Abstract
Alcohol exposure on postnatal days (PND) 4-9 in the rat adversely affects hippocampal anatomy and function and impairs performance on a variety of hippocampus-dependent tasks. Exposure during this developmental window reveals a linear relationship between alcohol dose and spatial learning impairment in the context preexposure facilitation effect (CPFE), a hippocampus-dependent variant of contextual fear conditioning (Murawski & Stanton, 2011). The purpose of the current report was to examine the effect of a range of alcohol doses administered during a narrower window, PND7-9, than previously reported (Experiment 1) and to begin to determine which memory processes involved in this task are impaired by developmental alcohol exposure (Experiment 2). In Experiment 1, rats pups received a single day binge alcohol dose of either 2.75, 4.00, 5.25 g/kg/day or were sham-intubated (SI) from PND7-9. Conditioned freezing during the test day was evident in all dosing groups, except for Group 5.25g, indicating no graded dose-related behavioral deficits with alcohol exposure limited to PND7-9. In Experiment 2, rat pups were exposed to the highest effective dose from Experiment 1 (5.25 g/kg/day) or were sham intubated over PND7-9. During training, rats remained in the conditioning context for 5-min following immediate shock delivery. During this test of post-shock freezing, both SI and alcohol-exposed rats given prior exposure to the conditioning context showed comparable freezing levels. Since alcohol-exposed rats showed normal post-shock freezing, deficits by these rats on the test day likely reflect a failure to consolidate or retrieve a context-shock association, rather than a deficit in hippocampal conjunctive processes (consolidation, pattern completion) that occur prior to shock on the training day. These findings illustrate the value of the CPFE for characterizing the separable memory processes that are impaired by neonatal alcohol exposure in this task.
Keywords: Context Preexposure Facilitation Effect (CPFE), Neonatal Alcohol Exposure, Fetal Alcohol Spectrum Disorders, Spatial Cognition, Hippocampus, Development
Introduction
Fetal alcohol spectrum disorder (FASD) describes a continuum of birth defects caused by maternal intake of alcohol during pregnancy. In humans, developmental alcohol exposure impairs the normal development of many brain regions including the cerebellum and hippocampus (Norman, Crocker, Mattson, & Riley, 2009; Willoughby, Sheard, Nash, & Rovet, 2008). These abnormalities are likely the cause of behavioral deficits in children in a variety of learning and memory tasks, such as eyeblink conditioning and spatial recognition memory (Hamilton, Kodituwakku, Sutherland, & Savage, 2003; Jacobson, Jacobson, Stanton, Meintjes, & Molteno, 2011; Jacobson et al., 2008; Spottiswoode et al., 2011; Uecker & Nadel, 1998). Importantly, the adverse effects of alcohol are largely a result of the timing, pattern and dosage of maternal ethanol consumption (Maier & West, 2001). Rodent model research has been useful in identifying the effects of these variables, especially the effects of different developmental windows of exposure which can’t be manipulated and are therefore difficult to study in human FASD. In rat models of FASD, the hippocampus, for example, is particularly vulnerable to damage when alcohol is administered during the neonatal period, which is equivalent to the brain growth spurt during the third-trimester of human pregnancy (Dobbing & Sands, 1979). Binge-like alcohol exposure during this period (PND4-9) produces hippocampal CA1 pyramidal cell loss, following a range of alcohol doses (Livy, Miller, Maier, & West, 2003; Marino, Aksenov, & Kelly, 2004; Murawski, Klintsova, & Stanton, 2012; Tran & Kelly, 2003).
When exposure is limited to PND7-9, CA1 pyramidal cell loss is also evident after administration with a high dose (5.25g/kg/day; Marino et al., 2004), suggesting hippocampal vulnerability during this narrow time window. At high alcohol doses, neonatal exposure produces behavioral deficits in a variety of hippocampus-dependent learning tasks such as spatial water maze and trace fear conditioning (Goodlett & Johnson, 1997; Hunt, Jacobson, & Torok, 2009). Despite high dose exposure, the behavioral deficits seen in these tasks tend to be modest. This may account for the limited data regarding dose-response effects of neonatal alcohol in spatial memory tasks (Murawski & Stanton, 2011). However, previous reports from our laboratory demonstrate that the context preexposure facilitation effect (CPFE) is especially sensitive to neonatal alcohol exposure (Murawski & Stanton, 2010; Murawski & Stanton, 2011). The CPFE is a variant of contextual fear conditioning which requires the hippocampus and emphasizes learning of conjunctive representations of context that is incidental rather than reinforcement-driven (Matus-Amat, Higgins, Barrientos, & Rudy, 2004; Rudy, 2009). The CPFE is abolished by alcohol administered during both the PND4-9 and PND7-9 periods of exposure. Additionally, following exposure of either 2.75, 4.00 and 5.25 g/kg/day from PND4-9, the CPFE reveals a linear dose-response curve and a significant negative correlation between test performance and blood alcohol concentration (BAC, Murawski & Stanton, 2011). The current report extends this previous work by examining dose-response effects on CPFE performance when alcohol exposure occurs during a narrower (PD7-9) period of neonatal development. Because the CPFE is particularly sensitive to neonatal alcohol and factors such as the window of exposure greatly determine alcohol effects on the developing brain (Gil-Mohapel, Boehme, Kainer, & Christie, 2010), it is of interest to examine dose-response functions with a more limited window of ethanol exposure using the CPFE as a comparison with dose-response effects reported with other tasks (Goodlett & Johnson, 1997; Murawski, Jablonski, Brown, & Stanton, 2013).
