Abstract
2CC-NBOMe (4-chloro-2,5-dimethoxyphenethyl-N-[(2-methoxyphenyl) methyl] ethanamine) and 25I-NBOMe (2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl) methyl] ethanamine) are of a class of N-benzyl phenethylamine derivatives whose synthesis was first reported in the scientific literature in 2011. Recent reports from “personal drug experience websites” and in the popular press indicate these drugs are the latest in a series of designer “Bath Salt” drugs of abuse. The presented high performance liquid chromatography triple quadrupole mass spectrometry (HPLC/MS/MS) method was developed for the detection and quantification of 2CC-NBOMe and 25I-NBOMe in serum of intoxicated emergency department patients. The assay applies 2-(2,5-dimethoxyphenyl)-N-(2-methoxybenzyl) ethanamine (25H-NBOMe) as the internal standard (ISTD). Samples were extracted using solid phase extraction (SPE) columns. The chromatographic separation was performed on a Luna 3μ C8 (2)100Å 100×2.0 mm, column. Detection was accomplished by multiple-reaction monitoring (MRM) via electrospray ionization (ESI) source operating in the positive ionization mode. The calibration curves were linear over the investigated concentration range, 30 to 2000 pg/mL, with a lower limit of detection (LOD) of 10 pg/mL for both 2CC-NBOMe and 25I-NBOMe. The method proved suitable for serum clinical toxicology testing. Two severely intoxicated emergency department patients were determined to have serum concentrations of 250 pg/mL and 2780 pg/mL of 25I-NBOMe using the presented method.
Introduction
2CC-NBOMe (4-chloro-2,5-dimethoxyphenethyl-N-[(2-methoxyphenyl) methyl] ethanamine) and 25I-NBOMe (2-(4-iodo-2,5-dimethoxyphenyl)-N-[(2-methoxyphenyl) methyl] ethanamine) (figure 1) are of a class of N-benzyl phenethylamine derivatives whose synthesis was first reported in the scientific literature in 2011 (Ettrup et al., 2011). In vitro binding studies have demonstrated that these compounds are potent serotonin 2A (5-HT2A) receptor agonists (Nichols et al., 2008; Zuba et al., 2012; 2013). The 5-HT2A receptor has been closely linked to complex behaviors including working memory, cognitive processes and affective disorders such as schizophrenia. These receptors are believed to mediate the primary effects of hallucinogenic drugs (Braden et al., 2006). Little to no pharmacokinetic or pharmacological data concerning man or whole animals is presently available in the professional literature. Recent reports from “personal drug experience websites” and in the popular press indicate these drugs are the latest in a series of designer “Bath Salt” drugs of abuse. These drugs may serve as replacements for the previously popular beta-keto derivatives of amphetamine: methcathinone and methedrone, as well as the methylenedioxy ring derivatives similar to methylenedioxymethamphetamine (MDMA, “Ecstasy”), methylone and methylenedioxypyrovalerone (MDPV), which are now controlled substances in Europe and the United States (Gibbons, 2012). As with earlier “Bath Salts” both 2CC-NBOMe and 25I-NBOMe can be obtained easily over the internet. Blotter papers containing 25I-NBOMe appeared on the market in 2011 (Zuba et al., 2013). These compounds are sometimes sold under the name 25I, N-bomb, or Smiles.
Fig 1.
Chemical Structure of 2CC-NBOMe, 25I-NBOMe and 25H-NBOMe
Only a single case of a 25I-NBOMe overdose has been published to date. The patient displayed initial signs and symptoms consistent with sympathomimetic toxidrome (tachycardia, hypertension, mydriasis, agitation, and hypokalemia) plus hallucinations and bizarre behavior likely associated with serotonergic toxicity (Rose et al., 2013). Two abstracts of presentations have been published describing case series of 25I-NBOMe exposures of 4 and 10 patients, respectively (Kelly et al., 2012; Rose et al., 2012). The most common effects in these patients were tachycardia (13 of 14), agitation (10 of 14), and hypertension (8 of 14). Five of 14 patients experienced tonic/clonic seizures and one suffered a cerebral hemorrhage. However, toxicological analysis for 25I-NBOMe in patient specimens was performed in only one of the 14 reported cases (Rose et al., 2013).
