Abstract
Glutamate transporter type 3 (EAAT3) may play a role in cognition. Isoflurane enhances EAAT3 trafficking to the plasma membrane. Thus, we used isoflurane to determine how EAAT3 might regulate learning and memory and the trafficking of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors, such as GluR1, to the plasma membrane, a fundamental biochemical process for learning and memory. Here, isoflurane increased EAAT3 but did not change GluR1 levels in the plasma membrane of wild-type mouse hippocampus. Isoflurane increased protein phosphatase activity in wild-type and EAAT3−/− mouse hippocampus. Also, isoflurane reduced GluR1 in the plasma membrane and decreased phospho-GluR1 in EAAT3−/− mice. The phosphatase inhibitor okadaic acid attenuated these effects. Finally, isoflurane inhibited context-related fear conditioning in EAAT3−/− mice but not in wild-type mice. Thus, isoflurane may increase GluR1 trafficking to the plasma membrane via EAAT3 and inhibit GluR1 trafficking via protein phosphatase. Lack of EAAT3 effects leads to decreased GluR1 trafficking and impaired cognition after isoflurane exposure in EAAT3−/− mice.
Keywords: cognition, GluR1, glutamate transporter, hippocampus, isoflurane, trafficking
Introduction
Glutamate is the major excitatory neurotransmitter in the central nervous system. Taking up extracellular glutamate by glutamate transporters (also named excitatory amino acid transporters, EAAT) is a main process to maintain the extracellular glutamate levels within the physiological ranges (Rothstein et al., 1996; Danbolt, 2001). Glutamate transporter type 3 (EAAT3) is the major neuronal EAAT (Rothstein et al., 1994; Danbolt, 2001). In addition to taking up glutamate, EAAT3 has been shown to have other functions. We and others have found that EAAT3 knockout mice have learning and memory deficits (Aoyama et al., 2006; Lee et al., 2012). Fear conditioning is associated with increased trafficking of EAAT3 to the plasma membrane in the hippocampus (Levenson et al., 2002). These findings suggest a role of EAAT3 in the learning and memory. However, it is no clear how this effect of EAAT3 is exerted.
A fundamental biochemical process underlying learning and memory is to increase synaptic strength by enhancing the trafficking of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) to the neuronal plasma membrane (Rumpel et al., 2005; Miyamoto, 2006; Whitlock et al., 2006; Man et al., 2007). GluR1 is an AMPAR subunit, whose trafficking to the plasma membrane is enhanced after its phosphorylation by protein kinases, such as protein kinase A (PKA) (Esteban et al., 2003; Man et al., 2007). However, protein phosphatases dephosphorylate GluR1 and inhibit its trafficking to the plasma membrane (Mansuy and Shenolikar, 2006). The role of EAAT3 in these complex biochemical processes remains obscure.
To explore the role of EAAT3 in regulating GluR1 trafficking and cognition, we used isoflurane, a commonly used volatile anesthetic in clinical practice, in this study. This use is because isoflurane enhances EAAT3 trafficking to the plasma membrane (Huang and Zuo, 2005; Huang et al., 2006; Huang et al., 2011). If EAAT3 trafficking to the plasma membrane plays a role in regulating synaptic strength, it should be expected that isoflurane would enhance learning and memory. However, the effect of isoflurane on learning and memory has been controversial. Some studies indicate improved learning and memory of rodents after exposure to isoflurane (Rammes et al., 2009), while others do not observe this effect (Butterfield et al., 2004; Bekker et al., 2006; Lee et al., 2012) or found a detrimental effect on learning and memory (Lin and Zuo, 2011; Valentim et al., 2010). Different animal models, anesthetic exposure methods and testing schedules for assessing learning and memory may have contributed to the contradictory findings. These inconsistent findings also suggest that isoflurane may affect many biochemical processes that are involved in learning and memory and may be a good reagent to use for identifying the interaction of EAAT3 with the known biochemical processes for learning and memory. In addition, determining the effects of isoflurane on these biochemical processes will improve our understanding on how anesthetics/anesthesia may affect the learning and memory. Thus, we designed ex vivo and in vivo experiments using wild-type and EAAT3 knockout mice to determine the possible role of EAAT3 in regulating GluR1 trafficking and cognition and the effects of isoflurane on this regulation.
Methods
These studies were conducted following protocols that were approved by Institutional Animal Care and Use Committee of the University of Virginia (Charlottesville, VA, USA). All animal experiments were performed according to the latest National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. We strived to minimize the number of animals and their suffering.
