, 2- and 3-stimulus trials (Figure 2A), suggesting that the drug effect did not interact with trial difficulty in contrast to the effects of scopolamine. It remains possible, however, that a dose × difficulty related effect might have been observed at a dose of ketamine between the 0.3 and 1.0 mg/kg doses employed here. The lack of a ketamine effect on the initial-attempt success for 4-stimuli trials is most likely related to a greater inter-subject variability under this condition. Specifically, several subjects completed very few or none of these trials on the first attempt under baseline conditions and therefore any decrease in performance would not be apparent in those subjects. Variability of drug response in addition to reduced efficacy (compared with scopolamine) might therefore have interacted with this baseline intersubject variation to obscure an effect of ketamine on the most difficult trials. It must be acknowledged, however, that the lack of effect of ketamine on the 4-stimulus trials may also be attributed to an artifact of the experimental design. Ketamine has a relatively rapid pharmacokinetic profile in monkeys and a recent study of non-operant behaviors has demonstrated that effects of single subanaesthetic doses peak approximately 10 minutes post injection and last approximately 40–50 minutes (Shiigi and Casey 1999). Since the vsPAL difficulty conditions are evaluated in sequential order it is possible that the more difficult trials were consistently completed at lower CNS drug concentrations compared with the easier trials. This artifact is unlikely to completely explain the current results, however, since ketamine consistently produced a dose-related effect on the BMS task run after the vsPAL session (data not shown) indicating that behaviorally active levels of the drug persisted.
As with the scopolamine study, the monkeys' performance was graded by task difficulty and improved with repeated attempts at a given trial (main effects of completion measure, F1,6 =152.25, p < .05; trial type, F3,18 = 35.43, p < .05). The significant interaction between completion measure and trial type (F3,18 = 17.77; p < .05) is most likely attributable to near-ceiling initial-attempt trial success for the easiest trial type (Figure 2A) since the post hoc analysis confirmed that the 3- and 4-stimulus overall trial completion was significantly higher than initial-attempt success for baseline, vehicle and each ketamine dose. Overall trial success was significantly better than initial-attempt success for 2-stim following the 1.0 and 1.78 mg/kg doses as well. Consistent with this and in contrast to the effect of scopolamine, overall trial completion success was essentially unaffected by ketamine in that post hoc analysis confirmed significant changes from vehicle performance only for 2-stim trials following the 1.78 mg/kg dose (Figure 2B).
The ancillary measures of vsPAL performance suggest that monkeys’ task persistance was not significantly altered by any dose of ketamine (Figure 3), in contrast to the effects of scopolamine. There was no significant effect of ketamine on the percent of task completed (F4,24 = 0.16; p = .95; Figure 3B) and furthermore, monkeys made significantly more incorrect responses (F4,24 = 5.79; p < .05) and exhibited a trend for increased numbers of total correct responses (F4,24 = 2.56; p =.06; Figure 3A) after ketamine. These data are consistent with the pattern of decreased initial-attempt trial completion success and preserved overall trial success shown in Figure 2. The fact that there was no significant increase in the number of attempts required to learn an initially-failed trial following ketamine challenge (F4,24 = 1.72, p = .12; Figure 3B) suggests that monkeys were able to learn an initially-failed trial quite rapidly under all doses of ketamine even while the number of initial-attempt failures increased. Finally, as with scopolamine, ketamine produced significant increases in both sample latency (F4,24 = 9.21; p < .05) and choice latency (F4,24 = 12.19; p < .05; Figure 3C). Unlike scopolamine, however, the slowing of responses was equivalent for sample and choice responses which is not consistent with any effect of the drug on distractability or attention. Therefore, in total, the pattern of the ancillary measures is quite distinct from the profile produced by scopolamine and suggests that task persistance and/or sustained attention was well-preserved following all doses of ketamine evaluated.