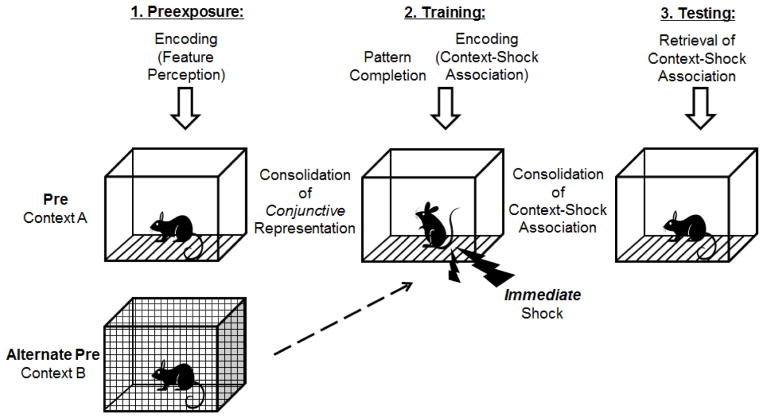
The CPFE is a 3-day procedure, requiring separable memory processes for each phase (Figure 1). In the CPFE, preexposure to the context occurs on the first day. This involves encoding the features of the context into a single unified representation (Jablonski, Schiffino, & Stanton, 2012; Rudy & O’Reilly, 1999; Rudy, 2009). Twenty-four hours later, during training, animals given prior exposure to the preexposure/training context use “pattern completion” in which a subset of the contextual features, experienced prior to immediate shock delivery, trigger recall of the entire contextual representation (or “context memory”) from the preexposure day, to associate that representation of the context with immediate foot shock (Rudy, 2009). Following consolidation of the context-shock association, a test of contextual freezing occurs on the final day (Fanselow, 1990). During the testing session, those rats pre-exposed to the conditioning context retrieve the context-shock association and freeze more than rats exposed to an alternate context. The latter group shows the “immediate shock deficit,” a failure to associate the training context with shock because of insufficient time to encode the context (Fanselow, 1990). In the CPFE, consolidation of the context memory itself can be examined apart from consolidation of the contextual fear memory, since the spatial learning and affective learning processes occur on separate days. This task, then, can be used to determine which memory processes are disrupted by neonatal alcohol exposure. One hypothesis is that alcohol-exposed rats can form conjunctive representations on the preexposure day and context-shock associations on the training day but fail to freeze on the test day because they cannot consolidate the context-shock association after training or cannot retrieve it during testing. This hypothesis can be tested by measuring post-shock freezing on the training day, which indicates that the context-shock association has been encoded at the time of training (Kim, Fanselow, DeCola, & Landeira-Fernandez, 1992; Rudy & Morledge, 1994). We have recently shown that developing rats given prior preexposure to the conditioning context display an increase in post-shock freezing on the training day relative to animals exposed to an alternate context (Jablonski et al., 2012), indicating intact post-shock freezing during the CPFE in developing rats. However, no studies have examined the influence of developmental alcohol exposure on post-shock freezing in the CPFE. The current report extends previous findings (Murawski & Stanton, 2010; Murawski & Stanton, 2011), by examining the effects of a range of alcohol doses (2.75, 4.00 and 5.25 g/kg/day) administered from PND7-9 on the CPFE. Experiment 2 extends a test of post-shock freezing to alcohol exposed animals in order to determine which memory processes involved in the CPFE are impaired by developmental alcohol exposure.
Figure 1.
Schematic diagram of the context preexposure facilitation effect (CPFE) paradigm and associated memory processes. On the first day (‘Preexposure’) rats are placed in the training context for 5-min (Group Pre) or are exposed to an alternate context (Group Alt-pre). During preexposure, the individual features of the context are bound together in a single-unified conjunctive representation of the context. Following consolidation of the conjunctive representation in memory twenty-four hours later (‘Training’), rats from both preexposure conditions are placed in the training context. Here, pattern completion occurs prior to immediate shock delivery in which a subset of the features are able to elicit retrieval from long-term memory of the entire conjunctive representation. Following shock delivery, consolidation of the association of context with shock occurs. After consolidation of the context-shock representation, twenty-four hours later (‘Testing’), all rats are returned to the training context at which point freezing behavior (fear to the context) is assessed for 5-min. Because animals preexposed to the training context are able to retrieve the contextual representation memory previously associated with shock, these animals (Group Pre) show an increase in freezing to the context compared to Group Alt-Pre.
Materials & Methods
Subjects
For Experiment 1 Subjects were 113 Long-Evans rats derived from 16 litters. For Experiment 2 Subjects were 73 Long-Evans rats derived from 9 litters.
As described previously (Murawski & Stanton, 2011), time-mated females were housed with breeder males in the animal housing facility of the Office of Laboratory Animal Medicine (OLAM) at the University of Delaware. The next day, rats were examined for an ejaculatory plug and, if found, that day was designated as gestational day (GD) 0. Pregnant females were housed in polypropylene cages (45 × 42 × 21 cm) with standard bedding and ad libitum access to food and water. The animal housing facility was maintained on a 12:12 hour light/dark cycle. Offspring date of birth was designated as postnatal day (PND) 0 and occurred on GD22 (between 09:00 and 17:00). Litters remained in the animal housing facility until PND2, at which point they were housed in the lab’s local colony room. On PND3, litters were culled to 8 pups per litter (typically 4 males and 4 females) and received a subcutaneous injection of a non-toxic black ink into one or more paws for identification. Pups were weaned on PND21 and housed in 45 × 24 × 17 cm cages with same-sex littermates, with ad libitum access to water and food. On PND29, rats were housed individually in small white polypropylene cages (24 × 18 × 13 cm) until the completion of behavioral testing. All subjects were treated in accordance with guidelines of the Institutional Animal Care and Use Committee at the University of Delaware.
Alcohol Dosing
For Experiment 1, rat pups were randomly assigned to be given one of three alcohol doses (2.75, 4.00, or 5.25 g/kg/day) or to receive sham intubations (SI). Whenever possible, one male and one female per litter were assigned to the same dosing or SI group (Treatment Condition). If same sex littermates were assigned to the same Treatment Condition, they were placed in distinct behavioral groups (Pre vs. Alt. Pre; Behavioral Group) so that no more than one same sex pup from the same litter was assigned to any given experimental condition (Treatment Condition x Behavioral Group).
Alcohol was administered via intragastric intubation from PND7 through PND9 as described previously (Murawski & Stanton, 2011). On PND7, pups were separated from the dam and placed in large anti-static weigh boats placed over a heating pad (low setting), to compensate for the lack of thermoregulation. Pups from each Treatment Condition were weighed prior to the first intubation (usually around 09:00). For alcohol-exposed pups, alcohol was delivered in a custom milk formula (see Kelly & Lawrence, 2008), in a single-binge dose. The milk formula was delivered in a volume of 0.02778 ml/g body weight at 12.53% (Group 2.75g), 18.19% (Group 4.00g), 23.94% (Group 5.25g) v/v. The intubation process involved gently passing PE10 tubing lubricated with corn oil down the esophagus and into the stomach at which point the milk formula was slowly released (about 10–15 sec). Group SI received the identical intubation process; however, no milk formula was given. Following completion of the intubation procedure (about 20 min per litter), pups were returned to the dam and colony room. Approximately 2 hours (+/− 10 min) after the first alcohol dose, pups were again separated from the dam for a second dosing session. Prior to intubation, pups received a small tail-clip in which blood samples were collected with a heparinized capillary tube. Blood sampled from Group SI were immediately discarded and samples from all alcohol treatment conditions were stored for further analysis (see ‘Blood Alcohol Analysis’). The second dosing session was identical to the first; however, pups from each alcohol treatment condition (Group 2.75g, 4.00g and 5.25g) received an infusion of milk only, without alcohol. Pups from Group SI received a sham intubation. A third milk-only dose or sham intubation occurred following the second dosing session. Alcohol-exposed pups received additional milk-only doses in order to help maintain normal body weight throughout the dosing period (Marino et al., 2004). Within daily dosing sessions, each pup received its subsequent alcohol or milk dose within 2 hours (+/− 10 min). Dosing continued in the same manner from PND8 through PND9 except no blood samples were collected and only 1 additional milk-only dose was given following the first daily alcohol administration.
