Abstract
Aims/hypothesis
Adiponectin is an adipocyte-derived hormone that plays an important role in energy homeostasis. The main objective of this study was to investigate whether or not adiponectin regulates brown adipose tissue (BAT) activation and thermogenesis.
Methods
Core body temperatures (CBTs) of genetic mouse models were monitored at room temperature and during cold exposure. Cultured brown adipocytes and viral vector-mediated gene transduction were used to study the regulatory effects of adiponectin on Ucp1 gene expression and the underlying mechanisms.
Results
The CBTs of adiponectin knockout mice (Adipoq−/−) were significantly higher than those of wild type (WT) mice both at room temperature and during the cold (4°C) challenge. Conversely, reconstitution of adiponectin in Adipoq−/− mice significantly blunted β adrenergic receptor agonist-induced thermogenesis of interscapular BAT. After 10 days of intermittent cold exposure, Adipoq−/− mice exhibited higher UCP1 expression and more brown-like structure in inguinal fat than WT mice. Paradoxically, we found that the anti-thermogenic effect of adiponectin requires neither AdipoR1 nor AdipoR2, two well-known adiponectin receptors. In sharp contrast to the anti-thermogenic effects of adiponectin, AdipoR1 and especially AdipoR2 promote BAT activation. Mechanistically, adiponectin was found to inhibit Ucp1 gene expression by suppressing β3-adrenergic receptor expression in brown adipocytes.
Conclusions/interpretation
This study demonstrates that adiponectin suppresses thermogenesis, which is likely to be a mechanism whereby adiponectin reduces energy expenditure.
Keywords: Adiponectin, Brown adipose tissue, Energy expenditure, Thermogenesis
Introduction
Fat depots have been generally classified as white adipose tissue (WAT) and brown adipose tissue (BAT). In humans and most other animals, WAT is the primary depot that stores chemical energy as triacylglycerol-enriched lipid droplets. By contrast, BAT is smaller in mass and specialised for thermogenesis. In humans, BAT was previously believed to regress after birth and vanish in adults. However, this dogma has been convincingly refuted by studies using [18F]fluorodeoxyglucose positron emission tomography scans [1–4]. These studies uncovered metabolically active BAT depots in adult humans that are quickly activated by cold exposure [2–4]. More importantly, human BAT mass and activity are inversely correlated with BMI, suggesting that BAT is an important player in energy metabolism in humans and may be harnessed to increase energy expenditure [2, 3, 5].
BAT is enriched in mitochondria that use uncoupling protein 1 (UCP1)-mediated uncoupling to convert significant amounts of chemical energy to heat [6]. UCP1 is exclusively expressed in brown adipocytes. Two types of brown adipocytes have been distinguished: (1) classical brown adipocytes that develop from myogenic factor 5 (Myf5)-positive, myoblast-like precursors and reside in the interscapular and perirenal regions [7]; (2) brown-like adipocytes that develop from a Myf5-negative precursors and are embedded in WAT [8, 9]. Differentiation into these brown-like adipocytes, generally referred to as ‘browning’, can be induced by prolonged cold exposure and β-adrenergic receptor (βAR) agonists [9–11]. In contrast to white adipocytes, brown-like adipocytes express UCP1 and harbour high levels of mitochondria and multilocular lipid droplets [8].
Adiponectin (also known as Adipoq or adipocyte complement-related protein of 30 kDa [Acrp30]) is an adipocyte-secreted hormone. It exhibits many characteristics of a starvation hormone in that it suppresses energy expenditure and enhances energy conservation [12–15]. Adiponectin overexpression increases fat mass in wild type (WT) mice and leads to a super-obese phenotype in the ob/ob background [12, 16]. Conversely, adiponectin ablation leads to a lean phenotype in two independent lines of Adipoq gene knockout (Adipoq−/−) mice as well as in transgenic mice expressing antisense mRNA against adiponectin [13, 14, 17]. Adiponectin suppresses basal and catecholamine-induced lipolysis in white adipocytes [17, 18]. We have reported that protein kinase A (PKA), an essential mediator of βAR signalling in BAT, is downregulated in WAT by adiponectin treatment [17]. Although an inhibitory effect of adiponectin on Ucp1 gene expression has been observed in mouse BAT [13, 15, 19], it is not clear whether or not, or indeed how, adiponectin regulates BAT activation and thermogenesis.
Using genetic mouse models and cold challenge tests, we found that Adipoq−/− mice maintained a higher core body temperature (CBT) than WT mice, and were protected from hypothermia during acute cold exposure. After prolonged intermittent cold exposure, these mice harbour a greater number of brown-like adipocytes in the inguinal fat than WT control mice. Interestingly, the present study revealed that the anti-thermogenic effect of adiponectin is independent of two of its receptors, AdipoR1 and AdipoR2. In addition, we found that adiponectin inhibits UCP1 expression by reducing β3 adrenergic receptor (also known as Adrb3) expression in brown adipocytes. Therefore, this study demonstrated that adiponectin reduces BAT thermogenesis in mice.
Methods
Mouse colonies and cold exposure
All mice were in the C57BL/6 background. AdipoR2+/− mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Adipoq−/− mice were generated by P. Scherer’s laboratory (The University of Texas Southwestern Medical Center, Dallas, TX, USA) and had been backcrossed to C57BL/6 background for nine generations. WT littermates of the last round of backcrossing served as controls. AdipoR1−/− mice were obtained from the National Institutes of Health-supported Mutant Mouse Regional Resource Centers (Chapel Hill, NC, USA). AdipoR1 and AdipoR2 gene knockout (AdipoR1−/− and AdipoR2−/−) mice were generated by crossing heterozygous breeders using their respective AdipoR1+/+ and AdipoR2+/+ littermates as controls. All mice were housed under standard conditions and handled according to the Association for Assessment and Accreditation of Laboratory Animal Care guidelines with approval from the University of California San Diego Animal Care and Use Committee.
