Abstract
Objective
We assessed the risk of adverse fetal outcomes following exposure to individual immunosuppressive drugs in pregnant women with chronic immune mediated diseases.
Methods
We used health plan data from Tennessee Medicaid and Kaiser Permanente Northern California and Southern California linked with vital records and medical records. Women with inflammatory arthropathies, systemic lupus erythematosus, and inflammatory bowel disease who filled prescriptions for immunosuppressive treatments during pregnancy were included. Major congenital malformations, fetal deaths, and life-threatening neonatal complications were identified from electronic data and validated with medical record review.
Results
The cohort included 608 infants, including 437 with exposure during pregnancy (402 first trimester, 35 second and third trimester only) and 171 whose mothers filled prescriptions for immunosuppressives before, but not during, pregnancy. There were 25 pregnancies (4.1% of the cohort) with confirmed major congenital malformations, 10 fetal deaths (1.6%), 23 life-threatening neonatal complications among preterm infants (20.4%), and 10 (2.1%) life-threatening complications among term infants. Compared to the reference group (medication treatment before, but not during, pregnancy), the risk ratios for adverse fetal outcomes associated with immunosuppressive use during pregnancy by exposure category included: methotrexate [risk ratio 1.39 (95% confidence interval 0.43,4.53)], tumor necrosis factor inhibitors [0.98 (0.38,2.55)], hydroxychloroquine [1.33 (0.69,2.55)], and other immunosuppressives [0.98, (0.48,1.98)].
Conclusions
We found no evidence of a large increase in risk of adverse fetal outcomes from first trimester exposure to immunosuppressive medications, though confidence intervals for risk ratios were wide. Further studies will be needed as use of these medications increases over time.
Chronic immune mediated diseases, including inflammatory arthropathies, connective tissue disorders, and inflammatory bowel disease, affect 3.5–5.5 million persons in the US(1, 2) and occur more commonly in women.(3–5) Because the onset of many of these diseases is during childbearing years(1, 2) and up to 50% of pregnancies in the US are unplanned,(6) it is plausible that many women taking medications to treat these conditions may become pregnant inadvertently and discover the pregnancy while taking the medication. In addition, many chronic immune mediated diseases might require treatment during pregnancy.
However, there is limited information on the fetal effects of the medications prescribed for treatment of chronic immune mediated diseases during pregnancy.(7, 8) Many of the studies to date assessing fetal outcomes have been uncontrolled case series, measured outcomes after knowledge of exposure, and included pregnancies with exposures to multiple medications at a time, limiting the ability to understand the effects of individual medications. Thus, we conducted an observational study in three large health plans which provide coverage for over 8 million individuals each year with considerable geographic and sociodemographic diversity. We assessed the relative proportion of adverse fetal outcomes following exposure to individual immunosuppressive medications during pregnancy for women with chronic immune mediated diseases.
PATIENTS and METHODS
Data Sources
We obtained study data from computerized claims, vital records, electronic medical records, and hard copy medical records for three geographically diverse health plans (Tennessee Medicaid, Kaiser Permanente Northern California, and Kaiser Permanente Southern California). All three health plans have automated databases that have been used previously to conduct similar studies.(9, 10) We have found excellent concordance between vital records and medical records for the key variables used to conduct the study, including last menstrual period (LMP), demographic variables, smoking, and alcohol use.(10) The initiation of the study differed according to site based on the earliest availability of the site’s computerized data (1995 for Tennessee Medicaid, 1998 for the Kaiser sites). Follow-up included deliveries/fetal deaths occurring through 2007.
Cohort
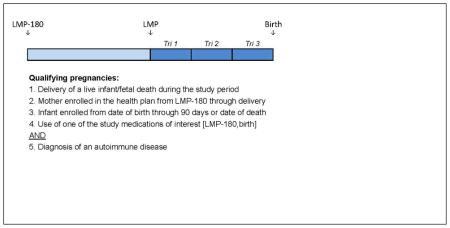
To assemble the retrospective cohort (Appendix A), we identified women and infants in the health plans who met all of the following criteria: 1) diagnosis of an immune mediated condition: inflammatory arthropathies (rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis), connective tissue disorders (systemic lupus erythematosus, scleroderma, inflammatory myopathies, and mixed connective tissue disorders), and inflammatory bowel disease, in the 180 days preceding the LMP (Appendix B); 2) prescription for one of the immunosuppressive medications of interest (Appendix C) or 30 days of consecutive corticosteroids between 180 days prior to the LMP and the date of delivery or date of fetal death; 3) continuous enrollment of the mother from 180 days prior to the LMP through the date of delivery/fetal death; 4) continuous enrollment of the infant from birth through 90 days of life or the date of death (including fetal death); and, 5) singleton birth. Births with maternal prescriptions during the first trimester for non-study medications thought to be teratogenic (valproic acid, chemotherapy medications, lithium misoprostol, and warfarin) were excluded.(10)
Medication Exposures
All births in the study group were classified according to maternal immunosuppressive medication use during pregnancy (Appendix D). To facilitate comparisons between individual medications, mutually exclusive categories of fetal exposure during the first trimester were created and included any methotrexate exposure, any TNF-I exposure in the absence of methotrexate, hydroxychloroquine in the absence of methotrexate or TNF-I, and other immunosuppressives in the absence of methotrexate, TNF-I, and hydroxychloroquine. The primary comparison group included women with immune mediated diseases treated with immunosuppressive medications in the 180 days before, but not during, pregnancy. For these women, last use of one of the study medications was a mean 2.4 months before the LMP.