For Experiment 2, all alcohol dosing procedures, including BAC analyses, were identical to Experiment 1, however, only the highest dose (5.25g/kg/day) was included. Thus, each litter typically consisted of 4 pups assigned to Group 5.25g and 4 pups to Group SI. Whenever possible, two males and two females were assigned to the same Treatment Condition. Same sex littermates that were assigned to the same treatment condition, were placed in different behavioral groups (Pre vs. Alt. Pre; Behavioral Group) so that no more than one same sex pup from the same litter was assigned to any given experimental condition (Treatment Condition x Behavioral Group).
Blood Alcohol Concentration
Blood samples from PND7 alcohol-exposed pups were centrifuged and plasma was stored at −20°C. Blood alcohol concentrations (BACs) were determined using an Analox GL5 Analyzer (Analox Instruments, Luneburg, MA), as previously described (Brown, Calizo, Goodlett, & Stanton, 2007; Murawski & Stanton, 2011). Briefly, the rate of oxidation of alcohol was measured from each plasma sample. BACs were determined by comparing the alcohol concentration (mg/dl) to the known values of an alcohol standard.
Apparatus & Stimuli
The apparatus, stimuli, and procedure were as described in previous studies from our laboratory (e.g., Murawski & Stanton, 2010; Schiffino, Murawski, Rosen, & Stanton, 2011). Context A consisted of four clear Plexiglas chambers (16.5 × 21.1 × 21.6 cm) situated under a fume hood, which provided overhead lighting as well as low-level background noise. The sides and ceilings of the chambers were transparent except for an opaque wall, which prevented viewing of adjacent rats. Each chamber floor consisted of stainless steel bars (.5-cm diameter situated 1.25-cm apart) connected to a shock generator. The unconditioned stimulus (US) was a 2 s 1.5 mA footshock. Context B was located in a separate room from those of Context A and differed from the conditioning chambers in size and texture (Jablonski et al., 2012). It consisted of separate wire mesh cages (22 × 22 × 26 cm) enclosed in larger sound-attenuated chambers (BRS/LVE, Laurel, MD) normally used for eyeblink conditioning (Brown, Calizo, & Stanton, 2008). Freezing behavior on the test day was recorded with a video camera connected to a Dell computer, which ran the FreezeFrame software (Wilmette, IL). This program provided offline analysis of animal movement determined by measuring changes in pixel luminance.
Design & Procedures
Preexposure
For Experiment 1, as described previously (Dokovna, Jablonski, & Stanton, 2013), a “multiple preexposure” CPFE protocol was conducted during the light cycle (between 2 and 7pm). Beginning on PND31, rats were weighed and placed individually in individual Plexiglas cages (11 × 11 × 18 cm) covered with opaque orange paper on 4 sides. For each “load” of animals which underwent behavior at the same time, rats were transported (typically 8 at a time) to either Context A (Group Pre, n=4) or Context B (Group Alt-Pre, n=4). Rats in the Alt-Pre group were transported to Context B by another experimenter. Once Group Pre arrived to the behavioral testing area, rats waited while the experimenter cleaned each chamber with 5% ammonium hydroxide (approximately 2-min). Rats were then brought into the testing room and were placed in Context A in which they were able to explore for 5-min. Rats were then removed from the chamber and returned to the transport container. After 1-min, rats were placed back in the identical chamber for 1-min. Placement in and out of the chamber occurred for 4 more cycles (5 1-min cycles total) and included no alteration to the chambers between placements. The timing of placement in Context B was yoked to chamber placement for Context A. Rats were returned to the colony room immediately following the end of the preexposure phase. Preexposure chamber location was counterbalanced across Treatment Condition.
Training
Twenty-four (+/−1) hours following preexposure, rats were again weighed and transported to the behavioral testing area in a manner identical to the previous day. Following the 2-min wait time, rats were brought into the testing room one at a time and were placed in their respective training chamber. For Group Pre, training occurred in the identical chamber from the preexposure phase. Upon placement, rats received an immediate (< 5s) 2s, 1.5 mA footshock. Rats were immediately removed following US offset and returned to the colony room.
Testing
Twenty-four (+/−1) hours following training, all rats were returned to the same chamber in which they received immediate shock on the training day. During testing, freezing to the context was measured during a 5-min period. All weighing, transport and chamber cleaning procedures were identical to the preexposure and training phases.
Experiment 2 Preexposure, Training & Testing
For Experiment 2 All CPFE phases were conducted in an identical manner to Experiment 1, except for the training day. Following immediate shock delivery during training, rats remained in the conditioning chamber for 5-min during which freezing behavior was recorded.
Data Analysis
As described previously (Schiffino et al., 2011) data were recorded and analyzed using FreezeFrame software (Wilmette, IL). Behavioral freezing bout length was set to .75. The freezing threshold for each subject (determined by changes in pixel luminance) was initially set by the program. However, a human observer blind to the conditions of the subjects, inspected the threshold and made adjustments if necessary. Adjustments were made offline in order to ensure that small motor movements were not recorded as freezing. Freezing was defined as the cessation of all movement aside from respiration. Freezing behavior was analyzed as the time spent freezing over the 5-min testing phase.
For Experiment 1, data were imported into Statistica 12 analysis software. Weight gain during the dosing period (PND7-9) was analyzed using a repeated measures ANOVA with Sex and Treatment Condition as between subject factors and Age as the within subjects factor. Body weights on PND31 were examined using a factorial ANOVA with Sex and Treatment Condition as factors. Since an absence of learning to the training context was expected in behavioral control group Alt-Pre, (Murawski & Stanton, 2010; Murawski & Stanton, 2011), this group consisted of animals from each Treatment Condition, but was not factored with each Treatment Condition. Thus, freezing behavior was analyzed with a 2 (Sex) x 5 Treatment-Behavioral Condition (Alt-Pre, SI, Group 2.75g, Group 4.00g, Group 5.25g) factorial ANOVA. Post-hoc Dunnett’s test were performed to compare each Treatment Condition with the Alt-Pre Group.
For Experiment 2, all data collection and weight analyses were analyzed identically to Experiment 1; however, only two Treatment Conditions were included (SI vs. EtOH). Behavioral data was analyzed with a 2 (Sex) x 2 (Treatment Condition) x 2 (Behavioral Group) factorial ANOVA on both Training and Testing day freezing scores, with post-hoc (Newman-Keuls) analyses included where necessary. Animals were considered outliers if both their training and testing scores exceeded +/− 1.96 standard deviations from the group mean.
Results
Experiment 1
The purpose of Experiment 1 was to examine learning and performance of the CPFE following a range of alcohol doses administered during a narrower window, PND7-9, than previously reported (Murawski & Stanton, 2011).
