Abstract
We propose pretreatment using microneedles to increase perianal skin permeability for locally targeted delivery of phenylephrine (PE), a drug that increases resting anal sphincter pressure to treat fecal incontinence. Microneedle patches were fabricated by micromolding poly-lactic-acid. Pre-treatment of human cadaver skin with microneedles increased PE delivery across the skin by up to 10-fold in vitro. In vivo delivery was assessed in rats receiving treatment with or without use of microneedles and with or without PE. Resting anal sphincter pressure was then measured over time using water-perfused anorectal manometry. For rats pretreated with microneedles, topical application of 30% PE gel rapidly increased the mean resting anal sphincter pressure from 7 ± 2 cm H2O to a peak value of 43 ± 17 cm H2O after 1 h, which was significantly greater than rats receiving PE gel without microneedle pretreatment. Additional safety studies showed that topically applied green fluorescent protein–expressing E. coli penetrated skin pierced with 23- and 26-gauge hypodermic needles, but E. coli was not detected in skin pretreated with microneedles, which suggests that microneedle-treated skin may not be especially susceptible to infection. In conclusion, this study demonstrates local transdermal delivery of PE to the anal sphincter muscle using microneedles, which may provide a novel treatment for fecal incontinence.
1. Introduction
Fecal incontinence is described as the loss of regular control of hard feces, loose feces, and gases of the bowels in an individual [1]. Incontinence occurs in about 2-18% of the adult population and in about 15% of healthy independent adults over the age of 50 [2, 3]. The frequency of fecal incontinence is considerably higher in patients with neurological problems [4]. For instance, fecal incontinence is the second leading cause of placement in nursing homes in the United States [5, 6]. Individuals with fecal incontinence suffer from an embarrassing condition that causes social isolation and difficulties in employment. In 2004 patients spent US$400 million on adult diapers, and an additional $1.5–7 billion was spent on care for the incontinence of institutionalized patients [6]. Nevertheless, these persons are frequently reluctant to visit a hospital to discuss their symptoms [7, 8].
The most common cause of fecal incontinence appears to be a degenerative disorder of the anal sphincter [9]. Fecal incontinence is also experienced by patients with an ileoanal reservoir [10], and it can also develop after low anterior resection in patients with rectal cancer [11]. Especially in women, fecal incontinence is mainly caused by structural anal sphincter damage associated with childbirth [12, 13]. Damage to the anal sphincter muscle leads to loss of control, causing the urgent fecal incontinence [14].
Treatment of fecal incontinence can be determined and categorized based on its pathogenesis [15]. There are many surgical ways to treat fecal incontinence [16], including sphincteroplasty, postanal repair, total pelvic floor repair, dynamic graciloplasty, and artificial bowel sphinctery. Sphincteroplasty is carried out when the anal sphincter is damaged. An improvement in anal pressure was found in up to 88% of patients in the short term after surgery. However, only 6% of patients experience improvement 10 years after surgical treatment. Postanal repair is a method that recovers bowel movement by decreasing an anorectal angle, but there are no reports of a well-designed study. Dynamic graciloplasty involves transferring the gracilis muscle into the anus, with implantation of stimulating electrodes and a pulse generator. Unfortunately, this surgery often leads to infection, pain, and constipation. An artificial bowel sphinctery is an implantable device used to treat patients with severe fecal incontinence who are not candidates for less-invasive forms of restorative therapy. If this device is inserted improperly, it can cause infection, skin erosion, and pain after the surgery [17, 18].
Drug treatment can be administered in two ways. One is oral administration and the other is topical application of drugs to the anal canal. Oral drugs that control bowel mobility are loperamide, diphenoxylate, difenoxin, and amitriptyline. Loperamide acts directly on the intestine to inhibit peristalsisby controlling muscle contractions in the intestine [19]. Diphenoxylate and difenoxin inhibit intestinal motility and propulsion. Amitriptyline is an antipsychotic drug that affects the functions of the colon, rectum, and anal sphincter and increases colonic transit time, leading to the formation of a firmer stool [20]. However, these drugs have side effects as well. Loperamide is a typical narcotic anti-diarrheal drug and makes stools harder. An overdose of loperamide may result in central nervous system (CNS) depression and paralytic ileus. Diphenoxylate and difenoxin in high doses can cause adverse CNS effects, and the adjunctive atropine may produce constipation. Amitriptyline can cause the common side effects of antipsychotic drugs [21, 22].
Recently, topical delivery of the drug phenylephrine (PE) into the sphincter muscle without surgery has been studied. PE is a selective alpha-1 agonist that causes sphincter contraction in vitro [23, 24] and PE elevates anal resting pressure in animal studies [25]. In current clinical practice, phenylephrine is widely used as a decongestant. The hydrochloride salt form of phenylephrine has been used because of physiological acceptance of the chlorine ion and increased solubility [26]. The hydrochloride salt of phenylephrine has water solubility greater than 1000 mg/ml and a melting point of 144 °C [27]. The internal anal sphincter exists in a state of tonic contraction and is the main factor responsible for the generation of anal resting pressure [28]. When the sphincter becomes fibrotic and weak, maximum anal canal resting pressure is reduced, causing episodes of passive fecal incontinence. In several studies, a high concentration (10% – 30%) of PE gel was applied to the anal canal for delivery of PE into the sphincter muscle [17, 22, 29, 30]. In some cases, topical application of PE gel increased maximum resting anal sphincter pressure [17, 31, 32]. However, in another study, high concentrations (10%–30%) of PE did not significantly increase resting anal pressure [30]. A study of patients who had a low anterior resection found that topical application of PE gel did not help relieve the patients’ fecal incontinence [29].
Transdermal drug delivery is a method of delivering drugs through the skin without injection or oral administration. It is becoming a popular system different from conventional methods. To improve transdermal drug delivery, iontophoresis, ultrasound, and chemical enhancers have been studied, but either require secondary equipment or have slow onset times [33, 34]. Microneedles, in contrast, have been shown to painlessly and quickly pierce the skin’s outer barrier, the stratum corneum, and can enable self-administration [35-37]. In addition, microneedles are inexpensive and disposable [38]. Microneedle patches can deliver a variety of drugs that cannot usually be transferred because of the impermeability of the skin [39]. For example, microneedles can increase transdermal delivery of small-molecule drugs, proteins [40], DNA [41], and vaccines [42, 43]. Previous studies with microneedles have focused primarily on systemic drug delivery via the skin or for vaccination. In this study, we introduce the use of microneedles for locally targeted delivery of drugs to the anal sphincter muscles by piercing holes in the perianal skin.
Because most patients with fecal incontinence are advanced in age, treatments based on drugs are preferred to surgery. However, there is no efficient drug therapy to repair fecal incontinence, which is especially induced by weakness of the anal muscle. We propose the novel treatment of fecal incontinence by topical application of PE on perianal skin pretreated using simple and economical treatment of microneedles. This treatment can carry PE into the local sphincter muscle efficiently with minimum pain and without resistance of the stratum corneum of the perianal skin. We performed the delivery study of PE across the perianal skin by pretreating it with microneedles (Figure 1). The drug delivery property was investigated as a function of the number of needles and concentrations of PE qualitatively and quantitatively. Also, the change in resting anal sphincter pressure of rats was measured in vivo after treatment. Efficiency of various treatments was compared to demonstrate the feasibility of combining microneedle pretreatment with topical application of PE on the perianal skin as a potential treatment of fecal incontinence.
Figure 1.
