Abstract
Objective
To compare asthma history and pulmonary function in adolescents born prematurely with very low birth weight with and without antenatal steroid exposure.
Study Design
We studied 188 fourteen-year-olds (94 exposed, 84 male). We used parent report to ascertain asthma and asthma-related symptoms and spirometry to assess pulmonary function. Steroid-exposed and unexposed groups were compared using Mann-Whitney U tests (continuous variables), chi-square analysis (categorical variables), and logistic regression (multivariate analyses).
Results
The steroid-exposed group had greater prevalence of larger airway obstruction (35%v. 21%), and steroid-exposed adolescents with birth weights < 1000 grams had 4.5-fold higher odds of larger airway obstruction. Wheezing in the last 12 months was twice as prevalent in steroid-exposed adolescents with birth weights between 1000–1500 g.
Conclusion
Antenatal steroid exposure does not provide long-term benefits for pulmonary outcomes in adolescents born prematurely with very low birth weight in the era of surfactant therapy.
Keywords: Corticosteroids, Prematurity, Lung Function, Asthma
Introduction
Approximately 1.5% of infants in the US are born each year with very low birth weight (VLBW; < 1500 g).1 In a recent multi-center study,2 44% of VLBW infants had respiratory distress syndrome and 22% developed neonatal chronic lung disease, increasing the risk for asthma and reduced pulmonary function in later years. When preterm delivery is anticipated, treatment of the mother with antenatal corticosteroid therapy (ANCS) is given to promote fetal lung maturation.3 Currently, nearly 80% of VLBW infants are exposed to ANCS,2 which decreases the risk for respiratory distress syndrome (RDS) and improves neonatal lung function and survival.4
Studies examining the long-term effects of ANCS on pulmonary outcomes are limited, and results are conflicting. Four follow-up studies revealed no difference between those exposed and not exposed to ANCS with regard to lung volumes, flow rates and/or lung mechanics, as well as prevalence of asthma and/or wheeze in the past 12 months.5–9 However, these studies were conducted in the 1970s and 1980s, prior to the availability of exogenous surfactant, a therapy that increased the survival rate for VLBW infants, particularly those born with extremely low gestational age and birth weight.10,11 Two studies of children in the exogenous surfactant era report lower risk of asthma in those exposed compared to those not exposed to ANCS.12,13 However, few infants received surfactant (11 and 30%), ANCS exposure varied greatly (24 and 68%), and pulmonary function was not studied. In view of these limitations and the likely differences in neonatal characteristics of samples born before compared to samples born after introduction of surfactant treatment, we evaluated the long-term effects of ANCS on pulmonary outcomes in a cohort of VLBW adolescents born in the surfactant era of whom 66% were treated with surfactant, a level more comparable to current treatment rates in VLBW infants.2 Our objective was to compare pulmonary function and asthma history and related symptoms between VLBW adolescents exposed to ANCS with those not exposed, with consideration of other factors such as birth weight and obesity which may affect the association between ANCS and these outcomes.
Methods
Participants
Participants were 188 fourteen-year-old adolescents recruited from a cohort of 479 survivors born prematurely with VLBW who met the following criteria: 1) birth at Forsyth Medical Center between January 1, 1992 and June 30, 1996; 2) birth weight < 1500 grams; 3) singleton birth; 4) no major congenital anomaly; and 5) follow-up clinical evaluation at one-year adjusted-age. The study was approved by the institutional review boards of Wake Forest University Baptist Medical Center and Forsyth Medical Center. Written informed consent was obtained from a parent or legal guardian, and written assent was obtained from the adolescent.
Methods
Participants, accompanied by a parent or legal guardian, reported to the Clinical Research Unit at Wake Forest Baptist Medical Center. Height and weight were measured in triplicate, and body mass index (BMI) was calculated (average weight in kilograms divided by average height in meters squared). Age- and gender-specific BMI percentiles and z-values were determined from National Center for Health Statistics 2000 reference data.14 A BMI percentile ≥ 85th percentile was considered overweight or obese.
History of asthma and asthma-related symptoms was obtained from parental report using the International Study of Asthma and Allergies in Childhood (ISAAC) Questionnaire.15 Parents also reported any asthma medications taken by their child in the previous two weeks.