Subjects
Seventeen subjects were excluded from final analyses. Eight were lost due to experimenter error and nine were removed for meeting the criteria as a statistical outlier (Alt Pre n=2; SI n=1; 2.75 n=3; 4.00 n=2; 5.25 n=1). Outliers were defined a priori as % freezing scores being +/− 1.96 standard deviations from the group mean. Statistical significance was set to p ≤ 0.05. Analyses were conducted on the remaining 96 subjects (Alt Pre n=23; SI n=20; 2.75 n=17; 4.00 n=18; 5.25 n=18).
Body Weights & BACs
Table 1: Body weights from Treatment Conditions SI, 2.75g, 4.00g and 5.25g on PND 7, 9 and 31 are summarized in Table 1. All groups gained a significant amount of weight over the dosing period (PND7-9). A 4 (Treatment Condition) x 2 (Sex) x 2 (Days) repeated measures ANOVA on PND7 and 9 body weights revealed significant main effects of Treatment Condition (F3,88 = 2.73, p<.049) and Days (F1,88 = 853.77, p<.001), as well as a significant Treatment Condition x Days interaction (F3,88 = 61.34, p<.001). Newman-Keuls performed on the interaction indicated weights did not differ across groups on PND7 (ps>.462). On PND9, body weights for group 5.25g significantly differed from all other groups (all ps<.001) and Groups SI, 2.75g and 4.00g did not differ from one another (ps >.079).
Table 1.
Body Weights and BACs for Experiments 1 and 2
| Exp. | Dose | Body Weight (g)
|
BACs (mg/dL)
|
|||||
|---|---|---|---|---|---|---|---|---|
| PD7 (males) | PD7 (females) | PD9 (males) | PD9 (females) | PD31 (males) | PD31 (females) | PD7 | ||
| 1 | SI | 14.05 ± 0.58 | 15.33 ± 0.50 | 18.62 ± 0.79 | 19.59 ± 0.65 | 100.46 ± 3.70 | 96.93 ± 2.30 | n/a |
| 2.75g | 14.89 ± 0.45 | 14.69 ± 0.33 | 19.46 ± 0.60 | 19.26 ± 0.39 | 110.00 ± 2.22 | 96.27 ± 2.34 | 210.61 ± 7.35 | |
| 4.00g | 15.87 ± 0.46 | 14.57 ± 0.59 | 18.76 ± 0.58 | 17.51 ± 0.64 | 106.75 ± 3.29 | 94.75 ± 2.36 | 322.00 ± 7.03 | |
| 5.25g | 15.23 ± 0.43 | 15.17± 0.38 | 15.56 ± 0.52* | 16.52 ± 0.68* | 99.23 ± 3.45 | 95.50 ± 1.87 | 414.00 ± 13.41 | |
| 2 | SI | 15.99 ± 0.29 | 15.15 ± 0.31 | 21.06 ± 0.41 | 19.58 ± 0.46 | 106.21 ± 1.56 | 96.82 ± 1.10 | n/a |
| EtOH | 15.78 ± 0.42 | 15.09 ± 0.36 | 15.60± 0.50* | 15.59 ± 0.37* | 98.25 ± 2.37* | 89.80 ± 1.74* | 426.81 ± 6.56 | |
Average body weights (± SE) are shown from 4 Treatment Conditions in Experiment 1 (SI, sham intubated; 2.75; 4.00; and 5.25 g) taken from the first and last day of the dosing period (PND7 and PND9, respectively). For Experiment 2, body weights were recorded for the 2 Treatment Conditions (SI, sham intubated; and Group EtOH). Significant differences in body weight compared with SI are noted with an *. Body weights taken at the beginning of preexposure (PND31) are also summarized for each experiment (Exp 1; n=94/96 rats; Exp 2; n=65). In all experiments, PND31 weights for females were significantly lower than males (Exp 1; n=47 males, 47 females; Exp 2; n=33 males, 32 females) BACs were taken from blood samples collected on the first day of dosing (PND7) from all ethanol-exposed groups (reported in mg/dl). (Exp 1 n=33 males, 35 females; Exp 2 n=16 males, 15 females) BAC - blood alcohol concentration.
A 4 (Treatment Condition) x 2 (Sex) factorial ANOVA on PND31 body weights revealed only a main effect of sex with females weighing less than males [F1,86 = 19.13, p<.001 (means and SEs for males:103 ± 1.75; females: 95.28 ± 1.13)]. Thus, even though Group 5.25g showed attenuated growth at PND9 compared to the other groups, this effect was transient as there were no weight differences between groups at the time of behavioral training.
BACs for Treatment Conditions 2.75g, 4.00g and 5.25g are depicted in Table 1. BACs are included from 68 of the 69 alcohol-exposed pups (1 sample from Group 2.75g was lost). A 3 (Treatment Condition) x 2 (Sex) factorial ANOVA on BACs revealed a main effect of Treatment Condition (F2,62 = 101.68 p<.001), with no main effect or interaction involving Sex (Fs<1.2).
Behavioral Measures
Testing
In the Alt-Pre behavioral control group, differences in testing freezing scores between treatment conditions (SI, 2.75g, 4.00g, 5.25g) were examined by a one-way ANOVA conducted on testing freezing scores between each Treatment Condition. Results indicated no significant Treatment effect [F3,19 = 1.41, p>.260 (means + SEs for each Treatment Condition: SI, 12.63 ± 3.92 n=7; 2.75g, 11.4 ± 3.32 n=4; 4.00g, 4.4 ± 1.39 n=7; 5.25g, 8.88 ± 3.40 n=5)] and so scores from animals in the Alt-Pre group were collapsed across this variable to create a single Alt-Pre group for subsequent analyses.
Test freezing for each Treatment-Behavioral condition in Experiment 1 is shown in Figure 2. The CPFE was impaired only in pups exposed to the highest alcohol dose from PND7-9, as indicated by a non-significant difference in context freezing from the Alt-Pre control group (Figure 1). A 5 (Treatment Group) x 2 (Sex) factorial ANOVA revealed only a main effect of Treatment-Behavioral Condition (F4,86 = 5.77, p<.001). Dunnett’s post-hoc analyses run on Treatment-Behavioral Condition demonstrated that all Treatment Groups differed from the Alt-Pre group (p’s <.006) except for Group 5.25g (p>.18). Thus, these findings demonstrate the CPFE at all doses except the highest dose and no graded dose-response function in CPFE performance with the PND7-9 exposure window.
Figure 2.