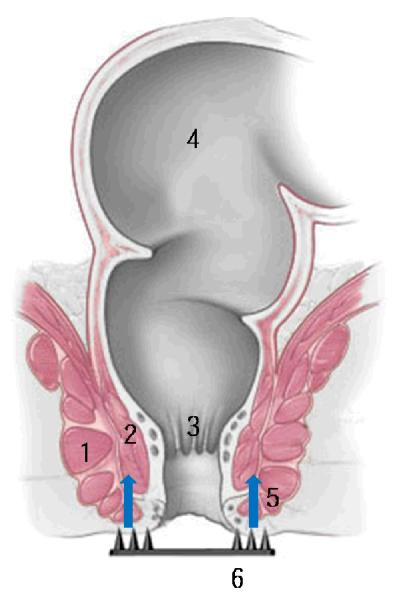
Diagram of locally targeted delivery of PE into anal sphincter muscle through perianal skin using microneedles. Microneedles generate holes on perianal skin to make local delivery pathways for PE to the sphincter muscles. 1. external sphincter muscle. 2. internal sphincter muscle. 3. anal canal. 4. rectum. 5. path of PE delivery. 6. microneedles. (The picture of the anus is copyrighted by the Mayo Foundation for Medical Education and Research and is used with permission.)
2. Materials and Methods
2.1. Fabrication of microneedles for drug delivery to perianal skin
As described previously [44], a female master microneedle mold was prepared using a photolithographic process with SU-8 epoxy photoresist (MicroChem, Newton, MA) and used to prepare a male master structure. Next, a PDMS female mold was prepared from the male master structure using the molding process again.
To make the final microneedle devices, the female molds were covered with pellets of poly-lactic acid (L-PLA, 1.1 dL/g, Lakeshore Biomaterials, AL) and placed in a vacuum oven (Eyeler, Tokyo Rikakikai, Japan) under –70 kPa vacuum for 5 min at 190 °C. PLA microneedles on a PLA solid base were removed from the PDMS mold after the sample cooled. A 6 mm × 6 mm square base on which the array of microneedles were placed was cut into a frame structure using laser cutting equipment (CO2 laser, Femto Science, Korea).
2.2. Anatomy of anus and needle insertion into the perianal skin
All animal experiments were performed according to the guidelines of laboratory animal care of Kangbuk Samsung Hospital and College of Bionano Technology, Kyungwon University. A rat (SD, 250-300 g, female, Charles River Technology, Yokohama, Japan) was placed in the prone position in a rodent restrainer (Universal holder, JEUNGDO Bio & Plant Co., Seoul, Korea) and an array of PLA microneedles were inserted into the perianal skin and then removed. The microneedle arrays used in the in vivo rat experiment were 600 μm tall and mounted on a 250-μm-thick square base.
To investigate the anatomic structure of the perianal skin and confirm the similarity of the stratum corneum of perianal skin with that of skin from the rat’s back, the cross-sectioned perianal skin was investigated after H&E staining. Perianal skin and back skin were obtained after euthanizing the rat with Beuthanasia (390 mg pentobarbital sodium and 50 mg phenytoin sodium per 1 ml, 1 ml/4.5 kg body weight administered intraperitoneally, Schering-Plough Animal Health, Boxmeer, Netherlands). The perianal skin specimen was embedded in Tissue-Tek O.C.T. (Sakura® Finetechnical Co., Tokyo, Japan). A freezing block was filled with Optimal Cutting Temperature (OCT) solution and the freezing block was frozen with liquid nitrogen. The frozen block was sectioned using a cryostat microtome (Microm HM 550P, Thermo Scientific, Wilmington, MA), and the section of the block was investigated to define the calcein diffusion profile (see below) in the rat anus using a fluorescence microscope (Eclipse 80i, Nikon, Tokyo, Japan).
Slides containing 30-μm-thick cryo-sections were placed in a slide holder and dehydrated twice, first in pure ethanol for 2 min and then in 95% ethanol for 2 min. The samples were stained in Mayer’s hematoxylin solution (Sigma-Aldrich, Pittsburgh, PA) for 15 min, washed in running tap water for 15 min, and then washed briefly in distilled water. Slides were counterstained in eosin-Y solution (Sigma-Aldrich) for 45 s and then dehydrated first with 95% ethanol for 2 min and then with pure ethanol for 2 min. Then the samples were cleared in xylene for 2 min and checked under a microscope.
2.3. Drug delivery property across the perianal skin
2.3.1. Qualitative in vitro model of formation of pathway across the perianal skin using microneedles
To view disruption of the skin barrier, the in vitro perianal skin from a rat was placed on 6 layers of tissue paper (Kimwipe). The polymeric microneedles were pressed into the skin with a force of 9.8 N force and then removed. A blue hydrophobic dye (Trypan blue solution (0.4 %), Sigma Chemical Corp.) was applied on the top of perianal skin. After 5 min, the blue dye was removed and the skin surface was patted dry with a wipe. The top side of the skin was viewed by light microscopy (Olympus SZX12; Melville, NY) to determine if dye stained holes made in the skin by the microneedles.
A 10 mM calcein solution (Sigma-Aldrich) was applied to the in vitro perianal skin of the rat after insertion and immediate removal of the microneedles, and was left for 15, 30, and 60 min to visually observe the diffusion profile of calcein across the perianal skin as a function of time. Samples were sectioned using a cryostat microtome (Microm HM 550P, Thermo Scientific, MA) and the sections of the block were investigated to define the calcein diffusion profile in the rat anus using a fluorescence microscope (Eclipse 80i, Nikon). As a negative control, a calcein solution with the same concentration was applied on a non-treated perianal skin for 60 min, and the fluorescence image of calcein in perianal skin was compared with the treated samples.
2.3.2. Quantitative in vitro model of delivery of phenylephrine into full thickness human cadaver skin using microneedles
An array of microneedles was applied to human cadaver skin (back, 60 year old female, Hans Biomed Co., Seoul, Korea) with 9.8 N of force and then removed. The applied force was measured using a push-pull gauge (Digital force gauge, BYTECC, Seoul, Korea. The apex end of the plastic pipette tip body with a 7.2 mm-diameter inner base (1 ml, Molecular BioProducts, San Deiego, CA) was cut and glued to skin by epoxy adhesive (Hunstman, Seoul, Korea) as a drug solution donor chamber after skin treatment with 0, 50 and 100 microneedles. A total of 0.1 ml of 0%, 10%, and 30% (w/w) concentrations of PE gel were put in the donor chamber and stored at 32 °C in an incubator (BF-25B, BiOFREE Co., Seoul, Korea) for 3 h [45]. The hydrochloride salt form of phenylephrine was used. A gel with 30% PE was prepared by mixing 30% of PE, 45% of petrolatumsferty yellow, 25% of distilled water, and carbopol® 940, and a gel with 10% PE was prepared by mixing 10% of PE, 65% of petrolatum yellow, and 25% of distilled water. A placebo gel containing no PE was prepared by mixing 75% of petrolatum yellow and 25% of distilled water. All gels were supplied in identical coded foil tubes by Il-Dong Pharm (Seoul, Korea).
After treatment, the full thickness skin was cut into pieces and frozen in liquid nitrogen. A frozen skin sample was pulverized into particles of a few hundred microns diameter using a tissue pulverizer (Shinko Seiki, Fukuoka, Japan), and the particles were put in a solution of 50% distilled water and 50% methanol (Sigma-Aldrich) for 12 h at room temperature to extract PE from the skin. The concentration of extracted PE was quantified by high performance liquid chromatography (HPLC, Agilent 1200, Agilent Technology, Santa Clara, CA). Twenty microliters of the supernatant in the extracted solution was separated by a 3.9 mm × 300 mm RP-Lichrosorb® C18 (Agilent) and the mobile phase consisting of 70% methanol and 30% of 20 mM phosphate buffer at a flow rate of 1.2 ml/min. Detection was carried out at 269 nm wavelength. The retention time for PE was about 3 min. The amount of PE was determined by measuring its peak height and comparing it with a calibration curve. As positive controls, 5 mg and 10 mg of PE solution (10%) were injected into full-thickness cadaver skin, and concentrations measured by HPLC after the same extraction method were compared.
