Abstract
The p53 tumor suppressor governs multiple cell-intrinsic programs, including cell-cycle arrest and apoptosis, to curb neoplastic growth. A new study reveals that p53 also acts through a novel non-cell-autonomous mechanism, by stimulating the innate immune system to maintain tissue homeostasis and suppress tumorigenesis.
Inactivation of the p53 tumor suppressor gene is one of the most frequent alterations in human cancers, and p53 deficiency causes a fully penetrant cancer predisposition in mice, together underscoring the crucial role for p53 in tumor suppression [1,2]. p53 is a cellular stress sensor that can restrain neoplastic growth in a cell-autonomous manner by inducing cell-cycle arrest or apoptosis, or by regulating metabolism, in response to stress signals [1-3]. p53 can also inhibit nascent tumor growth through cellular senescence, a permanent cell-cycle arrest response [1,4]. Cellular senescence is accompanied by a host of gene expression changes, including a program known as the senescence-associated secretory phenotype (SASP), in which numerous cytokines and chemokines secreted from senescent cells promote communication with cells in the microenvironment [5,6]. The pivotal role for p53 in senescence has thus suggested that p53 may exert some of its biological effects by stimulating interplay between cells of the local milieu. Indeed, such a function for p53 was previously illuminated by studies showing that p53 can promote tissue homeostasis after liver damage by inducing senescence and the SASP in hepatic stellate cells (HSCs), which in turn provokes the recruitment of natural killer (NK) immune cells, clearance of senescent cells, and resolution of fibrotic lesions [7]. While highlighting a role for p53 as a ‘guardian of the tissue’, what remained unclear was whether this p53 function contributes to suppressing tumorigenesis — an important question, given conflicting reports that the SASP could both promote and inhibit tumorigenesis [5,8].
In a recent manuscript published in Cell, Lujambio et al. [9] have now investigated how the p53-triggered SASP in HSCs affects carcinogenesis in the liver (Figure 1). Wild-type mice and mice with conditional deletion of p53 in HSCs (HSC-p53Δ/Δ) were treated with the fibrosis-inducer carbon tetrachloride (CCl4) to cause chronic liver damage. The HSC-p53Δ/Δ animals displayed increased liver cirrhosis, liver failure, and mortality relative to wild-type controls, underscoring a key role for p53 in preserving organ integrity. In addition, the HSC-p53Δ/Δ mice also developed small tumors on the surface of the liver, often of wild-type p53 status, suggesting that p53 inactivation in HSCs might promote tumorigenesis in other cells. To solidify the notion that p53 action in the HSCs promotes a restrictive environment for tumorigenesis in the liver, the authors treated mice with the carcinogen diethylnitrosamine, which induces hepatocellular carcinoma, and then examined whether CCl4-induced fibrosis could enhance the development of hepatocellular carcinoma. Indeed, HSC-p53Δ/Δ mice developed more tumors than wild-type controls. Importantly, all of the tumors analyzed retained intact p53 genes, suggesting again that they did not arise from the p53-null HSC compartment. These striking findings reveal that p53 acts in a non-cell-autonomous manner to suppress tumor development.
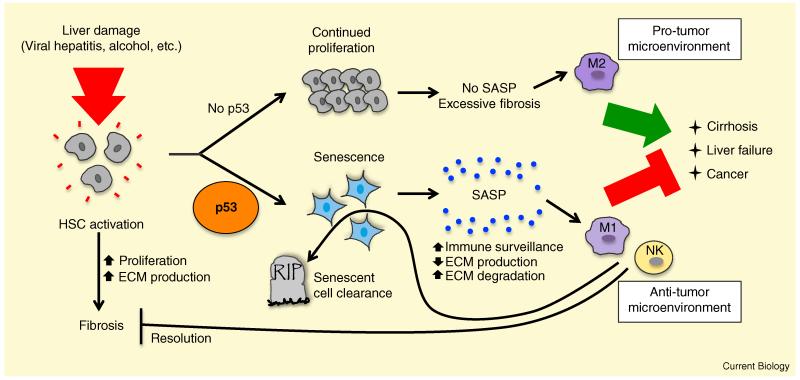
Figure 1.
p53 promotes senescence in hepatic stellate cells to maintain organ integrity and suppress cancer upon liver damage.