Animals
Eight- to twelve-week old male EAAT3 knockout mice and their wild-type CD1 littermates were used in these studies. The EAAT3 knockout mice were from the strain as described by Peghinni et al (Peghini et al., 1997). The CD-1 wild-type mice were from Charles River Laboratories (Wilmington, MA, USA). The EAAT3 knockout mice have a disrupted exon 1 of the EAAT3 gene. They were backcrossed with wild-type CD-1 mice for at least 10 generations before they were used in our study. Our previous studies showed that these mice did not express EAAT3 proteins (Lee et al., 2010; Li and Zuo, 2011). To prevent genetic drift and as recommended by the Banbury Conference (Silva et al., 1997), the EAAT3 knockout mice were backcrossed with CD-1 wild-type mice at least once every eight generations
Hippocampal slices preparation
Similar to what we have reported (Huang and Zuo, 2005; Jung et al., 2008), fresh hippocampal slices were prepared from 8- to 12-week old EAAT3 male knockout mice and their wild-type littermates. Mice were euthanized by 5% isoflurane and then decapitated immediately. The brain was removed rapidly and placed in ice-cold artificial cerebrospinal fluid (ACSF) containing 116 mM NaCl, 26.2 mM NaHCO3, 5.4 mM KCl, 1.8 mM CaCl2, 0.9 mM MgCl2, 0.9 mM NaH2PO4, and 5.6 mM glucose (pH 7.4). Hippocampal slices at 300 μm in thickness were cut by a vibrating tissue slicer (Microslicer DTK 1500E, TED Pella, Inc., Redding, CA) in cold cutting solution (260 mM sucrose, 26.2 mM NaHCO3, 3 mM KCl, 1.2 mM NaH2PO4, 5 mM MgCl2, and 9 mM glucose, pH 7.4). The solution was bubbled with 5% CO2 and 95% O2. The slices were then kept for 0.5 h at 4°C in the ACSF gassed with 5% CO2 and 95% O2 before they were used for experiments.
Isoflurane exposure
ACSF at 1 ml per well in 24-well cell culture plate was bubbled with 2% isoflurane in oxygen for 5 min at 37°C before freshly prepared hippocampal slices were place in the ACSF. The ACSF was then bubbled with the isoflurane containing gases for additional 5 min. The concentrations of gases including isoflurane were monitored continuously by a Date™ infrared analyzer (Capnomac, Helsinki, Finland). The exposure to 2% isoflurane for 5 min was chosen because this condition significantly increased EAAT3 trafficking to the plasma membrane.13, 14
In the in vivo experiment, mice were exposed to isoflurane by placing them in a chamber gassed with 2% isoflurane in oxygen for 5 min. To maintain the body temperature of the mice, part of the chamber was submerged in a water-bath at 37°C.
Reagent application during isoflurane treatment
Some hippocampal slices were incubated with or without isoflurane in the presence or absence of 2 μM KT5720, a PKA inhibitor, or 1 μM okadaic acid (OA), an inhibitor for protein phosphatase 1 and 2A, at 37°C. Some hippocampal slices from EAAT3 knockout mice were incubated with 400 μM acetoxymethyl ester of N6-benzoyl-cAMP (6-BNZ-cAMP-AM), a PKA activator, for 5 min at 37°C. KT5720 and 6-BNZ-cAMP were initially dissolved in dimethyl sulfoxide (DMSO) and then diluted with ACSF. The highest DMSO concentration in the final incubation buffer containing KT5720 and 6-BNZ-cAMP were 1% and 2%, respectively. This amount of DMSO was used in the corresponding vehicle experiments. OA was dissolved in ACSF. KT5720 and OA were added at the same time as isoflurane was introduced to the slices.
Biotinylation
Fresh slices were incubated with or without isoflurane or other agents for 5 min in ACSF containing 1 mg/ml sulfosuccinimidyl-2-(biotinamido)ethyl-1,3-dithiopropionate (sulfo-NHS-SS-biotin; Thermo Scientific, Rockford, IL) at 37°C with gentle shaking (Huang and Zuo, 2005; Huang et al., 2006). The ACSF was removed and ice-cold phosphate buffered saline (PBS)-Ca2+/Mg containing 100 mM glycine was added to the slices for 10 min twice to quench unreacted sulfo-NHS-SS-biotin. The slices were homogenized and kept in lysis buffer (PBSCa 2+/Mg containing 0.1% SDS, 1% Triton X-100 and protease inhibitors) for 1 h. The homogenates were centrifuged at 14,000 g for 10 min at 4°C to remove nuclei and debris. An aliquot of the supernatant (100 μl) was kept aside for determination of phospho-GluR1 by western blotting. About 300 μl supernatant was affinity-purified overnight at 4°C on NeutrAvidin beads and the purified biotinylated proteins was re-suspended in 200 μl of 2 × Laemmli buffer containing 5% 2-mercaptoethanol and 50 mM dithiothreitol for 30 min at 4°C.
Immunoprecipitation
Immunoprecipitation was performed as we described previously (Huang and Zuo, 2005; Huang et al., 2006). After being exposed or not being exposed to 2% isoflurane for 5 min, hippocampal slices were lysed in 300 μl of lysis buffer containing 50 mM Tris-HCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 50 mM NaCl and complete protease inhibitors for 1 h at 4°C. The lysates were centrifuged at 14,000 g for 10 min to remove cell debris. The resulting supernatants were incubated overnight with 2 μg of affinity-purified polyclonal rabbit anti-EAAT3 antibody (Alpha Diagnostics International, San Antonio, TX) at 4°C. The mixture was then incubated with 40 μl of Protein A/G Plus-Agarose beads for 1 h at 4°C with gentle shaking. The sample was then centrifuged at 500 g for 2 min at 4°C. The beads were washed four times with lysis buffer, and the immune complexes were then eluted by incubation with 100 μl of Laemmli buffer at 90°C to 95°C for 5 min.