2.4. In vivo measurement of rat resting anal sphincter pressure
Resting anal sphincter pressure in each subject was measured using a four-channel water-perfused anorectal manometry system (Arndorfer, Inc., Greendale, WI, USA) (Figure 2). A 3 mm-diameter manometric catheter with four channels was fabricated from polyvinylchloride (PVC) tubing for measuring rodent resting anal pressure (Mui Scientific, Ontario, Canada). A four-channel hydraulic capillary infusion system was used with four pressure transducers (Medex Inc., Hilliard, Ohio). The catheter was left in-situ to establish a stable pressure. Mechanical pressure was transmitted to a PC polygraph HR (Synnetics Medical Inc., Irving, TX) via pressure transducers (Medex Inc., Hilliard, Ohio), and the resulting electrical pulses were displayed on an IBM-compatible computer using a Polygram 2.0 for Windows anorectal manometry analysis module (Medtronics Inc, Minneapolis, MN). The catheter was inserted to place holes of four channels on the external anus of the rats, and each catheter was perfused with distilled, sterile water at a constant pressure of 7 psi. Four tubes were used to record pressure, and the pressure catheters were radially oriented at a 90° angle and were arranged to measure anal sphincter pressure. The measurements were repeated with six rats, and the mean pressures over the high pressure zone were calculated.
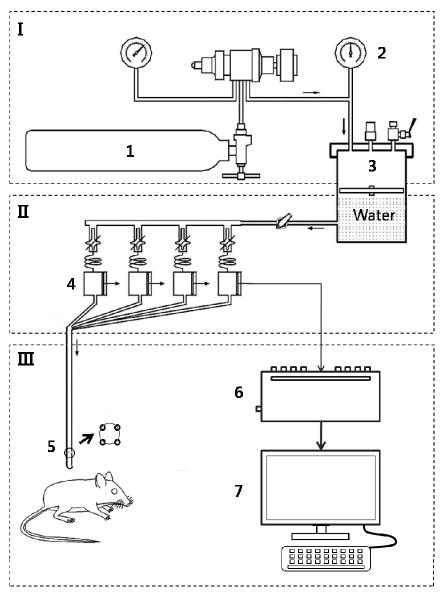
Figure 2.
Diagram of measurement of resting anal sphincter pressure using four-channel water perfused anorectal manometry system with catheter for rat anus. I. gas system: 1. nitrogen tank, 2. pressure controller. II. liquid system: 3. pressure pump, 4. pressure sensor. III. measurement and signal process: 5. catheter, 6. transducer, 7. computer.
The change in resting anal sphincter pressure of the rats was compared among five rat groups. Group A (N = 6) was a non-treated negative control group, and Group B (N = 6) was rats treated with gel without PE. Group C (N = 6) was treated with PLA microneedles only without PE gel. Group D (N = 6) was treated around the anus and on the anal canal with 30% PE gel only. For Group E (N = 12), PLA microneedles were inserted on the perianal skin and removed, and then 30% PE gel (PE, company Il-Dong Pharm) was applied. In summary, Groups A, B and C did not receive treatment with PE; Group D received PE treatment but microneedles were not applied; and Group E received PE treatment with the pretreatment of microneedles.
Rats were kept in the rat holder (Universal holder, Jeungdo Bio & Plant Co., Ltd.). The resting anal sphincter pressure measurement was performed for two intervals. Anal sphincter pressure was first measured through a catheter at 5, 10, 20, 30, and 40 min for 30 s to observe short-term response to treatments. A second observation was carried out for 30 s at 1, 2, 4 and 6 h after treatments to observe the long-term change in maximum resting anal sphincter pressure. The change in anal sphincter pressures was compared statistically among groups.
This study examined the use of phenylephrine gel with pretreatment using microneedles and with placebo for outcome of maximum anal resting pressure during measured periods. Analysis of variance was performed for percentage subjective improvement. p<0.05 was considered statistically significant. A sample size 12 rats was used for Group E and 6 rats were used for the other Groups (A, B, C and D) were used to observe the difference between means to be shown at 95% confidence level for trials. All data were collected prospectively. Student’s t-test was used to compare the mean values between pretreatment and treatment in a group; a p-value < 0.05 was considered statistically significant.
2.5 Safety test of skin barrier to E. coli after microneedle pretreatment
To partially assess safety, the possibility of infection with E. coli introduced through residual holes in the skin after microneedle pretreatment was investigated by confocal microscopy. E. coli was selected as a model pathogen because the presence of E. coli is indicative of fecal contamination that could occur in the perianal region.
E. coli was grown at 37 °C in Luria-Bertani (LB) medium supplemented with ampicillin to an optical density of 0.6 to 0.7 at 600 nm (OD600). After that, E. coli was grown at 37 °C under the medium with isopropyl β-D-thiogalactopyranoside solution (IPTG) (Sigma-Aldrich, Pittsburgh, PA) for 3 h in a shaking incubator (Biofree, Seoul, Korea). E. coli cells were collected by centrifugation at 10,000 rpm for 2 min and then washed in PBS (Sigma-Aldrich).
The confocal microscopy study was performed after applying green fluorescent protein (GFP)– expressing E. coli suspension for 2 h on the treated skin of 6-wk-old hairless mouse (CAnN.Cg-Foxn1 nu/Crlj0rr, female, Charles River Technology). Rats were too difficult to study in large enough numbers to permit statistical comparisons. Hairless mice have been used for a variety of studies of E. coli contamination. The mice were anesthetized with ether and then their back skin was pretreated using microneedles, a 23-gauge hypodermic needle, or a 26-gauge hypodermic needle (Sungshim Medical Co., Gyeonggi-do, Korea). Then 10 μl of GFP-expressing E. coli suspension (1×109/ml) was applied to the back skin for 2 h. After 2 h, the E. coli suspension was cleaned from the skin surface and the mice were sacrificed under ether anesthesia. The treated skin was biopsied from the sacrificed mice and observed at 100× magnification by confocal microscopy (650LP, Nikon C1sil TE2000-E, Nikon).
3. Results and Discussion
3.1. Microneedles for delivery of drug into perianal skin
We first fabricated microneedles designed specifically for drug delivery to perianal skin. The master structures were fabricated using an inclined rotation for angled UV light exposure and laser cutting technique. The master structures were used to form PDMS molds that can be utilized in a conventional plastic replication process. The microneedles were 600 μm in height with 10-μm diameter tips and were placed on a 250-μm-square base as shown in Figure 3(a). The 600 μm length of microneedles was chosen to enable simple manual insertion into skin with minimal pain [46]. The needles were positioned in a 10 × 10 array with center-to-center spacing of 600 μm. A 1.4 × 1.4 mm opening was cut in the center in order not to irritate the anus and to generate holes only on the perianal skin close to the sphincter muscle. This resulted in a final structure containing 88 microneedles on a 6 mm square frame for in vivo test, as shown in Figure 3(b). As previous studies have demonstrated [47], the number of holes determines the flux of drug delivery. Thus, the number of holes should be considered for the delivery of a therapeutic dose of the drug. This prototype microneedle design is suitable for laboratory studies. With design improvements, microneedles could be adapted for clinical use, ideally for self-administration by patients themselves.
Figure 3.