Discussion
The outcome of this investigation demonstrates that monkeys’ performance of a task which has been found to be sensitive to, and specific for, cognitive symptoms arising early in AD (Fowler et al. 1997; Swainson et al. 2001) is disrupted by either acute muscarinic or NMDA blockade in a manner that is consistent with previous observations in monkeys trained on other tests of spatial or pattern memory (e.g., Buffalo et al. 1994; Ogura and Aigner 1993; Schulze et al. 1992). In particular, the measure most similar in form to traditional D(N)MS and spatial delayed-response (DR) procedures, i.e., the initial-attempt trial success, was impaired in a dose-dependent manner by each drug. The present vsPAL procedure, while not identical to either D(N)MS or DR memory procedures, incorporates aspects of each of these tasks and therefore the demonstration of impaired vsPAL performance following NMDA and muscarinic blockade represents a necessary and important pharmacological validation of the behavioral test. Beyond this basic validation, the present findings illustrate both similarities and differences in the way that muscarinic and NMDA antagonists interfere with memory performance thus permitting an interpretation of distinct roles. Therefore the a priori working hypothesis that the two drugs would produce similar effects, based on indirect comparison of previous findings, was not supported. This description of the manner in which muscarinic and NMDA antagonists interfere with vsPAL performance may therefore be helpful in inferring a more specific brain insult from human neuropsychological test results.
In the present results, scopolamine produced a dose × difficulty dependent impairment of performance as measured by either initial-attempt or overall completion success. For example, following the 10 µg/kg dose performance on the 1- and 2-stimuli trials was not significantly decreased whereas performance on the more difficult trials was significantly impaired. These results demonstrate that scopolamine interferes with both the formation of a stimulus-location association (initial-attempt success) and the incremental strengthening of that association (overall success). The difficulty-dependent dose-effect suggests a highly specific effect of scopolamine on the memory-load aspects of the task. In contrast, while ketamine clearly impaired the strength of a stimulus-location association formed on the initial sample presentation for each trial (lowered initial-attempt trial success), the drug did not block incremental trial acquisition, or learning, since animals were able to achieve overall success equivalent to untreated performance. Similarly, there was no evidence that ketamine impaired initial-attempt trial success in a dose × difficulty dependent manner suggesting an effect on memory that did not depend on memory load. Therefore the present results fail to support the working hypothesis that scopolamine and ketamine effects on memory are identical.
The differences observed between the effects of scopolamine and ketamine on vsPAL performance may be interpretable post hoc under a single model of memory function. Hasselmo has proposed a model (Hasselmo 1995; 1999), based on evidence from multiple levels of analysis, describing the manner in which muscarinic and NMDA mechanisms of the hippocampus may contribute to memory function. In short, the model proposes that NMDA mechanisms strengthen associative links between discontinuous stimuli whereas muscarinic activity switches that associative activity between intrinsic and extrinsic input. Thus in the present vsPAL task, NMDA mechanisms would be involved in the association of visual pattern information with spatial location information on any given sample presentation (e.g., "blue square" goes with "right side"). Muscarinic systems would be predicted to contribute to the temporal, or episodic, ordering of associations. For example, on the third sample of a three-stimulus trial, the animal is faced with three "correct" target spatial locations to associate with the present stimulus pattern. The current "correct" location is derived from extrinsic information (perceptual) but two other "correct" locations are derived from intrinsic information (remembered from the first two sample locations within the trial). The Hasselmo model predicts that muscarinic activity enhances the salience of extrinsic information over intrinsic information, thereby allowing the (NMDA-dependent) linkage of current-pattern with current-location instead of erroneously linking the current pattern with a previously-correct location. Thus, muscarinic antagonists such as scopolamine or atropine are predicted to interfere most severely with memory tests featuring high levels of proactive interference from intrinsic sources such as limited-set D(N)MS or spatial delayed-response procedures (e.g., Bartus and Johnson 1976; Penetar and McDonough 1983). In contrast, the effect of NMDA antagonists would be predicted to be independent of such interference. The Hasselmo model therefore offers an explanation for two aspects of the present data. First, the model attributes the relatively stronger trial difficulty-dependent effect of scopolamine on initial-attempt accuracy to an increase in proactive interference on multi-stimulus trials as outlined above. Second, the model attributes impaired initial attempt accuracy combined with a preserved ability to learn a set of associations with repeated attempts under ketamine to an attenuation of the stimulus-location associative process on each attempt without disrupting the identity of the location to be associated with a given stimulus. In contrast, the model attributes the impaired ability to improve with practice under scopolamine to a disruption of the linkage of a correct stimulus with a correct location because of interference from previous "correct" spatial or pattern information. Therefore, while the present study was not designed as an explicit test of the Hasselmo proposals (Hasselmo 1995; 1999), the current results support that model of hippocampal muscarinic and NMDA contributions to memory function.