We present a high performance liquid chromatography triple quadrupole mass spectrometry (HPLC/MS/MS) method for the identification and quantification of 2CC-NBOMe and 25I-NBOMe in human serum. The assay was developed in response to an outbreak of N-benzyl-phenethylamine derivative abuse and non-fatal overdose cases in our state during early 2012. The method is novel in that presently there are no published methods for the analysis of 2CC-NBOMe in biological specimens and our initial method involving liquid/liquid extraction of 25I-NBOMe from serum is only the procedure in the literature to date (Rose et al., 2013).
Materials and Methods
Reagents
The phenethylamine derivative primary reference materials for 25H-NBOMe, 2CC-NBOMe and 25I-NBOMe were purchased from Cayman Chemical Company (Ann Arbor, Michigan) as hydrochloride salts. Acetic acid, acetonitrile, ammonium acetate, dichloromethane, ethanol, formic acid, isopropanol, methanol and water were purchased from Fisher Scientific (Hanover Park, Illinois). Ammonia was purchased from Macron Chemicals (Charlotte, North Carolina). Hydrochloric acid and sodium phosphate dibasic were purchased from J.T. Baker (Phillipsburg, New Jersey). Sodium phosphate monohydrate was purchased from Sigma Aldrich (St. Louis, Missouri). All reagents were ACS grade or better. Medical grade nitrogen was purchased from National Welders Supply Company (Richmond, Virginia) and the Clean Screen ZSDUA020 sold phase extraction (SPE) columns were purchased from UCT (Horsham, PA). Liquichek™ controls were purchased from Bio-Rad Laboratories, Inc. (Hercules, California).
Stock solution preparation and dilutions
A series of working standard solutions of 1 and 10 ng/mL were prepared by appropriate dilution with ethanol of the stock standard solution of 2CC-NBOMe and 25I-NBOMe. An internal standard (ISTD) working solution of 10 ng/mL 25H-NBOMe was prepared by appropriately diluting the internal standard stock solution with ethanol. Working standards were stored at −20°C.
Preparation of calibrators and quality control specimens
In-house drug-free serum provided the matrix for all prepared calibrators, quality control (QC) and other study specimens. Drug-free out of date serum was obtained in-house from the Department of Transfusion Medicine. The drug-free serum specimens were analyzed by gas chromatography/mass spectrometry and found not to contain common drugs of abuse, phenethylamine derivatives of interest in this study or their metabolites. Appropriate volumes of the working solutions of 2CC-NBOMe and 25I-NBOMe were added to serum to obtain an eight-point calibration curve of 0, 30, 50, 100, 250, 500, 1000 and 2000 pg/mL of each analyte. Calibrators were prepared fresh in duplicate before analysis of each batch of samples. The following QC serums specimens for 25CC-NBOMe and 25I-NBOMe were prepared and analyzed with each batch of test specimens: limit of quantification quality control (LOQC), target concentration of 30 pg/mL; low control (LQC), target concentration of 75 pg/mL; medium control (MQC), target concentration of 750 pg/mL; and high control (HQC), target concentration of 1500 pg/mL. A drug free control (negative control) that contained neither 2CC-NBOMe nor 25I-NBOMe with ISTD added and a double negative control containing neither 2CC-NBOMe and 25I-NBOMe nor ISTD were also analyzed with each test batch. All QC samples were stored at -20°C until testing.
Specimen extraction
To 1 mL aliquots of calibrators, QC specimens and patient serums was added 50 μL of ISTD consisting of 10 ng/mL (500 pg total) of 25H-NBOMe followed by the addition of 1 mL of 100 mM phosphate buffer (pH 6). The samples were then mixed for 5 minutes and centrifuged for 10 minutes at 3000 rpm. The Clean Screen ZSDUA020 sold phase SPE columns were conditioned with 3 mL of methanol followed by 3 mL of Di water and lastly 1 mL of 100 mM phosphate buffer (pH 6). The samples were added to the columns and aspirated under gravity. The columns were then washed with 3 mL of DI water followed by 1 mL 100mM acetic acid and finally 3 mL of methanol. Columns were dried under vacuum. 2CC-NBOMe, 25I-NBOMe and ISTD were then eluted with 3 mL of 78:20:2 dichloromethane/isopropanol/ammonia (v:v:v). One hundred microliters of 1% HCl in methanol (v:v) and 200 μL of DI water were added to the eluate. The samples were evaporated under nitrogen leaving approximately 200 μL of DI water. This solution was transferred to auto-sampler vials for analysis.