Identification of Possible Study Outcomes
Adverse fetal outcomes included congenital malformations, fetal death, and life-threatening neonatal complications. Possible congenital malformations were identified from birth certificate checkboxes and infant claims in the first 90 days of life, fetal death or death certificates (deaths during the first 90 days of life), and the maternal hospitalization associated with the delivery or fetal death. Life-threatening neonatal complications were identified from infant claims and included respiratory failure, seizure, moderate to severe jaundice, or sepsis during the first 28 days of life.
Validation of Study Outcomes
Medical records and autopsy reports were reviewed to confirm all adverse fetal outcomes. Trained chart abstractors obtained the pertinent medical records, including transfer hospitalizations, which were de-identified and then reviewed by two physician adjudicators (WOC, STC), who were unaware of maternal drug exposure, to confirm that outcomes met the study clinical definitions. (10) For example, congenital malformations met definitions for individual malformations used in the Metropolitan Atlanta Congenital Defects Program.(11)
Analysis
Proportions of study endpoints for each exposure category were calculated by dividing the number of infants with the study endpoints by the corresponding number of births. Comparisons of study endpoints by exposure group were performed by calculating risk ratios for exposures to medications of interest compared to the reference group.
In order to control for potential confounding, we used data from the 180 days prior to LMP to create a propensity score, constructed as the linear predictor of a binary logistic regression model in which the dependent variable indicated any use or non-use of immunosuppressives (Appendix E). We then used Poisson regression to estimate the risk ratios for the effect of immunosuppressives on study endpoints adjusting for the propensity score as a model covariate. We chose Poisson regression without an offset variable because the outcomes of interest do not occur at an even rate during follow-up and because this approach yields risk ratio estimates, which are more intuitive to quantify the exposure effect.(12) We utilized the generalized estimating equation (GEE) method with Huber-White sandwich estimator to provide correct estimates of standard errors of the log of the risk ratio, preventing the model from over-dispersion and accounting for dependency among repeated measures. (13)
Human Subjects Protection
The study was carried out in compliance with the Helsinki Declarations. Permission to perform the study was obtained by the institutional review boards for each site, as well as the health plans that provided the data.
RESULTS
The final cohort included 608 mother-infant pairs occurring in 573 unique women, including 402 pregnancies with first trimester exposure to the immunosuppressive medications of interest (23 methotrexate, 56 TNF-I, 194 hydroxychloroquine, 129 other immunosuppressives), 35 with exposures to immunosuppressives in the second and/or third trimester only, and 171 with no exposure to immunosuppressive medications during pregnancy (Table 1). The maternal age, race, ethnicity, education, Medicaid enrollment, and smoking status for mothers in the cohort varied by the medication exposure group (Table 1). The distribution of maternal chronic immune mediated diseases varied by medication exposure group as well (Table 1), reflecting the clinical use of these medications for the conditions included. The mean year of birth was comparable for all of the exposure groups with the exception of TNF-I, in which infants had a later mean year of birth, reflecting the more recent approval and marketing of these medications. There were no clinically significant variations in mean birth weight or gestational age across the medication exposure groups.
Table 1.
Characteristics of women and infants with exposures to immunosuppressive medications during the first trimester of pregnancy.