The comparison between the effects of scopolamine and ketamine challenge in the present results highlight another advantage of the vsPAL procedure in studying memory (dys)function. Observations limited to the initial-attempt trial completion success would appear consistent with a previous study which demonstrated that short-term visual recognition memory was impaired by dizocilpine (MK-801) in a manner approximately equivalent to (and additive with) the effects of scopolamine (Matsuoka and Aigner 1996) and therefore consideration only of the initial-attempt success in the present study would appear to simply extend those results to short-term visuo-spatial association recognition memory. The addition of the incremental learning component of the vsPAL task, however, allows an important contrast to be drawn between the effect of the two drugs. Specifically, monkeys were able to achieve overall trial success equivalent to baseline levels following all doses of ketamine whereas overall success was impaired by scopolamine in a dose × difficulty dependent manner. In contrast, monkeys made fewer correct responses, fewer errors and missed more trials after scopolamine. This difference might be most parsimoniously attributed to a preserved ability to remain "on-task" (i.e., preserved “sustained attention“ or persistance) following ketamine administration but not after scopolamine.
The present work therefore recommends consideration of multiple performance variables in future clinical investigations. The initial human studies which employed this task contrasted the proportion of subjects reaching a priori performance criteria between groups (e.g., Sahakian et al. 1988; Sahgal et al. 1991), an approach constrained by a limited ability to describe performance changes over time in a single individual. Later longitudinal studies (Fowler et al. 1997; Swainson et al. 2001) have employed an "error-to-criterion" measure (analogous to the "total incorrect responses" measure used in the present study) of performance which is limited by the assumption that subjects can successfully complete all trials with a given number of allowed attempts (analogous to a consistent overall trial success in the present study). This approach makes it difficult to compare intact and compromised performance since some individuals may not reach criterion within the alotted (or any number of) trial, as was found for monkeys following scopolamine. Swainson and colleagues (Swainson et al. 2001) partially satisfy these concerns by providing both error-to-criterion and percent-failure measures. Interestingly, in a scopolamine challenge study of normal volunteers only a trend for impaired vsPAL performance was observed at the highest dose administered (Robbins et al. 1997). The present results would suggest that effects on the most-difficult trial types were obscured by combining all trial-difficulties in the analysis. Consequently, contrast of the effects of scopolamine and ketamine in the present study illustrates the importance of considering multiple aspects of vsPAL performance and, by extension any neuropsychological measure.
In summary, previous observations from studies of visuo-spatial paired-associates learning in elderly and AD human populations have suggested that this nonverbal task may offer improved specificity for identifying age-related cognitive dysfunction (Fowler et al. 1997; Rabbitt and Lowe 2000; Robbins et al. 1994; Swainson et al. 2001). The results of the present study demonstrate that rhesus monkeys perform the task in a manner analogous to humans and that their performance is disrupted by administration of scopolamine in a manner consistant with previous anticholinergic models of memory disruption. Furthermore, challenge of the monkeys with ketamine demonstrated that while muscarinic and NMDA blockade may have similar detrimental effects on some measures of memory function, differences were readily apparent when considering all aspects of vsPAL performance. Different patterns of vsPAL impairment in individual patients may therefore recommend or contraindicate particular drug therapies.
Acknowledgements
We are grateful to Ilham Polis and Bob Lintz for expert technical assistance. This work was supported by funds from the Universitywide AIDS Research Program, University of California, Grant No. F99-SRI051 (MAT) and by USPHS grants DA13390 (MAT), DA05831 (MRW), DA09111 (LHG) and MH47680 (LHG). This is publication #13905-NP from The Scripps Research Institute.