Intraperitoneally implanted emitters, ER4000 receiver and the Vital View data acquisition system (Mini Mitter, Bend, OR, USA) were used to wirelessly monitor CBT. CBTs were recorded at 1 min intervals. During fasting, CBTs were recorded from 20:00 to 07:30. Mice were singly caged for all cold exposure studies, which started at 08:00 or 08:30. For acute cold exposure, mice were transferred to a 4°C room. A Bio-Thermo Lifechip (Destron Fearing, South St Paul, MN, USA) was implanted underneath the interscapular BAT (iBAT) to measure iBAT temperature at 5 min intervals after i.p. injection of isoprenaline (ISO, 0.1 μmol/g of body weight) or saline control. For intermittent cold exposure, the exposure to 4°C was progressively extended as follows: 3 h on the first day and one additional hour each day up to 6 h/day for 7 days.
In vivo gene transduction
Adenovirus-encoded adiponectin (Ad-Acrp30) was used to overexpress or reconstitute adiponectin in mice [20, 21]. Purified adenovirus vector (1×109 pfu per mouse) was injected through the tail vein. Experiments and tissue sample collection were performed 3 days later when blood adiponectin levels were increased 10–12-fold without overt signs of adenovirus-induced hepatitis [21, 22]. As we previously reported [21, 22], transduction with Ad-Acrp30 significantly reduced blood FFA and triacylglycerol levels without affecting body weight or composition (data not shown).
Mitochondrial respiration assay
Mitochondria were purified from freshly collected tissues [23], and oxygen consumption was measured in a Mitocell Miniature respirometer (model 782, Strathkelvin Instruments Limited, Motherwell, UK) using pyruvate or palmitoyl-dl-carnitine (Sigma-Aldrich, St Louis, MO, USA) as substrates [24, 25].
Citrate synthase activity assay
Citrate synthase activity was measured spectrophotometrically using tissue homogenates [21]. After an initial 2 min absorbance reading taken at 412 nm, the reaction was initiated with the addition of 3.0 mmol/L acetyl-CoA, and the change in absorbance was measured every 10 s for 2 min.
Immunoblotting and quantitative real-time PCR
Previously described procedures were used [21, 22]. Briefly, protein samples (50 μg) were separated using NuPAGE 4–12% Bis-Tris gels (Life Technologies, Carlsbad, CA, USA). Proteins were transferred to polyvinylidene difluoride membranes, which were then blotted with primary antibodies overnight and visualised using ECL Plus substrate (Thermo Scientific, Waltham, MA, USA). The density of the bands was quantified using Quantity One 1-D Analysis Software (Bio-Rad Laboratories, Hercules, CA, USA). Trizol (Life Technologies) was used to isolate total mRNA. Relative PCR quantification was performed using a Stratagene Mx3000P (Agilent Technologies, Santa Clara, CA, USA). Expression data were normalised to the amount of 18S rRNA.
Brown adipocyte culture
Brown adipocytes were differentiated from either immortalised mouse brown pre-adipocytes (obtained from Y.-H. Tseng’s laboratory, Joslin Diabetes Center, Harvard Medical School, Boston, MA, USA) or the stromal vascular fraction of mouse iBAT [26]. Specifically, cells were cultured in a differentiation medium (DMEM with 10% FBS, 20 nmol/l insulin and 1 nmol/l T3) for 3 days and then switched to induction medium (differentiation medium plus 0.125 mmol/l indometacin, 5 μmol/l dexamethasone and 0.5 mmol/l IBMX). Two days later, the cells were switched back to differentiation medium for 5 days. The co-culture system was used for in vitro adiponectin treatment [17, 21]. For in vitro lipolysis assays, a co-culture system was used to treat brown adipocytes (derived from the stromal vascular fraction of iBAT from Adipoq−/− mice) with adiponectin for 24 h [17, 21]. The cells were then treated with the selective β3-adrenergic agonist BRL37344 (10 ng/ml) or PBS for 1 h. Glycerol levels in culture medium were measured as previously described [17].
Statistical analysis
Data are expressed as mean ± SEM. Statistical analyses were performed using the Student’s t test or ANOVA, followed by Bonferroni post-hoc tests using GraphPad Prism version 6.00 for Mac, GraphPad Software, La Jolla, CA, USA. A p value of <0.05 was considered to be statistically significant.
Results
Adiponectin inhibits thermogenesis and energy expenditure in mice
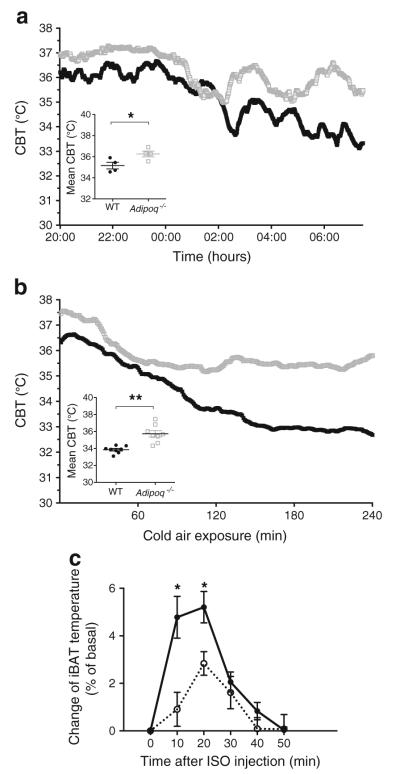
Thermogenesis is the main function of BAT, especially in rodents. To study the effects of adiponectin on BAT activation, CBTs were compared between Adipoq−/− and WT mice using intraperitoneally implanted wireless sensors. As shown in Fig. 1a, the CBTs of Adipoq−/− mice housed at room temperature (RT) were significantly higher than those of WT mice. During acute cold exposure (4°C air), Adipoq−/− mice were remarkably more resistant to hypothermia than WT mice (Fig. 1b) and their average CBTs significantly exceeded those of WT control mice (Fig. 1b inset). The resistance of Adipoq−/− mice to hypothermia was abolished when their iBAT was excised (Electronic supplementary material [ESM] Fig. 1a). Conversely, using adenoviral vectors to express adiponectin in the liver of Adipoq−/− mice, we found significantly lower CBTs in adiponectin-reconstituted mice than adenovirus-encoded green fluorescent protein-transduced control mice during acute cold exposure (ESM Fig. 1b).
Fig. 1.