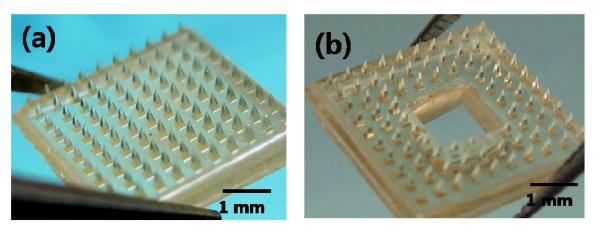
Diagram of poly-lactic acid microneedle arrays designed to apply to perianal skin. (a) Microneedle arrays with 600 μm height on a 250 μm-square base prepared by micromolding. (b) Microneedles on the frame structure after laser cutting of the base layer.
3.2. Analysis of drug delivery through the perianal skin
3.2.1. Qualitative analysis in vitro and in vivo
The anatomic study of perianal skin and the study of drug delivery properties were conducted to apply the strategy of transdermal drug delivery using microneedles to perianal local drug delivery of PE. Because of the lack of histological information about perianal skin, a histological study of the perianal skin, the anal entrance, was performed. The sectioned entrance of a rat anus shows a 20 μm thick stratum corneum layer as seen in Figure 4(a). The entrance of the anus has an outer stratum corneum layer that functions as a barrier mechanically, physically, and chemically like skin. A comparison of the histological structure of the perianal skin, as seen in Figure 4(a), and of the back skin of the rat and human cadaver skin, as seen in Figure 4(b) and 4(c), shows that they have similar stratum corneum structures. The anus itself is lined by wet epithelium and the perianal skin is around the entrance of the anus. The sphincter muscle is a few millimeters below the surface of the perianal skin. This suggests that microneedle treatment, which has shown efficacy for transdermal drug delivery in skin on other sites of the body, could be utilized for drug delivery through the perianal skin based on the anatomic similarity between back skin and perianal skin. The puncturing of perianal skin with microneedles can generate the drug delivery path to the sphincter muscle. In contrast, the delivery of drugs into the anal canal can have adverse effects related to systemic circulation because the descending colon, rectum and anus do not have first pass liver effects and only the GI track above the descending colon does [48].
Figure 4.
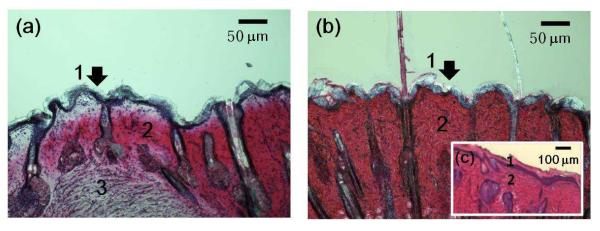
Histological images by optical microscope at 100× after H&E staining of (a) perianal skin of rat (b) back skin of rat and (c) human cadaver skin (back, 60 year old female). 1. stratum corneum. 2. dermis. 3. muscle.
We qualitatively assessed the ability of polymer microneedles to pierce perianal skin and increase skin permeability to drugs. Trypan blue does not stain the stratum corneum, but will stain the epidermis below [49]. Figure 5 shows an optical photomicrograph of an array of blue dots on the perianal skin showing Trypan blue transport through the skin via pathways created when polymeric microneedles were inserted into the perianal skin and then removed. This suggests that microneedles can make pathways for uptake of PE and other drugs.
Figure 5.
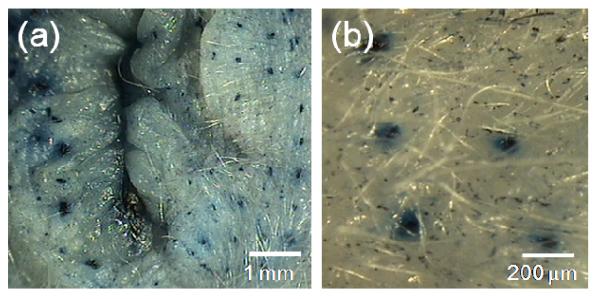
Optical microscope images of Trypan blue dots on a rat’s perianal skin at (a) 40× and (b) 100×. The rat’s perianal skin was pierced by an array of PLA microneedles and subsequently stained with Trypan blue dye to show the sites of microneedle penetration.
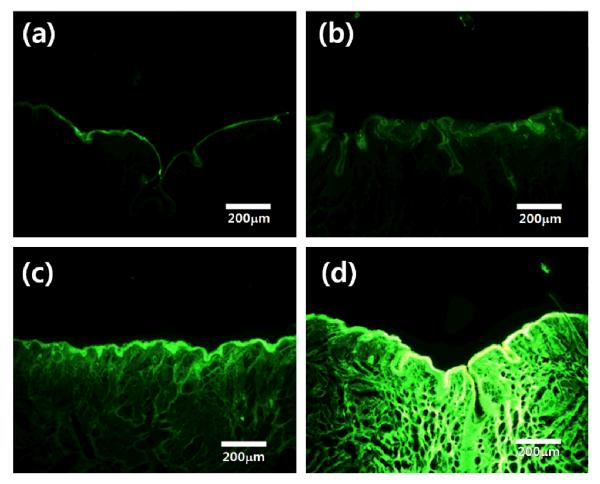
The diffusion of green-fluorescent calcein into perianal skin was investigated histologically as a function of the duration of application for visual analysis of drug diffusion. The diffusion of calcein through holes in perianal skin was investigated at 15, 30, and 60 min after application of calcein qualitatively, and it was compared with the application of calcein for 60 min without microneedle treatment. As shown in Figure 6(a), the diffusion of calcein into perianal skin at 60 min after application was not found when microneedles were not used. When microneedles were used, calcein remained near the surface of the perianal skin after 15 min, and it diffused to a depth of a few hundred microns after 60 min (Figure 6(d)). From this qualitative experiment by visualization of diffusion, we can confirm that the drug went across the perianal skin after first crossing the stratum corneum layer of the perianal skin with the use of microneedles. This qualitative experiment demonstrates that transdermal drug delivery using microneedles can enhance the locally targeted delivery of therapeutic doses across the perianal skin at the desired delivery rate.
Figure 6.
Fluorescence microscopic images of skin histological sections after different exposure durations of calcein in rat perianal skin: (a) at 60 min after calcein application without use of microneedles, and (b) at 15 min, (c) 30 min, and (d) 60 min after treatment using microneedles. The images were taken by fluorescence microscopy with the same photographic exposure conditions.
Most studies of PE delivery into the sphincter muscle have examined delivery through the anal canal. There has been no study of local targeted drug delivery into the sphincter muscle through the perianal skin. Qualitative study can provide the outline of drug delivery using this pathway. Calcein of 622 Da has a higher molecular weight than 203 Da of PE. Thus, PE applied to the anus for the same length of time as calcein may diffuse still faster and deeper than calcein.
3.2.2. Quantitative analysis in vitro
From this qualitative experiment, the duration of PE application ranging from 15 to 120 min was used to quantitatively measure the amount of PE delivered into human cadaver skin in vitro and to investigate the change in resting anal sphincter pressure in vivo. For the in vitro study, full thickness human cadaver skin was used as a substitute for perianal skin due to the insufficient surface area of perianal skin for in vitro analysis in a standard diffusion cell. Although human skin is somewhat thicker than rat skin and rat skin has been used a model membrane for permeation study of drug [50], it is nonetheless a good model that is often used. Moreover, the perianal skin of a rat has little hair compared to other parts of the rat’s body (Figure 5(a)), which is similar to the hair density on human cadaver skin which we used. Even though PE has a low molecular weight of 203 Da, it is a very hydrophilic molecule. Thus the amount of PE delivered into the subcutaneous layer is limited primarily by the nonviable stratum corneum layer [33]. Thus, cadaver skin can be a good alternative model of perianal skin for evaluating the efficacy of microneedle treatment.