| First Trimester Exposures to Immunosuppressive Medicationsa
|
No use of immunosuppressives during pregnancy (n=171)f | ||||
|---|---|---|---|---|---|
| Methotrexateb (n=23) | TNF-Ic (n=56) | Hydroxychloroquined (n=194) | Other immunosuppressivee (n=129) | ||
| Maternal Characteristics | |||||
| Age, years mean, (s.d.) | 30.8 (5.9) | 29.4 (6.3) | 30.8 (5.8) | 28.8 (6.5) | 29.0 (6.2) |
| Black race, n (%) | 7 (30.4) | 7 (12.5) | 34 (17.5) | 27 (20.9) | 33 (19.3) |
| Hispanic ethnicity, n (%) | 4 (17.4) | 15 (26.8) | 46 (23.7) | 21 (16.3) | 34 (19.9) |
| ≤12 years education, n (%) | 10 (43.5) | 27 (48.2) | 65 (33.5) | 58 (45.0) | 94 (55.0) |
| Medicaid enrollment, n (%) | 10 (43.5) | 26 (46.4) | 59 (30.4) | 58 (45.0) | 80 (46.8) |
| Smoking, n (%) | 3 (13.0) | 10 (17.9) | 14 (7.2) | 11 (8.5) | 24 (14.0) |
| Maternal conditions, n (%) | |||||
| Rheumatoid arthritis | 21 (91.3) | 32 (57.1) | 52 (26.8) | 27 (20.9) | 66 (38.6) |
| Systemic lupus erythematosus | 1 (4.3) | 3 (5.4) | 139 (71.6) | 16 (12.4) | 44 (25.7) |
| Inflammatory bowel disease | 1 (4.3) | 16 (28.6) | 0 (0) | 64 (49.6) | 35 (20.5) |
| Autoimmune hepatitis | 0 (0) | 0 (0) | 0 (0) | 12 (9.3) | 1 (0.6) |
| Other autoimmune condition | 0 (0) | 5 (8.9) | 3 (1.5) | 9 (7.0) | 20 (11.7) |
| No autoimmune diagnoses | 0 (0) | 0 (0) | 0 (0) | 1 (0.8) | 5 (2.9) |
| Infant characteristics | |||||
| Year of birth (mean) | 2003 | 2005 | 2003 | 2003 | 2003 |
| Birth weight, grams (mean) | 3141 | 2930 | 2912 | 3105 | 3034 |
| Gestational age, weeks (mean) | 37.7 | 38.1 | 37.5 | 38.0 | 37.9 |
Includes pregnancies with first trimester exposures to medications of interest and the reference group. Thirty-five pregnancies with second/third trimester exposure only are described in the text.
Methotrexate users may have filled prescriptions for other immunosuppressive medications.
TNF-I=tumor necrosis factor inhibitors and included etanercept, infliximab, and adalimumab, in the absence of methotrexate.
In the absence of methotrexate or TNF-I.
Other immunosuppressive medications: gold, sulfasalazine, leflunomide, azathioprine, and minocycline (no methotrexate, TNF-I, or hydroxychloroquine).
Includes pregnancies with exposures to immunosuppressive medications before, but not during pregnancy. Group includes women with pre-pregnancy only use of methotrexate (n=39), TNF-I in the absence of methotrexate use (n=16), hydroxychloroquine in the absence of methotrexate or TNF-I (n=51), and other immunosuppressive medications in the absence of the above medications (n=65).
Adverse fetal outcomes according to medication exposure group are shown in Table 2. There were 25 pregnancies (4.1% of the cohort including all exposure groups) with confirmed major congenital malformations, including 2.3% in the 171 mothers with no pregnancy exposures and 3.1–6.7% in the four groups with first trimester exposures to the medications of interest. There were 10 fetal deaths (1.6%) in the cohort, including 2.3% in the reference group and 0%–8.7% in the first trimester exposure groups. Because of the known strong association between preterm birth and adverse post-delivery outcomes, we considered life-threatening neonatal complications for preterm and term births separately. Among the 113 preterm infants, there were 23 (20.4%) who had neonatal complications. These included respiratory failure in 21 infants and sepsis in 13 infants. Among the 485 term infants, 10 (2.1%) had life-threatening neonatal complications following delivery.
Table 2.
Adverse fetal outcomes according to exposure to immunosuppressive medications during pregnancy.
| First Trimester Exposures to Immunosuppressive Medicationsa
|
No use of immunosuppressives during pregnancy (n=171) | ||||
|---|---|---|---|---|---|
| Methotrexateb (n=23) | TNF-Ic (n=56) | Hydroxychloroquined (n=194) | Other immunosuppressivee (n=129) | ||
| Malformations, n (%) | 1 (4.3) | 2 (3.6) | 13 (6.7) | 4 (3.1) | 4 (2.3) |
| Cardiac | 1 (4.3) | 1 (1.8) | 4 (2.1) | 0 (0) | 0 (0) |
| Specific defects | Ventricular septal defect (1) | Aortic insufficiency (1) | Pulmonic stenosis (1), complete heart block (1), ventricular septal defect (2) | ||
| Central nervous system | 0 (0) | 0 (0) | 1 (0.5) | 1 (0.8) | 0 (0) |
| Specific defects | Congenital hydrocephalus (1) | Porencephalic cyst (1) | |||
| Musculoskeletal | 0 (0) | 0 (0) | 1 (0.5) | 1 (0.8) | 0 (0) |
| Specific defects | Club feet (1) | Polydactyly (1) | |||
| Gastrointestinal | 0 (0) | 1 (1.8) | 1 (0.5) | 0 (0) | 1 (0.6) |
| Specific defects | Diaphragmatic hernia (1) | Pyloric stenosis (1) | Pyloric stenosis (1) | ||
| Genitourinary | 0 (0) | 1 (1.8) | 6 (3.1) | 2 (1.6) | 2 (1.2) |
| Specific defects | Chordee (1) | Hypospadias (4), meatal stenosis (1), ambiguous genitalia (1) | Hypospadias (1), hydronephrosis (1) | Hydronephrosis (1), Ureteropelvic junction obstruction (1) | |
| Orofacial | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.6) |
| Specific defects | Cleft palate (1) | ||||
| Fetal deaths | 2 (8.7) | 0 (0) | 2 (1.0) | 2 (1.6) | 4 (2.3) |
| Gestational age, weeks (mean) | 33.5 | -- | 34.5 | 22.0 | 28.0 |
| Autopsy findings, n | |||||
| Malformations | 0 | 0 | -- | 1 | 0 |
| Nuchal cord | 1 | 0 | -- | 1 | 0 |
| Chorioamnionitis | 0 | 0 | -- | 1 | 0 |
| Neonatal complicationsf | |||||
| Preterm infants (n=113), n (%)g | 0 (0) | 3 (33.3) | 10 (22.2) | 4 (21.0) | 6 (18.8) |
| Term infants (n=485), n (%)h | 1 (5.6) | 1 (2.1) | 4 (2.7) | 2 (1.8) | 1 (0.7) |
Outcomes for 35 pregnancies with exposure to study medications in the 2nd or 3rd trimesters only are included in the text.