Adiponectin inhibits thermogenesis in mice. (a, b) An emitter was implanted into the peritoneal cavity of 2-month-old male WT (black squares) and Adipoq−/− mice (grey outlined squares). After 1 week of recovery, CBTs were monitored during (a) overnight fasting (n=4 per group) or (b) 4°C cold exposure (n=7–9 per group). Average CBT (±SEM) is displayed in the insets. *p<0.05, **p<0.01 vs WT. (c) male Adipoq−/− mice implanted with a Bio-Thermo Lifechip (Destron Fearing, South St Paul, MN, USA) underneath iBAT were injected i.v. with purified Ad-GFP (black circles) or Ad-Acrp30 (white circles) virus. Three days later, iBAT temperature was remotely measured before and after ISO injection (n=5 per group). Increase of iBAT temperature (% of basal ± SEM) is displayed. *p<0.05 vs Ad-Acrp30-injected mice
The sympathetic nervous system (SNS) plays a critical role in BAT activation, a process mediated primarily by βARs, especially β3 [7, 27]. We measured the β agonist ISO-induced changes of iBAT temperature to assess the effect of adiponectin on thermogenesis in Adipoq−/− mice. Adiponectin reconstitution significantly attenuated ISO-induced increase of iBAT temperature (Fig. 1c), an effect consistent with the cold exposure findings (ESM Fig. 1b). Together, these results suggest that adiponectin inhibits BAT activation and thermogenesis in mice.
Given the importance of BAT in energy metabolism, we used metabolism cages to investigate the effects of adiponectin on energy expenditure. Consistent with previous reports [12–15], Adipoq−/− mice exhibited significantly higher energy expenditures than WT mice, particularly at the onset of the dark cycle (ESM Fig. 1c). These results further confirm the inhibitory effects of adiponectin on energy expenditure.
Adiponectin reduces Ucp1 expression in iBAT
iBAT is the largest BAT in mice. Although CBTs were significantly increased in Adipoq−/− mice, their iBAT mass was indistinguishable from that of WT mice (n=8, 101.56±6.5 vs 94.29±6.2 mg; p=0.44). In addition, there was no obvious change in their iBAT morphology as assessed by hematoxylin and eosin staining (ESM Fig. 2).
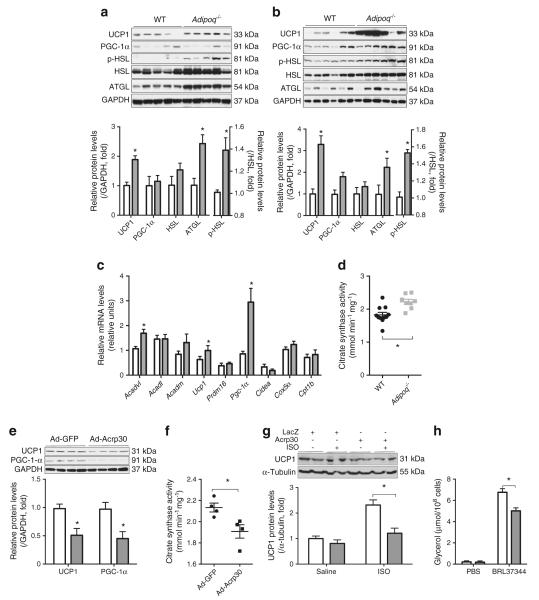
UCP1 is essential for thermogenesis in BAT. Its expression in iBAT was compared between Adipoq−/− and WT mice. Similar to previous reports [13, 15], we found significantly more UCP1 protein in the iBAT of Adipoq−/− mice than WT controls housed at RT (Fig. 2a). The difference became striking after 4 h of cold air exposure (Fig. 2b). In addition, cold exposure significantly increased PGC-1α gene expression in the iBAT of Adipoq−/− mice compared with WT control mice (Fig. 2b, c).
Fig. 2.
Adiponectin reduces UCP1 expression in iBAT. iBATs were collected from 8-week-old WT and Adipoq−/− mice at (a) RT or (b–d) after 4 h of cold exposure. (e, f) WT mice were injected i.v. with Ad-GFP or Ad-Acrp30 3 days before iBAT collection. (d, f) Citrate synthase activity in iBAT homogenates was determined based on the acetylation of oxaloacetic acid. (g, h) brown adipocytes differentiated from stromal vascular fraction of the iBAT of Adipoq−/− mice were treated overnight with Acrp30 (grey bar) using a co-culture system. Protein or medium samples were collected after treatment with ISO (10 μmol/l, 4 h) or BRL37344 (10 ng/ml, 1 h). Levels of (g) UCP1 protein and (h) glycerol in medium were determined. In (a–c): white bar, WT, grey bar, Adipoq−/−; (e): white bar, Ad-GFP, grey bar, Ad-Acrp30. n=6–8, *p<0.05 vs control mice
Citrate synthase is the first enzyme of the tricarboxylic acid cycle. Its activity is often used to assess the oxidative status of mitochondria, which is key to thermogenesis. We found significantly higher citrate synthase activity in the iBAT of Adipoq−/− mice than that of WT control mice (Fig. 2d).
Taking an alternative approach, we used an adenoviral vector to overexpress adiponectin in WT mice to investigate the effect of adiponectin on UCP1 expression and citrate synthase activity in iBAT. As shown in Fig. 2e, f, adiponectin overexpression significantly decreased UCP1 and peroxisome proliferator–activated receptor γ co-activator 1 α (PGC-1α) protein levels, as well as citrate synthase activity in iBAT. These in vivo effects were recapitulated in vitro using cultured brown adipocytes, showing that adiponectin treatment significantly reduced ISO-induced UCP1 expression (Fig. 2g). Together, these results indicate that adiponectin reduces Ucp1 gene expression and citrate synthase activity in mouse iBAT.
Similar to our previous observation in WAT [17], phosphorylation of hormone-sensitive lipase was robustly elevated in iBAT of Adipoq−/− mice at RT and after cold exposure (Fig. 2a, b). Interestingly, remarkably increased protein levels of adipose triacylglycerol lipase were also found in the iBAT of Adipoq−/− mice (Fig. 2a, b), but not in WAT [17]. Furthermore, by using cultured brown adipocytes from Adipoq−/− mice, we found that glycerol release induced by the selective β3 agonist BRL37344 was significantly decreased by adiponectin treatment (Fig. 2h). Together, these results indicate that adiponectin inhibits lipolysis in brown adipocytes similar to its effect on white adipocytes [17, 18]. Lipolysis provides fatty acids to fuel thermogenesis in BAT. Therefore, we postulated that increased lipolysis and fuel supply contributed to the cold-resistant phenotype of Adipoq−/− mice.