Pulmonary function testing was conducted in accordance with standard guidelines16 using a Viasys Vmax Encore Metabolic Cart equipped with a heated-wire pneumotachometer and spirometry software. Forced expiratory volumes and flow rates were obtained. Primary variables of interest were forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), and the ratio of FEV1 to FVC (FEV1/FVC). Values were expressed as % of predicted based on reference data specific for gender, race, age, and height, and values below the 5th percentile were considered below normal.17 In order to minimize risk to study participants, they were not told to refrain from taking any of their usual medications.
Airway reactivity was determined in response to progressive maximal exercise and inhaled bronchodilator therapy. Spirometry was repeated five minutes post-exercise and exercise-induced bronchoconstriction was defined as a 15% decrease in FEV1 from pre-exercise values.18 Spirometry was also performed 20 minutes following inhalation of two puffs of albuterol, and a 12% increase in FEV1 from baseline was considered a positive bronchodilator response.19
Information about tobacco smoke exposure was also obtained because of its association with asthma risk20 by asking the participants to complete a form in private regarding whether they smoked or not. Environmental smoke exposure was determined by asking the parents to complete a form indicating if the father or mother currently smoked. Antenatal smoke exposure was assessed by asking if the mother smoked during her pregnancy with the participant.
Neonatal characteristics of the participants were obtained from a research database by research nurses and included: birth weight; gestational age; exposure to ANCS, postnatal dexamethasone, and surfactant; number of days of mechanical ventilation and supplemental oxygen; and diagnosis of bronchopulmonary dysplasia (BPD) based on supplemental oxygen requirement at 36 weeks post-menstrual age.21
Data analysis
Descriptive statistics were computed, and based on measures of central tendency and dispersion, nonparametric statistical analyses were deemed appropriate. Between-group comparisons (ANCS-exposed v. not exposed) were made using Mann-Whitney U tests for continuous variables and Chi-square analysis for categorical variables. Logistic regression analysis was used to adjust for potential confounders (variables associated with both ANCS and pulmonary outcomes using p ≤ 0.2) and also to examine possible mediators and moderators of the association between ANCS and pulmonary outcomes. In view of potential survivor bias associated with ANCS exposure in those with lower birth weight, stratum-specific odds ratios (OR) were computed for birth weight groups (<1000 g v. ≥1000 g).
Results
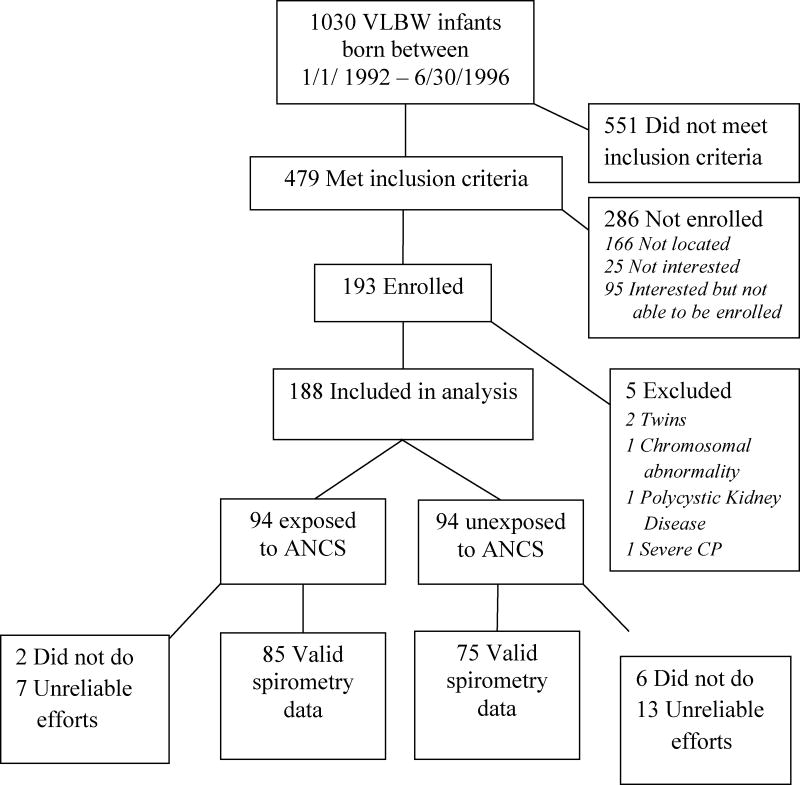