Methotrexate users may have filled prescriptions for other immunosuppressive medications.
TNF-I=tumor necrosis factor inhibitors included etanercept, infliximab, adalimumab, in the absence of methotrexate. One infant had two defects (diaphragmatic hernia and aortic insufficiency).
In the absence of methotrexate or TNF-I.
Other immunosuppressive medications included gold, sulfasalazine, leflunomide, azathioprine, and minocycline in the absence of methotrexate, TNF-I, or hydroxychloroquine.
Neonatal complications included respiratory failure, seizures, jaundice, or sepsis.
Included 3 preterm infants with methotrexate exposure, 9 with TNF-I exposures, 45 with hydroxychloroquine exposures, 19 with other immunosuppressive exposures, and 32 infants with no exposure to the medications of interest during pregnancy. Results for exposures during the 2nd or 3rd trimester are shown in the text.
Included 18 term infants with methotrexate exposures, 47 with TNF-I exposures, 147 with hydroxychloroquine exposures, 108 with other immunosuppressive exposures, and 135 infants with no exposure to the medications of interest during pregnancy. Results for exposures during the 2nd or 3rd trimester are shown in the text.
There was no statistically significant difference in the study outcomes among first trimester exposure groups and the reference group of mothers with immune mediated diseases who used medications before, but not during, pregnancy for any of the medications of interest (Table 3). However, confidence intervals were wide for some risk ratios due to small numbers of first trimester exposures.
Table 3.
Adverse fetal outcomes according to exposure to immunosuppressive medications during the first trimester of pregnancy.
| First Trimester Exposures to Immunosuppressive Medicationsa
|
No use of immunosuppressive medications (n=171) (Reference) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methotrexateb (n=23) | TNF-Ic (n=56) | Hydroxychloroquined (n=194) | Other immunosuppressivee (n=129) | |||||||||||
|
| ||||||||||||||
| N | % | Risk Ratiof (95% CI) | N | % | Risk Ratio (95% CI) | N | % | Risk Ratio (95% CI) | N | % | Risk Ratio (95% CI) | N | % | |
|
|
||||||||||||||
| Congenital malformations | 1 | 4.3 | 1.96 | 2 | 3.6 | 1.59 | 13 | 6.7 | 3.11 | 4 | 3.1 | 1.42 | 4 | 2.3 |
| (0.23,17.00) | (0.30,8.47) | (0.99,9.77) | (0.38,5.40) | |||||||||||
| Fetal death | 2 | 8.7 | 3.18 | 0 | 0 | - | 2 | 1.0 | 0.35 | 2 | 1.6 | 0.55 | 4 | 2.3 |
| (0.54,18.62) | - | (0.05,2.44) | (0.11,2.68) | |||||||||||
| Neonatal complicationsg | ||||||||||||||
| Preterm (n=108)h | 0 | 0 | - | 3 | 33.3 | 1.74 | 10 | 22.2 | 1.09 | 4 | 21.1 | 1.05 | 6 | 18.8 |
| - | (0.54,5.61) | (0.41,2.91) | (0.34,3.24) | |||||||||||
| Term (n=455)i | 1 | 5.6 | 5.90 | 1 | 2.1 | 2.60 | 4 | 2.7 | 2.77 | 2 | 1.9 | 2.01 | 1 | 0.7 |
| (0.34,103.86) | (0.19,36.27) | (0.26,29.06) | (0.19,21.28) | |||||||||||
| Any adverse fetal outcomej | 3 | 13.0 | 1.39 | 5 | 8.9 | 0.98 | 25 | 12.9 | 1.33 | 12 | 9.3 | 0.98 | 15 | 8.8 |
| (0.43,4.53) | (0.38,2.55) | (0.69,2.55) | (0.48,1.98) | |||||||||||
Outcomes for 35 pregnancies with exposure to study medications in the 2nd or 3rd trimesters only are included in the text.
Methotrexate users may have filled prescriptions for other immunosuppressive medications.
TNF-I=tumor necrosis factor inhibitors included etanercept, infliximab, adalimumab, in the absence of methotrexate. One infant had two defects (diaphragmatic hernia and aortic insufficiency).
In the absence of methotrexate or TNF-I.