Adiponectin reduces mitochondrial respiration in iBAT without overtly altering mitochondrial biogenesis
Mitochondrial enrichment is a hallmark of brown adipocytes. We and others have reported that adiponectin enhances mitochondrial biogenesis in skeletal muscle [21, 28, 29]. However, the current study shows that in BAT, adiponectin reduces expression of PGC-1α (Fig. 2b, c, e), a key regulator of mitochondrial biogenesis. We, therefore, set out to investigate the effects of adiponectin on mitochondrial biogenesis in brown adipocytes. We found no significant difference in mitochondrial DNA copy number or the mitochondrial marker Cox5a between Adipoq−/− and WT or between adiponectin-overexpressing and control mice (ESM Fig. 3a, b). In addition, by comparing the ultrastructure of iBAT, we found no significant difference in mitochondrial structure or density between Adipoq−/− and WT mice (ESM Fig. 3c, d). These results indicate that despite inhibiting PGC-1α expression in iBAT, adiponectin does not significantly affect mitochondrial biogenesis in brown adipocytes. Given the important role of PGC-1α in mitochondrial biogenesis, there was an apparent discordance between the effects of adiponectin on PGC-1α expression, mitochondrial biogenesis and citrate synthase activity in brown adipocytes. This discordance is reminiscent of mice with Lkb1 deficiency in skeletal muscle, where exercise increases mitochondrial protein expression and citrate synthase activity without changing PGC-1α or mitochondrial DNA [30]. Moreover, PGC-1α deficiency fails to abolish exercise-induced adaptive mitochondrial protein expression in muscle [31].
Oxygen consumption is a readout for mitochondrial metabolic state. We compared the respiration rates of mitochondria purified from iBAT of Adipoq−/− and adiponectin-overexpressing mice in comparison with their respective controls. We found no difference in any of the four states of respiration when pyruvate was used as substrate (ESM Fig. 3e). However, in the presence of the lipid substrate palmitoyl carnitine, the respiration rate of Adipoq−/− mitochondria was significantly higher than that in WT controls (Fig. 3a). Conversely, the rate was decreased by adiponectin reconstitution of Adipoq−/− mice (Fig. 3b). These results suggest that despite the lack of a measurable effect on iBAT mitochondrial biogenesis, adiponectin suppresses fatty acid combustion in brown adipocytes.
Fig. 3.
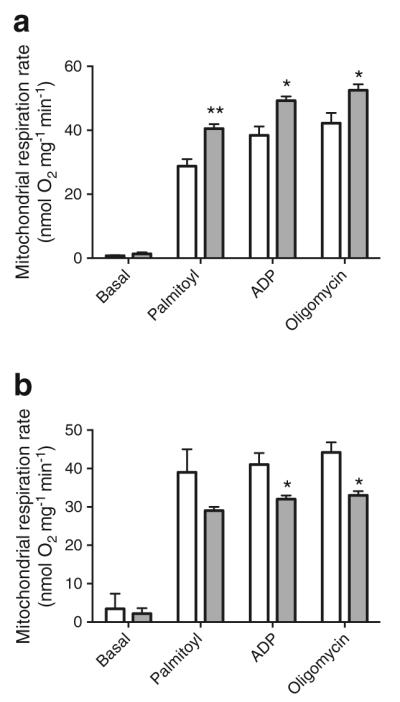
Adiponectin reduces mitochondrial respiration rate in iBAT. Mitochondria were purified from (a) freshly isolated iBAT of Adipoq−/− (grey bars) and WT mice (white bars) or (b) adiponectin-reconstituted mice (Ad-GFP, white bars; Ad-Acrp30, grey bars), all housed at RT. Respiration rates were determined using palmitoyl carnitine (10 μmol/l) as substrate. Data are presented as mean ± SEM. n=6–8 per group of mice. *p<0.05, **p<0.01 vs control mice
Adiponectin decreases browning of subcutaneous fat
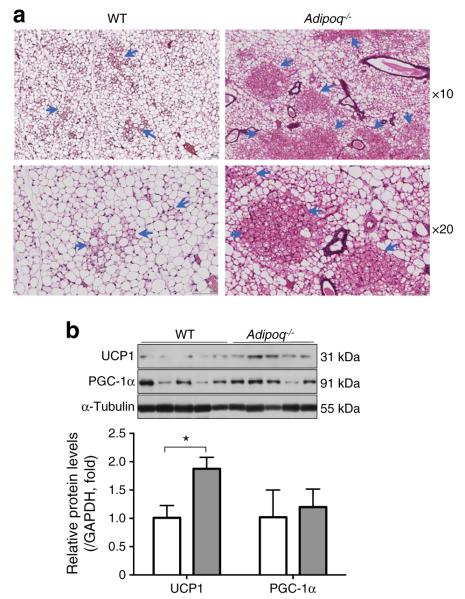
Browning has been observed in rodent models [8, 9]. Hyperplasia of beige cells in WAT has been suggested to enhance thermogenesis and energy expenditure [8–11]. By comparing inguinal fat from mice that were housed under regular conditions, we found no significant difference in UCP1 expression or histology structure between WT and Adipoq−/− mice (data not shown). We then exposed Adipoq−/− and WT mice to cold air intermittently for 10 days prior to comparing the histology of and gene expression in inguinal fat. As expected, cold exposure significantly increased brown adipocyte-like structure in inguinal fat of both WT and Adipoq−/− mice (data not shown). Figure 4 shows that Adipoq−/− mice contained significantly more brown adipocyte-like regions (Fig. 4a) and higher UCP1 protein levels (Fig. 4b) than WT control mice. These results indicate that the inguinal fat of Adipoq−/− mice is more prone to cold-induced browning, suggesting that adiponectin normally inhibits cold-induced browning of subcutaneous fat.
Fig. 4.