Instrumental Analysis
The HPLC/MS/MS analysis was performed with an Applied Biosystems 3200 Q trap with a turbo V source for TurbolonSpray attached to a Shimadzu SCL HPLC system controlled by Analyst 1.4.2 software. The chromatographic separation was performed on a Luna 3μ C8(2)100Å 100x2.0 mm, column (Phenomenex, Torrance, California). The mobile phase consisted of A: Water with 10 mM ammonium acetate and 0.1% formic acid and B: Acetonitrile. The following gradient was applied: 0.00-1.10 min, 20% B, a linear gradient to 33% B at 8.00 min, hold for 6.90 min, then return to 20% B at 8.00 min. The source temperature was set at 650°C and had a curtain gas flow rate of 30 mL/min. The ionspray voltage was 5000 V, with the ion source gases 1 and 2 at flow rates of 25 mL/min. The acquisition mode used was multiple reaction monitoring (MRM). The retention times were: 2CC-NBOMe, 4.4 min; 25I-NBOMe, 4.8 min; and 25H-NBOMe, 3.8 min. 2CC-NBOMe and 25I-NBOMe had a declustering potential of 38 eV and 25H-NBOMe had a declustering potential of 19 eV. The following transition ions (m/z) were monitored in MRM mode with their corresponding collection energies (eV) in parentheses: 2CC-NBOMe: 336>121 (26) and 336>91 (60); 25I-NBOMe: 428>121 (26) and 428>91 (50); and 25H-NBOMe: 302>121 (28) and 302>91 (70). The chromatographic separation is presented in figure 2. The total run time for the analytical method was 10 minutes.
Fig 2.
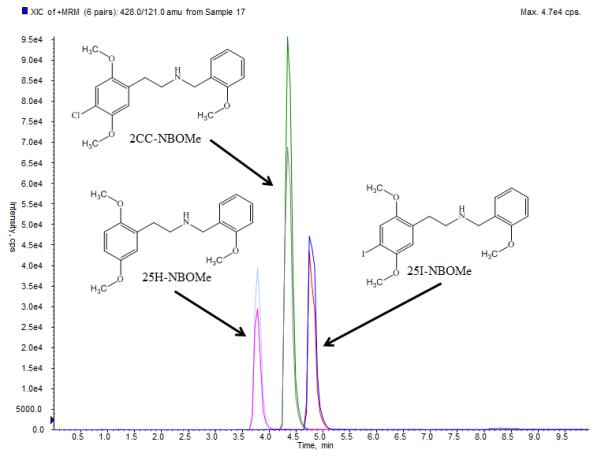
Chromatographic separation of 2CC-NBOMe, 25I-NBOMe and 25H-NBOMe
Assay Performance
The evaluation of the assay was conducted over five separate days. The samples batches were analyzed as recommended for biomedical assay validation (Bioanalytical Method Validation., 2001) for linearity, LOQ, accuracy/bias, precision, selectivity, freeze and thaw stability, bench-top stability, long-term stability and post-preparative stability. Validation sample batches contained calibrators in duplicate, drug-free samples with internal standard added, drug-free samples without internal standard and replicates of the prepared LOQC, LQC, MQC and HQC samples.
Linearity, LOQ and LOD
An eight-point calibration of 0, 30, 50, 100, 250, 500, 1000 and 2000 pg/mL in duplicate of 2CC-NBOMe and 25I-NBOMe was prepared in drug-free serum. The calibration curve was constructed by a linear regression plot of the ratio of the peak area of the abundance quantification ion of 2CC-NBOMe and 25I-NBOMe to the peak area abundance quantification ion of 25H-NBOMe ISTD, versus the calibrator concentrations. The lower limit of quantification (LOQ) of 30 pg/mL and the lower limit of detection (LOD) of 10 pg/mL for 2CC-NBOMe and 25I-NBOMe were administratively set. LOQC samples were used to verify the LOQ was within +20% of the 30 pg/mL target value and had a response at least five times greater than the signal to noise ratio of the response to drug-free serum. Samples prepared at the LOD of 10 pg/mL were analyzed with each batch to verify that there was a response at least three times greater than the signal to noise ratio of the response to drug-free serum.