References
- Bartus RT. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol. 2000;163:495–529. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Johnson HR. Short-term memory in the rhesus monkey: disruption from the anti- cholinergic scopolamine. Pharmacol Biochem Behav. 1976;5:39–46. doi: 10.1016/0091-3057(76)90286-0. [DOI] [PubMed] [Google Scholar]
- Beats BC, Sahakian BJ, Levy R. Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychol Med. 1996;26:591–603. doi: 10.1017/s0033291700035662. [DOI] [PubMed] [Google Scholar]
- Blesch A, Grill RJ, Tuszynski MH. Neurotrophin gene therapy in CNS models of trauma and degeneration. Prog Brain Res. 1998;117:473–484. doi: 10.1016/s0079-6123(08)64033-9. [DOI] [PubMed] [Google Scholar]
- Boyce S, Rupniak NM, Steventon MJ, Cook G, Iversen SD. Psychomotor activity and cognitive disruption attributable to NMDA, but not sigma, interactions in primates. Behav Brain Res. 1991;42:115–121. doi: 10.1016/s0166-4328(05)80002-6. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Gillam MP, Allen RR, Paule MG. Acute behavioral effects of MK-801 in rhesus monkeys: assessment using an operant test battery. Pharmacol Biochem Behav. 1994;48:935–940. doi: 10.1016/0091-3057(94)90203-8. [DOI] [PubMed] [Google Scholar]
- Coull JT, Middleton HC, Robbins TW, Sahakian BJ. Clonidine and diazepam have differential effects on tests of attention and learning. Psychopharmacology (Berl) 1995;120:322–332. doi: 10.1007/BF02311180. [DOI] [PubMed] [Google Scholar]
- Flicker C, Ferris SH, Reisberg B. Mild cognitive impairment in the elderly: predictors of dementia. Neurology. 1991;41:1006–1009. doi: 10.1212/wnl.41.7.1006. [DOI] [PubMed] [Google Scholar]
- Fowler KS, Saling MM, Conway EL, Semple JM, Louis WJ. Computerized delayed matching to sample and paired associate performance in the early detection of dementia. App Neuropsych. 1995;2:72–78. doi: 10.1207/s15324826an0202_4. [DOI] [PubMed] [Google Scholar]
- Fowler KS, Saling MM, Conway EL, Semple JM, Louis WJ. Computerized neuropsychological tests in the early detection of dementia: prospective findings. J Int Neuropsychol Soc. 1997;3:139–146. [PubMed] [Google Scholar]
- Frederick DL, Gillam MP, Allen RR, Paule MG. Acute behavioral effects of phencyclidine on rhesus monkey performance in an operant test battery. Pharmacol Biochem Behav. 1995;52:789–797. doi: 10.1016/0091-3057(95)00182-v. [DOI] [PubMed] [Google Scholar]
- Ghoneim MM, Hinrichs JV, Mewaldt SP, Petersen RC. Ketamine: behavioral effects of subanesthetic doses. J Clin Psychopharmacol. 1985;5:70–77. [PubMed] [Google Scholar]
- Harder JA, Aboobaker AA, Hodgetts TC, Ridley RM. Learning impairments induced by glutamate blockade using dizocilpine (MK-801) in monkeys. Br J Pharmacol. 1998;125:1013–1018. doi: 10.1038/sj.bjp.0702178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. Neuromodulation and cortical function: modeling the physiological basis of behavior. Behav Brain Res. 1995;67:1–27. doi: 10.1016/0166-4328(94)00113-t. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. Neuromodulation: acetylcholine and memory consolidation. Trends Cogn Sci. 1999;3:351–359. doi: 10.1016/s1364-6613(99)01365-0. [DOI] [PubMed] [Google Scholar]
- Hetem LA, Danion JM, Diemunsch P, Brandt C. Effect of a subanesthetic dose of ketamine on memory and conscious awareness in healthy volunteers. Psychopharmacology (Berl) 2000;152:283–288. doi: 10.1007/s002130000511. [DOI] [PubMed] [Google Scholar]
- Huang EP, Stevens CF. The matter of mind: molecular control of memory. Essays Biochem. 1998;33:165–178. doi: 10.1042/bse0330165. [DOI] [PubMed] [Google Scholar]