Enhanced browning of inguinal fat of Adipoq−/− mice. Adipoq−/− and WT mice were intermittently exposed to cold air for 10 days. (a) inguinal fat pads were collected for haematoxylin and eosin staining, and brown adipocyte-like structures are marked (blue arrows). (b) UCP1 and PGC-1α protein levels were determined by western blotting. White bars, WT; grey bars, Adipoq−/−. The data shown are mean ± SEM, n=5 per group. *p<0.05
Adiponectin inhibits thermogenesis independently of adiponectin receptors 1 and 2
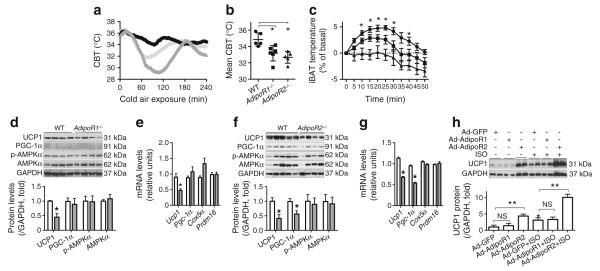
AdipoR1 and AdipoR2 have been reported to be the main cell-surface receptors that mediate adiponectin signalling [32]. To investigate the roles of these receptors in the inhibition of BAT activation by adiponectin, we challenged AdipoR1−/− and AdipoR2−/− mice to cold air. To our surprise, both groups of mice were more susceptible than WT to cold-induced hypothermia (Fig. 5a, b). In addition, ISO-induced iBAT hyperthermia was significantly attenuated in AdipoR1−/− mice and, more strikingly, abolished in AdipoR2−/− mice (Fig. 5c). These results indicate that in contrast to the cold resistance of Adipoq−/− mice, both AdipoR1−/− and AdipoR2−/− mice were cold intolerant.
Fig. 5.
AdipoR1−/− and AdipoR2−/− mice are cold intolerant. (a, b) Mice of three genotypes were exposed to 4°C 1 week after emitter implantation and CBTs were monitored. The data are presented as mean or mean ± SEM (n=5–6 per group). (a) Black circles, WT, light grey triangles; AdipoR1−/−; dark grey triangles, AdipoR2−/−. (c) temperatures of iBAT in mice kept at RT were remotely recorded before and after ISO injection. Black circles, WT; black squares, AdipoR1−/−; black triangles, AdipoR2−/−. The basal ± SEM percentage temperature changes are presented. *p<0.05 vs AdipoR1−/− or AdipoR2−/− mice. (d–g) iBATs were collected from AdipoR1−/− or AdipoR2−/− mice after 4 h of cold air exposure. Levels of protein and mRNA (d, e grey bars, AdipoR1−/−; f, g grey bars, AdipoR2−/−) were measured by immunoblotting or real-time PCR (n=5–8 per geno-type). *p<0.05 vs WT. (h) Differentiated brown adipocytes from immortalised pre-adipocytes were transduced for 24 h with adenovirus vectors encoding AdipoR1 or AdipoR2 (Ad-AdipoR1 and Ad-AdipoR2). Four hours after ISO treatment, protein levels of UCP1 were measured by immunoblotting (n=8). *p<0.05 vs Ad-GFP-transduced cells without ISO treatment. **p<0.01 vs indicated group
Receptor deletion could conceivably lead to compensatory increases in ligand levels. We determined adiponectin levels in serum and iBAT to investigate why AdipoR1−/− and AdipoR2−/− mice were impaired in thermogenesis. Remarkably, serum and iBAT adiponectin levels were decreased in AdipoR1−/− mice (ESM Fig. 4a, b) but remained unaffected in AdipoR2−/− mice (ESM Fig. 4c, d). Given the anti-thermogenic effect of adiponectin, these results suggest that the cold intolerance of AdipoR1−/− and AdipoR2−/− mice is not due to changes in adiponectin levels.
Given that thermogenesis is increased in Adipoq−/− mice but decreased in both AdipoR1−/− and AdipoR2−/− mice, we created double knockouts to further explore this paradox. Upon cold challenge, both AdipoR1−/− and AdipoR2−/− mice became hypothermic faster than WT mice (ESM Fig. 4e). Remarkably, the cold intolerance of both groups of knockout mice was alleviated by concomitant deletion of adiponectin (ESM Fig. 4e). These results suggest that adiponectin regulates thermogenesis through a mechanism distinct from AdipoR1 and AdipoR2.
In line with their cold intolerant phenotype, the iBAT of AdipoR1−/− and AdipoR2−/− mice expressed less UCP1 protein and Ucp1 mRNA than WT controls (Fig. 5d–g). In cultured brown adipocytes, we found that AdipoR2 overexpression robustly increased UCP1 protein levels regardless of ISO treatment (Fig. 5h). These results indicate that AdipoR2 enhances UCP1 expression in brown adipocytes.
AMP-activated protein kinase (AMPK) is an effector of AdipoR1 and AdipoR2 [32]. However, we detected no significant alteration in protein levels or phosphorylation of AMPK in iBAT of AdipoR1−/− or AdipoR2−/− mice (Fig. 5d, f), suggesting that AMPK may not be responsible for AdipoR1- or AdipoR2-dependent thermogenesis.
Adiponectin inhibits Ucp1 expression by downregulating β3 adrenergic receptor in adipocytes
To study the effects of adiponectin on Ucp1 gene expression and its underlying mechanism, in vitro differentiated brown adipocytes were treated with adiponectin using a co-culture system [21]. Interestingly, despite a minor effect on basal Ucp1 gene expression, adiponectin treatment robustly diminished UCP1 induction by the β3-specific agonist BRL37344 (Fig. 6a) and the nonselective β agonist ISO (Fig. 2g). Given the critical role of the SNS in BAT development and activation, the results of this in vitro study suggest that the SNS may mediate the inhibitory effect of adiponectin on UCP1 expression and BAT thermogenesis. To test this hypothesis, sympathetic denervation of iBATwas performed by injecting 6-hydroxydopamine into the iBAT of both Adipoq−/− and adiponectin-reconstituted mice [33, 34]. A significant decrease of tyrosine hydroxylase mRNA in iBAT was apparent (ESM Fig. 5a). Despite the denervation, adiponectin robustly inhibited UCP1 expression in iBAT (ESM Fig. 5b). We, therefore, concluded that adiponectin acted directly on iBAT to secondarily modulate the SNS effect.
Fig. 6.