Absolute recovery and ion suppression
The percent recovery was determined by first extracting and preparing residues of drug free serum. These residues were then reconstituted with water to prepare test samples containing the target concentrations of the 750 pg/mL of 2CC-NBOMe and 25I-NBOMe and 500pg/mL of the ISTD. The addition of the drugs to the residue of extracted serum mitigated any matrix effects on recovery studies. The absolute recovery of the assay was determined by comparing the absolute area of the extracted aliquots of 750 pg/mL 2CC-NBOMe and 25I-NBOMe and 500 pg/mL ISTD compared to the absolute peak area of reconstituted matrix with unextracted 750 pg/mL 2CC-NBOMe and 25I-NBOMe and 500 pg/mL ISTD, multiplied by 100. The ion suppression of the assay was determined by comparing the absolute area of the reconstituted matrix with unextracted 750 pg/mL 2CC-NBOMe and 25I-NBOMe and 500 pg/mL ISTD added compared to the absolute peak area of unextracted 750 pg/mL 2CC-NBOMe and 25I-NBOMe and 500 pg/mL ISTD samples, multiplied by 100 and then minus 100.
Accuracy/Bias and Precision
Accuracy/bias and precision of the method were determined from analysis of five different batches of the prepared QC samples. The percent accuracy/bias of the method was calculated as the ratio of the mean 2CC-NBOMe and 25I-NBOMe concentration of five aliquots of each QC sample analyzed in the same batch of samples, to the target concentration of the QC samples times 100. The criteria for acceptable assay mean accuracy/bias were quantified 2CC-NBOMe and 25I-NBOMe results within +15% of the target value of the prepared QC samples. The intra-day precision of the method was determined by quantified results of replicate analysis of three aliquots of the four different prepared QC samples. The inter-day precision was determined from quantified results of the three intra-day aliquots and triplicate analysis of the four prepared controls on five different days.
Stability
Stability experiments in serum were performed at three QC concentrations, the LQC, MQC and HQC with three replicas at each concentration. The stability in serum was assessed during storage and after three freeze-thaw cycles at -20°C with 24 hr passing in-between two cycles. The bench-top stability at room temperature was assessed in serum for 48 hr to cover the processing time of the samples including transportation. Long term storage was assessed by keeping the QC frozen at -20°C for one month before analysis. These QC samples were then run against freshly prepared calibrators. The post-preparative stability or the auto-sampler stability was assessed by re-injection reproducibility after storage of the samples in the auto-sampler for 72 h at room temperature. Under these conditions 2CC-NBOMe and 25I-NBOMe were considered to be stable in serum as their concentrations were within 20% of the target values for the QC samples tested.
Results
Each calibrator concentration for each of five calibration curves, of the duplicate curves, was determined to be within + 15% of the expected value except the LOQ which was determined to be within 20%. The linear regression correlation coefficients (r2) for the calibration curves of 2CC-NBOMe and 25I-NBOMe in the five batches yielded a mean r2 of 0.996 + 0.003 and 0.996 + 0.002, respectively with a range of 0.992 – 0.999. LOQ and LOD for 2CC-NBOMe and 25I-NBOMe were administratively set at 30 pg/mL and 10 pg/mL, respectively. The LOD had a response greater than 10 times the signal to noise ratio of the response to drug-free serum. 10 pg/mL samples were analyzed in triplicate and were found to a have at least five times the signal to noise ratio of the drug-free serum.
The absolute recovery of the assay for 2CC-NBOMe and 25I-NBOMe at the 750 pg/mL concentration (n=3) was 103% and 97%, respectively. The absolute recovery for the 25H-NBOMe, ISTD, at 500 pg/mL (n=3) was 86%. The ion suppression for 2CC-NBOMe and 25I-NBOMe at the 750 pg/mL (n=3) was 8% and 7%, respectively. The ion suppression for 25H-NBOMe, ISTD, at 500 pg/mL (n=3) was 3% (table 1).
Table 1.