To quantify changes in delivery property caused by polymer microneedles, the amount of PE delivered into human cadaver skin was measured as a function of the number of microneedles and the concentration of PE gel. PE gel is suitable for retention on the surface of the anus for the required duration and sustained release over a few hours. As seen in Figure 7, when a 30% concentration of PE gel was applied on cadaver skin in 3 h experiment, pretreatment using an array of 50 polymer microneedles increased the amount of PE delivered by 6 times more than that delivered without microneedle pretreatment, and a 100 needle array increased the amount delivered by 10 times. There is a statistically significant difference in the amount of PE delivered between 50 and 100 microneedles (ANOVA, P < 0.05). This quantitative analysis of PE delivered into cadaver skin can provide the boundary of the dose of PE delivered with respect to duration for efficient anal drug delivery. The in vivo result of change in resting anal sphincter pressure by treatments (presented below) is compared with in vitro quantitative results to obtain the relationship between resting anal sphincter pressure change and dose of PE.
Figure 7.
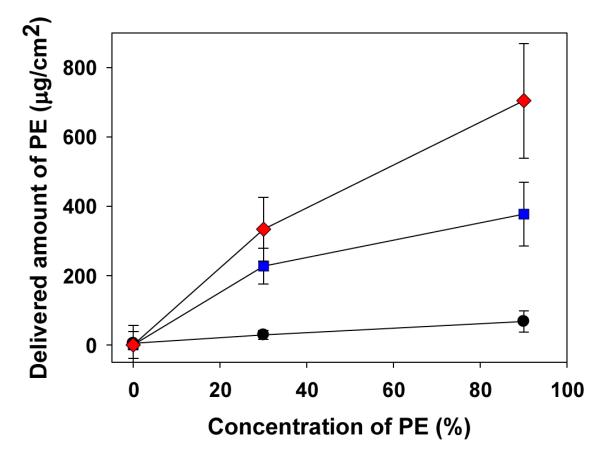
Amount of PE delivered into full thickness human cadaver skin for 3 h as a function of the number of microneedles and concentration of PE. Non-treatment ( ), 50 microneedles (
), 50 microneedles ( ), and 100 microneedles (
), and 100 microneedles ( ). Error bars show the standard deviation.
). Error bars show the standard deviation.
Also, PE concentration affects the delivery rate into cadaver skin layer as shown in Figure 7. PE gel formulations with 10% and 30% concentrations were used to investigate the effect of PE concentration on the delivery rate of PE. A higher concentration of PE can deliver more PE into the subcutaneous layer. When microneedle pretreatment was performed, the amount of PE delivered with a 30% concentration of PE gel was almost two times greater than the amount delivered with a 10% concentration of PE gel in 3 h experiment (Student’s t-test, P < 0.05). A 30% concentration of PE was selected for in vivo evaluation of this new treatment because the topical application of 30% PE gel on the anal canal has been developed for treating fecal incontinence, and the possible high therapeutic dose of PE is suitable for fast local delivery into the sphincter muscle to obtain the evident difference in efficacy of microneedle treatment as a feasibility test of the new treatment. Previous studies have shown that there were no serious side effects with application of 30% PE on the anus [30].
3.3. The change in resting anal sphincter pressure in rats after topical treatments with PE and microneedles
The goal of this new treatment is to raise resting anal sphincter pressure resulting from the contraction of the anal sphincter muscle by locally targeted delivery of PE. When the sphincter muscle becomes weak, anal resting pressure subsides, resulting in fecal incontinence. Resting anal sphincter pressure was observed as a function of duration of the application of 30% concentration of PE gel. The delivery of PE into the sphincter muscle was expected to increase resting anal sphincter pressure. The measurement of the resting tone of the sphincter muscle is a critical parameter determining the status of fecal incontinence [51]. Figure 2 depicts a system for the measurement of resting anal sphincter pressure in rats using a water-perfused anorectal manometry system. The change in measured pressure reflects the effect on resting anal sphincter pressure of each pretreatment using microneedles and topical application of PE.
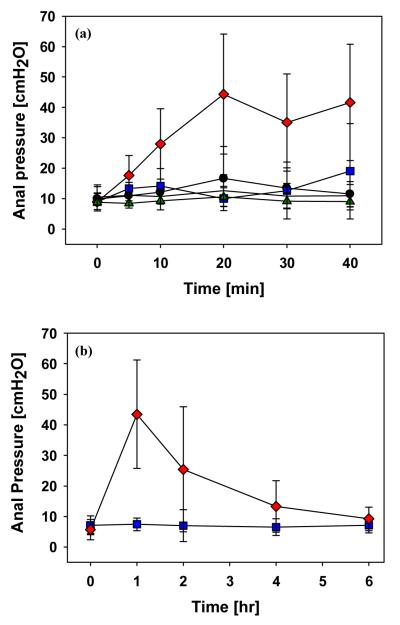
Our study had five experimental groups, which varied based on the use of microneedles and application of PE. To investigate the initial response of the sphincter muscle by locally targeted delivery of PE into the muscle, resting sphincter muscle pressure was measured every 10 min for 45 min. As shown in Figure 8(a), the results from negative control Groups A (non-treatment), B (treatment with only microneedles), and C (application of placebo gel) show that there was no effect on the increase of resting anal sphincter pressure by physical stimulation such as the insertion of microneedles and gel application. Also, no increase was observed in resting anal sphincter pressure after application of PE gel on the anal canal and perianal skin (Group D) compared to that before application (Student’s t-test, p=0.14). This result shows that the application of PE on the anal canal was not effective in increasing resting anal sphincter pressure.
Figure 8.
(a) Measurement of resting anal sphincter pressure for 40 min without treatment, Group A ( ); treatment with microneedles only, Group B (
); treatment with microneedles only, Group B ( ); application of PE gel only on anal canal and perianal skin (Group D (
); application of PE gel only on anal canal and perianal skin (Group D ( ); and treatment with PE gel and microneedles on perianal skin, Group E (
); and treatment with PE gel and microneedles on perianal skin, Group E ( ). (N of each group = 6). Error bars show the standard deviation (b) Measurement of resting anal sphincter pressure for 6 h after application of PE gel only on anal canal and perianal skin, Group D (
). (N of each group = 6). Error bars show the standard deviation (b) Measurement of resting anal sphincter pressure for 6 h after application of PE gel only on anal canal and perianal skin, Group D ( ); and treatment with PE gel and microneedles on perianal skin, Group E (
); and treatment with PE gel and microneedles on perianal skin, Group E ( ). (N of each group = 12). Error bars show the standard deviation
). (N of each group = 12). Error bars show the standard deviation
Comparison of Group E to Group D shows that pretreatment with microneedles enhanced the delivery of PE into the sphincter muscle by increasing PE permeability to the skin. As a result, there is a statistical difference in resting anal sphincter pressure at 45 min between a group treated with both PE gel and microneedles (Group E) versus the other groups (Groups A, B, C, and D; ANOVA, p< 0.05). This result confirms that the local delivery of PE into the sphincter muscle through holes generated by microneedles on the perianal skin produced a significant increase in resting anal sphincter pressure.
The significant increase in anal sphincter muscle pressure by drug therapy was the main target of in vivo measurement to test the feasibility of a new microneedle-based method for delivering PE through perianal skin. In addition to demonstrating the increase in muscle pressure, this study provided the first observation of local targeted delivery into subcutaneous muscle using microneedles. The targeted delivery of PE into the sphincter muscle was effective in increasing muscle pressure resulting from muscle contraction.
Application of PE gel was previously reported to increase the maximum resting anal sphincter pressure when the gel was applied to the distal anal canal because PE can be delivered passively into the inner sphincter muscle through the anal canal [17, 31]. Another study of the application of PE on the anal canal of patients who had rectal excision did not show an improvement in the patients’ fecal incontinence [29, 30]. Our study showed that the application of a 30% concentration of PE gel on the anal canal (Group D) did not significantly increase resting anal sphincter pressure compared to a negative control (Group A) under the conditions used in this study. Thus, the delivery of PE through the anal canal could not confirm the effectiveness of PE, which may be due to the insufficient dose delivery and inappropriate delivery rate of PE into the sphincter muscle.