- Iversen SD. Behavioural evaluation of cholinergic drugs. Life Sci. 1997;60:1145–1152. doi: 10.1016/s0024-3205(97)00059-3. [DOI] [PubMed] [Google Scholar]
- Kinscherf R, Deigner HP, Haberkorn U. Apoptosis modulators in the therapy of neurodegenerative diseases. Expert Opin Investig Drugs. 2000;9:747–764. doi: 10.1517/13543784.9.4.747. [DOI] [PubMed] [Google Scholar]
- Lichtenberg PA, Ross T, Millis SR, Manning CA. The relationship between depression and cognition in older adults: a cross-validation study. J Gerontol B Psychol Sci Soc Sci. 1995;50:P25–P32. doi: 10.1093/geronb/50b.1.p25. [DOI] [PubMed] [Google Scholar]
- Massoud F, Devi G, Moroney JT, Stern Y, Lawton A, Bell K, Marder K, Mayeux R. The role of routine laboratory studies and neuroimaging in the diagnosis of dementia: a clinicopathological study. J Am Geriatr Soc. 2000;48:1204–1210. doi: 10.1111/j.1532-5415.2000.tb02591.x. [DOI] [PubMed] [Google Scholar]
- Masur DM, Sliwinski M, Lipton RB, Blau AD, Crystal HA. Neuropsychological prediction of dementia and the absence of dementia in healthy elderly persons. Neurology. 1994;44:1427–1432. doi: 10.1212/wnl.44.8.1427. [DOI] [PubMed] [Google Scholar]
- Matsuoka N, Aigner TG. Cholinergic-glutamatergic interactions in visual recognition memory of rhesus monkeys. Neuroreport. 1996;7:565–568. doi: 10.1097/00001756-199601310-00045. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McMahon PM, Araki SS, Neumann PJ, Harris GJ, Gazelle GS. Cost-effectiveness of functional imaging tests in the diagnosis of Alzheimer disease. Radiology. 2000;217:58–68. doi: 10.1148/radiology.217.1.r00se1358. [DOI] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC. Expression mechanisms underlying NMDA receptor-dependent long-term potentiation. Ann N Y Acad Sci. 1999;868:515–525. doi: 10.1111/j.1749-6632.1999.tb11320.x. [DOI] [PubMed] [Google Scholar]
- Ogura H, Aigner TG. MK-801 impairs recognition memory in rhesus monkeys: comparison with cholinergic drugs. J Pharmacol Exp Ther. 1993;266:60–64. [PubMed] [Google Scholar]
- Oye I, Paulsen O, Maurset A. Effects of ketamine on sensory perception: evidence for a role of N- methyl-D-aspartate receptors. J Pharmacol Exp Ther. 1992;260:1209–1213. [PubMed] [Google Scholar]
- Penetar DM, McDonough JH., Jr Effects of cholinergic drugs on delayed match-to-sample performance of rhesus monkeys. Pharmacol Biochem Behav. 1983;19:963–967. doi: 10.1016/0091-3057(83)90399-4. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Hodges JR. Fate of patients with questionable (Very mild) Alzheimer's disease: longitudinal profiles of individual Subjects' decline. Dement Geriatr Cogn Disord. 2000;11:342–349. doi: 10.1159/000017264. [DOI] [PubMed] [Google Scholar]
- Rabbitt P, Lowe C. Patterns of cognitive ageing. Psychol Res. 2000;63:308–316. doi: 10.1007/s004269900009. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Baker HF. A critical evaluation of monkey models of amnesia and dementia. Brain Res Brain Res Rev. 1991;16:15–37. doi: 10.1016/0165-0173(91)90018-4. [DOI] [PubMed] [Google Scholar]
- Robbins TW, James M, Lange KW, Owen AM, Quinn NP, Marsden CD. Cognitive performance in multiple system atrophy. Brain. 1992;115(Pt 1):271–291. doi: 10.1093/brain/115.1.271. [DOI] [PubMed] [Google Scholar]