Other immunosuppressive medications included gold, sulfasalazine, leflunomide, azathioprine, and minocycline in the absence of methotrexate, TNF-I, or hydroxychloroquine.
Risk ratios estimated with Poisson regression adjusting for the propensity score. Reference group for all analyses is women who filled immunosuppressive medications before pregnancy but not during. Generalized estimating equations with Huber-White sandwich estimators were used to account for multiple pregnancies for a woman and for multiple gestations.
Adverse neonatal complications included respiratory failure, seizures, jaundice, or sepsis.
Included 3 preterm infants with methotrexate exposure, 9 with TNF-I exposures, 45 with hydroxychloroquine exposures, 19 with other immunosuppressive exposures, and 32 infants with no exposure to the medications of interest during pregnancy. Results for exposures during the 2nd or 3rd trimester are shown in the text.
Included 18 term infants with methotrexate exposures, 47 with TNF-I exposures, 147 with hydroxychloroquine exposures, 108 with other immunosuppressive exposures, and 135 infants with no exposure to the medications of interest during pregnancy. Results for exposures during the 2nd or 3rd trimester are shown in the text.
Infants may have experienced more than one outcome; thus, numbers of outcomes in the columns may not sum to the total of any adverse fetal outcome.
We performed sensitivity analyses which included exposures to drugs with long half-lives in the 30 days prior to LMP, which were not materially different from our primary analyses. We also performed comparisons between infants with prenatal exposure to immunosuppressives in the second or third trimester, but none in the first trimester (n=35) with our reference group of no immunosuppressive medication use during pregnancy. In these comparisons, we found no material differences in the proportion of infants with each of the specified study outcomes. To assess whether the mother’s disease process might influence the associations between medication exposure and adverse outcomes, we compared the proportion of outcomes occurring in medication exposure groups by maternal condition (Appendix E). Small sample size precluded statistical comparisons of these groups.
DISCUSSION
In this retrospective cohort study drawn from three large health plans with considerable geographic, socioeconomic, racial, and ethnic diversity, adverse fetal outcomes for infants whose mothers had exposure to immunosuppressive medications during pregnancy were uncommon events. Though confidence intervals were wide and statistical power was limited, we found no significant increase in risk for congenital malformations, fetal death, or neonatal complications in pregnancies with first trimester exposures to immunosuppressive medications. Although the rates of these adverse pregnancy outcomes in the cohort were greater than previously published rates in all pregnancies in the United States,(14) this may reflect different ascertainment methodologies, the underlying chronic immune mediated diseases treated by the medications, as well as exposure to these drugs.
Our study addresses many limitations of prior research in this area. Rather than including patients from a single institution or registry, we identified women with immune mediated diseases from three large health plans which included large numbers of racial and ethnic minorities, groups which have been understudied in previous work. Because some of the autoimmune conditions increase the risk for adverse fetal outcomes, we restricted the cohort to women with treated autoimmune conditions and compared risk for women with treatment during pregnancy to women with treatment before, but not during, pregnancy. Exposures to the medications of interest were identified from filled prescriptions, avoiding recall bias inherent in interviewing mothers or requesting information from providers. In addition, by identifying study outcomes from claims and vital records and reviewing medical records and autopsy reports to confirm all outcomes, we avoided ascertainment bias present in voluntary post-marketing databases. Finally, while many previous studies included women with exposure to multiple medications at a time, we constructed mutually exclusive exposure groups, allowing us to ascertain outcomes for each medication group of interest.
We did not find a statistically significant increase in the proportion of congenital malformations among infants whose mothers had exposure to methotrexate in the first trimester. The one malformation among 23 exposed women was not one of the classically described malformations. Many of the previous reports linking methotrexate to malformations included women with higher doses used to treat cancer as opposed to the lower doses used to treat rheumatoid arthritis in our cohort.(15) In addition, it is possible that some women with methotrexate exposure terminated the pregnancy prior to 20 weeks. Thus, our study findings do not provide reliable evidence that methotrexate is safe for use during pregnancy.
Limitations of our study include identification of maternal autoimmune conditions with ICD-9 codes, though our review of medical records for cases identified concordance between claims diagnoses and medical records 85% of the time. In addition, we could not identify spontaneous or therapeutic pregnancy terminations. We could not determine if women filled prescriptions for methotrexate for intended termination of pregnancy, though we found that most of the women filled several prescriptions during pregnancy, suggesting that this was not the case. While smoking and alcohol use may be underreported in 20–25% of pregnancies,(10) underreporting is unlikely to be differential by exposure group. In addition, despite the combination of data from three large health plans, there were small numbers of outcomes, which limited study power. As a result, confidence intervals around our risk ratios were wide for many comparisons. However, even if risk is increased, these estimates suggest that the magnitude of risk would not be on the order of other important teratogens, such as thalidomide.
In conclusion, while these data provide safety information on the magnitude of potential risk of adverse fetal outcomes for women with chronic immune mediated diseases and their healthcare providers, further follow-up studies with greater sample size will be needed. In addition, further monitoring for potential adverse fetal effects will be needed as use of some of these medications increases over time.