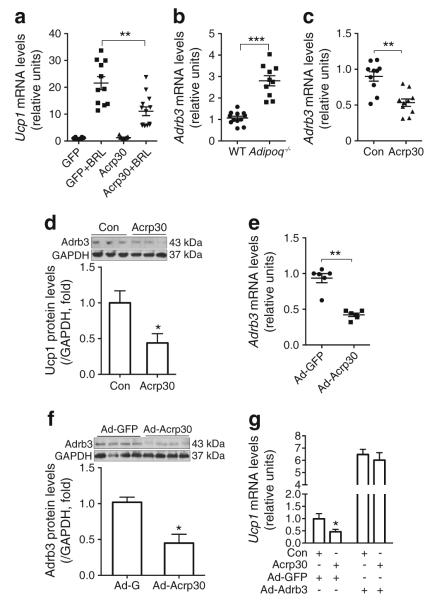
Adiponectin inhibits UCP1 expression in brown adipocytes through downregulating Adrb3. (a) Brown adipocytes differentiated from the stromal vascular fraction of iBAT from Adipoq−/− mice were treated overnight with Acrp30 using a co-culture system. BRL (50 ng/ml) was added to the medium 4 h before sample collection. Expression levels of Ucp1 were determined by real-time PCR (n=8–13 per group). (b) Adrb3 mRNA levels of the iBAT of Adipoq−/− and WT mice housed at RT (n=10–12 per group). (c, d) differentiated brown adipocytes were treated overnight with Acrp30 using a co-culture system. Levels of (c) Adrb3 mRNA and (d) Adrb3 protein were determined by real-time PCR and immunoblotting (n=10 per group). (e, f) Adipoq−/− mice were transduced with Acrp30-expressing adenovirus to reconstitute adiponectin, with Ad-GFP as control. iBATs were collected 3 days later to quantify (e) Adrb3 mRNA by real-time PCR and (f) Adrb3 protein by immunoblotting (n=8 per group). (g) Adipocytes differentiated from immortalised brown preadipocytes were transduced with an adenovirus vector overexpressing Adrb3 for 24 h. ISO was added 4 h before sample collection. A co-culture system was used for adiponectin treatment. Data are presented as mean ± SEM, n=8 per group. *p<0.05 vs cells treated with control medium (d) or cells transduced with Ad-GFP without Ad-Adrb3. **p<0.01 vs indicated group. Con, control
The β3-adrenergic receptor Adrb3 is highly expressed in rodent adipocytes and is pivotal to SNS-mediated lipolysis and thermogenesis in BAT. We found a remarkable upregulation of Adrb3 mRNA levels in iBAT (Fig. 6b) and inguinal fat (ESM Fig. 6a) of Adipoq−/− mice compared with WT controls. In brown adipocytes differentiated from iBAT stromal vascular fraction, adiponectin treatment significantly reduced Adrb3 mRNA and protein expression (Fig. 6c, d). In line with these in vitro results, adiponectin reconstitution in Adipoq−/− mice also robustly reduced Adrb3 mRNA and protein levels in iBAT (Fig. 6e, f). PKA is a main effector of Adrb3 in adipocytes. Consistent with the changes in Adrb3 expression, phosphorylation of PKA substrates was significantly reduced in adiponectin-treated brown adipocytes and iBAT of adiponectin-reconstituted Adipoq−/− mice (ESM Fig. 6b, c). These results clearly indicate that adiponectin inhibits Adrb3 gene expression in mouse brown adipocytes.
To verify whether or not Adrb3 downregulation mediates the inhibitory effects of adiponectin on UCP1 expression, Adrb3 was overexpressed in cultured brown adipocytes. The overexpression prevented adiponectin from inhibiting UCP1 expression in ISO-treated brown adipocytes (Fig. 6g), supporting the hypothesis that adiponectin inhibits UCP1 expression in brown adipocytes by suppressing Adrb3.
Discussion
Emerging evidence suggests that adiponectin plays an important role in energy homeostasis beyond its insulin-sensitising effect. The current study shows that adiponectin inhibits BAT activation and thermogenesis by suppressing both Ucp1 gene expression in brown adipocytes and browning of subcutaneous fat. This study also reveals that adiponectin suppresses BAT activation by inhibiting βAR signalling in that tissue. To our knowledge, this is the first study to date that systematically demonstrates the regulatory effect of adiponectin on BAT activation and thermogenesis. Identification of the inhibitory effects of adiponectin on BAT provides mechanistic insights into how this fat-secreted starvation hormone reduces energy expenditure and modulates energy metabolism.
Thermogenesis is the main function of BAT. By monitoring the CBT of genetically manipulated mouse models with and without acute cold stress, our study demonstrated that adiponectin inhibits thermogenesis in mice. This anti-thermogenic effect likely has two components: the extent of mitochondrial uncoupling and the availability of fuels. UCP1 is expressed only in brown adipocytes, where it promotes thermogenesis by uncoupling fuel oxidation from ATP formation. Although iBAT in our Adipoq−/− mice showed no obvious alterations in tissue mass or histology, it expresses more UCP1 protein than in WT mice both in the basal state and after cold exposure. Conversely, Ucp1 gene expression is down-regulated in adiponectin-overexpressing mice. These results confirm previous reports that adiponectin inhibits Ucp1 gene expression in brown adipocytes [13, 15, 19]. Fatty acids are an important fuel for thermogenesis. In response to cold exposure, the SNS turns on both lipolysis and thermogenesis. Lipolysis releases fatty acids for mitochondrial oxidation and thermogenesis. Previous studies have demonstrated that adiponectin inhibits lipolysis in white adipocytes [17, 18]. The current study shows that adiponectin also inhibits lipolysis in brown adipocytes. In both types of adipocyte, the inhibition of lipolysis should reduce the supply of fatty acids as thermogenic substrates. Additionally, we found that adiponectin suppresses browning of subcutaneous fat in mice repetitively exposed to cold. Although beige cells can contribute to thermogenesis, their limited presence in WAT under physiological conditions suggests a minor role in adiponectin-inhibited thermogenesis.