Western Blot Analysis
About 30 – 50 μg proteins per lane were subjected to 10% SDS-polyacrylamide gel electrophoresis and then were electrotransferred to nitrocellulose membranes. The protein bands were probed with the following primary antibodies over-night at 4°C: rabbit polyclonal anti-EAAT3 at 0.5 μg/ml, goat polyclonal anti-GluR1 at 1:1000 dilution, goat polyclonal anti-phospho-GluR1-ser845 at 1:500 dilution, rabbit polyclonal anti-PKA at 1:2000 dilution or rabbit polyclonal anti-glyceraldehydes 3-phosphate dehydrogenase (GAPDH) at 1:3000 dilution. The anti-GluR1 and anti-phospho-GluR1 antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). The anti-PKA antibody was from Cell Signaling (Danvers, MA). The anti-GAPDH antibody was from Sigma-Aldrich (St. Louis, MO, USA). The membrane was then incubated with a horseradish peroxidase-conjugated goat anti-rabbit (1:4000 dilution) or donkey anti-goat (1:4000 dilution) antibody for 2 h at room temperature. The protein bands were visualized by the enhanced chemiluminescence method and quantified using the G:Box equipped with Gene tools analysis software (Syngene, Frederick, MD). The densities of protein bands were normalized to those of GAPDH or EAAT3 (the immunoprecipitation experiments) in the same sample and then normalized by the same day control results. The hippocampal slices for the control and experimental conditions on the same day were from the same mice.
Phosphatase activity assays
After being exposed or not being exposed to 2% isoflurane for 5 min, hippocampal slices were sonicated and placed in 300 μl lysis buffer for 30 min at 4°C. The lysates were centrifuged at 14,000 g for 10 min. The supernatants were used to detect the phosphatase activity according to the protocol of phosphatase assays kit (Cat number: 786453, G-Biosciences, St Louis, MO).
Fear conditioning test
Fear conditioning was performed by using the Freeze Monitor from San Diego Instruments (San Diego, CA) as we described before (Lin and Zuo, 2011; Li et al., 2013). Briefly, each animal was placed in a test chamber wiped with 70% alcohol and subjected to three tone-foot shock pairings (tone: 2000 Hz, 75 db, 30 s; foot shock: 0.3 mA, 2 s) with an inter-trial interval of 1 min in a relatively dark room. Animal was removed from the test chamber 30 s after the conditioning training. Five minutes after this training, mice were exposed to 2% isoflurane in oxygen or only oxygen for 5 min. Mice were placed back in the chamber 24 h later for 5 min in the absence of tone and shock. The amount of time with freezing behavior was recorded in this 5 min. The animal was placed 2 h later in a test chamber that had a different context and smell environment from the first test chamber (this second chamber was wiped with 1% acetic acid) in a relatively light room. After a 2-min acclimatization time, the auditory stimulus then was turned on for three cycles, each cycle for 30 s followed by a 1-min inter-cycle interval (4.5 min in total). The freezing behavior in the 4.5 min period was recorded. Freezing behavior was defined as absence of all movements except for respiration. Freezing behavior assessment was scored from the video by one observer blinded to group assignment. These tests test hippocampus-dependent (context-related) and hippocampus-independent (tone-related) learning and memory functions (Kim and Fanselow, 1992).
Statistics Analysis
Results are presented as mean ± S.E.M. (n ≥ 5). The n indicates the number of animals used in each condition. Statistical analysis was performed by paired t-test or repeated-measures ANOVA followed by Tukey test for post hoc comparison as appropriate for the data of experiments using hippocampal slices or by t-test or Mann-Whitney Rank Sum test as appropriate for the fear conditioning data. A P < 0.05 was considered statistically significant.