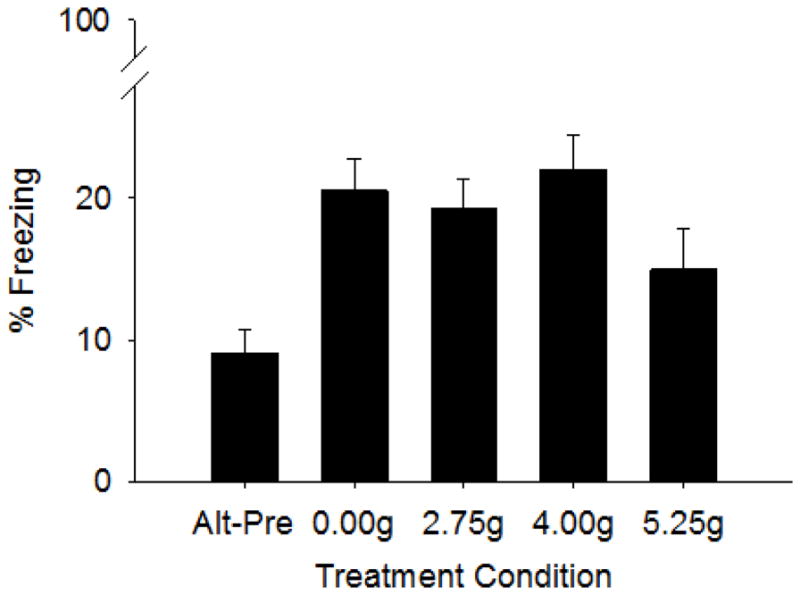
Mean (+SE) percent freezing during testing for the context preexposure facilitation effect (CPFE) in Experiment 1. Treatment Conditions include Group Alt-Pre (n=23, comprised of each EtOH dose, 2.75g, n=4, 4.00g, n=7, 5.25, n=5 and SI sham intubated, 0.00g, n=7), Group SI (n=20), Group 2.75g (n=17), Group 4.00g (n=18), and Group 5.25g (n=18). Rats from each group were preexposed to Context A except for Group Alt-Pre which was preexposed to the alternate context (Context B). Compared to Group Alt Pre, preexposure to Context A facilitated freezing for all groups, except the group given the highest ethanol dose (5.25 g/kg).
Experiment 2
The purpose of Experiment 2 to was to examine the separable memory processes involved in the CPFE following PND7-9 alcohol exposure during a test of post-shock freezing. If alcohol-exposed rats show normal post-shock freezing, then freezing deficits by these rats on the test day (Experiment 1) likely reflect a failure to consolidate or retrieve a context-shock association, rather than a deficit in conjunctive processes (learning, consolidation, pattern completion, encoding of the context-shock association) that occur prior to, or on the training day. Alternatively, deficits in post-shock freezing by these rats suggest a deficit in one or more of these processes.
Subjects
Data from same-sex littermates assigned to identical Treatment Conditions and Behavioral Groups were averaged and analyzed as a single data point. Oversampling occurred on three occasions (SI, Alt-Pre, Female n=1; SI, Pre, Female n=1; SI, Pre, Male n=1). Five subjects were excluded from final analyses. Data from three subjects were lost due to equipment failure and two subjects were removed for meeting the criteria as a statistical outlier (see ‘Data Analysis’; Alt-Pre, SI n-1; Pre, SI n=1). Analyses were conducted on the remaining 65 subjects (Alt Pre, SI n=17; Alt-Pre, EtOH n=15; Pre, SI n=17; Pre, EtOH n=16).
Body Weights & BACs
Body weights from the EtOH and SI Treatment Conditions are summarized in Table 1. A 2 (Treatment Condition) x 2 (Sex) x 2 (Days) repeated measures ANOVA on PND7 and 9 body weights revealed significant main effects of Sex (F1,61 = 4.12, p<.047), Treatment Condition (F1,61 = 43.24, p<.001) and Days (F1,61= 396.07, p<.001), as well as a significant Treatment Condition x Days (F1,61 = 346.38, p<.001) and Treatment x Sex x Days interaction (F1,61 = 7.01, p<.011). Newman-Keuls tests performed on the Treatment x Days interaction indicated weights did not differ across groups on PND7 (p>0.75). On PND9, body weights for Group EtOH significantly differed from Group SI (p<0.001). Further Newman-Keuls analyses on the Treatment x Sex x Days interaction indicated significantly greater weights on PND9 for males compared to females from Group SI (p<.01) and significantly greater PND9 weights compared to PND7 weights for Group SI for both males and females (ps<.001).
A 2 (Treatment Condition) x 2 (Sex) factorial ANOVA on PND31 body weights revealed a main effect of sex with females weighing less than males [F1,86 = 19.13, p<.001 (means and SEs for males:103 ± 1.75; females: 95.28 ± 1.13)] and a main effect of Treatment Condition (F1,61 = 26.42, p<.001) but no interaction of Treatment Condition and Sex (p>.78).
BACs for Group EtOH are depicted in Table 1. A one-way ANOVA on BACs between Sex indicated no effect (p>.33).
Behavioral Measures
Training
Contextual fear on the Training day was assessed following immediate shock delivery on PND32. Compared to their Alt-Pre counterparts, both Group SI and Group EtOH displayed significant fear to the training context (Figure 3). A 2 (Treatment Condition) x 2 (Behavioral Group) x 2 (Sex) factorial ANOVA revealed only a main effect of Behavioral Group (F1,57 = 41.90 p<.001) with no interaction between Treatment Condition or Sex (ps>.55). Means and SEs for Group Pre: 25.56 ± 2.94; Group Alt-Pre: 4.23 ± 1.06.
Figure 3.
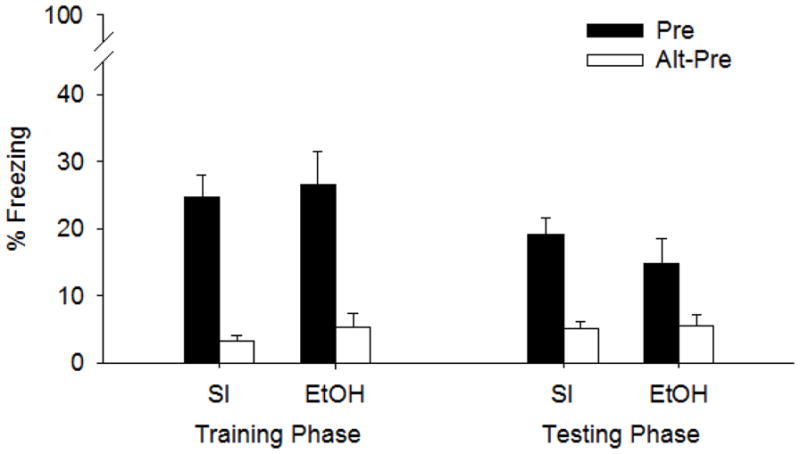
Mean (+SE) percent freezing during the training and testing phases of the CPFE in Experiment 2. Treatment conditions include Group SI and Group EtOH. Rats from each group were preexposed to either Context A (Pre, filled bars) or Context B (Alt-Pre, clear bars). All rats were given an immediate shock 24 hours later in Context A and remained in the chamber for 5-min during the Training Phase (left panel). Male, SI, Pre, n=8; Female, SI, Pre, n= 9; Male SI, Alt-Pre, n=9; Female, SI, Alt-Pre, n=8; Male, EtOH, Pre, n=8; Female, EtOH, Pre, n=8; Male, Etoh, Alt-Pre, n=8, Female, EtOH, Alt-Pre, n=7). Rats were tested 24 hours later for contextual freezing in Context A during the testing phase (right panel). Male, SI, Pre, n=8; Female, SI, Pre, n= 9; Male SI, Alt-Pre, n=9; Female, SI, Alt-Pre, n=8; Male, EtOH, Pre, n=8; Female, EtOH, Pre, n=8; Male, Etoh, Alt-Pre, n=8, Female, EtOH, Alt-Pre, n=7). Preexposure to Context A facilitated freezing for each Treatment Condition (SI, Group 5.25g) during both phases of the CPFE paradigm. Alt-Pre Groups significantly differed from their Alt-Pre counterparts.