In contrast to leptin, adiponectin exhibits several characteristics of a starvation hormone. For example, it inhibits energy expenditure and enhances fat accumulation in several mouse studies [12–15]. Although other mechanisms may be involved, our discovery of adiponectin-mediated BAT inhibition provides mechanistic insights into how this hormone suppresses energy expenditure and enhances fat accumulation. We realise that the effects of adiponectin on BAT activation and fat accumulation are modest under our experimental conditions. However, the effects could be more pronounced in different settings since the importance of UCP1 is highly context dependent. Specifically, Ucp1 knockout mice are obese at thermoneutrality but not at RT [6]. Our mice were housed at RT, which is below thermoneutrality for mice. Therefore, the magnitude of adiponectin-inhibited energy expenditure may be attenuated by counter-regulatory factors in energy metabolism and BAT activation due to the constant cold stress of RT.
Several plasma membrane proteins have been described as adiponectin receptors [32, 35]. Although detailed signalling pathways have not been elucidated, AdipoR1 and AdipoR2 have been reported to transduce the effects of adiponectin on both glucose and lipid metabolism [32]. We were surprised to find that neither AdipoR1 nor AdipoR2 is necessary for adiponectin to inhibit BAT activation. Our study showed that AdipoR2 knockout dramatically impaired thermogenesis (Fig. 5a–c), while concomitant deletion of adiponectin in these mice attenuated the phenotype (ESM Fig. 4e), suggesting that adiponectin and AdipoR2 regulate thermogenesis through different pathways. Lending support to the notion that AdipoR2 modulates thermogenesis independently of adiponectin is the nematode Caenorhabditis elegans. Despite the absence of an adiponectin homologue in this worm, deletion of its AdipoR2 homologue impairs both cold adaptation and lipid metabolism [36]. While we were preparing this paper, Kajimura et al reported that the regulatory effect of adiponectin on bone mass is independent of AdipoR1 and AdipoR2 [15]. Therefore, we propose that AdipoR1 and AdipoR2 may also serve as receptors for other ligands or may have ligand-independent activities that control BAT activation and energy metabolism. Further investigations are warranted to determine the signalling pathways that mediate the inhibitory effects of adiponectin on thermogenesis.
The SNS plays a critical role in BAT development and thermogenesis. Adrb3, a member of the βAR family, is predominantly expressed in adipose tissues in rodents. Our study found that sympathetic denervation of iBAT did not prevent the inhibitory effect of adiponectin on UCP1 expression in that tissue (ESM Fig. 5b). This is in contrast to cultured brown adipocytes, where the inhibitory effect of adiponectin on UCP1 requires the presences of adrenergic agonists (Figs. 2g and 6a). These results suggest that adiponectin inhibits UCP1 and thermogenesis through a pathway within adipocytes and not through the SNS. Indeed, overexpression of Adrb3 in brown adipocytes abolished this inhibitory effect (Fig. 6g), supporting the hypothesis that Adrb3 downregulation mediates inhibition of BAT activation and thermogenesis in mice by adiponectin. Our study does not address mechanistically how adiponectin downregulates Adrb3 in adipocytes. Given that the phenotype of Adipoq−/− mice is the opposite to that of AdipoR1−/− and AdipoR2−/− mice, it seems unlikely that adiponectin inhibits Adrb3 through AdipoR1 or AdipoR2. It is noteworthy that Adrb3 expression and function in humans, unlike rodents, is not restricted to adipocytes and is uncertain. Caution should be taken in extrapolating the anti-thermogenic effect of adiponectin in mice to that in humans.
In summary, this study demonstrates that adiponectin inhibits BAT activation and thermogenesis in mice by suppressing UCP1 expression, lipolysis and brown adipocyte recruitment. The findings expand our understanding of how adiponectin regulates energy homeostasis.
Supplementary Material
Acknowledgements
We thank Y.-H. Tseng (Joslin Diabetes Center, Harvard Medical School, Boston, MA, USA) and P. Scherer (The University of Texas Southwestern Medical Center, Dallas, TX, USA) for providing brown pre-adipocytes and Adipoq−/− mice, respectively.
Funding This work was supported by grants DK080418 (JS), HD069634 (JS), DK095132 (JS) and R01DK075916 (GF) from the National Institutes of Health and 5-I01-BX000702 (N-WC) from the Department of Veterans Affairs.
Abbreviations
- Acrp30
Adipocyte complement-related protein of 30 kDa (adiponectin)
- Ad-Acrp30
Adenovirus-encoded adipocyte complement-related protein of 30 kDa (adiponectin)
- Ad-GFP
Adenovirus-encoded green fluorescent protein
- AMPK
AMP-activated protein kinase
- ATGL
Adipose triacylglycerol lipase
- βAR
β-adrenergic receptor
- BAT
Brown adipose tissue
- CBT
Core body temperature
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- HSL
Hormone-sensitive lipase
- iBAT
Interscapular brown adipose tissue
- ISO
Isoprenaline
- Myf5
Myogenic factor 5
- PGC-1α
Peroxisome proliferator–activated receptor γ co-activator 1 α
- PKA
Protein kinase A
- RT
Room temperature
- SNS
Sympathetic nervous system
- UCP1
Uncoupling protein 1
- WAT
White adipose tissue
- WT
Wild type
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00125-014-3180-5) contains peer-reviewed but unedited supplementary material, which is available to authorised users.
Duality of interest The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement LQ, HY, CB and BL performed experiments, analysed data and revised the article. GF, N-WC and JSc contributed study design, reviewing and editing of this manuscript. JSh is responsible for the integrity of this work as a whole and conceived and supervised the study and wrote the manuscript. All authors approved the final version of this manuscript.
Contributor Information
Liping Qiao, Department of Pediatrics, University of California San Diego, 9500 Gilman Drive, MC 0983, La Jolla, CA 92093, USA.
Hyung sun Yoo, Department of Pediatrics, University of California San Diego, 9500 Gilman Drive, MC 0983, La Jolla, CA 92093, USA.
Chris Bosco, Department of Pediatrics, University of California San Diego, 9500 Gilman Drive, MC 0983, La Jolla, CA 92093, USA.
Bonggi Lee, Department of Pediatrics, University of California San Diego, 9500 Gilman Drive, MC 0983, La Jolla, CA 92093, USA.
Gen-Sheng Feng, Department of Pathology, University of California San Diego, La Jolla, CA, USA.
Jerome Schaack, Department of Microbiology, University of Colorado at Denver and Health Sciences Center, Aurora, CO, USA.