Recovery and Ion Suppression
| Recovery & Suppression (n=3) | ||
|---|---|---|
| Designer Drug | Conc. (750 pg/mL) | % Mean +/− % SD |
| 2CC-NBOMe | Recovery (%) | 100 +/− 3 |
| Suppression (%) | 8+/−14 | |
| 25I-NBOMe | Recovery (%) | 97+/−10 |
| Suppression (%) | 7+/−14 | |
|
| ||
| ISTD | Conc. (500 pg/mL) | % Mean +/− % SD |
|
| ||
| 25H-NBOMe | Recovery (%) | 86+/−14 |
| Suppression (%) | 3+/−14 | |
The accuracy/bias of the assay for 2CC-NBOMe and 25I-NBOMe over the linear range of the assay varied from a low of 92% at a concentration of 1500 pg/mL exhibited by 2CC-NBOMe to 110% at a concentration of 30 pg/mL exhibited by 25I-NBOMe (table 2). The inter-day precision at the four different QC concentrations ranged between 97% to 103% for 2CC-NBOMe and 99 to 110% 25I-NBOMe of their target values. The intraday precision of the 2CC-NBOMe QC specimens ranged between 90% and 104%, while the intra-day precision for 25I-NBOMe QC specimens ranged between 93% and 100% of their target values (table 3).
Table 2.
Accuracy/Bias
| Accuracy/Bias (n=5) | Mean Conc. +/− SD | Average | |
|---|---|---|---|
| Designer Drug | Control | (pg/mL) | (%) |
| 2CC-NBOMe | LOQ (30 pg/mL) | 30 +/− 3 | 100 |
| LQC (75 pg/mL) | 72 +/−5 | 96 | |
| MQC (750 pg/mL) | 718 +/− 25 | 96 | |
| HQC (1500 pg/mL) | 1375 +/− 107 | 92 | |
|
| |||
| 25I-NBOMe | LOQ (30 pg/mL) | 34 +/− 2 | 111 |
| LQC (75 pg/mL) | 74 +/− 6 | 98 | |
| MQC (750 pg/mL) | 755 +/− 95 | 101 | |
| HQC (1500 pg/mL) | 1481 +/− 200 | 99 | |
Table 3.
Precision
| Precision | Intra Day (n=3) | |||
|---|---|---|---|---|
| Designer Drug | Control | Mean Conc. (pg/mL) | CV | (%) Accuracy/Bias |
| 2CC-NBOMe | LOQ (30 pg/mL) | 27 | 9 | 90 |
| LQC (75 pg/mL) | 72 | 11 | 96 | |
| MQC (750 pg/mL) | 733 | 1 | 98 | |
| HQC (1500 pg/mL) | 1563 | 9 | 104 | |
|
| ||||
| 25I-NBOMe | LOQ (30 pg/mL) | 30 | 15 | 100 |
| LQC (75 pg/mL) | 71 | 6 | 95 | |
| MQC (750 pg/mL) | 694 | 7 | 93 | |
| HQC (1500 pg/mL) | 1473 | 12 | 98 | |
|
| ||||
| Inter Day, 5 days (n=15) | ||||
| Designer Drug | Control | Mean Conc. (pg/mL) | CV | (%) Accuracy/Bias |
|
| ||||
| 2CC-NBOMe | LOQ (30 pg/mL) | 31 | 12 | 103 |
| LQC (75 pg/mL) | 75 | 11 | 100 | |
| MQC (750 pg/mL) | 712 | 10 | 95 | |
| HQC (1500 pg/mL) | 1460 | 12 | 97 | |
|
| ||||
| 25I-NBOMe | LOQ (30 pg/mL) | 33 | 9 | 110 |
| LQC (75 pg/mL) | 76 | 12 | 101 | |
| MQC (750 pg/mL) | 742 | 10 | 99 | |
| HQC (1500 pg/mL) | 1536 | 11 | 102 | |
The selectivity of the assay was determined using six different lots of drug-free serum. Each individual lot was analyzed with and without internal standard. No peaks were detected that co-eluted with the targeted 2CC-NBOMe and 25I-NBOMe or with the internal standard. This ensured that endogenous serum components did not interfere with the assay. No interferences were observed from compounds in the following commercially available controls; Liquichek™ Immunoassay Plus Control, level 3; Liquichek™ Therapeutic Drug Monitoring, level 3; and Liquichek™ Urine Toxicology Control, Level C3 (table 4).
Table 4.