As shown in the figure, 193 of 479 eligible VLBW adolescents were enrolled in the study. After enrollment, we found that four participants did not meet eligibility criteria and one was unable to participate due to impaired motor function; consequently, their data were excluded from analysis. Demographic and neonatal characteristics were similar among eligible VLBW survivors who did or did not participate (data not shown) except for gestational age which was lower (p = 0.01) in participants [27 (24, 32) v. 28 (24, 33) weeks for median (5th, 95th percentiles)]. The neonatal characteristics of the remaining 188 participants (94 exposed and 94 not exposed to ANCS) are shown in Table 1. More ANCS-exposed children were Caucasian and exposed postnatally to dexamethasone, but the other neonatal characteristics did not differ between the two groups. Forty-seven percent of the participants (47 ANCS-exposed, 41 unexposed) had extremely low birth weight (ELBW; < 1000 g). In the ANCS-exposed group, 92 of the 94 mothers received betamethasone, one received dexamethasone, and one received both betamethasone and dexamethasone. More detailed information about number of doses and time of dose relative to birth was available on 63 of 94 ANCS-exposed women. The majority of women (62%) received two doses (one full course) and the majority of births (62%) occurred at least 24 hours after initiation of treatment but less than 7 days. No differences in neonatal or current characteristics were found between offspring of ANCS-exposed women with and without detailed data.
Figure 1.
Figure Follow-up details of VLBW participants.
Table 1.
Neonatal characteristics by ANCS exposure expressed as median (5th, 95th percentile) or n (%).
| Unexposed to ANCS (n = 94) |
Exposed to ANCS (n = 94) |
|
|---|---|---|
| Gestational Age, wks | 27 (23, 33) | 28 (24, 32) |
| Male Gender, n (%) | 39 (42) | 45 (48) |
| Caucasian Race, n (%) | 39 (42) | 64 (68)a |
| Birth Weight, g | 1043 (595, 1461) | 1003 (618, 1466) |
| Birth Weight z-value | −0.164 (−2.013, 1.115) | −0.228 (−1.849, 0.706) |
| Mechanical ventilation, days | 4.0 (0, 60.3) | 5.0 (0, 55.3) |
| Supplemental oxygen, days | 25.0 (0, 209.0) | 29.0 (0, 191.3) |
| + Surfactant Therapy, n (%) | 59 (63) | 58 (62) |
| + Postnatal Dexamethasone Exposure, n (%)b | 16 (17) | 24 (26) |
| + BPD diagnosis, n (%) | 26 (28) | 23 (25) |
| + Antenatal smoke exposure, n (%)c | 17 (20) | 11 (12) |
p <0.05, ANCS-exposed > unexposed
Postnatal dexamethasone exposure unknown in 2 ANCS-exposed and 1 unexposed
Antenatal smoke exposure unknown in 1 ANCS-exposed and 1 unexposed
Participants’ characteristics at the follow-up evaluation are presented for the two groups in Table 2. Median height z-value at 14 years of age was significantly higher in the ANCS-exposed compared to the unexposed group. Weight and BMI z-values did not differ between groups, but 29% of the ANCS-exposed group and 37% of the unexposed group had a BMI >85th percentile making them overweight or obese. Only five participants, but nearly half of parents in both groups, reported smoking.
Table 2.