In our next set of experiments, the long-term response to PE delivery was measured for 6 h to study the duration of efficacy by a single treatment. As seen in Figure 8(b), the application of PE gel on the treated area with the use of microneedles in 1 h demonstrates that the maximum resting anal sphincter pressure by the combination of microneedle pretreatment and PE gel application (Group E) was 43 ± 17 cmH2O compared to 7 ± 2 cmH2O for PE gel application only (Group D, Student’s t-test, p<0.001). Supposing the size of opening holes to be unchanged, the dose of PE delivered into the sphincter muscle with microneedle pretreatment for 1 h was expected to be in the range of 80 to 160 μg through 88 microneedles based on the in vitro delivery test with full thickness human cadaver skin. The resting anal sphincter pressure of Group E diminished slowly after 1 h, with a significant increase in anal sphincter pressure still evident after 2 h, but a significant difference was no longer observed at 4 h between Group D and Group E (Student’s t-test, p=0.08). This result seems to be caused by the resealing of hole openings, leading to reduction of the diffusion rate [44].
While these data provide proof that microneedles can be used to increase PE delivery to the anal sphincter muscles, 2–4 h of continued effectiveness using this new treatment is not long enough for effective long-term treatment of fecal incontinence clinically. A previous clinical test of PE application to the anal canal was performed based on the application of PE every 12 h for 4 weeks [30], and longer efficient delivery over 12 h of PE into the sphincter muscle is recommended for clinical application. Building off the present study, microneedles might be further adapted for controlled release drug delivery to extend the effects of PE for much longer times [52]. Moreover, the skin could be more fully occluded with a patch during PE delivery, rather than covered only with a gel formulation. Occlusion with a patch has been shown to keep skin pores open for much longer times [53], which could increase the extent and duration of PE absorption across the skin.
Reflecting further, there has been no consistent drug therapy to date to increase resting sphincter muscle pressure. The result from this study showing muscle contraction by locally targeted drug delivery with easy and economical microneedle pretreatment has potential clinical applicability to treat fecal incontinence. Microneedles with various formulations have been developed using biodegradable polymers for long-term delivery [52]. We believe that an attractive formulation of microneedles for perianal skin puncture can achieve long-term delivery of PE by leaving the tip part (i.e., “arrowhead”) of the needle encapsulating the drug after insertion [54]. PE has been used primarily as a decongestant, as an agent to dilate the pupil, and as a means to increase blood pressure. PE is currently delivered by oral administration and via nasal spray. However, these delivery methods have low bioavailability and efficacy due to rapid metabolism of PE in the gastrointestinal tract. A microneedle system can provide effective transdermal delivery of PE in addition to the treatment of fecal incontinence. Previous work reported that an increase in resting anal pressure was effective to improve sphincter function [17]. The normal range of resting anal pressure in humans is known to be above 60 mm H2O and patients with fecal incontinence were reported below 60 mm H2O [17]. However, there is a relative lack of normative data stratified for age and gender [55], and the relevant pressures in the rat model used in this study are also not known. The aim of this study is restricted to exploring the feasibility of using active transdermal drug delivery to increase anal resting pressure. By clinical test in the future, we will test whether the increase of resting anal pressure using microneedles and PE will be effective for treatment of fecal incontinence in humans.
3.4 E-coli diffusion through holes
While microneedle treatment can increase skin permeability to PE, it might also increase skin permeability to pathogens leading to infection, such as E. coli. We assessed this possibility by applying GFP-expressing E. coli to skin of hairless mice after treatment with microneedles or with hypodermic needles as a positive control. To investigate the distribution of E. coli in the skin after microneedle pretreatment, a series of confocal microscopy images were taken, which allowed depth profiling of the GFP-expressing E. coli to be performed. The areas treated with 23-gauge and 26-gauge hypodermic needles had a fluorescence response as shown in Figure 9. This confirms that E. coli diffuses into skin through holes made by hypodermic needles. Strong signals were detected more than 100 μm deep into the skin, and the intensity of fluorescence decreased gradually.
Figure 9.
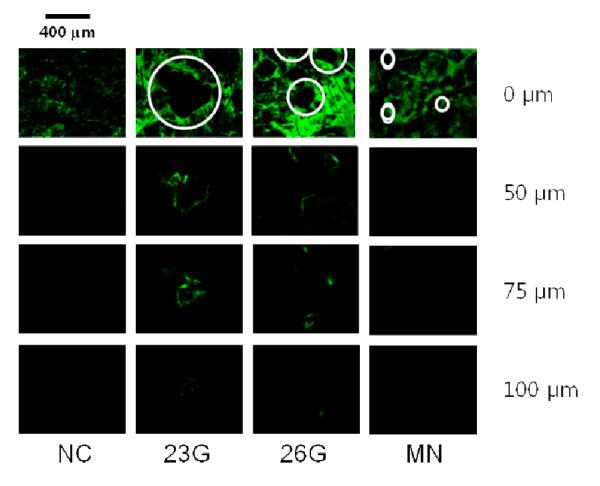
The distribution of E. coli in the skin. A series of confocal microscopy images (surface, 50 μm, 75 μm, and 100 μm below surface) were taken at 100× magnification, which allowed depth profiling of the GFP-expressing E. coli to be performed after diffusion through holes. Skin treated with 23-gauge (23G) and 26-gauge (26G) hypodermic needles before application of GFP-expressing E. coli for 2 h in-vivo had fluorescence responses deep into the skin. But no evidence of E. coli penetration into the skin was found in microneedle-treated skin (MN) or non-treated skin (NC).
In contrast, a fluorescent signal was not observed in confocal images of microneedle-treated skin, which appeared similar to the negative control skin that was not treated with any kind of needles before application of E. coli. This experiment suggests that microneedle treatment will not cause microbial delivery through holes made using the microneedles. In a previous study using an in vitro quantitative test, the application of microneedles in an appropriate manner was reported to allow much lower microbial penetration than hypodermic needles [56]. Further tests will be needed to more fully assess the safety of microneedles, including the possibility of infection.
Subcutaneous injection of over 5×107 of E. coli into mouse was reported to result in subcutaneous swelling and abscess [57]. Long exposure of generated channels to E. coli can induce formation of infection including abscess. Thus, fast recovery of holes in skin right after microneedle treatment is desirable to prevent microbial infection. In addition, there is a chance of minimal irritation by microneedles. The minimal irritation by 400 μm length microneedles was reported to last less than 2 h [58].
4. Conclusion
This study presents the first locally targeted delivery of a drug to muscle and to the perianal region using microneedles. This new delivery method can be used to increase resting anal sphincter pressure, which is important in treating fecal incontinence, by delivering PE into the sphincter muscle. This study also demonstrates the feasibility of this new delivery method. Microneedle-treated skin is more permeable than the anal mucosa, resulting in better bioavailability, faster onset, and stronger effects. In contrast, drug delivery into the anal canal leads to undesired drug absorption into the systemic circulating system [59-61]. Thus, locally targeted delivery to the sphincter muscle through the perianal skin using microneedles can reduce the required drug dose and thereby reduce side effects associated with systemic absorption, such as increased blood pressure. The hypothesized reduction in size effects needs to be addressed directly in additional experiments.
