Abstract
Breast cancer is predominantly a disease of older women, yet there is a knowledge gap due to the persisting misalignment between the age distribution of women with breast cancer and the age distribution of participants in clinical trials. The purpose of this report is to state the U13 conference breast cancer panel’s recommendations regarding therapeutic clinical trials that will fill gaps in knowledge regarding the care of older patients with breast cancer. The U13 conference was a collaboration between the Cancer and Aging Research Group and the National Institute on Aging and the National Cancer Institute (NCI). Clinical trials should be developed for frail and vulnerable patients who would not enroll on the standard phase III trials, as well as efforts need to be made to increase enrollment of fit older patients on standard phase III trials. As a result of this conference, panel members are working with the NCI and cooperative groups to address these knowledge gaps. With the aging population and increasing incidence of breast cancer with age, it is essential to study the feasibility, toxicity, and efficacy of cancer therapy in this at-risk population.
Keywords: Breast cancer, Aging, Elderly
Introduction
In the USA, over 200,000 women are diagnosed with breast cancer each year [1]. Breast cancer incidence and mortality rates increase with age and nearly one half of all new breast cancer diagnoses occur in women age 65 and older [2]. By 2030, it is estimated that 20 % of the population in the USA will be older than 65 years of age resulting in an exponential growth of older women with breast cancer [3]. National treatment guidelines do not set an upper age limit for the use of chemotherapy, but acknowledge that comorbid medical conditions and life expectancy must be considered when prescribing chemotherapy [4]. However, many comorbid medical conditions in older patients are well controlled and treated [5, 6]. Subsequently, the average life expectancy is increasing, and as a result, the risk of breast cancer relapse during the remaining lifespan becomes an important consideration. On average, 65- and 75-year-old patients have an anticipated life expectancy of 20 and 12 years, respectively [7]. Furthermore, the number and proportion of people at very old ages is increasing (Fig. 1).
Fig. 1.
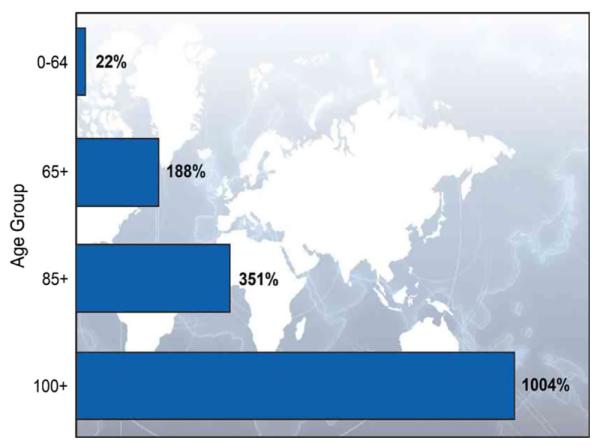
Percentage Change in the World’s Population by Age: 2010–2050. United Nations, World Population Prospects: The 2010 Revision. Available at: http://esa.un.org/unpd/wpp
Chemotherapy has been shown to improve breast cancer-related health outcomes, including survival, in women with breast cancer [8]. Women aged 50–65 years with early stage breast cancer will achieve a 20 % proportional reduction in the risk of recurrence and an 11 % proportional reduction in the risk of death with adjuvant chemotherapy [8]. Furthermore, a recent clinical trial in women with estrogen receptor-positive breast cancer reported that breast cancer-specific mortality was 25 % higher for women between the ages of 65 and 74 (HR 1.25; 95 % CI 1.01–1.54), and 63 % higher for women 75 years of age or older (HR 1.63; 95 % CI 1.23–2.16), compared with women under the age of 65 (p < 0.001) [9]. The investigators speculated the significant increase in breast cancer-specific mortality in older women was potentially secondary to difference in age-related treatment patterns, with older adults less likely to receive standard treatments. In particular, only 5.2 % of patients aged 75 and over received adjuvant chemotherapy despite 48 % of these patients having node positive disease.