Acknowledgments
We acknowledge the data partners who provided data needed to conduct the study: TennCare Bureau, Tennessee Department of Health, Kaiser Permanente Northern California, and Kaiser Permanente Southern California. We acknowledge the following individuals who provided assistance with the study: Vanderbilt University School of Medicine: Patricia A. Gideon (chart reviews and abstraction), Michelle DeRanieri (chart reviews and abstraction), Leanne Balmer (chart reviews and abstraction), Shannon D. Stratton (chart reviews and abstraction), Judith A. Dudley (programming), Lynne Caples (project management), Tracy Crowley (data entry), Kaiser Permanente Northern California: Hong Chen (programming), and Roxana Odouli (medical record retrieval and abstraction); Kaiser Permanente Southern California: Fang Niu (programming) and Felicia Bixler (medical record retrieval and abstraction); SABER Collaboration: AHRQ: Parivash Nourjah; Brigham and Women’s Hospital: Robert Glynn, Mary Kowal, Joyce Lii, Jeremy Rassen, Sebastian Schneeweiss, Daniel Solomon; Fallon Medical Center and University of Massachusetts: Leslie Harrold; FDA, David Graham, Carolyn McCloskey, Rita Ouellet-Hellstrom, Kristin Phucas; Kaiser Permanente Northern California: Lisa Herrinton, Liyan Liu, Kaiser Permanente Colorado: Marcia Raebel; University of Alabama at Birmingham: Lang Chen, Jeffrey Curtis, Elizabeth Delzell, Nivedita Patkar, Kenneth Saag, Fenglong Xie; University of Pennsylvania: Kevin Haynes, James Lewis, Vanderbilt University: Carlos Grijalva, Ed Mitchel.
This project was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (P60AR056116); Agency for Healthcare Research and Quality and the Food and Drug Administration (HS17919 and HS10384) through the Centers for Education and Research on Therapeutics program and the Safety Assessment of Biologic Therapy (SABER) collaboration. The authors of the report are responsible for its content. Statements in the report should not be construed as endorsement by AHRQ, FDA, or DHHS. T. Craig Cheetham received funding support from Merck Pharmaceuticals for a study that did not involve any of the medications studied in this manuscript or any competing products. Thomas Morgan served on a Gaucher Disease advisory board for Shire Pharmaceuticals in 2012 and as a consultant in 2013 on litigation for medications unrelated to the present study.
Appendix
A. Cohort Formation
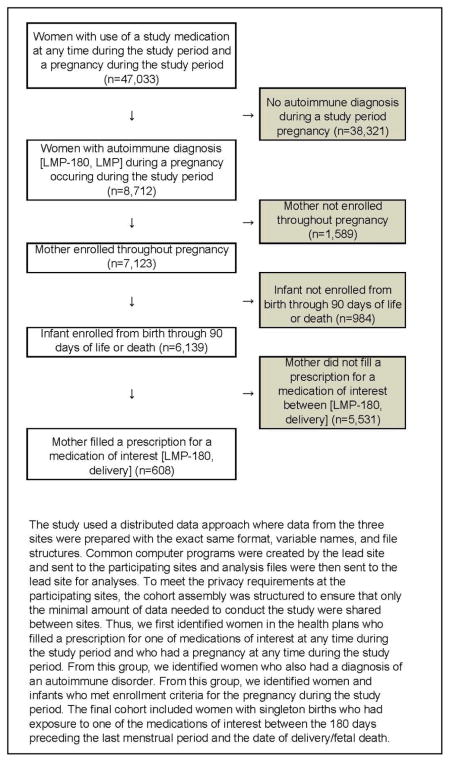
B. Immune mediated Diagnoses of Interest
We identified women with immune mediated diagnoses preceding pregnancy (LMP-180 through LMP) by searching health plan claims for diagnoses in any claim type for conditions shown in Table A.1. and no evidence of an organ transplant between LMP-180 and date of delivery/fetal death (ICD-9 codes: V42.0, V42.1, V42.6, V42.7, V42.81, V42.83, 996.8x. CPT codes of 50320, 50360, 50365, 50370, 50380; 33935, 33940, 33945; 32851, 32852, 32853, 32854; 47135, 47136; 38240, 38241; 48554, 48556 ICD-9-CM procedure codes of 33.5; 33.6; 37.5; 41.0; 50.5; 52.8; 55.6).
Table A.1.