Nai-Wen Chi, Veterans Affairs San Diego Healthcare System, and Department of Medicine, University of California San Diego, La Jolla, CA, USA.
Jianhua Shao, Department of Pediatrics, University of California San Diego, 9500 Gilman Drive, MC 0983, La Jolla, CA 92093, USA.
References
- 1.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 2.Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 4.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 5.Saito M, Okamatsu-Ogura Y, Matsushita M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Virtanen KA, Nuutila P. Brown adipose tissue in humans. Curr Opin Lipidol. 2011;22:49–54. doi: 10.1097/MOL.0b013e3283425243. [DOI] [PubMed] [Google Scholar]
- 8.Boström P, Wu J, Jedrychowski MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cinti S. Adipocyte differentiation and transdifferentiation: plasticity of the adipose organ. J Endocrinol Invest. 2002;25:823–835. doi: 10.1007/BF03344046. [DOI] [PubMed] [Google Scholar]
- 10.Fisher FM, Kleiner S, Douris N, et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 2012;15:395–404. doi: 10.1016/j.cmet.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J-Y, van de Wall E, Laplante M, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kubota N, Yano W, Kubota T, et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007;6:55–68. doi: 10.1016/j.cmet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Saito K, Arata S, Hosono T, et al. Adiponectin plays an important role in efficient energy usage under energy shortage. Biochim Biophys Acta. 2006;1761:709–716. doi: 10.1016/j.bbalip.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Kajimura D, Lee HW, Riley KJ, et al. Adiponectin regulates bone mass via opposite central and peripheral mechanisms through FoxO1. Cell Metab. 2013;17:901–915. doi: 10.1016/j.cmet.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Combs TP, Pajvani UB, Berg AH, et al. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004;145:367–383. doi: 10.1210/en.2003-1068. [DOI] [PubMed] [Google Scholar]
- 17.Qiao L, Kinney B, Schaack J, Shao J. Adiponectin inhibits lipolysis in mouse adipocytes. Diabetes. 2011;60:1519–1527. doi: 10.2337/db10-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wedellová Z, Dietrich J, Siklová-Vítková M, et al. Adiponectin inhibits spontaneous and catecholamine-induced lipolysis in human adipocytes of non-obese subjects through AMPK-dependent mechanisms. Physiol Res. 2011;60:139–148. doi: 10.33549/physiolres.931863. [DOI] [PubMed] [Google Scholar]
- 19.Dong M, Yang X, Lim S, et al. Cold exposure promotes atherosclerotic plaque growth and instability via UCP1-dependent lipolysis. Cell Metab. 2013;18:118–129. doi: 10.1016/j.cmet.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiao L, MacLean PS, You H, et al. Knocking down liver ccaat/enhancer-binding protein alpha by adenovirus-transduced silent interfering ribonucleic acid improves hepatic gluconeogenesis and lipid homeostasis in db/db mice. Endocrinology. 2006;147:3060–3069. doi: 10.1210/en.2005-1507. [DOI] [PubMed] [Google Scholar]
- 21.Qiao L, Kinney B, Yoo HS, et al. Adiponectin increases skeletal muscle mitochondrial biogenesis by suppressing mitogen-activated protein kinase phosphatase-1. Diabetes. 2012;61:1463–1470. doi: 10.2337/db11-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiao L, Zou C, van der Westhuyzen DR, Shao J. Adiponectin reduces plasma triglyceride by increasing VLDL triglyceride catabolism. Diabetes. 2008;57:1824–1833. doi: 10.2337/db07-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc. 2007;2:287–295. doi: 10.1038/nprot.2006.478. [DOI] [PubMed] [Google Scholar]
- 24.Yeh T-YJ, Beiswenger KK, Li P, et al. Hypermetabolism, hyperphagia, and reduced adiposity in tankyrase-deficient mice. Diabetes. 2009;58:2476–2485. doi: 10.2337/db08-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zechner C, Lai L, Zechner JF, et al. Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell Metab. 2010;12:633–642. doi: 10.1016/j.cmet.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tseng Y-H, Kokkotou E, Schulz TJ, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blaak EE, van Baak MA, Kempen KP, Saris WH. Role of alpha- and beta-adrenoceptors in sympathetically mediated thermo-genesis. Am J Physiol. 1993;264:E11–E17. doi: 10.1152/ajpendo.1993.264.1.E11. [DOI] [PubMed] [Google Scholar]
- 28.Iwabu M, Yamauchi T, Okada-Iwabu M, et al. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca(2+) and AMPK/SIRT1. Nature. 2010;464:1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 29.Civitarese AE, Ukropcova B, Carling S, et al. Role of adiponectin in human skeletal muscle bioenergetics. Cell Metab. 2006;4:75–87. doi: 10.1016/j.cmet.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanner CB, Madsen SR, Hallowell DM, et al. Mitochondrial and performance adaptations to exercise training in mice lacking skeletal muscle LKB1. Am J Physiol Endocrinol Metab. 2013;305:E1018–E1029. doi: 10.1152/ajpendo.00227.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leick L, Wojtaszewski JFP, Johansen ST, et al. PGC-1alpha is not mandatory for exercise- and training-induced adaptive gene responses in mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2008;294:E463–E474. doi: 10.1152/ajpendo.00666.2007. [DOI] [PubMed] [Google Scholar]
- 32.Yamauchi T, Kamon J, Ito Y, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 33.Chao P-T, Yang L, Aja S, et al. Knockdown of NPYexpression in the dorsomedial hypothalamus promotes development of brown adipocytes and prevents diet-induced obesity. Cell Metab. 2011;13:573–583. doi: 10.1016/j.cmet.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thureson-Klein A, Mill-Hyde B, Barnard T, Lagercrantz H. Ultrastructural effects of chemical sympathectomy on brown adipose tissue. J Neurocytol. 1976;5:677–690. doi: 10.1007/BF01181581. [DOI] [PubMed] [Google Scholar]
- 35.Hug C, Wang J, Ahmad NS, et al. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Svensson E, Olsen L, Mörck C, et al. The adiponectin receptor homologs in C. elegans promote energy utilization and homeostasis. PLoS ONE. 2011;6:e21343. doi: 10.1371/journal.pone.0021343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.