In response to liver damage, triggered by conditions such as viral hepatitis, alcohol abuse, and fatty liver disease, hepatic stellate cells (HSCs) become activated, proliferating and secreting extracellular matrix (ECM) components, leading to fibrosis. In the presence of p53 (bottom), HSCs undergo senescence, associated with the induction of the senescence-associated secretory phenotype (SASP). Secreted factors include those that regulate the extracellular matrix (ECM) and those that stimulate immune surveillance by polarizing macrophages to an anti-tumorigenic M1 phenotype and activating natural killer (NK) cells. Together, these responses inhibit cancer, trigger senescent cell clearance, and promote the resolution of fibrosis. In the absence of p53 activity (top), HSCs continue to proliferate and secrete ECM components, resulting in excessive fibrosis, cirrhosis, liver failure, and mortality. In addition, p53-deficient HSCs secrete factors that polarize macrophages to a pro-tumorigenic M2 fate, thereby promoting liver cancer.
What is the mechanism by which p53 in HSCs suppresses tumorigenesis in neighboring cells? To address this question, the investigators performed transcriptional profiling and functional annotation clustering, comparing senescent HSCs with proliferating ones, and these experiments revealed an enrichment for immune response, NF-κB activation, and protein secretion signatures in senescent HSCs. By defining factors preferentially secreted from senescent HSCs, the investigators identified interferon γ (IFNγ) and interleukin 6 (IL-6), cytokines that stimulate macrophage activation, a notable observation given that macrophages are known to modulate liver fibrosis [10]. In elegant experiments to define the effects of the p53-driven SASP program on macrophages, the investigators co-cultured macrophages with conditioned media from either proliferating p53-null or senescent p53-positive HSCs. They discovered that proliferating p53-deficient HSCs secrete factors that polarize macrophages towards a pro-tumorigenic M2 phenotype, while factors secreted by p53-expressing senescent HSCs induce an M1 phenotype, which is associated with antitumor activity. Macrophages polarized to an M1 phenotype were in turn able to eliminate the senescent HSCs but not the proliferating HSCs upon co-culture, suggesting that the p53-induced SASP signals macrophages to trigger HSC clearance along with the resolution of fibrosis. Additionally, the M2-polarized, but not M1-polarized, macrophages enhanced the proliferation of premalignant hepatoblasts, suggesting that p53-deficient proliferating HSCs induce a pro-proliferative microenvironment. Consistent with previous observations that p53 and NF-κB cooperate to induce senescence and the SASP, NF-κB was required for this p53 SASP response in HSCs, as attenuation of NF-κB, or of its transcriptional targets Il6, Ifn, and Icam, blocked the cytotoxic effects of macrophages on senescent HSCs. Based on these collective observations, the authors conclude that p53 activity in HSCs promotes an antitumor microenvironment by inducing a SASP that stimulates tumor-combating M1 macrophages, while keeping tumor-promoting M2 macrophages in check.
This study shows a clear non-cell-autonomous role for p53 in tumor suppression in vivo, in keeping with observations made previously by Campisi and colleagues when they originally described the SASP [6]. In that study, they showed that the SASP generated by senescent human cells can promote certain malignant phenotypes in cultured cells — epithelial-to-mesenchymal transition and invasiveness — and that both the SASP response and these malignant phenotypes are greatly exacerbated by p53 inhibition. These observations indicate that p53 normally acts to restrain the SASP and associated pro-tumorigenic activities. This quantitative augmentation of the SASP response in the absence of p53 is in striking contrast with the results presented here, where a clear switch in cytokine profiles ensues from p53 loss, resulting in differential immune cell activation. Thus, depending on the setting, the SASP can promote either anti-proliferative or pro-tumorigenic phenotypes, and p53 can differentially modulate the SASP, emphasizing the importance of investigating such responses in each particular context of interest.