The lack of clinical trial data in older women with breast cancer and the growing number of older women with breast cancer are a significant challenge to medical oncologists, not only because of the increasing numbers, but also because of physiologic changes due to aging, which may increase the risk of treatment toxicity and compromise the ability to deliver therapy [10]. To compound this problem, less evidence-based data are available to guide the care of the growing number of older women with breast cancer as older patients are disproportionately underrepresented in breast cancer clinical trials [11]. To bridge this knowledge gap, a U13 conference grant (U13 AG038151), “Geriatric Oncology Research to Improve Clinical Care,” a cooperative conference grant between the Cancer and Aging Research Group in collaboration with the Geriatrics and Clinical Gerontology branch of the National Institute on Aging (NIA) and the National Cancer Institute (NCI) was formed.
The U13 conference, “Design and Implementation of Therapeutic Clinical Trials for Older and/or Frail Adults with Cancer,” brought together multidisciplinary investigators from geriatrics and oncology to identify and address the areas of highest research priorities in cancer and aging and therapeutic clinical trials for older and/or frail adults with cancer. Here, we report the U13 conference breast cancer panel’s recommendations regarding therapeutic clinical trials that will fill gaps in knowledge regarding the care of older patients with breast cancer.
Breast cancer and aging: treatment in the adjuvant setting
Age is no longer a valid eligibility criterion in and of itself, and the majority of the NCI’s clinical trial cooperative groups no longer specify an upper age limit. Data suggest that older patients who enroll in clinical trials tolerate the standard chemotherapy regimens, and even intensive regimens, although older adults are at increased risk for treatment toxicity [12, 13]. In addition, data demonstrate a significant survival benefit for standard chemotherapy regimens in healthy older patients that meet stringent eligibility criteria for these trials [13].
Age bias plays a major role in offering clinical trials to patients, even in major cooperative group institutions [11]. In a review of patient accrual to three breast cancer adjuvant chemotherapy trials in the Cancer and Leukemia Group B (CALGB), none of which had an upper age limit that excluded older women, only 8 % of patients were older than 65 years, and only 4 % were older than age 70 [11]. Interestingly, data support similar willingness to enroll in clinical trials when research studies are offered to both older and younger patients; however, older adults were less likely to be offered clinical trial participation [11]. While data have shown that standard chemotherapy regimens improve treatment outcomes in older patients with breast cancer, the potential for increased chemotherapy-related toxic effects is an important concern. For example, renal function and bone marrow reserve decrease with age and can increase the risk of toxic effects from treatment regimens that include myelosuppressive agents or agents that are renally excreted [14]. Hence, there is a need to identify the frail older individual who is apt to experience undue side effects and requires a modified treatment plan as well as the fit older individual likely to benefit from and tolerate standard therapy.
To fill this need and bridge the knowledge gap of the toxicity and feasibility of established adjuvant therapies in older breast cancer patients, the U13 breast cancer panel recommended designing therapeutic clinical trials of established adjuvant chemotherapy regimens for older women with breast cancer. These studies would incorporate a comprehensive geriatric assessment in order to identify the factors other than age for increased risk of toxicity from therapy, in order to guide future interventions to decrease this risk. A geriatric assessment evaluates functional status, comorbidity, cognition, psychologic state, social support, and nutritional status, which are independent predictors of the risk of morbidity/mortality in older adults (Fig. 3) [15, 16].
Fig. 3.