Participants’ characteristics at 14 years of age expressed as medians (5th, 95th percentiles) or n (%).
| Unexposed to ANCS n=94 |
Exposed to ANCS n=94 |
|
|---|---|---|
| Weight Z-value | 0.509 (−1.731, 2.302) | 0.319 (−1.458, 2.185) |
| Height Z-value | −0.382 (−2.567, 1.157) | −0.152a (−2.016, 1.337) |
| BMI Z-value | 0.630 (−1.590, 2.398) | 0.333 (−1.299, 2.142) |
| + Current Smoking Status, n (%) | 3 (3) | 2 (2) |
| + Parental Smoking Statusb, n (%) | 43 (49) | 38 (41) |
p < 0.05, ANCS-exposed > unexposed
Either parent smoked; unknown in 7 unexposed and 2 ANCS-exposed
History of Asthma and Related Symptoms
Parental responses to the ISAAC questionnaire regarding the participant’s history of asthma and related symptoms are presented in Table 3. Although history of asthma, wheeze in the last 12 months, and nocturnal cough were reported frequently by both groups, no significant group differences were found. Furthermore, reported use of asthma medications in the past two weeks did not significantly differ between groups (12.8% and 16% for unexposed and ANCS-exposed participants, respectively).
Table 3.
Prevalence of asthma and related symptoms as indicated by parental responses to the ISAAC questionnaire.a
| Unexposed to ANCS n=94 |
Exposed to ANCS n=94 |
|
|---|---|---|
| Asthma everb, n (%) | 33 (36) | 39 (42) |
| Wheeze last 12 mos, n (%) | 13 (14) | 22 (23) |
| Wheeze with exercise, n (%) | 17 (18) | 19 (20) |
| Sleep disturbed by SOBc, n (%) | 7 (7) | 7 (7) |
| Nocturnal cough, n (%) | 21 (23) | 23 (25) |
p>0.05 for all between-group comparisons
Question not completed by parent of one unexposed participant
SOB: shortness of breath
Pulmonary Function
As shown in the figure, eight children did not perform pulmonary testing and 20 did not give reliable efforts. The pulmonary function test results for the 75 unexposed and 85 ANCS-exposed subjects with acceptable efforts are provided in Table 4. There were no group differences for median values of FVC or FEV1 (% predicted). Only two participants in each group had FVC values below normal (< 5th percentile), whereas 8% of the unexposed and 14% of the ANCS-exposed groups had FEV1 values < 5th percentile. Compared to those unexposed, ANCS-exposed participants had a lower median FEV1/FVC, and a greater percentage(35 v. 21%, respectively; p=0.06) had FEV1/FVC values below normal (< 5th percentile). The below normal FEV1/FVC values were accompanied by a positive history of asthma or related symptoms in 81% of unexposed and 83% of exposed participants. It should be noted that of the participants who reported recent use of asthma medications, only two participants reported long-acting bronchodilator use within 12 hours of testing and none reported short-acting bronchodilator use within 4 hours of testing. The test results of one participant (ANCS-exposed) were within normal limits with no evidence of airway obstruction. However, the other participant, (unexposed), despite medication, exhibited both larger and smaller airway obstruction as well as a positive response to bronchodilator therapy given as part of testing. Exclusion of their data from analysis did not alter the results appreciably.
Table 4.
Forced expiratory volumes and flow rates at 14 years of age. Values are medians (5th, 95th percentiles).
| Unexposed to ANCS n = 75 |
Exposed to ANCS n = 85 |
|
|---|---|---|
| FVC, % predicted | 103 (81, 122) | 102 (84, 125) |
| FEV1, % predicted | 95 (73, 119) | 94 (73, 121) |
| FEV1/FVC, % predicted | 96 (78, 107) | 94* (77, 104) |
p<0.05, ANCS-exposed < unexposed
Airway Reactivity
The prevalence of exercise-induced bronchoconstriction was similar between groups with 9% of unexposed and 6% of ANCS-exposed groups exhibiting ≥ 15% decrease in FEV1 following exercise. A positive response to bronchodilator therapy was observed in 14% of unexposed and 16% of ANCS-exposed groups. Of those with a baseline FEV1/FVC value < 5th percentile, 37% exhibited ≥ 12% improvement.