Compounds found in the commercially available Liquichek™ controls;
| Liquichek™ Immunoassay Plus Control | |||
| Acetaminophen | Digoxin | Insulin | SHBG |
| AFP | Disopyramide | Iron | Somatomedin-C |
| Aldosterone | Estradiol | Iron (TIBC) | T3 (Free) |
| 17-Alpha-Hydroxyprogesterone | Estriol (Free) | LH | T3 (Total) |
| Amikacin | Estriol (Total) | Lidocaine | T3 Uptake/T-Uptake |
| Amiodarone | Estrogens (Total) | Lithium | T4 (Free) |
| Amitriptyline | Ethosuximide | NAPA | T4 (Total) |
| Androstenedione | Ferritin | Netilmicin | TBG |
| Angiotensin I | Flecainide | Nortriptyline | Testosterone |
| Anti-TG Ab | Folate | PAP | Testosterone (Free) |
| Anti-TPO Ab | Fructosamine | Phenobarbital | Theophylline |
| Caffeine | FSH | Phenytoin | Thyroglobulin |
| Carbamazepine | Gentamicin | Phenytoin (Free) | Tobramycin |
| Carbamazepine (Free) | hCG | Primidone | Tricyclic Antidepressants |
| CEA | hCG-Beta Subunit | Procainamide | (TCA) Screen |
| Chloramphenicol | hGH | Progesterone | TSH |
| CK-MB Isoenzyme | 25-Hydroxy Vitamin D | Prolactin | Valproic Acid |
| Cortisol | Ibuprofen | Propranolol | Valproic Acid (Free) |
| Cyclosporine | Imipramine | PSA | Vancomycin |
| 11-Deoxycortisol* | IgA | PSA (Free) | Vitamin B12 |
| Desipramine | IgE | PTH-MM | |
| DHEA | IgG | Quinidine | |
| DHEA Sulfate | IgM | Salicylate | |
|
| |||
| Liquichek™ Therapeutic Drug Monitoring | |||
| Acetaminophen | Digoxin | Nortriptyline | T4 (Free) |
| Amikacin | Disopyramide | Phenobarbital | T4 (Total) |
| Amitriptyline | Estriol (Total) | Phenytoin | Theophylline |
| Caffeine | Ethosuximide | Phenytoin (Free) | Tobramycin |
| Carbamazepine | Flecainide | Primidone | Tricyclic Antidepressant (TCA) |
| Carbamazepine (Free) | Gentamicin | Procainamide | Screen |
| Chloramphenicol | Haloperidol | Propranolol | TSH |
| Clonazepam | Imipramine | Quinidine | Valproic Acid |
| Cortisol | Lidocaine | Salicylate | Valproic Acid (Free) |
| Cyclosporine | Lithium | T3 (Free) | Vancomycin |
| Desipramine | Methotrexate | T3 (Total) | |
| Diazepam | NAPA | T3 Uptake/T-Uptake | |
|
| |||
| Liquichek™ Urine Toxicology Control | |||
| d-Amphetamine | Pentobarbital | Benzoylecgonine | Codeine |
| d-Methamphetamine | Phenobarbital | Ethanol | Morphine-3-β-d-Glucuronide |
| MDMA | Secobarbital | Lysergic Acid Diethylamide (LSD) | Phencyclidine (PCP) |
| MDA | α-Hydroxyalprazolam | Methadone | Propoxyphene |
| MDEA | Nordiazepam | Methadone Metabolite | Norpropoxyphene |
| Amobarbital | Oxazepam | Methaqualone | |
| Butalbital | THC-COOH | 6-Monoacetylmorphine | |
Sample carryover was evaluated in each of the five validation batches using two different procedures. Immediately following the injection of the 2000 pg/mL 2CC-NBOMe and 25I-NBOMe calibrator, an extract of a drug-free serum was injected. The rejection criterion for carryover was set at the detection of 2CC-NBOMe and 25I-NBOMe at a concentration less than 20% of the 10 pg/mL LOD. Neither 2CC-NBOMe nor 25I-NBOMe carried over to into the injected aliquot drug-free serum. As an additional measure to evaluate possible carryover, an injection of the extracted HQC (1500 pg/mL) sample was immediately followed by injection of the LQC (75 pg/mL) sample. This procedure was routinely applied each time HQC and LQC samples were analyzed. The rejection criterion for carryover was set at a concentration with a bias of less than 20% of the target value of the LQC. Lack of carryover was confirmed as none of the 2CC-NBOMe and 25I-NBOMe LQC samples demonstrated a significant quantified bias.