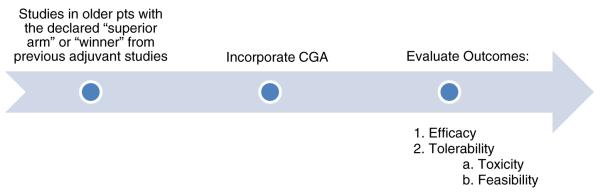
Recommendations to bridge the knowledge gap in older women with breast cancer in the adjuvant setting
An example of this approach would be studying dose-dense therapy, as described in the Intergroup Trial C9741, which has been widely adopted in the USA for the adjuvant treatment of patients with node positive breast cancer [17]. This prospective clinical trial had 4 arms and tested two hypotheses (Fig. 2). The first hypothesis was that the efficacy of adjuvant chemotherapy may be improved with sequential drug administration, not combination drug administration. The second hypothesis was that the efficacy of adjuvant chemotherapy may be improved by increasing the dose density of the regimen by delivering standard-dose chemotherapy with shorter intervals (2 rather than 3 weeks) between treatment cycles. At a median follow-up of 36 months, dose-dense treatment improved the primary end points, disease-free survival (DFS) [risk ratio (RR) = 0.74; p = 0.010], and overall survival (OS) [RR 0.69; p = 0.013]. There was no difference in either DFS or OS between the concurrent and sequential treatment schedules. Subsequently, dose-dense adjuvant chemotherapy has become an internationally recognized option for patients with high-risk breast cancer (Fig. 3).
Fig. 2.
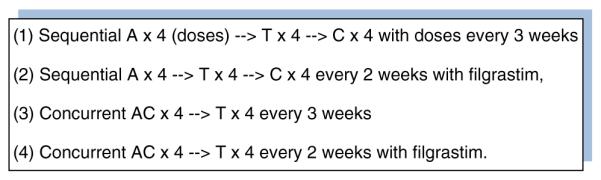
The treatment arms of the Intergroup Trial C9741/cancer and leukemia group B Trial 974117
The fact that sequential versus combination therapy yields comparable outcomes provides a scientific basis for studying the efficacy of other therapies sequentially in a potentially curative manner. Furthermore, the dose and number of chemotherapy drugs used are risk factors for older adults with cancer in developing grade 3–5 toxicities [18]. Therefore, sequential therapy may be better tolerated in the older patients, allowing the ability to deliver optimal dose intensity. Unfortunately, only two percent of patients enrolled in Intergroup Trial C9741 were aged 70 or older, and geriatric assessments were not performed. Therefore, the U13 conference breast cancer panel proposes a dose-dense sequential chemotherapy as used in C9741 for older patients with breast cancer who are at a high risk of recurrence to evaluate the feasibility of completing this adjuvant chemotherapy regimen, as well as to identify the risk factors for toxicity. This study may be particularly useful in a frail or vulnerable population who is deemed to be at high risk of side effects from combination chemotherapy. Incorporation of a geriatric assessment pretreatment would be utilized to understand factors other than (or in addition to) chronological age, which place patients at increased risk for treatment toxicity. Incorporation of geriatric assessment in longitudinal follow-up would be utilized to identify which patients are at risk for short- or long-term declines in physical function, as well as to evaluate the impact of treatment toxicity on these parameters.
With solid evidence showing that older women receiving anthracycline-based adjuvant chemotherapy have higher rates of congestive heart failure after completing treatment, a second recommendation for a therapeutic adjuvant clinical trial for older patients with breast cancer is to evaluate a nonanthracycline chemotherapy regimen in patients with a high risk of breast cancer recurrence, utilizing the same framework as described above [19]. As well as confirming breast cancer outcomes with an established nonanthracycline chemotherapy regimen, investigators are encouraged to incorporate a geriatric assessment to evaluate factors other than or in addition to age that might identify those individuals who are most likely to tolerate the cytotoxic regimen or which patient need additional support.
These suggested research studies are examples of designing therapeutic clinical trials integrating geriatric and oncology principles. These studies could fill critical gaps in the knowledge of adjuvant chemotherapy feasibility, tolerability, and toxicity in older patients with breast cancer.
Breast cancer and aging: treatment in metastatic setting
Despite the compelling demographics of cancer and aging and the recommendations of expert societies, the enrollment of older adults in registration trials remains dismal. (REF) Since 2007, six drugs have been approved by the US food and Drug Administration for the treatment of breast cancer (Table 1). According to the geriatric usage sections of the drug package inserts, on average, only 20 % of patients included in the registration trials were aged 65 years or older and 4 % of patients included were aged 75 years or older. As approximately half of all breast cancer diagnoses occur in women over the age of 60, the patients enrolled in the registration trials are likely not representative of the patient population where these agents will be prescribed.