Multivariate Analyses
Potential confounding by ANCS group differences in height and race was eliminated by expressing pulmonary function data as % of predicted. Logistic regression analysis revealed that participants exposed to ANCS had increased odds (OR = 2.01, 95% CI: 0.99, 4.09; p=0.05) of having an FEV1/FVC < 5th percentile compared to those unexposed, and this association persisted when adjusting for postnatal dexamethasone exposure and current BMI z-value (variables correlated with both ANCS and FEV1/FVC at p ≤ 0.2). Further analysis, stratified by birth weight (< 1000 grams or ≥ 1000 grams), revealed that ANCS-exposure was associated with below normal FEV1/FVC only among those with birth weight < 1000 grams (OR = 4.5; CI: 1.32, 15.36, p=0.02).
ANCS was not associated with history of asthma in the unadjusted analysis as well as analyses that adjusted for group differences in race, postnatal dexamethasone exposure, current height, or birth weight. In contrast to history of asthma, the odds of wheezing in the last 12 months was higher in the ANCS-exposed group (OR=1.90; CI: 0.89, 4.05, p=0.095). This association was accentuated with adjustment for postnatal dexamethasone exposure (adjusted OR = 2.06; CI: 0.96, 4.42, p=0.065). Analysis stratified by birth weight revealed that ANCS was associated with wheeze only in the higher birth weight group (1000–1500 g) (OR= 2.6; CI: 0.98, 6.88, p=.054).
Discussion
Based on improvements in survival and short-term morbidity, ANCS has become standard practice for preterm labor and conducting a randomized controlled trial of ANCS would be unethical. With nearly 80% of VLBW infants currently exposed,2 our sample provided us with the unique opportunity to examine the long-term effects of ANCS exposure in a cohort with a 50% exposure rate, primarily due to year of birth relative to the NIH consensus conference promoting ANCS use.22 Our results suggest that ANCS exposure is not associated with improved pulmonary outcomes in adolescents born prematurely with VLBW. The lack of long-term benefits of ANCS on pulmonary function has been described previously in persons born prior to the development of exogenous surfactant as a treatment,5–9 but ours is the first to report no benefit in a cohort born in the era of surfactant therapy. Our finding that ANCS exposure in ELBW adolescents was associated with greater odds of airway obstruction (FEV1/FVC < 5th percentile) was somewhat unexpected, but most likely reflects the positive effects of ANCS on survival in ELBW infants.10,11
Although no association between ANCS exposure and history of asthma was evident in our study, we found higher odds of wheeze in the last 12 months in higher birth weight ANCS- exposed adolescents compared to their unexposed peers. Palta et al.12 likewise reported no association between ANCS-exposure and history of asthma; however, unlike our study, they found lower odds (OR=0.56, 95% CI: 0.29, 1.10) of wheezing in the last 12 months in ANCS-exposed 8 year-old VLBW children. In contrast, Hung et al.13 reported lower odds (OR=0.38, 95% CI: 0.15, 0.97) of asthma in ANCS-exposed v. unexposed 2–8 year-old VLBW children, but they excluded children who had BPD. The inconsistencies between findings may reflect possible recall error and bias associated with questionnaires, as well as differences in study samples. For example, over 60% of our sample received surfactant therapy compared to only 30% in the study by Palta et al.12 and 11% in the study by Hung et al.13 History of asthma was also higher in our study (38%) compared to only 19%12 and 11%13 in the two previous studies. Furthermore, our participants were older at follow-up (14 vs. ≤ 8 years old) and perhaps the effects of ANCS exposure on pulmonary outcomes change with growth and maturation.
Irrespective of ANCS exposure and its effects, the overall high prevalence of asthma and below normal larger airway function is concerning and may reflect the high rates of overweight and obesity in our sample.23 The positive bronchodilator response observed in about 1/3 of participants with below normal FEV1/FVC suggests that the airway obstruction may be reversed or at least reduced by pharmacotherapy, and lack of a positive response in the laboratory does not preclude the possibility of a clinical response to therapy.19 It is also possible that a reduced FEV1/FVC may reflect abnormal airway development associated with both prenatal and postnatal factors (eg. ANCS, mechanical ventilation, impaired growth).19
Animal studies suggest that ANCS promotes fetal lung maturation by inducing septation and thinning of alveolar walls, resulting in larger but fewer alveoli.24 In humans, alveolarization may be assessed by plethysmography combined with newer technologies such ashelium-3 magnetic resonance which suggests that alveolarization continues into childhood and adolescence.25 Although previous studies revealed no differences in lung volumes, flow rates and/or mechanics,5–9 these measurements as well as diffusion capacity are more accessible and would have provided insight into the long-term effects of ANCS on other measures of lung function and pulmonary gas exchange.