Results
Isoflurane did not affect the trafficking of GluR1 to the plasma membrane of wild-type mouse hippocampus but decreased GluR1 trafficking to the plasma membrane of EAAT3 knockout mouse hippocampus
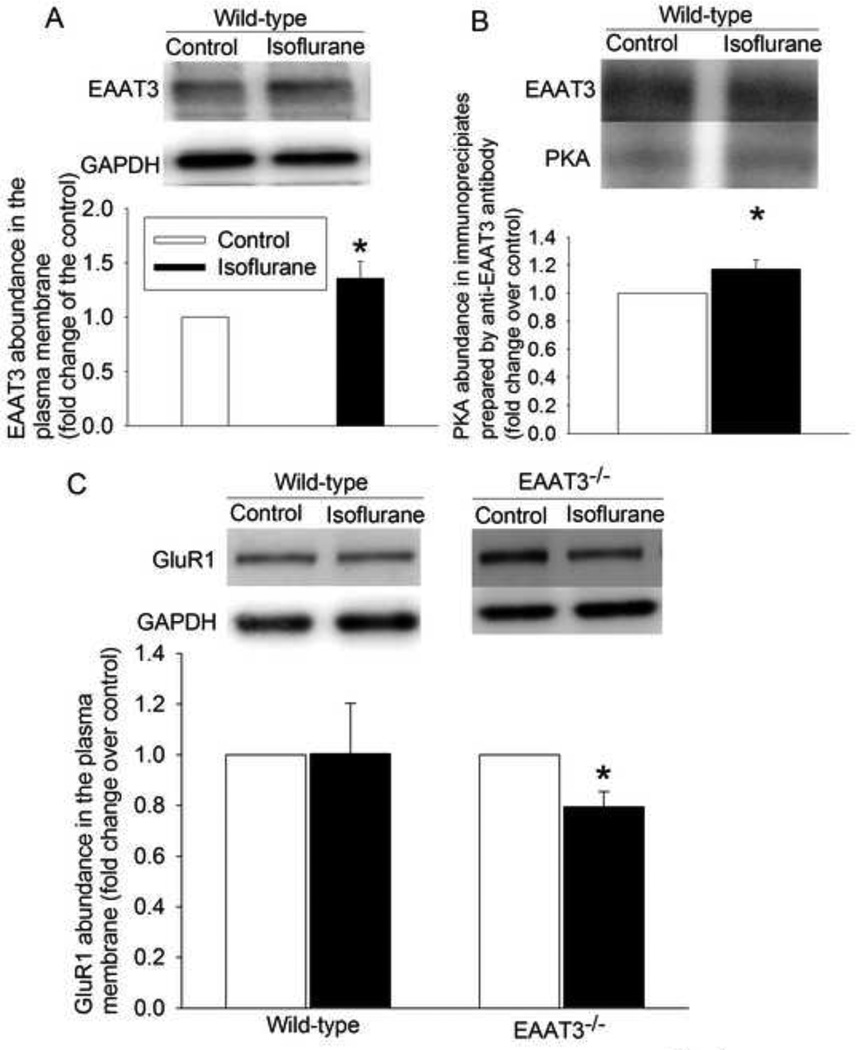
Similar to our previous results (Huang and Zuo, 2005; Huang et al., 2006), exposure of mouse hippocampus to 2% isoflurane for 5 min significantly increased the trafficking of EAAT3 to the plasma membrane of wild-type mice (Fig. 1A). Isoflurane also increased the amount of PKA in EAAT3 immunoprecipitates prepared from hippocampal lysates (Fig. 1B). However, isoflurane did not change the trafficking of GluR1 to the plasma membrane in the wild-type mouse hippocampus but decreased GluR1 trafficking to the plasma membrane of EAAT3 knockout mouse hippocampus (Fig. 1C).
Fig. 1.
Isoflurane induced EAAT3 but not GluR1 trafficking to the plasma membrane in the CD1 wild-type mouse hippocampus. A: the amount of biotinylated EAAT3 normalized by GAPDH. B: the amount of PKA normalized by EAAT3 in the immunoprecipitates prepared by an anti-EAAT3 antibody. C: the amount of biotinylated GluR1 normalized by GAPDH. Results are mean ± S.E.M. (n = 6 for panels A, = 9 for panel B, and = 5 – 6 for panel C). * P < 0.05 compared with the corresponding control. Statistical analysis was performed by paired t-test.
PKA inhibition did not affect isoflurane-induced inhibition of GluR1 trafficking to the plasma membrane of EAAT3 knockout mouse hippocampus
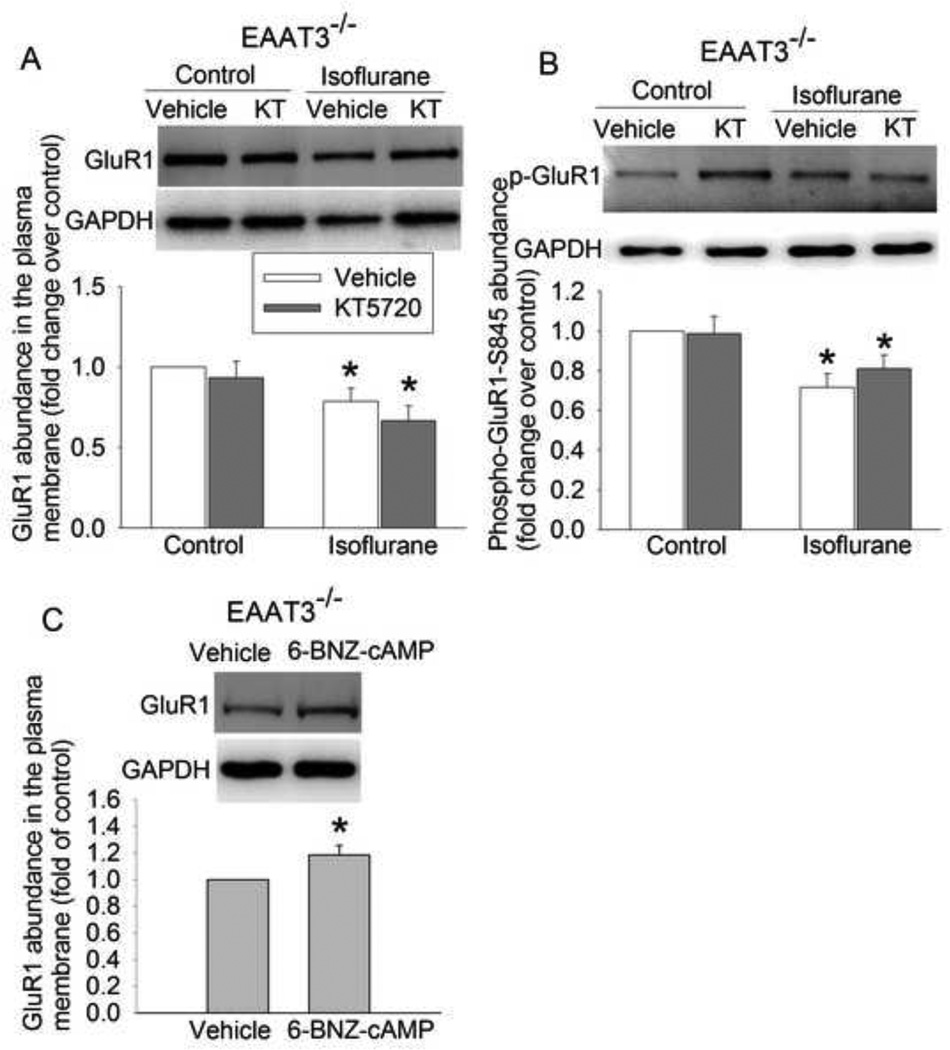
The PKA inhibitor KT5720 did not affect the GluR1 trafficking to the plasma membrane of EAAT3 knockout mouse hippocampus under control and isoflurane exposure condition (Fig. 2A). Consistent with this finding, KT5720 also did not affect the phosphorylation of GluR1 at ser845 (Fig. 2B), a PKA phosphorylation site (Esteban et al., 2003; Man et al., 2007). However, 6-BNZ-cAMP-AM, an agonist of PKA, increased GluR1 trafficking to the plasma membrane in the EAAT3 knockout mouse hippocampus (Fig. 2C).