Table 1.
FDA approved agents in breast cancer since 2007
| Agents | Date approved |
Indication | Age |
|
|---|---|---|---|---|
| >65 (%) | >75 (%) | |||
| Ixabepilone [22] | 10/2007 | Combination with capecitabine in metastatic or locally advanced breast cancer resistant to treatment with an anthracycline and a taxane |
10 | <1 |
| 10/2007 | Monotherapy in metastatic or locally advanced breast cancer resistant to anthracyclines, taxanes, and capecitabine |
13 | 3 | |
| Eribulin [23] | 11/2010 | Third-line treatment metastatic breast cancer | 15 | 2 |
| Everolimus [21] | 7/2012 | Advanced hormone receptor-positive, HER2-negative breast cancer in combination with exemestane, after failure of treatment with letrozole or anastrozole |
40 | 15 |
| Ado-trastuzumab emtansine [24] |
2/2013 | HER2-positive breast cancer following treatment with trastuzumab and a taxane |
13 | 2 |
| Pertuzumab [25] | 6/2012 | Combination with trastuzumab and docetaxel in HER2-positive metastatic breast cancer who have not received prior anti-HER2 therapy |
15 | 1 |
| Lapatinib [26, 27] | 1/2010 | Combination with capecitabine in metastatic HER2-positive breast cancer |
17 | 1 |
| Combination with letrozole in metastatic HER2-positive breast cancer | 44 | 12 | ||
You should organize the agents alphabetically or by the date of approval
The study was supported by the U13 conference grant (U13 AG038151)
The study sponsors were not involved in the writing of the article or the decision to submit it for publication. The authors were independent from study sponsors
Ixabepilone in combination with capecitabine was granted FDA approval based on a statistically significant improvement in median progression-free survival of 5.7 versus 4.1 months (HR 0.69; 95 % CI, 0.58–0.83; p < 0.001) with the combination of ixabepilone plus capecitabine compared with capecitabine monotherapy, in the treatment of patients with metastatic breast cancer who progress after an anthracycline and taxane [20]. Ten percent of the enrolled patients were aged 65 and older and <1 % of patients enrolled were aged 75 and older. The overall incidence of grade 3 and 4 adverse events were significantly higher in patients over age 65, compared with patients under age 65 (82 vs. 68 %) including grade 3 and 4 stomatitis (9 vs. 1 %), febrile neutropenia (9 vs. 3 %), and fatigue (16 vs. 12 %) [20].
In the randomized study of patients with advanced hormone receptor-positive, HER2-negative breast cancer study, treated with exemestane ± everolimus, the incidence of deaths due to any cause within 28 days of the last everolimus dose was 6 % in patients over 65 years of age compared with 2 % in patients under 65 years of age [21]. Adverse reactions leading to permanent treatment discontinuation occurred in 33 % of patients over 65 years of age compared with 17 % in patients under 65 years of age.
The U13 breast cancer panel recommended performing a series of phase II studies in recently FDA approved drugs in older women with metastatic breast cancer. These studies should incorporate a geriatric assessment to identify predictors of toxicity based on geriatric variables and evaluate tolerability (toxicity and feasibility). If there is a concern regarding the potential toxicity of the therapy in older adults, then clinical trial schemes can be modified to build in additional safety criteria. For example, the study could include an initial dose reductions followed by dose escalations to standard doses in older patients that tolerate therapy. This approach would be favored in patients in metastatic disease where a key goal was palliation and maintenance of quality of life. It would not be recommended in the adjuvant setting where decreased dose intensity could impact the curative intent of the regimen.
The biggest challenge to fill the knowledge gaps on treatment of metastatic breast cancer is to get an adequate representation of older adults on clinical trials. One way of accomplishing this is to prespecify the inclusion of a specific proportion of older patients (which mirrors the percentage of individuals within the age group with the disease) in the study design. Alternatively, if there is concern that this would slow the accrual to clinical trials, then the study could be expanded postcompletion to obtain an adequate number of older adults in order to fill the gap in knowledge regarding the efficacy and toxicity of the treatment in this patient population.