- Robbins TW, James M, Owen AM, Sahakian BJ, McInnes L, Rabbitt P. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5:266–281. doi: 10.1159/000106735. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Semple J, Kumar R, Truman MI, Shorter J, Ferraro A, Fox B, McKay G, Matthews K. Effects of scopolamine on delayed-matching-to-sample and paired associates tests of visual memory and learning in human subjects: comparison with diazepam and implications for dementia. Psychopharmacology (Berl) 1997;134:95–106. doi: 10.1007/s002130050430. [DOI] [PubMed] [Google Scholar]
- Rupniak NM, Duchnowski M, Tye SJ, Cook G, Iversen SD. Failure of d-cycloserine to reverse cognitive disruption induced by scopolamine or phencyclidine in primates. Life Sci. 1992;50:1959–1962. doi: 10.1016/0024-3205(92)90525-t. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Downes JJ, Eagger S, Evenden JL, Levy R, Philpot MP, Roberts AC, Robbins TW. Sparing of attentional relative to mnemonic function in a subgroup of patients with dementia of the Alzheimer type. Neuropsychologia. 1990;28:1197–1213. doi: 10.1016/0028-3932(90)90055-s. [DOI] [PubMed] [Google Scholar]
- Sahakian BJ, Morris RG, Evenden JL, Heald A, Levy R, Philpot M, Robbins TW. A comparative study of visuospatial memory and learning in Alzheimer- type dementia and Parkinson's disease. Brain. 1988;111:695–718. doi: 10.1093/brain/111.3.695. [DOI] [PubMed] [Google Scholar]
- Sahgal A, Sahakian BJ, Robbins TW, Wray CJ, Lloyd S, Cook JH, McKeith IG, Disley JCA, Eagger S, Boddington S, Edwardson JA. Detection of Visual Memory and Learning Deficits in Alzheimer's Disease Using the Cambridge Neuropsychological Test Automated Battery. Dementia. 1991;2:150–158. [Google Scholar]
- Schulze GE, Gillam MP, Paule MG. Effects of atropine on operant test battery performance in rhesus monkeys. Life Sci. 1992;51:487–497. doi: 10.1016/0024-3205(92)90025-k. [DOI] [PubMed] [Google Scholar]
- Shiigi Y, Casey DE. Behavioral effects of ketamine, an NMDA glutamatergic antagonist, in non-human primates. Psychopharmacology (Berl) 1999;146:67–72. doi: 10.1007/s002130051089. [DOI] [PubMed] [Google Scholar]
- Swainson R, Hodges JR, Galton CJ, Semple J, Michael A, Dunn BD, Iddon JL, Robbins TW, Sahakian BJ. Early detection and differential diagnosis of Alzheimer's disease and depression with neuropsychological tasks. Dement Geriatr Cogn Disord. 2001;12:265–280. doi: 10.1159/000051269. [DOI] [PubMed] [Google Scholar]
- Taffe MA, Weed MR, Gold LH. Scopolamine alters rhesus monkey performance on a novel neuropsychological test battery. Brain Res Cogn Brain Res. 1999;8:203–212. doi: 10.1016/s0926-6410(99)00021-x. [DOI] [PubMed] [Google Scholar]
- Thompson DM, Mastropaolo J, Winsauer PJ, Moerschbaecher JM. Repeated acquisition and delayed performance as a baseline to assess drug effects on retention in monkeys. Pharmacol Biochem Behav. 1986;25:201–207. doi: 10.1016/0091-3057(86)90253-4. [DOI] [PubMed] [Google Scholar]
- Weed MR, Taffe MA, Polis I, Roberts AC, Robbins TW, Koob GF, Bloom FE, Gold LH. Performance norms for a rhesus monkey neuropsychological testing battery: acquisition and long-term performance. Brain Res Cogn Brain Res. 1999;8:185–201. doi: 10.1016/s0926-6410(99)00020-8. [DOI] [PubMed] [Google Scholar]
- Welsh KA, Butters N, Hughes JP, Mohs RC, Heyman A. Detection and staging of dementia in Alzheimer's disease. Use of the neuropsychological measures developed for the Consortium to Establish a Registry for Alzheimer's Disease. Arch Neurol. 1992;49:448–452. doi: 10.1001/archneur.1992.00530290030008. [DOI] [PubMed] [Google Scholar]
- Zakzanis KK, Leach L, Kaplan E. On the nature and pattern of neurocognitive function in major depressive disorder. Neuropsychiatry Neuropsychol Behav Neurol. 1998;11:111–119. [PubMed] [Google Scholar]