Testing
Contextual fear assessed in the same rats during testing on PND33 revealed similar results to freezing measured on the Training day (Figure 3.). A 2 (Treatment Condition) x 2 (Behavioral Group) x 2 (Sex) factorial ANOVA revealed a main effect of Behavioral Group (F1,57 = 25.3 p<.001), with Group Pre (17.09 ± 2.16) displaying an increase in freezing compared to Group Alt-Pre (5.35 ± 0.9). No main effect of Sex, Treatment Condition, or interaction of Treatment Condition x Behavioral Group were found (Fs < 1). However, significant interactions involving Sex x Treatment (F1,57 = 4.1 p<.05) and Sex x Behavioral Group x Treatment Condition (F1,57 = 5.0 p<.03) were found. Newman-Keuls on the Sex x Behavioral Group x Treatment Condition revealed SI females from Group Pre showed significantly more freezing compared to SI males from the same behavioral group (p<.05) and alcohol reduced freezing in Pre females (p < .021) but not Pre males (Pre, SI, males 13.32 ± 3.02, females 24.33 ± 3.08; Alt-Pre, SI, males 5.07 ± 1.78, females 5.28 ± 1.21; Pre, EtOH, males 19.13 ± 4.96, females 10.69 ± 5.04; Alt-Pre, EtOH males 5.01 ± 2.41, females 6.2 ± 2.0).
Discussion
In Experiment 1, alcohol exposure at the 5.25g/kg/day dose produced a disruption of the CPFE, while rats exposed to lower doses (4.00g/kg and 2.75g/kg) did not differ significantly from the Alt-Pre control group. In Experiment 2, a test of post-shock freezing revealed that SI and alcohol-exposed animals from Group-Pre indicate comparable freezing levels during the training phase of the CPFE and this effect was also seen during testing 24 hours later in male rats. Female rats were impaired by alcohol exposure during the testing phase (similar to both sexes in Experiment 1).
The current set of experiments examined the effect of binge-like neonatal alcohol exposure on incidental contextual fear learning by 1) examining the effects of a range of doses administered during a narrow time window and 2) investigating the effects of neonatal alcohol on the separable memory processes involved in the CPFE. In accordance with previous reports from our laboratory (Dokovna et al., 2013; Murawski & Stanton, 2010; Murawski & Stanton, 2011), control (SI) animals given prior exposure to the conditioning context (Group Pre) show an increase in time spent freezing during testing, compared to those exposed to an alternate context (Group Alt-Pre), which demonstrate the immediate shock deficit (Fanselow, 1990). In an effort to increase the amount of freezing during the testing phase by SI-Pre animals, the current set of experiments involved a multiple preexposure paradigm consisting of 5-min plus 5 single 1 min exposures to the conditioning chamber during the preexposure phase (Dokovna et al., 2013; Matus-Amat et al., 2004). The findings of Experiment 1 are in accord with previous studies indicating PND7-9 exposure to 5.25 g/kg/day alcohol impairs the CPFE despite multiple exposures to the conditioning context, when freezing is assessed only on the test day (Dokovna et al., 2013).
In Experiment 1, rat pups administered an alcohol dose of 5.25g/kg from PND7-9 show an absence of the CPFE, in agreement with our earlier reports (Dokovna et al., 2013; Murawski & Stanton, 2011). Impairment of the CPFE by alcohol exposure on PND4-9 yields a linear dose-response function whereby 2.75g/kg is ineffective, 4.00g/kg impairs performance, and 5.25g/kg abolishes performance (Murawski & Stanton, 2011). With the narrower, PND7-9 exposure window, the 5.25g/kg dose of alcohol also abolishes the CPFE, when either a single-preexposure protocol (Murawski & Stanton, 2011) or a multiple-preexposure protocol is used (Dokovna et al., 2013). In the present study, the CPFE was impaired to a lesser extent by the highest dose than in these previous studies, despite the use of the same protocol as Dokovna et al.(2013). This may reflect the proportion of littermates (25% vs. 50%) receiving the highest dose in this study relative to the previous ones that only involved SI and high-dose rats. In any event, the present findings indicate that, when a narrower exposure window (PND7-9) is used, graded dose-response effects are replaced by more modest effects at only the highest dose.
Although this is the first study to address the question of how neonatal alcohol exposure window influences alcohol dose-response effects in contextual conditioning, this issue has been addressed in the literature using other tasks. For example, a high (5.25g/kg) and intermediate (4.5g/kg) dose administered over PND4-9 or PND7-9, but not PND4-6, produced spatial water maze deficits in PND26 to 31 rats (Goodlett & Peterson, 1995; Goodlett & Johnson, 1997). Because these studies suggest the sensitivity of PND7-9 ethanol exposure to later behavioral performance and greater behavioral impairments result with an increase in ethanol dose (Goodlett & Johnson, 1997; Hunt et al., 2009; Murawski et al., 2012; Murawski & Stanton, 2011; Tomlinson, Wilce, & Bedi, 1998), it was hypothesized that rats would show a linear dose response effect following ethanol exposure from PND7-9. However, the low and intermediate treatment groups (2.75g and 4.00g) from the current study indicated no impairment during CPFE testing. The last critical period of hippocampal development in the rat occurs from GD18 to PND9; during which synaptogenesis, dendritic arborization, the proliferation of glial cells and neurogenesis occurs in the dentate gyrus of the hippocampus (Gil-Mohapel et al., 2010). The alcohol insult from the current report not only occurs during a limited time window but also occurs at the end of this critical period. This timeframe, in addition to small doses, may partially account for the lack of behavioral impairment. Even though ethanol exposure (5.25g/kg) from PND4-9 induces reductions in CA1 pyramidal cells at PND31 (Murawski et al., 2012), it may be that lower doses of alcohol limited to PND7-9 do not induce as severe anatomical effects especially to the hippocampus, which is critically involved in the CPFE (see below). In support of this assertion, (Ikonomidou et al., 2000) found that in rat pups exposed at different developmental ages to ethanol via subcutaneous injection in a total dose of 5.00g/kg, and assessed 24-hr later, the greatest amount of hippocampal neurodegeneration was found when ethanol was administered on PND3. The number of degenerating neurons drastically decreased from PND7-14, suggesting a lessened response of hippocampal neurons to ethanol during later ages of exposure. Indeed, some studies do show a lack of ethanol impairment in hippocampus-dependent tasks following high or intermediate neonatal administration from PND7-9 (Hunt et al., 2009; Jablonski, Schreiber, Westbrook, Brennan, & Stanton, 2013; Murawski et al., 2013). Thus, even though alcohol exposure from PND7-9 produces dose-response effects in some spatial learning tasks, limiting the window of exposure to PND7-9 decreases the sensitivity of the CPFE to lower alcohol doses; possibly by inducing less damage to the developing hippocampus and/or other areas such as the medial prefrontal cortex (mPFC) which may also play an important role in the CPFE (Asok, Schreiber, Jablonski, Rosen, & Stanton, 2013).