Conclusion
Breast cancer is predominantly a disease of older women, yet there is a knowledge gap due to the persisting misalignment between the age distribution of women with breast cancer and the age distribution of participants in clinical trials. To address these gaps, future clinical research for older patients with breast cancer should include a geriatric assessment that can be utilized to identify individuals who may be at risk for treatment-related side effects, which require a modified treatment plan. Trials should be developed for frail and vulnerable patients who would not enroll on the standard phase III trials, as well as efforts need to be made to increase enrollment of fit older patients on standard phase III trials. Ultimately, enhanced research collaborations between oncologists, geriatricians, and a multidisciplinary team are needed to address issues pertinent to older patients. As a result of this conference, panel members are working with the NCI and cooperative groups to address these knowledge gaps.
Acknowledgments
The study was supported by the U13 conference grant (U13 AG038151). The study sponsors were not involved in the writing of the article or the decision to submit it for publication. The authors were independent from study sponsors.
Contributor Information
M. F. Barginear, Hurria Hofstra-North Shore LIJ School of Medicine, North Shore-LIJ Cancer Institute, 450 Lakeville Road, Lake Success, NY 11042, USA
H. Muss, Hurria Hofstra-North Shore LIJ School of Medicine, North Shore-LIJ Cancer Institute, 450 Lakeville Road, Lake Success, NY 11042, USA; UNC Lineberger Comprehensive Cancer Center, Chapel Hill, NC, USA
G. Kimmick, Hurria Hofstra-North Shore LIJ School of Medicine, North Shore-LIJ Cancer Institute, 450 Lakeville Road, Lake Success, NY 11042, USA; Duke University Medical Center, Durham, NC, USA
C. Owusu, Hurria Hofstra-North Shore LIJ School of Medicine, North Shore-LIJ Cancer Institute, 450 Lakeville Road, Lake Success, NY 11042, USA; Case Western Reserve University School of Medicine, Cleveland, Ohio, USA
E. Mrozek, Hurria Hofstra-North Shore LIJ School of Medicine, North Shore-LIJ Cancer Institute, 450 Lakeville Road, Lake Success, NY 11042, USA; The Ohio State University, Columbus, OH, USA
A. Shahrokni, Hurria Hofstra-North Shore LIJ School of Medicine, North Shore-LIJ Cancer Institute, 450 Lakeville Road, Lake Success, NY 11042, USA; Memorial Sloan Kettering Cancer Center, NY, USA
K. Ballman, Hurria Hofstra-North Shore LIJ School of Medicine, North Shore-LIJ Cancer Institute, 450 Lakeville Road, Lake Success, NY 11042, USA; Mayo Clinic, Rochester, MN, USA
A. Hurria, Hurria Hofstra-North Shore LIJ School of Medicine, North Shore-LIJ Cancer Institute, 450 Lakeville Road, Lake Success, NY 11042, USA; City of Hope Comprehensive Cancer Center, Duarte, CA, USA
References
- 1.Desantis C, Siegel R, Bandi P, et al. Breast cancer statistics. CA Cancer J Clin. 2011;61:409–418. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 3.Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 4.McDermott AM, Toelle TR, Rowbotham DJ, et al. The burden of neuropathic pain: results from a cross-sectional survey. Eur J Pain. 2006;10:127–135. doi: 10.1016/j.ejpain.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 5.McDonald M, Hertz RP, Unger AN, et al. Prevalence, awareness, and management of hypertension, dyslipidemia, and diabetes among United States adults aged 65 and older. J Gerontol A Biol Sci Med Sci. 2009;64:256–263. doi: 10.1093/gerona/gln016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owusu C, Hurria A, Muss H. Adjuvant therapy for older women with early-stage breast cancer: treatment selection in a complex population. Am Soc Clin Oncol Educ Book. 2012;32:3–9. doi: 10.14694/EdBook_AM.2012.32.69. [DOI] [PubMed] [Google Scholar]