The notion that p53 can promote a dialogue between neighboring cells recalls a previous study showing that during prostate cancer development in mice, p53 activation in epithelial tumor cells promotes a non-cell-autonomous, p53-dependent proliferative arrest in tumor-supportive stromal fibroblasts [11]. Interestingly, as the tumors progressed, the stromal fibroblast growth arrest was overcome by the selection of fibroblasts that had inactivated p53 by genetic mutation. This observation raised the intriguing possibility that p53-induced paracrine signals emanating from one cell may impose a selection pressure for p53 mutation in other cells within the microenvironment, which could ultimately further fuel tumor development. These findings suggest that cells may have evolved strategies to use p53 both to generate and to sense non-cell-autonomous tumor-suppressive signals.
The non-cell-autonomous role of p53 may have evolved to support the natural wound-healing process in damaged tissues. Perhaps the primary function of the p53-induced SASP in the liver is to maintain the senescent state as a means of shutting down fibrosis-causing HSC proliferation. Additionally, this response would fulfill the immediate need to halt the proliferation of potentially tumorigenic cells. However, since this response is only cytostatic in nature and allows the persistence of damaged cells in the milieu, a second step involving the recruitment of the innate immune system may have evolved to orchestrate the elimination of senescent cells. As a by-product of this process, the p53-induced SASP establishes an anti-tumor microenvironment. Thus, this response serves the dual purposes of wound healing and inhibition of tumor growth in the surrounding tissue.
Together, these studies have uncovered a novel non-cell-autonomous mechanism of p53-mediated tumor suppression in which p53 action in one cell influences tumor development in another cell. An obvious implication of these findings is that in Li-Fraumeni patients, who are heterozygous for a p53 mutant allele, or in p53−/− mice, the observed tumor predisposition may be fueled in part by lack of intact non-cell-autonomous p53 tumor suppressor mechanisms. Continued analysis of the p53-induced SASP program and its interplay with the immune system will further advance our understanding of p53 tumor suppression mechanisms. For example, in which tumor types are non-cell-autonomous mechanisms of p53 action most relevant? How is p53 induced in such settings as activated HSCs? How do these non-cell-autonomous p53 functions affect cancer therapy and can they be leveraged to enhance tumor treatment? It is known that senescence contributes to the cytotoxicity of anticancer drugs and that reactivation of endogenous p53 in p53-deficient murine liver carcinomas provokes cellular senescence followed by an innate immune response that triggers tumor clearance [12,13]. Thus, a deeper understanding of the crosstalk between p53-expressing senescent cells and other cells of the microenvironment will ultimately facilitate the development of novel chemotherapeutic approaches.
References
- 1.Vousden KH, Prives C. Blinded by the light: The growing complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 2.Brady CA, Attardi LD. p53 at a glance. J. Cell Sci. 2010;123:2527–2532. doi: 10.1242/jcs.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maddocks OD, Vousden KH. Metabolic regulation by p53. J. Mol. Med. 2011;89:237–245. doi: 10.1007/s00109-011-0735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nat. Rev. Cancer. 2010;10:51–57. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu. Rev. Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cahu J, Bustany S, Sola B. Senescence-associated secretory phenotype favors the emergence of cancer stem-like cells. Cell Death Dis. 2012;3:e446. doi: 10.1038/cddis.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lujambio A, Akkari L, Simon J, Grace D, Tschaharganeh DF, Bolden JE, Zhao Z, Thapar V, Joyce JA, Krizhanovsky V, et al. Non-cell-autonomous tumor suppression by p53. Cell. 2013;153:449–460. doi: 10.1016/j.cell.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin. Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill R, Song Y, Cardiff RD, Van Dyke T. Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell. 2005;123:1001–1011. doi: 10.1016/j.cell.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 12.Schmitt CA, Fridman JS, Yang M, Lee S, Baranov E, Hoffman RM, Lowe SW. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell. 2002;109:335–346. doi: 10.1016/s0092-8674(02)00734-1. [DOI] [PubMed] [Google Scholar]
- 13.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–660. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]