Fig. 2.
Isoflurane reduced GluR1 phosphorylation and trafficking in the hippocampus of EAAT3−/− mice. The vehicle group contained dimethyl sulfoxide that was used to dissolve KT5720 and 6-BNZ-cAMP. A: the amount of biotinylated GluR1 normalized by GAPDH, B: total cellular phospho-GluR1 normalized by GAPDH, C: the amount of biotinylated GluR1 normalized by GAPDH. Results are mean ± S.E.M. (n = 6 for panels A, = 7 for panel B, and = 8 for panel C). * P < 0.05 compared with corresponding control. Statistical analysis was performed by repeated-measures ANOVA followed by Tukey test for post hoc comparison (for data in panels A and B) or paired ttest (for data in panel C). KT: KT5720.
Protein phosphatase inhibition attenuated isoflurane-induced inhibition of GluR1 trafficking to the plasma membrane of EAAT3 knockout mouse hippocampus
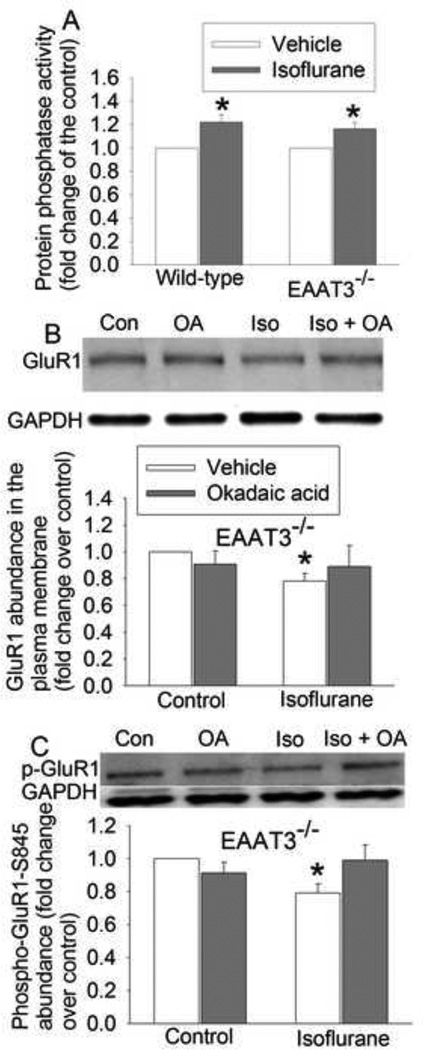
Protein phosphatases can dephosphorylate GluR1, which then inhibits GluR1 trafficking to the plasma membrane (Mansuy and Shenolikar, 2006). To determine the role of phosphatases in the isoflurane effects on GluR1 trafficking, we first determine the effects of isoflurane on phosphatase activity. Isoflurane significantly increased phosphatase activity in the hippocampus of wild-type and EAAT3 knockout mice (Fig. 3A). OA, a phosphatase inhibitor, attenuated the isoflurane-induced inhibition of GluR1 trafficking and phosphorylation at ser845 in the hippocampus of EAAT3 knockout mice, although OA did not affect GluR1 trafficking and phosphorylation under control condition (Figs. 3B and 3C).
Fig. 3.