- 7.Minino AM, Murphy SL. Death in the United States, 2010. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- 8.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 9.van de Water W, Markopoulos C, van de Velde CJ, et al. Association between age at diagnosis and disease-specific mortality among postmenopausal women with hormone receptor-positive breast cancer. JAMA. 2012;307:590–597. doi: 10.1001/jama.2012.84. [DOI] [PubMed] [Google Scholar]
- 10.Lichtman SM, Wildiers H, Chatelut E, et al. International Society of Geriatric Oncology Chemotherapy Taskforce: evaluation of chemotherapy in older patients–an analysis of the medical literature. J Clin Oncol. 2007;25:1832–1843. doi: 10.1200/JCO.2007.10.6583. [DOI] [PubMed] [Google Scholar]
- 11.Kemeny MM, Peterson BL, Kornblith AB, et al. Barriers to clinical trial participation by older women with breast cancer. J Clin Oncol. 2003;21:2268–2275. doi: 10.1200/JCO.2003.09.124. [DOI] [PubMed] [Google Scholar]
- 12.Kimmick GG, Muss HB. Systemic therapy for older women with breast cancer. Oncology (Williston Park) 2001;15:280–291. discussion 291–292, 295–296, 299. [PubMed] [Google Scholar]
- 13.Muss HB, Woolf S, Berry D, et al. Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA. 2005;293:1073–1081. doi: 10.1001/jama.293.9.1073. [DOI] [PubMed] [Google Scholar]
- 14.Lichtman SM, Skirvin JA. Pharmacology of antineoplastic agents in older cancer patients. Oncology (Williston Park) 2000;14:1743–1755. discussion 1755, passim. [PubMed] [Google Scholar]
- 15.Ramjaun A, Nassif MO, Krotneva S, et al. Improved targeting of cancer care for older patients: a systematic review of the utility of comprehensive geriatric assessment. J Geriatr Oncol. 2013;4:271–281. doi: 10.1016/j.jgo.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Wildes TM, Ruwe AP, Fournier C, et al. Geriatric assessment is associated with completion of chemotherapy, toxicity, and survival in older adults with cancer. J Geriatr Oncol. 2013;4:227–234. doi: 10.1016/j.jgo.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 18.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinder MC, Duan Z, Goodwin JS, et al. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol. 2007;25:3808–3815. doi: 10.1200/JCO.2006.10.4976. [DOI] [PubMed] [Google Scholar]
- 20.Thomas ES, Gomez HL, Li RK, et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol. 2007;25:5210–5217. doi: 10.1200/JCO.2007.12.6557. [DOI] [PubMed] [Google Scholar]
- 21.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bunnell C, Vahdat L, Schwartzberg L, et al. Phase I/II study of ixabepilone plus capecitabine in anthracycline-pretreated/resistant and taxane-resistant metastatic breast cancer. Clin Breast Cancer. 2008;8:234–241. doi: 10.3816/CBC.2008.n.026. [DOI] [PubMed] [Google Scholar]
- 23.Cortes J, O’Shaughnessy J, Loesch D, et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;377:914–923. doi: 10.1016/S0140-6736(11)60070-6. [DOI] [PubMed] [Google Scholar]
- 24.Blackwell KLMD, Gianni L, et al. Primary results from EMILIA, a phase 3 study of trastuzumab emtansine (T-DM1) vs capecitabine and lapatinib in HER2-positive locally advanced or metastatic breast cancer previously treated with trastuzumab and a taxane. ASCO Annual Meeting; June 3; 2012. 2012. Abstract LBA1. Presented. [Google Scholar]
- 25.Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 27.Schwartzberg LS, Franco SX, Florance A, et al. Lapatinib plus letrozole as first-line therapy for HER-2 + hormone receptor-positive metastatic breast cancer. Oncologist. 2010;15:122–129. doi: 10.1634/theoncologist.2009-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]