ICD-9 codes for immune mediated diseases
| Diagnosis | ICD-9 code |
|---|---|
| Inflammatory arthropathies | |
|
| |
| Rheumatoid arthritis | 714.0 |
| Felty’s Syndrome | 714.1 |
| Other RA with visceral or systemic involvement | 714.2 |
| Juvenile chronic polyarthritis | 714.3 |
| Chronic post rheumatic arthropathy | 714.4 |
| Rheumatoid arthritis lung | 714.81 |
| Inflammatory polyarthropathy | 714.9 |
| Ankylosing spondylitis/inflammatory spondylopathies | 720.x |
| Psoriatic arthritis or psoriasis | 696.0 |
|
| |
| Connective Tissue Disorders | |
|
| |
| Systemic lupus erythematosus | 710.0, 695.4 |
| Systemic sclerosis | 710.1 |
| Inflammatory myopathy | 359.6, 359.8x, 359.9 |
| Connective tissue disorder, diffuse and mixed | 710.8, 710.9 |
|
| |
| Inflammatory Bowel Disease | |
|
| |
| Crohn’s Disease | 555.x |
| Ulcerative colitis | 556.x |
| Other and nonspecified noninfectious colitis | 558.x |
| Gastroenteritis and duodenitis | 535.9 |
| Anal fistula | 565.1 |
| Ulcer anus, rectum | 569.41 |
| Granuloma of rectum | 569.49 |
| Ulceration of intestine | 569.82 |
|
| |
| Autoimmune Hepatitis | |
|
| |
| Chronic hepatitis | 571.4x |
C. Immunosuppressive Medications of Interest
We identified women from the three health plans who filled prescriptions for the immunosuppressive medications of interest based on computerized health plan data. Because of the dosing frequencies and extremely long half-life of some of the immunosuppressive medications, we estimated an exposure window based on the days of supply for each prescription, the dosing interval for medications administered by infusion, and the pharmacologic properties of the medications of interest (Table A.2). We defined the exposure window as the period of active medication exposure and included the days of supply for drugs with shorter half-lives and one dosing interval for medications with longer half-lives. Because of the complexities of determining biologic effects of many of these medications, we were conservative in our definition and considered the risk to the fetus greatest when there were high plasma concentrations of drug for the mother.
Table A.2.
Immunosuppressive drugs for immune mediated disease
| Medication | Half-life (range) | Exposure Window |
|---|---|---|
| Antimetabolites | ||
| Methotrexate | 8–15 hours | Days supply |
| Tumor necrosis factor antagonist (TNF-I) | ||
| Etanercept | 3–5.5 days | Days supply |
| Infliximab | 8–10 days | 56 days |
| Adalimumab | 10–18 days | Days supply |
| Anakinra | 4–6 hours | Days supply |
| Rituximab | 3–8 days | 14 days |
| Antimalarial drugs | ||
| Hydroxychloroquine | 40 days | Days supply |
| Other immunosuppressive medications | ||
| Azathioprine | 3 hours | Days supply |
| Cyclosporine | 10–27 hours | Days supply |
| Cyclophosphamide | 1–7 hours | Days supply |
| Mycophenolate | 16–18 hours | Days supply |
| D-penicillamine | 1–7 hours | Days supply |
| Minocycline | 11–22 hours | Days supply |
| Sulfasalazine | 6–10 hours | Days supply |
| Leflunomide | 4–28 days | Adjusted days supply |
We calculated an adjusted exposure window for leflunomide prescriptions, to account for its extremely long half-life. Clinical guidelines recommend a washout period of several weeks prior to pregnancy following pre-conception exposures to leflunomide, accompanied by an 11 day regimen of cholestyramine or activated charcoal, which is thought to reduce the plasma levels of leflunomide below that which is felt to represent risk for the developing fetus. (Temprano 2005) Thus, for women exposed to leflunomide where the prescribed days of supply plus 21 days extended past the last menstrual period (LMP), we identified prescribing of cholestyramine or activated charcoal between the last leflunomide prescription and the LMP. If prescribing of this prevention regimen was identified, the days of supply for leflunomide was terminated 11 days after the cholestyramine or activated charcoal filling date.
D. Classification of Maternal Medication Use
E. Propensity Score
In order to control for potential confounding, we performed Poisson regression with a propensity score constructed as the linear predictor of a binary logistic regression model in which the dependent variable was a binary variable indicating any use or non-use of immunosuppressives. Maternal factors included in the propensity score model were collected from the 180 days preceding LMP and included sociodemographic variables (maternal age, race, ethnicity, years of education, parity), chronic health diagnoses (smoking, diabetes, epilepsy, asthma, renal disease, cancer, heart disease, hypertension, HIV, substance use, obesity, migraine headaches), medications used to treat chronic diseases (angiotensin converting enzyme inhibitors, antihypertensives, anticoagulants, non-teratogenic anticonvulsants, anti-infectives, estrogen preparations, statins, insulin, oral hypoglycemics, HIV medications, beta-agonists), chronic immune mediated diseases (rheumatoid arthritis, autoimmune muscular diseases, psoriatic arthritis, systemic lupus erythematosus, inflammatory bowel disease, autoimmune hepatitis), geographic factors, and calendar year of pregnancy. The propensity score was multiply imputed, so there were no missing propensity scores. Propensity scores were constructed separately (using the same variables) for congenital malformations, fetal death, and adverse effects.