Isoflurane increased phosphatase activity and phosphatase inhibition attenuated isoflurane-induced decrease of GluR1 phosphorylation and trafficking in the hippocampus of EAAT3−/− mice. A: protein phosphatase activity of freshly prepared brain slices. B: the amount of biotinylated GluR1 normalized by GAPDH, C: total cellular phospho-GluR1 normalized by GAPDH. Results are mean ± S.E.M. (n = 8 for panel A, and = 5 for panels B and C). * P < 0.05 compared with corresponding control. Statistical analysis was performed by paired t-test (for data in panel A) or repeated-measures ANOVA followed by Tukey test for post hoc comparison (for data in panels B and C). Con: control; Iso: isoflurane.
Isoflurane impaired context-related fear conditioning in the EAAT3 knockout mice
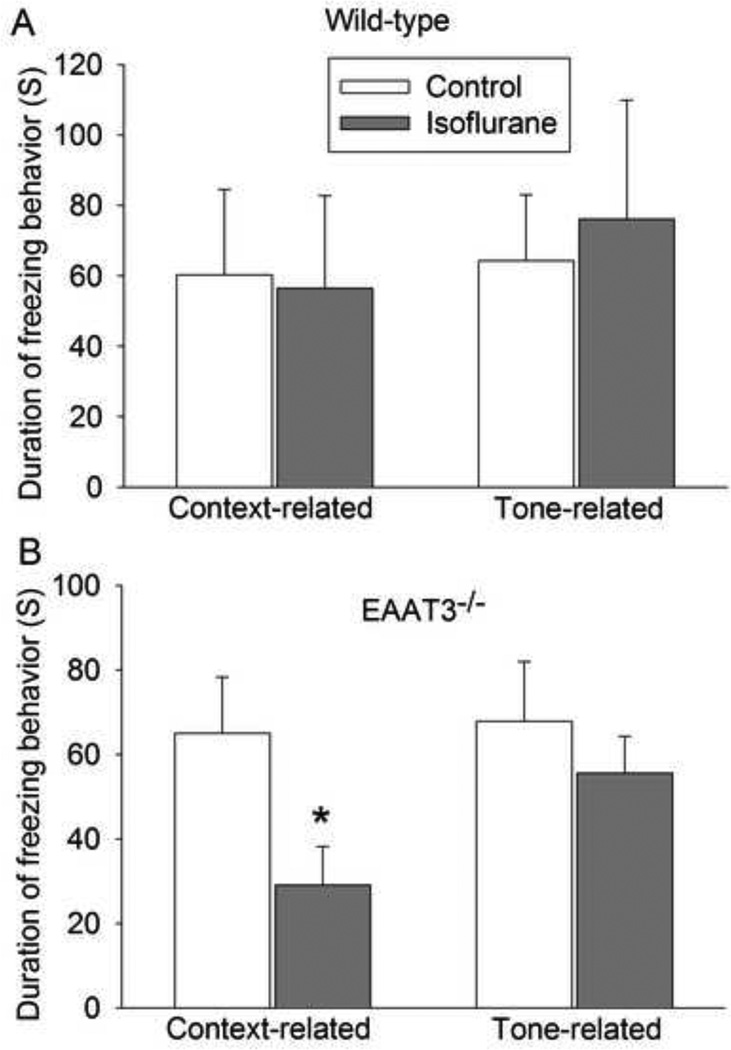
Although isoflurane did not affect the context- and tone-related fear conditioning in the wild-type mice (Fig. 4A), it significantly reduced context-related but not tone-related fear conditioning in the EAAT3 knockout mice (Fig. 4B).
Fig. 4.
Isoflurane impaired context-related fear conditioning in the EAAT3−/− mice. A: wild-type mice. B: EAAT3−/− mice. Results are mean ± S.E.M. (n = 8 for panel A, and = 7 for panels B). * P < 0.05 compared with corresponding control. Statistical analysis was performed by Mann-Whitney Rank Sum test (for data in panel A) or t-test (for data in panel B).
Discussion
Previous studies have implied a possible role of EAAT3 in learning and memory (Levenson et al., 2002; Aoyama et al., 2006; Lee et al., 2012). Consistent with our previous studies (Huang and Zuo, 2005; Huang et al., 2006), isoflurane increased EAAT3 trafficking to the plasma membrane in the hippocampus. Isoflurane also increased the amount of PKA in the immunoprecipitates prepared by an EAAT3 antibody, suggesting that EAAT3 may directly or indirectly be associated with PKA. PKA can phosphorylate/activate GluR1, which facilitates GluR1 trafficking to the plasma membrane (Esteban et al., 2003; Man et al., 2007). However, isoflurane did not affect GluR1 trafficking to the plasma membrane in the wild-type mouse hippocampus in our study. This outcome may be the results of countering effects from protein kinases and phosphatases on GluR1 phosphorylation status because isoflurane also increased protein phosphatase activity in the wild-type mouse hippocampus. This increased phosphatase activity may not be countered in the EAAT3 knockout mice because isoflurane decreased GluR1 phosphorylation and trafficking to the plasma membrane in these mice and phosphatase inhibition attenuated the effects of isoflurane on GluR1 phosphorylation and trafficking. PKA activation by 6-BNZ-cAMP-AM increased GluR1 trafficking to the plasma membrane in the EAAT3 knockout mice, suggesting that the signaling pathway downstream of PKA to lead to GluR1 trafficking is intact in these mice. PKA may not play an important role in the isoflurane effects on GluR1 phosphorylation and trafficking in the hippocampus of these mice because PKA inhibition did not affect GluR1 phosphorylation and trafficking. Together, our results suggest that isoflurane may work on protein kinases via EAAT3 to increase GluR1 phosphorylation and trafficking and increases phosphatase activity to decrease GluR1 phosphorylation and trafficking. These two effects counter each other in the wild-type mice but the inhibitory effect on GluR1 trafficking is predominant in the EAAT3 knockout mice under the current experimental conditions.