Under the stability tested conditions for the three freeze thaw cycles, room temperature for 48 hours and frozen for one month stability 2CC-NBOMe and 25I-NBOMe were determined to be stable the for all LQC, MQC and HQC samples. Stability of the extracted 2CC-NBOMe, 25I-NBOMe and the ISTD, 25H-NBOMe were also considered stable as the concentrations of the re-injected QC samples were within + 20% of their target concentrations (table 5).
Table 5.
Stability
| Freeze/Thaw | ||||
|---|---|---|---|---|
| Designer Drug | Control | Mean Conc. (pg/mL) | CV | (%) Accuracy/Bias |
| 2CC-NBOMe | LQC (75 pg/mL) | 84 | 5 | 112 |
| MQC (750 pg/mL) | 803 | 3 | 107 | |
| HQC (1500 pg/mL) | 1684 | 3 | 112 | |
|
| ||||
| 25I-NBOMe | LQC (75 pg/mL) | 77 | 13 | 103 |
| MQC (750 pg/mL) | 842 | 1 | 112 | |
| HQC (1500 pg/mL) | 1690 | 2 | 113 | |
|
| ||||
| BenchTop | ||||
| Designer Drug | Control | Mean Conc. (pg/mL) | CV | (%) Accuracy/Bias |
|
| ||||
| 2CC-NBOMe | LQC (75 pg/mL) | 64 | 1 | 86 |
| MQC (750 pg/mL) | 859 | 5 | 114 | |
| HQC (1500 pg/mL) | 1673 | 4 | 112 | |
|
| ||||
| 25I-NBOMe | LQC (75 pg/mL) | 66 | 4 | 88 |
| MQC (750 pg/mL) | 809 | 3 | 108 | |
| HQC (1500 pg/mL) | 1706 | 1 | 114 | |
|
| ||||
| 1 Month | ||||
| Designer Drug | Control | Mean Conc. (pg/mL) | CV | (%) Accuracy/Bias |
|
| ||||
| 2CC-NBOMe | LQC (75 pg/mL) | 84 | 5 | 112 |
| MQC (750 pg/mL) | 803 | 3 | 107 | |
| HQC (1500 pg/mL) | 1684 | 9 | 112 | |
|
| ||||
| 25I-NBOMe | LQC (75 pg/mL) | 34 | 5 | 113 |
| MQC (750 pg/mL) | 802 | 2 | 107 | |
| HQC (1500 pg/mL) | 1626 | 4 | 108 | |
|
| ||||
| Post Prep at 48 hours | ||||
| Designer Drug | Control | Mean Conc. (pg/mL) | CV | (%) Accuracy/Bias |
|
| ||||
| 2CC-NBOMe | LQC (75 pg/mL) | 74 | 16 | 99 |
| MQC (750 pg/mL) | 768 | 19 | 102 | |
| HQC (1500 pg/mL) | 1397 | 19 | 93 | |
|
| ||||
| 25I-NBOMe | LQC (75 pg/mL) | 73 | 18 | 97 |
| MQC (750 pg/mL) | 769 | 16 | 103 | |
| HQC (1500 pg/mL) | 1333 | 14 | 89 | |
Patient Serums
Emergency room serum samples collected from two intoxicated patients displaying symptoms as previously described by Rose et al. (2013) after the development of the presented method were determined to contain 250 pg/mL and 2,780 pg/mL 25I-NBOMe. During hospitalization, both patients admitted using 25I-NBOMe.
Discussion
The presented HPLC/MS/MS method demonstrated acceptable reliability and reproducibility for the detection and quantification of 2CC-NBOMe and 25I-NBOMe in serum specimens. During the development of this assay, we observed a consistent loss of both 2CC-NBOMe and 25I-NBOMe was observed if we the SPE elution solvent was evaporated to dryness. The addition of methanolic HCl and water to the elution solvent combined with limited evaporation of the solvent resulted in acceptable recoveries. Accuracy as well as intra-day and inter-day precisions were determined not to exceed CVs of <15% over the dynamic range of the assay. The variance in these parameters may be reduced with the use of deuterated internal standards. Tables 1, 2 and 3 present data from 202 different tests. The quantified results of these tests were within the acceptable performance of + 20% of their targeted values with application of only 25H-NBOMe as a single non-deuterated internal standard. This demonstrates the robustness of the assay. When the assay was developed, no deuterated internal standards were commercially available for 2CC-NBOMe or 25I-NBOMe. 25H-NBOMe was used as the ISTD because of it’s structurally similarity to 2CC-NBOMe and 25I-NBOMe, and it had not been reported as an abused designer drug. The assay was free of significant interference from matrix effects and free from significant analyte carryover. The 30 pg/mL LOQ for 2CC-NBOMe and 25I-NBOMe is well below concentrations detected in serum from intoxicated patients to date.