There are several other limitations with this study. Although we had access to data regarding ANCS exposure for our participants, maternal medical records with information regarding the amount and frequency of dose, and timing of exposure relative to birth was not available on all of the participants. Neonatal and current characteristics for ANCS-exposed offspring of women with and without detailed exposure information did not differ, and all women were treated by the obstetrical staff at one hospital. Additionally, a greater number of participants in the unexposed group did not do or gave unreliable efforts for pulmonary function testing which may have affected the results and limit representativeness. There are a number of genetic, epigenetic, and environmental factors (e.g. family history of asthma, air pollution, etc.) which may affect pulmonary outcomes and were not assessed or adjusted for in the current study.20 Finally, due to limited funding and time, we were only able to recruit and evaluate about 40% of the eligible sample. Nonetheless, other than slightly lower gestational age, the neonatal characteristics of those recruited were similar to the overall cohort.
While the benefits of ANCS for improving survival and short-term morbidity in preterm infants are well-documented, there is little evidence of its long-term benefits among adolescent VLBW survivors. The higher odds of wheeze in the last 12 months may simply reflect recall error or bias, but it is possible that the effects of ANCS exposure become more evident with growth and maturation. Others have reported adverse effects of ANCS-exposure on cardiometabolic outcomes in adolescents and young adults,26–29 thus warranting continued research in VLBW survivors beyond infancy and childhood. Furthermore, the high prevalence of asthma and below normal airway function observed in this cohort indicates the need for closer surveillance and clinical evaluation in this population as they mature.
Acknowledgments
We wish to acknowledge Ms. Alice Scott, RN, for her hard work in recruiting participants and coordinating the project, Ms. Patty Brown, RN, for her assistance in recruiting participants, the personnel of the Clinical Research Unit for their support with data collection and processing, and the participants and their families without whom this research would not be possible.
This research was funded by NICHD PO1 HD047584, the Clinical Research Unit of Wake Forest Baptist Medical Center grant MO1-RR07122, Forsyth Medical Center, and the Wake Forest School of Medicine Department of Pediatric Research Funds. The sponsors had no involvement in the design, collection, analysis, or interpretation of data, the writing of the manuscript, or the decision to submit the paper for publication.
Footnotes
CONFLICT OF INTEREST
The authors, Patricia A. Nixon, Lisa K. Washburn, and T. Michael O’Shea, declare no conflicts of interest.
References
- 1.Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary Data for 2010. Hyattsville, MD: National Center for Health Statistics; 2011. [PubMed] [Google Scholar]
- 2.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196:147.e1–8. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 3.ACOG Committee Opinion No. 475: Antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2011;117:422–424. doi: 10.1097/AOG.0b013e31820eee00. [DOI] [PubMed] [Google Scholar]
- 4.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3:CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Doyle LW, Ford GW, Rickards AL, Kelly EA, Davis NM, Callanan C, et al. Antenatal corticosteroids and outcome at 14 years of age in children with birth weight less than 1501 grams. Pediatrics. 2000;106:E2. doi: 10.1542/peds.106.1.e2. [DOI] [PubMed] [Google Scholar]
- 6.Dalziel SR, Rea HH, Walker NK, Parag V, Mantell C, Rodgers A, et al. Long term effects of antenatal betamethasone on lung function: 30 year follow up of a randomised controlled trial. Thorax. 2006;61:678–683. doi: 10.1136/thx.2005.051763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiebicke W, Poynter A, Chernick V. Normal lung growth following antenatal dexamethasone treatment for respiratory distress syndrome. Pediatr Pulmonol. 1988;5:27–30. doi: 10.1002/ppul.1950050107. [DOI] [PubMed] [Google Scholar]