Our results suggest a possible interaction between PKA and EAAT3. This interaction may be at the plasma membrane because many components of the signaling pathway to activate PKA, such as the G proteins, are at the plasma membrane. There is a significant PKA activity in the plasma membrane (Depry et al., 2011). Also, isoflurane increased EAAT3 trafficking to the plasma membrane. Activated PKA can then phosphorylate GluR1 to enhance its trafficking to the plasma membrane.
Consistent with our findings from Ex vivo hippocampal slice experiments, isoflurane did not affect the context- and tone-related fear conditioning of wild-type mice. However, isoflurane reduced context- but not tone-related fear conditioning in the EAAT3 knockout mice. Since context-related fear conditioning is hippocampus-dependent and tone-related fear conditioning is hippocampus-independent (Kim and Fanselow, 1992), our results suggest that hippocampus-dependent learning and memory is impaired by isoflurane in the EAAT3 knockout mice.
Our findings are significant. The best evidence so far in the literature for a possible role of EAAT3 in the learning and memory is that fear conditioning is associated with an increased EAAT3 trafficking to the plasma membrane (Levenson et al., 2002). Our current study provides additional evidence for this role. We also provide initial evidence for the increased phosphatase activity induced by isoflurane and the effects of isoflurane on GluR1 trafficking and phosphorylation. These findings improve our understanding of isoflurane effects on learning and memory.
There are limitations in our study. Our findings suggest a role of EAAT3 in activating protein kinases after isoflurane exposure. However, detailed molecular mechanisms for this role are not investigated. Further studies are needed to identify these mechanisms. Also, EAAT3 deletion in the EAAT3 knockout mice may induce compensatory changes that may contribute to the findings in this study. Identifying these changes may help determine this possibility. However, our results do not suggest a high possibility for the compensatory changes to contribute to our findings because protein phosphatase activity was equally increased by isoflurane in the hippocampal slices of wild-type and EAAT3 knockout mice. Increase of protein phosphatase activity is considered to be a mechanism for isoflurane to reduce GluR1 phosphorylation and trafficking to the plasma membrane in our study.
In summary, we have found that isoflurane may alter GluR1 phosphorylation and trafficking in the hippocampus via its effects with two opposite directions. EAAT3 may participate in the isoflurane effects to increase GluR1 phosphorylation and trafficking. Lack of EAAT3 leads to the decreased GluR1 trafficking and impaired learning and memory after isoflurane exposure in the EAAT3 knockout mice.
Highlights.
Isoflurane can increase and decrease GluR1 trafficking to the plasma membrane
Isoflurane-increased GluR1 trafficking to the plasma membrane may be via EAAT3
Isoflurane reduces GluR1 trafficking in the hippocampus of the EAAT3−/− mice
Isoflurane decreases hippocampus-dependent cognition in the EAAT3−/− mice
Acknowledgements
This study was supported by grants (GM065211 and GM098308 to Z Zuo) from the National Institutes of Health, Bethesda, Maryland, by a grant from the International Anesthesia Research Society (2007 Frontiers in Anesthesia Research Award to Z Zuo), Cleveland, Ohio, by a Grant-in-Aid from the American Heart Association Mid-Atlantic Affiliate (10GRNT3900019 to Z Zuo), Baltimore, Maryland, and the Robert M. Epstein Professorship endowment, University of Virginia.
Abbreviations
- ACSF
artificial cerebrospinal fluid
- 6-BNZ-cAMP-AM
acetoxymethyl ester of N6-benzoyl-cAMP
- DMSO
dimethyl sulfoxide
- EAAT3
glutamate transporter type 3
- GAPDH
glyceraldehydes 3-phosphate dehydrogenase
- OA
okadaic acid
- PBS
phosphate buffered saline
- PKA
protein kinase A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None.