Compared to topical drug application without microneedles, the faster local delivery of the drug into the sphincter muscle with microneedles is recommended for minimizing clearance of the medicine by urination and defecation and for enhancing a patient’s convenience. Thus, coated microneedles may be more suitable to deliver a therapeutic dose of medicine in a short time. Another concern of this system is infection resulting from the holes generated by microneedles. Thus, fast healing of holes or blocking of holes after treatment and sustained release of PE over a few days should be incorporated into the design of the next-generation system. Sustained release of PE from dissolvable microneedles can be a good candidate for a perianal skin delivery system because of long-term delivery of the drug and reduction in the number of microneedle treatments. Overall, local transdermal delivery of phenylephrine to the anal sphincter muscle can be achieved using microneedles and may provide the basis for improved treatment of fecal incontinence.
Acknowledgements
This work was supported by a grant from the Fundamental R&D Program for Core Technology of Materials funded by the Ministry of Knowledge Economy, Republic of Korea and by the GRRC program of Gyeonggi province [GRRC Kyungwon 2010-A01, Development of Microfluidic Chip for diagnosis of disease]
It was also supported in part by the U.S. National Institutes of Health. M.R.P. serves as a consultant and is an inventor on patents licensed to companies developing microneedle-based products. This potential conflict of interest has been disclosed and is being managed by Georgia Tech and Emory University.
References
- [1].Whitehead WE, Wald A, Norton NJ. Treatment options for fecal incontinence. Dis. Colon Rectum. 2001;44:131–142. doi: 10.1007/BF02234835. [DOI] [PubMed] [Google Scholar]
- [2].Crowell MD, Schettler VA, Lacy BE, Lunsford TN, Harris LA, DiBaise JK, Jones MP. Impact of anal incontinence on psychosocial function and health-related quality of life. Dig. Dis. Sci. 2007;52:1627–1631. doi: 10.1007/s10620-006-9249-3. [DOI] [PubMed] [Google Scholar]
- [3].Farage MA, Miller KW, Berardesca E, Maibach HI. Psychosocial and societal burden of incontinence in the aged population: a review. Arch. Gynecol. Obstet. 2008;277:285–290. doi: 10.1007/s00404-007-0505-3. [DOI] [PubMed] [Google Scholar]
- [4].Coggrave M, Wiesel PH, Norton C. Management of fecal incontinence and constipation in adults with central neurological diseases. Cochrane. DB. Syst. Rev. 2006;19:CD002115. doi: 10.1002/14651858.CD002115.pub3. [DOI] [PubMed] [Google Scholar]
- [5].Aslan E, Beji NK, Erkan HA, Yalcin O, Gungor F. The prevalence of and the related factors for urinary and fecal incontinence among older residing in nursing homes. J. Clin. Nursing. 2009;18:3290–3298. doi: 10.1111/j.1365-2702.2009.02936.x. [DOI] [PubMed] [Google Scholar]
- [6].Rao SSC. Diagnosis and management of fecal incontinence. Am. J. Gastroenterol. 2004;99:1585–1604. doi: 10.1111/j.1572-0241.2004.40105.x. [DOI] [PubMed] [Google Scholar]
- [7].Cotterill N, Norton C, Avery KNL, Abrams P, Donovan JL. A patient-centered approach to developing a comprehensive symptom and quality of life assessment of anal incontinence. Dis. Colon Rectum. 2008;51:82–87. doi: 10.1007/s10350-007-9069-3. [DOI] [PubMed] [Google Scholar]
- [8].Wald A. Fecal incontinence in adults. New Engl. J. Med. 2007;356:1648–1655. doi: 10.1056/NEJMcp067041. [DOI] [PubMed] [Google Scholar]
- [9].Vaizey CJ, Kamm MA, Bartram CI. Primary degeneration of the internal anal sphincter as a cause of passive faecal incontinence. Lancet. 1997;349:612–615. doi: 10.1016/S0140-6736(96)09188-X. [DOI] [PubMed] [Google Scholar]
- [10].Rao SS, Siddiqui J. Diagnosis of Fecal Incontinence. In: Ratto C, Doglietto GB, editors. Fecal Incontinence: Diagnosis and Treatment. Springer; Milan: 2007. p. 95. [Google Scholar]
- [11].Pucciani F, Ringressi MN, Redditi S, Masi A, Giani I. Rehabilitation of fecal incontinence after sphincter-saving surgery for rectal cancer: encouraging results. Dis. Colon Rectum. 2008;51:1552–1558. doi: 10.1007/s10350-008-9312-6. [DOI] [PubMed] [Google Scholar]
- [12].Varma MG, Brown JS, Creasman JM, Thom DH, Van Den Eeden SK, Beattie MS, Subak LL. Fecal incontinence in females older than aged 40 years: Who is at risk? Dis. Colon Rectum. 2006;49:841–851. doi: 10.1007/s10350-006-0535-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bharucha AE, Zinsmeister AR, Locke GR, Seide BM, McKeon K, Schleck CD, Melton LJ. Prevalence and burden of fecal incontinence: a population-based study in women. Gastroenterology. 2005;129:42–49. doi: 10.1053/j.gastro.2005.04.006. [DOI] [PubMed] [Google Scholar]
- [14].Jarrett MED, Dudding TC, Nicholls RJ, Vaizey CJ, Cohen CRG, Kamm MA. Sacral nerve stimulation for fecal incontinence related to obstetric anal sphincter damage. Dis. Colon Rectum. 2008;51:531–537. doi: 10.1007/s10350-008-9199-2. [DOI] [PubMed] [Google Scholar]
- [15].Bellicini N, Molloy PJ, Caushaj P, Kozlowski P. Fecal incontinence review. Dig. Dis. Sci. 2008;53:41–46. doi: 10.1007/s10620-007-9819-z. [DOI] [PubMed] [Google Scholar]
- [16].Madoff RD. Surgical treatment options for fecal incontinence. Gastroenterology. 2004;126:48–54. doi: 10.1053/j.gastro.2003.10.015. [DOI] [PubMed] [Google Scholar]
- [17].Cheetham MJ, Kamm MA, Phillips RKS. Topical phenylephrine increases anal canal resting pressure in patients with faecal incontinence. Br. Med. J. 2001;48:356. doi: 10.1136/gut.48.3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mellgren A. Results of Traditional Surgical Treatment for Fecal Incontinence. Elsevier; pp. 27–29. [Google Scholar]
- [19].Jafri S, Pasricha PJ. Agents used for diarrhea, constipation, and inflammatory bowel disease; agents used for biliary and pancreatic disease. McGraw-Hill; New York: 2001. [Google Scholar]
- [20].Farrar JT. The effects of drugs on intestinal motility. Clinics in gastroenterology. 1982;11:673. [PubMed] [Google Scholar]
- [21].Scarlett Y. Medical management of fecal incontinence. Gastroenterology. 2004;126:55–63. doi: 10.1053/j.gastro.2003.10.007. [DOI] [PubMed] [Google Scholar]
- [22].Ehrenpreis ED, Chang D, Eichenwald E. Pharmacotherapy for fecal incontinence: a review. Dis. Colon Rectum. 2007;50:641–649. doi: 10.1007/s10350-006-0778-9. [DOI] [PubMed] [Google Scholar]
- [23].Regadas F, De O B, Albuquerque J, Capaz F. Pharmacological study of the internal anal sphincter in patients with chronic anal fissure. Br. J. Surg. 1993;80:799–801. doi: 10.1002/bjs.1800800650. [DOI] [PubMed] [Google Scholar]