- 8.Smolders-de Haas H, Neuvel J, Schmand B, Treffers PE, Koppe JG, Hoeks J. Physical development and medical history of children who were treated antenatally with corticosteroids to prevent respiratory distress syndrome: a 10- to 12-year follow-up. Pediatrics. 1990;86:65–70. [PubMed] [Google Scholar]
- 9.Wong YC, Beardsmore CS, Silverman M. Antenatal dexamethasone and subsequent lung growth. Arch Dis Child. 1982;57:536–538. doi: 10.1136/adc.57.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Philip AG. Neonatal mortality rate: is further improvement possible? J Pediatr. 1995;126:427–433. doi: 10.1016/s0022-3476(95)70463-9. [DOI] [PubMed] [Google Scholar]
- 11.Horbar JD, Wright EC, Onstad L. Decreasing mortality associated with the introduction of surfactant therapy: an observational study of neonates weighing 601 to 1300 grams at birth. The Members of the National Institute of Child Health and Human Development Neonatal Research Network. Pediatrics. 1993;92:191–196. [PubMed] [Google Scholar]
- 12.Palta M, Sadek-Badawi M, Sheehy M, Albanese A, Weinstein M, McGuinness G, et al. Respiratory symptoms at age 8 years in a cohort of very low birth weight children. Am J Epidemiol. 2001;154:521–529. doi: 10.1093/aje/154.6.521. [DOI] [PubMed] [Google Scholar]
- 13.Hung Y-L, Hsieh W-S, Chou H-C, Yang Y-H, Chen C-Y, Tsao P-N. Antenatal steroid treatment reduces childhood asthma risk in very low birth weight infants without bronchopulmonary dysplasia. J Perinat Med. 2010;38:95–102. doi: 10.1515/jpm.2010.002. [DOI] [PubMed] [Google Scholar]
- 14.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 15.Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–491. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 16.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 17.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 18.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 19.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 20.Blumenthal MN. Genetic, epigenetic, and environmental factors in asthma and allergy. Ann Allergy Asthma Immunol. 2012;108:69–73. doi: 10.1016/j.anai.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Shennan AT, Dunn MS, Ohlsson A, Lennox K, Hoskins EM. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics. 1988;82:527–532. [PubMed] [Google Scholar]
- 22.Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995;273:413–418. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 23.Lu FL, Hsieh C-J, Caffrey JL, Lin M-H, Lin Y-S, Lin C-C, et al. Body mass index may modify asthma prevalence among low-birth-weight children. Am J Epidemiol. 2012;176:32–42. doi: 10.1093/aje/kwr484. [DOI] [PubMed] [Google Scholar]
- 24.Vyas J, Kotecha S. Effects of antenatal and postnatal corticosteroids on the preterm lung. Arch Dis Child Fetal Neonatal Ed. 1997;77:F147–150. doi: 10.1136/fn.77.2.f147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narayanan M, Owers-Bradley J, Beardsmore CS, Mada M, Ball I, Garipov R, et al. Alveolarization continues during childhood and adolescence: new evidence from helium-3 magnetic resonance. Am J Respir Crit Care Med. 2012;185:186–191. doi: 10.1164/rccm.201107-1348OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doyle LW, Ford GW, Davis NM, Callanan C. Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clin Sci. 2000;98:137–142. [PubMed] [Google Scholar]
- 27.Finken MJJ, Meulenbelt I, Dekker FW, Frolich M, Walther FJ, Romijn JA, et al. Abdominal fat accumulation in adults born preterm exposed antenatally to maternal glucocorticoid treatment is dependent on glucocorticoid receptor gene variation. J Clin Endocrinol Metab. 2011;96:E1650–1655. doi: 10.1210/jc.2011-0288. [DOI] [PubMed] [Google Scholar]
- 28.Kelly BA, Lewandowski AJ, Worton SA, Davis EF, Lazdam M, Francis J, et al. Antenatal glucocorticoid exposure and long-term alterations in aortic function and glucose metabolism. Pediatrics. 2012;129:e1282–1290. doi: 10.1542/peds.2011-3175. [DOI] [PubMed] [Google Scholar]
- 29.Dalziel SR, Walker NK, Parag V, Mantell C, Rea HH, Rodgers A, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365:1856–1862. doi: 10.1016/S0140-6736(05)66617-2. [DOI] [PubMed] [Google Scholar]