F. Outcomes by maternal disease and medication exposure group.a
| Methotrexate | TNF-Ib | Hydroxychloroquine | Other | None | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||
| N pregnancies | N adverse events | % | N pregnancies | N adverse events | % | N pregnancies | N adverse events | % | N pregnancies | N adverse events | % | N pregnancies | N adverse events | % | |
|
|
|||||||||||||||
| SLEc | 1 | 0 | 0.0% | 3 | 1 | 33.3% | 139 | 19 | 13.7% | 16 | 2 | 12.5% | 44 | 9 | 20.5% |
| RAd | 21 | 2 | 9.5% | 32 | 3 | 9.4% | 52 | 6 | 11.5% | 27 | 1 | 3.7% | 66 | 3 | 4.5% |
| IBDe | 1 | 1 | 100.0% | 16 | 1 | 6.3% | 0 | 0 | 0.0% | 64 | 6 | 9.4% | 35 | 0 | 0.0% |
| Other | 0 | 0 | 0.0% | 5 | 0 | 0.0% | 3 | 0 | 0.0% | 21 | 3 | 14.3% | 21 | 3 | 14.3% |
| TOTAL | 23 | 3 | 13.0% | 56 | 5 | 8.9% | 194 | 25 | 12.9% | 128 | 12 | 9.4% | 166 | 15 | 9.0% |
Excludes 6 pregnancies which had no autoimmune diagnoses.
TNF-I=tumor necrosis factor inhibitors
SLE=systemic lupus erythematosus
RA=rheumatoid arthritis
IBD=inflammatory bowel disease
Contributor Information
William O. Cooper, Email: william.cooper@vanderbilt.edu.
T. Craig Cheetham, Email: craig.t.cheetham@kp.org.
De-Kun Li, Email: de-kun.li@kp.org.
C. Michael Stein, Email: mike.stein@vanderbilt.edu.
S. Todd Callahan, Email: todd.callahan@vanderbilt.edu.
Thomas M. Morgan, Email: thomas.morgan@vanderbilt.edu.
Ayumi K. Shintani, Email: ayumi.shintani@vanderbilt.edu.
Ning Chen, Email: aimee.n.chen@gmail.com.
Marie R. Griffin, Email: marie.griffin@vanderbilt.edu.
Wayne A. Ray, Email: wayne.ray@vanderbilt.edu.
Reference List
- 1.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 2.Lakatos PL. Recent trends in the epidemiology of inflammatory bowel diseases: up or down? World J Gastroenterol. 2006;12(38):6102–8. doi: 10.3748/wjg.v12.i38.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaffo A, Saag KG, Curtis JR. Treatment of rheumatoid arthritis. Am J Health SystPharm. 2006;63(24):2451–65. doi: 10.2146/ajhp050514. [DOI] [PubMed] [Google Scholar]
- 4.D’Cruz DP, Khamashta MA, Hughes GR. Systemic lupus erythematosus. Lancet. 2007;369(9561):587–96. doi: 10.1016/S0140-6736(07)60279-7. [DOI] [PubMed] [Google Scholar]
- 5.Beaulieu DB, Kane S. Inflammatory bowel disease in pregnancy. Gastroenterol Clin North Am. 2011;40(2):399–413. ix. doi: 10.1016/j.gtc.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Henshaw SK. Unintended pregnancy in the United States. Fam Plann Perspect. 1998;30(1):24–9. 46. [PubMed] [Google Scholar]
- 7.Skomsvoll JF, Wallenius M, Koksvik HS, Rodevand E, Salvesen KA, Spigset O, et al. Drug insight: Anti-tumor necrosis factor therapy for inflammatory arthropathies during reproduction, pregnancy and lactation. Nat Clin Pract Rheumatol. 2007;3(3):156–64. doi: 10.1038/ncprheum0426. [DOI] [PubMed] [Google Scholar]
- 8.Carter JD, Ladhani A, Ricca LR, Valeriano J, Vasey FB. A safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration database. JRheumatol. 2009;36(3):635–41. doi: 10.3899/jrheum.080545. [DOI] [PubMed] [Google Scholar]
- 9.Li DK, Liu L, Odouli R. Exposure to non-steroidal anti-inflammatory drugs during pregnancy and risk of miscarriage: population based cohort study. BMJ. 2003;327(7411):368. doi: 10.1136/bmj.327.7411.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper WO, Hernandez-Diaz S, Arbogast PG, Dudley JA, Dyer S, Gideon PS, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med. 2006;354(23):2443–51. doi: 10.1056/NEJMoa055202. [DOI] [PubMed] [Google Scholar]
- 11.Metropolitan Atlanta Congenital Defects Program Coding Manual. Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 12.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 13.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44(4):1049–60. [PubMed] [Google Scholar]
- 14.MacDorman MF, Kirmeyer S. Fetal and perinatal mortality, United States, 2005. NatlVital StatRep. 2009;57(8):1–19. [PubMed] [Google Scholar]
- 15.Milunsky A, Graef JW, Gaynor MF., Jr Methotrexate-induced congenital malformations. J Pediatr. 1968;72(6):790–5. doi: 10.1016/s0022-3476(68)80430-5. [DOI] [PubMed] [Google Scholar]