Conclusion
A method for the determination of 2CC-NBOMe and 25I-NBOMe in serum was developed. The assay used a simple SPE extraction procedure prior to chromatographic analysis. The assay is particularly suited for analysis of serum samples form emergency room patients. Further, the assay may be easily adapted for the analysis of 2CC-NBOMe and 25I-NBOMe in research specimens.
Acknowledgments
This project was supported by the National Institute on Drug Abuse Center grant P50DA005274 and the Hubert H. Humphrey Fellowship Program. The authors wish to thank Sarah Carney for proof reading this manuscript.
References
- Center for Veterinary Medicine; May, 2001. Bioanalytical Method Validation: A Guidance for Industry. U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research. [Google Scholar]
- Braden MR, Parrish JC, Naylor JC, Nichols DE. Molecular interaction of serotonin 5-HT2A receptor residues Phe339(651) and Phe340(652) with superpotent N-benzyl phenethylamine agonists. Molecular Pharmacology. 2006;70:1956–1964. doi: 10.1124/mol.106.028720. [DOI] [PubMed] [Google Scholar]
- Ettrup A, Hansen M, Santoni MA, Paine J, Gillings N, Palner M, Lehel S, Herth MM, Madsen J, Kristensen J, Begtrup M, Knudsen GM. Radiosynthesis and in vivo evaluation of a series of substituted 11C-phenethylamines as 5-HT2A agonist PET tracers. European Journal of Nuclear Medicine and Molecular Imaging. 2011;38:681–693. doi: 10.1007/s00259-010-1686-8. doi: 10.1007/s00259-010-1686-8. [DOI] [PubMed] [Google Scholar]
- Gibbons S. “Legal Highs” – novel and emerging psychoactive drugs: a chemical overview for the toxicologist. Clinical Toxicology. 2012;50:15–24. doi: 10.3109/15563650.2011.645952. doi: 10.3109/15563650.2011.645952. [DOI] [PubMed] [Google Scholar]
- Kelly A, Eisenga B, Riley B, Judge B. Case series of 25I-NBOMe exposures with laboratory confirmation [abstract] Clinical Toxicology. 2012;50:702. [Google Scholar]
- Nichols DE, Frescasa SP, Chemela BR, Rehderb KS, Zhongb D, Lewin AH. High specific activity tritium-labeled N-(2-methoxybenzyl)-2,5-dimethoxy-4 iodophenethylamine (INBMeO): A high-affinity 5-HT2A receptor-selective agonist radioligand. Bioorganic and Medincinal Chemistry. 2008;16:6116–6123. doi: 10.1016/j.bmc.2008.04.050. doi: 10.1016/j.bmc.2008.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SR, Cumpston KL, Stromberg PE, Wills BK. Severe poisoning following self reported use of 25-I, a novel substituted amphetamine [abstract] Clinical Toxicology. 2012;50:707–708. [Google Scholar]
- Rose SR, Poklis JL, Poklis A. A case of 25I-NBOMe (25-I) intoxication: a new potent 5-HT2A agonist designer drug. Clinical Toxicology. 2013;51:174–177. doi: 10.3109/15563650.2013.772191. doi: 10.3109/15563650.2013.772191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuba D, Sekula K. Identification and characterization of 2,5-dimethoxy-3,4-dimethyl-B-phenethylamine (2C-G) – a new designer drug. Drug Testing Analysis. 2012 doi: 10.1002/dta.1396. doi: 10.1002/dta.1396. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Zuba D, Sekula K, Buczek A. 25C-NBOMe – new potent hallucinogenic substance identified on the drug market. Forensic Science International. 2013;227:7–14. doi: 10.1016/j.forsciint.2012.08.027. doi: 10.1016/j.forsciint.2012.08.027. [DOI] [PubMed] [Google Scholar]