In Experiment 2, alcohol-exposed and SI rats were examined for freezing behavior on the training day in the CPFE. In agreement with previous work, rats given exposure to the context 24-hour before immediate shock delivery show increased freezing compared to the Alt-Pre control group (Jablonski et al., 2012). Alcohol exposure during the identical time window (PND7-9) and dose (5.25g/kg) produces an absence of the CPFE during testing (Experiment 1, Dokovna et al., 2013; Murawski & Stanton, 2011). The purpose of Experiment 2 was to determine which memory processes involved in the CPFE are disrupted by neonatal alcohol exposure. We show no impairment in post-shock freezing to the context in neither Group SI nor Group EtOH. This finding suggests that the failure to perform in the CPFE likely reflects a failure to consolidate or retrieve a context-shock association, rather than a deficit in conjunctive processes (learning, consolidation, pattern completion) that occur prior to shock on the training day (Figure 1).
Twenty-four hours following training, the same rats were returned to the conditioning context for 5-min of testing and only Group 5.25g females were impaired in the CPFE relative to Group SI. Alcohol did not impair retention of the CPFE in male rats. It is possible that the elevation of freezing on the testing day in alcohol-exposed animals is partly a result of an increase in exposure to the chamber following the immediate shock. The presentation of the shock followed by immediate chamber exposure may create a stronger fear memory and/or a map-like representation of the context, which could account for the increase in fear to the context the next day. Why this effect would occur in males but not females requires further investigation. To our knowledge, this is the first report to examine both post-shock and testing day freezing as a repeated measure in the CPFE.
The current data suggest that the effect of alcohol during this narrow PD7-9 exposure window is not sufficient to disrupt either the acquisition of the context representation on the preexposure day, or pattern completion occurring on the training day. This finding contrasts with our previous work examining the ontogeny of post-shock freezing in the CPFE. We found that PND24 pups given preexposure to the context show elevated levels of post-shock freezing at training, relative to their Alt-Pre counterparts. PND17 pups, in contrast, showed low levels of freezing regardless of preexposure group. PND17 may thus be an age in which the hippocampus cannot support the context encoding, pattern completion, or context-shock processes that are required for the CPFE. Additional work from our lab suggests an impairment of the ability of PND4-9 alcohol-exposed animals to form a conjunctive representation of the preexposure day (Murawski et al., 2012). Murawski et al., (2012) showed that, following preexposure, juvenile rats exposed to alcohol over PND4–9 (5.25g/kg) showed reductions in the number of CA1 c-Fos+ cells compared to Group SI, suggesting that the CPFE impairments in rats receiving alcohol during this wider exposure window reflect a failure to learn about the context during the preexposure phase. In addition to variation in the window of alcohol exposure used in the current report, Experiment 2 utilized a multiple preexposure paradigm in which animals were given 5-min of chamber exploration following by 5, 1-min chamber placements. Murawski et al., (2012) used a single 5-min exposure to the context. It is possible that the additional context preexposure could facilitate encoding and/or consolidation of the context memory in alcohol-exposed animals (Murawski & Stanton, 2010) and thereby “shift” the mechanism of the alcohol-induced deficit from context encoding to consolidation or retrieval of the context-shock association. This difference in preexposure protocol could also contribute to differences in dose-response effects between the present study and our previous one (Murawski & Stanton, 2011).
The consolidation process required for intact post-shock freezing on the training day in the CPFE is the context experienced 24-hr prior. Thus, consolidation of the context representation along with the association of the retrieved representation of that memory with shock prior to training is necessary for post-shock freezing. In sCFC and cued-fear conditioning, however, long-term consolidation of two temporally separate events (preexposure and training) is not necessary and alcohol exposed animals are much less impaired relative to the CPFE (Murawski & Stanton, 2010). Even though the amygdala is critically involved in short- and long-term fear learning, intact sCFC and cued fear conditioning following neonatal alcohol exposure suggests this structure is not necessarily targeted by our exposure protocol. Also, novel object location recognition (OL) involving a short delay between the sample and testing phase (5-min) is also unaffected by PND7-9 alcohol exposure when testing occurs in the juvenile period (Jablonski et al., 2013). Thus, consolidation efficacy, rather than context acquisition or pattern completion, may account for the alcohol impairment in CPFE at testing in the present study.
The current set of experiments further characterizes the effects of neonatal alcohol exposure in context processing during development. Experiment 1 demonstrated that alcohol exposure during a narrow time window does not produce the graded dose-related behavioral deficits in the CPFE that are observed with longer exposure. Since alcohol-exposed rats showed normal post-shock freezing in Experiment 2, deficits in these rats on the test day probably reflect a failure to consolidate or retrieve a context-shock association. Understanding dose-response functions and the effects of neonatal alcohol exposure on specific memory functions during development may provide targets for interventions aimed at mitigating the cognitive impairments associated with human FASD.