References
- Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, Swanson RA. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- Bekker A, Shah R, Quartermain D, Li YS, Blanck T. Isoflurane preserves spatial working memory in adult mice after moderate hypoxia. Anesth Analg. 2006;102:1134–1138. doi: 10.1213/01.ane.0000198637.36539.c1. [DOI] [PubMed] [Google Scholar]
- Butterfield NN, Graf P, Ries CR, MacLeod BA. The effect of repeated isoflurane anesthesia on spatial and psychomotor performance in young and aged mice. Anesth Analg. 2004;98:1305–1311. doi: 10.1213/01.ane.0000108484.91089.13. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Depry C, Allen MD, Zhang J. Visualization of PKA activity in plasma membrane microdomains. Molecular bioSystems. 2011;7:52–58. doi: 10.1039/c0mb00079e. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Huang Y, Feng X, Sando JJ, Zuo Z. Critical Role of Serine 465 in Isoflurane-induced Increase of Cell-surface Redistribution and Activity of Glutamate Transporter Type 3. J Biol Chem. 2006;281:38133–38138. doi: 10.1074/jbc.M603885200. [DOI] [PubMed] [Google Scholar]
- Huang Y, Li L, Washington JM, Xu X, Sando JJ, Lin D, Zuo Z. Inhibition of isoflurane-induced increase of cell-surface redistribution and activity of glutamate transporter type 3 by serine 465 sequence-specific peptides. Eur J Pharmacol. 2011;655:16–22. doi: 10.1016/j.ejphar.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Zuo Z. Isoflurane induces a protein kinase C alpha-dependent increase in cell surface protein level and activity of glutamate transporter type 3. Mol Pharmacol. 2005;67:1522–1533. doi: 10.1124/mol.104.007443. [DOI] [PubMed] [Google Scholar]
- Jung HH, Lee JJ, Washington JM, Zuo Z. Inability of volatile anesthetics to inhibit oxygen-glucose deprivation-induced glutamate release via glutamate transporters and anion channels in rat corticostriatal slices. Brain Res. 2008;1227:234–239. doi: 10.1016/j.brainres.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Lee S, Park SH, Zuo Z. Effects of isoflurane on learning and memory functions of wild-type and glutamate transporter type 3 knockout mice. J Pharm Pharmacol. 2012;64:302–307. doi: 10.1111/j.2042-7158.2011.01404.x. [DOI] [PubMed] [Google Scholar]
- Lee SN, Li L, Zuo Z. Glutamate transporter type 3 knockout mice have a decreased isoflurane requirement to induce loss of righting reflex. Neurosci. 2010;171:788–793. doi: 10.1016/j.neuroscience.2010.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson J, Weeber E, Selcher JC, Kategaya LS, Sweatt JD, Eskin A. Long-term potentiation and contextual fear conditioning increase neuronal glutamate uptake. Nat Neurosci. 2002;5:155–161. doi: 10.1038/nn791. [DOI] [PubMed] [Google Scholar]
- Li L, Wang Z, Zuo Z. Chronic intermittent fasting improves cognitive functions and brain structures in mice. PLoS One. 2013;8:e66069. doi: 10.1371/journal.pone.0066069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zuo Z. Glutamate transporter type 3 knockout reduces brain tolerance to focal brain ischemia in mice. J Cereb Blood Flow Metab. 2011;31:1283–1292. doi: 10.1038/jcbfm.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Zuo Z. Isoflurane induces hippocampal cell injury and cognitive impairments in adult rats. Neuropharmacology. 2011;61:1354–1359. doi: 10.1016/j.neuropharm.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man HY, Sekine-Aizawa Y, Huganir RL. Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc Natl Acad Sci U S A. 2007;104:3579–3584. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansuy IM, Shenolikar S. Protein serine/threonine phosphatases in neuronal plasticity and disorders of learning and memory. Trends Neurosci. 2006;29:679–686. doi: 10.1016/j.tins.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Miyamoto E. Molecular mechanism of neuronal plasticity: induction and maintenance of long-term potentiation in the hippocampus. J Pharmacol Sci. 2006;100:433–442. doi: 10.1254/jphs.cpj06007x. [DOI] [PubMed] [Google Scholar]
- Peghini P, Janzen J, Stoffel W. Glutamate transporter EAAC-1-deficient mice develop dicarboxylic aminoaciduria and behavioral abnormalities but no neurodegeneration. EMBO J. 1997;16:3822–3832. doi: 10.1093/emboj/16.13.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rammes G, Starker LK, Haseneder R, Berkmann J, Plack A, Zieglgansberger W, Ohl F, Kochs EF, Blobner M. Isoflurane anaesthesia reversibly improves cognitive function and long-term potentiation (LTP) via an up-regulation in NMDA receptor 2B subunit expression. Neuropharmacology. 2009;56:626–636. doi: 10.1016/j.neuropharm.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Simpson EM, Takahashi JS, Lipp H-P, Nakanishi S, Wehner JM, Giese KP, Tully T, Abel T, Chapman PF, Fox K, Grant S, Itohara S, Lathe R, Mayford M, McNamara JO, Morris RJ, Picciotto M, Roder J, Shin H-S. Mutant mice and neuroscience: recommendations concerning genetic background. Banbury Conference on genetic background in mice. Neuron. 1997;19:755–759. doi: 10.1016/s0896-6273(00)80958-7. [DOI] [PubMed] [Google Scholar]
- Valentim AM, Di Giminiani P, Ribeiro PO, Rodrigues P, Olsson IA, Antunes LM. Lower isoflurane concentration affects spatial learning and neurodegeneration in adult mice compared with higher concentrations. Anesthesiology. 2010;113:1099–1108. doi: 10.1097/ALN.0b013e3181f79c7c. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]