Acknowledgments
The authors would like to thank the University of Delaware Office of Laboratory Animal Medicine (OLAM) for the care of the animals and Professor J.B. Rosen for the use of his fear conditioning equipment. The study was supported by 1-F31-AA021317-01 to SAJ and 1-R21-HD070662-01 to MES.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asok A, Schreiber WB, Jablonski SA, Rosen JB, Stanton ME. Egr-1 increases in the prefrontal cortex following training in the context preexposure facilitation effect (CPFE) paradigm. Neurobiology of Learning and Memory. 2013;106C:145–153. doi: 10.1016/j.nlm.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KL, Calizo LH, Goodlett CR, Stanton ME. Neonatal alcohol exposure impairs acquisition of eyeblink conditioned responses during discrimination learning and reversal in weanling rats. Developmental Psychobiology. 2007;49(3):243–257. doi: 10.1002/dev.20178. [DOI] [PubMed] [Google Scholar]
- Brown KL, Calizo LH, Stanton ME. Dose-dependent deficits in dual interstimulus interval classical eyeblink conditioning tasks following neonatal binge alcohol exposure in rats. Alcoholism: Clinical and Experimental Research. 2008;32(2):277–293. doi: 10.1111/j.1530-0277.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Human Development. 1979;3(1):79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Dokovna LB, Jablonski SA, Stanton ME. Neonatal alcohol exposure impairs contextual fear conditioning in juvenile rats by disrupting cholinergic function. Behavioural Brain Research. 2013;248(0):114–120. doi: 10.1016/j.bbr.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Factors governing one-trail contextual conditioning. Animal Learning and Behavior. 1990;18(3):264–270. [Google Scholar]
- Gil-Mohapel J, Boehme F, Kainer L, Christie BR. Hippocampal cell loss and neurogenesis after fetal alcohol exposure: Insights from different rodent models. Brain Research Reviews. 2010;64(2):283–303. doi: 10.1016/j.brainresrev.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Johnson TB. Neonatal binge ethanol exposure using intubation: Timing and dose effects on place learning. Neurotoxicology and Teratology. 1997;19(6):435–446. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Peterson SD. Sex differences in vulnerability to developmental spatial learning deficits induced by limited binge alcohol exposure in neonatal rats. Neurobiology of Learning and Memory. 1995;64(3):265–275. doi: 10.1006/nlme.1995.0009. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD. Children with fetal alcohol syndrome are impaired at place learning but not cued-navigation in a virtual morris water task. Behavioural Brain Research. 2003;143(1):85–94. doi: 10.1016/s0166-4328(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Jacobson SE, Torok EJ. Deficits in trace fear conditioning in a rat model of fetal alcohol exposure: Dose–response and timing effects. Alcohol. 2009;43(6):465–474. doi: 10.1016/j.alcohol.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287(5455):1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Jablonski SA, Schiffino FL, Stanton ME. Role of age, post-training consolidation, and conjunctive associations in the ontogeny of the context preexposure facilitation effect. Developmental Psychobiology. 2012;54:714–722. doi: 10.1002/dev.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski SA, Schreiber WB, Westbrook SR, Brennan LE, Stanton ME. Determinants of novel object and location recognition during development. Behavioural Brain Research. 2013;256:140–150. doi: 10.1016/j.bbr.2013.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Stanton ME, Meintjes EM, Molteno CD. Biobehavioral markers of adverse effect in fetal alcohol spectrum disorders. Neuropsychology Review. 2011;21(2):148–167. doi: 10.1007/s11065-011-9169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Jacobson JL. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcoholism: Clinical and Experimental Research. 2008;32(2):365–372. doi: 10.1111/j.1530-0277.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Lawrence CR. Intragastric intubation of alcohol during the perinatal period. Methods in Molecular Biology. 2008;447:101–110. doi: 10.1007/978-1-59745-242-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS, DeCola JP, Landeira-Fernandez J. Selective impairment of long-term but not short-term conditional fear by the N-methyl-D-aspartate antagonist APV. Behavioral Neuroscience. 1992;106(4):591–596. doi: 10.1037//0735-7044.106.4.591. [DOI] [PubMed] [Google Scholar]
- Livy DJ, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: Effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicology and Teratology. 2003;25(4):447–458. doi: 10.1016/s0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Maier SE, West JR. Drinking patterns and alcohol-related birth defects. Alcohol Research and Health. 2001;25(3):168–174. [PMC free article] [PubMed] [Google Scholar]
- Marino MD, Aksenov MY, Kelly SJ. Vitamin E protects against alcohol-induced cell loss and oxidative stress in the neonatal rat hippocampus. International Journal of Developmental Neuroscience. 2004;22(5–6):363–377. doi: 10.1016/j.ijdevneu.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. The Journal of Neuroscience. 2004;24(10):2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski NJ, Klintsova AY, Stanton ME. Neonatal alcohol exposure and the hippocampus in developing male rats: Effects on behaviorally induced CA1 c-fos expression, CA1 pyramidal cell number, and contextual fear conditioning. Neuroscience. 2012;206(0):89–99. doi: 10.1016/j.neuroscience.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski NJ, Jablonski SA, Brown KL, Stanton ME. Effects of neonatal alcohol dose and exposure window on long delay and trace eyeblink conditioning in juvenile rats. Behavioural Brain Research. 2013;236(0):307–318. doi: 10.1016/j.bbr.2012.08.025. [DOI] [PubMed] [Google Scholar]
- Murawski NJ, Stanton ME. Variants of contextual fear conditioning are differentially impaired in the juvenile rat by binge ethanol exposure on postnatal days 4–9. Behavioural Brain Research. 2010;212(2):133–142. doi: 10.1016/j.bbr.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murawski NJ, Stanton ME. Effects of dose and period of neonatal alcohol exposure on the context preexposure facilitation effect. Alcoholism: Clinical and Experimental Research. 2011;35(6):1160–1170. doi: 10.1111/j.1530-0277.2011.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AL, Crocker N, Mattson SN, Riley EP. Neuroimaging and fetal alcohol spectrum disorders. Developmental Disabilities Research Reviews. 2009 doi: 10.1002/ddrr.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Morledge P. Ontogeny of contextual fear conditioning in rats: Implications for consolidation, infantile amnesia, and hippocampal system function. Behavioral Neuroscience. 1994;108(2):227–234. doi: 10.1037//0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O’Reilly RC. Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behavioral Neuroscience. 1999;113(95):867–880. doi: 10.1037//0735-7044.113.5.867. [DOI] [PubMed] [Google Scholar]
- Rudy JW. Context representations, context functions, and the parahippocampal–hippocampal system. Learning & Memory. 2009;16(10):573–585. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffino FL, Murawski NJ, Rosen JB, Stanton ME. Ontogeny and neural substrates of the context preexposure facilitation effect. Neurobiology of Learning and Memory. 2011;95(2):190–198. doi: 10.1016/j.nlm.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spottiswoode BS, Meintjes EM, Anderson AW, Molteno CD, Stanton ME, Dodge NC, Jacobson SW. Diffusion tensor imaging of the cerebellum and eyeblink conditioning in fetal alcohol spectrum disorder. Alcoholism: Clinical and Experimental Research. 2011;35(12):2174–2183. doi: 10.1111/j.1530-0277.2011.01566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson D, Wilce P, Bedi KS. Spatial learning ability of rats following differing levels of exposure to alcohol during early postnatal life. Physiology & Behavior. 1998;63(2):205–211. doi: 10.1016/s0031-9384(97)00424-1. [DOI] [PubMed] [Google Scholar]
- Tran TD, Kelly SJ. Critical periods for ethanol-induced cell loss in the hippocampal formation. Neurotoxicology and Teratology. 2003;25(5):519–528. doi: 10.1016/s0892-0362(03)00074-6. [DOI] [PubMed] [Google Scholar]
- Uecker A, Nadel L. Spatial but not object memory impairments in children with fetal alcohol syndrome. American Journal of Mental Retardation. 1998;103(1):12–18. doi: 10.1352/0895-8017(1998)103<0012:SBNOMI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Willoughby KA, Sheard ED, Nash K, Rovet J. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. Journal of the International Neuropsychological Society. 2008;14(06) doi: 10.1017/S1355617708081368. [DOI] [PubMed] [Google Scholar]