- [24].O’Kelly T, Brading A. In vitro response of the human and canal longitudinal muscle layer to cholinergic and adrenergic stimulation: Evidence of sphincter specialization. Br. J. Surg. 1993;80:1337–1341. doi: 10.1002/bjs.1800801041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yamato S, Rattan S. Role of alpha adrenoceptors in opossum internal anal sphincter. J. Clin. Invest. 1990;86:424. doi: 10.1172/JCI114728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Thomas E, Rubino J. Solubility, melting point and salting-out relationships in a group of secondary amine hydrochloride salts. Int. J. Pharm. 1996;130:179–185. [Google Scholar]
- [27].Hamdani J, Moës AJ, Amighi K. Development and evaluation of prolonged release pellets obtained by the melt pelletization process. Int. J. Pharm. 2002;245:167–177. doi: 10.1016/s0378-5173(02)00348-4. [DOI] [PubMed] [Google Scholar]
- [28].Penninckx F, Lestar B, Kerremans R. The internal anal sphincter: mechanisms of control and its role in maintaining anal continence. Baillieres Clin. Gastroenterol. 1992;6:193–214. doi: 10.1016/0950-3528(92)90027-c. [DOI] [PubMed] [Google Scholar]
- [29].Carapeti EA, Kamm MA, Phillips RKS. Randomized controlled trial of topical phenylephrine in the treatment of faecal incontinence. Br. J. Surg. 2000;87:38–42. doi: 10.1046/j.1365-2168.2000.01306.x. [DOI] [PubMed] [Google Scholar]
- [30].Park JS, Kang SB, Kim DW, Namgung HW, Kim HL. The efficacy and adverse effects of topical phenylephrine for anal incontinence after low anterior resection in patients with rectal cancer. Int. J. Colorectal Dis. 2007;22:1319–1324. doi: 10.1007/s00384-007-0335-6. [DOI] [PubMed] [Google Scholar]
- [31].Carapeti EA, Kamm MA, Nicholls RJ, Phillips RKS. Randomized, controlled trial of topical phenylephrine for fecal incontinence in patients after ileoanal pouch construction. Dis. Colon Rectum. 2000;43:1059–1063. doi: 10.1007/BF02236550. [DOI] [PubMed] [Google Scholar]
- [32].Badvie S, Andreyev HJN. Topical phenylephrine in the treatment of radiation-induced faecal incontinence. Clin. Oncol. 2005;17:122–126. doi: 10.1016/j.clon.2004.07.011. [DOI] [PubMed] [Google Scholar]
- [33].Prausnitz MR, Langer R. Transdermal drug delivery. Nat. Biotechnol. 2008;26:1261–1268. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Singh S. An Overview of Transdermal Drug Delivery. Drug Delivery Report Autumn/Winter. 2005:35–40. [Google Scholar]
- [35].McAllister DV, Wang PM, Davis SP, Park JH, Canatella PJ, Allen MG, Prausnitz MR. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc. Nat. Acad. Sci. (USA) 2003;100:13755–13760. doi: 10.1073/pnas.2331316100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Coulman SA, Anstey A, Gateley C, Morrissey A, McLoughlin P, Allender C, Birchall JC. Microneedle mediated delivery of nanoparticles into human skin. Int. J. Pharm. 2009;366:190–200. doi: 10.1016/j.ijpharm.2008.08.040. [DOI] [PubMed] [Google Scholar]
- [37].You SK, Noh YW, Park HH, Han M, Lee SS, Shin SC, Cho CW. Effect of applying modes of the polymer microneedle-roller on the permeation of l-ascorbic acid in rats. J. Drug Target. 2009:1–6. doi: 10.3109/10611860903115274. [DOI] [PubMed] [Google Scholar]
- [38].Kim YC, Quan FS, Compans RW, Kang SM, Prausnitz MR. Formulation and coating of microneedles with inactivated influenza virus to improve vaccine stability and immunogenicity. J. Control. Release. 142:187–195. doi: 10.1016/j.jconrel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Li G, Badkar A, Kalluri H, Banga AK. Microchannels created by sugar and metal microneedles: Characterization by microscopy, macromolecular flux and other techniques. J. Pharm. Sci. 2009 doi: 10.1002/jps.21981. [DOI] [PubMed] [Google Scholar]
- [40].Banga AK. Microporation applications for enhancing drug delivery. Expert Opin. Drug Deliv. 2009;6:343–354. doi: 10.1517/17425240902841935. [DOI] [PubMed] [Google Scholar]
- [41].Coulman SA, Barrow D, Anstey A, Gateley C, Morrissey A, Wilke N, Allender C, Brain K, Birchall JC. Minimally invasive cutaneous delivery of macromolecules and plasmid DNA via microneedles. Curr. Drug Del. 2006;3:65–75. doi: 10.2174/156720106775197510. [DOI] [PubMed] [Google Scholar]
- [42].Ding Z, Verbaan FJ, Bivas-Benita M, Bungener L, Huckriede A, van den Berg DJ, Kersten G, Bouwstra JA. Microneedle arrays for the transcutaneous immunization of diphtheria and influenza in BALB/c mice. J. Control. Release. 2009;136:71–78. doi: 10.1016/j.jconrel.2009.01.025. [DOI] [PubMed] [Google Scholar]
- [43].Zhu Q, Zarnitsyn VG, Ye L, Wen Z, Gao Y, Pan L, Skountzou I, Gill HS, Prausnitz MR, Yang C. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc. Natl. Acad. Sci. U. S. A. 2009;106:7968. doi: 10.1073/pnas.0812652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Park J-H, Choi S-O, Seo S, Choy YB, Prausnitz MR. A microneedle roller for transdermal drug delivery. Eur. J. Pharm. Biopharm. 2010;76:282–289. doi: 10.1016/j.ejpb.2010.07.001. [DOI] [PubMed] [Google Scholar]
- [45].Martanto W, Davis SP, Holiday N, Wang J, Gill H, Prausnitz MR. Transdermal delivery of insulin using microneedles in vivo. Pharm. Res. 2004;21:947–952. doi: 10.1023/b:pham.0000029282.44140.2e. [DOI] [PubMed] [Google Scholar]
- [46].Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. Clin. J. Pain. 2008;24:585. doi: 10.1097/AJP.0b013e31816778f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Park JH, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J. Control. Rel. 2005;104:51–66. doi: 10.1016/j.jconrel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- [48].Dujovny N, Quiros RM, Saclarides TJ. FACS, FASCRS, Anorectal anatomy and embryology. Surg. Oncol. Clin. N. Am. 2004;13:277–293. doi: 10.1016/j.soc.2004.01.002. [DOI] [PubMed] [Google Scholar]
- [49].McAllister DV. Chem. Eng. Georgia Institute of Technology; Atlanta: 2000. Microfabricated Needles for Transdermal Drug Delivery. [Google Scholar]
- [50].Özgüney IS, Karasulu HY, Kantarci G, Sözer S, Güneri T, Ertan G. Transdermal delivery of diclofenac sodium through rat skin from various formulations. AAPS PharmSciTech. 2006;7:39–45. doi: 10.1208/pt070488. [DOI] [PubMed] [Google Scholar]
- [51].Eckardt VF, Elmer T. Reliability of anal pressure measurements. Dis. Colon Rectum. 1991;34:72–77. doi: 10.1007/BF02050212. [DOI] [PubMed] [Google Scholar]
- [52].Park JH, Allen MG, Prausnitz MR. Polymer Microneedles for Controlled-Release Drug Delivery. Pharm. Res. 2006;23:1008–1019. doi: 10.1007/s11095-006-0028-9. [DOI] [PubMed] [Google Scholar]
- [53].Gupta J. Chemical and Biomolecular Engineering. Georgia Institute of Technology; Atlanta: 2009. Microneedles for transdermal drug delivery in human subjects; p. 198. [Google Scholar]
- [54].Chu LY, Prausnitz MR. Separable Arrowhead Microneedles. J. Control. Release. 149:242–249. doi: 10.1016/j.jconrel.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Rao S, Azpiroz F, Diamant N, Enck P, Tougas G, Wald A. Minimum standards of anorectal manometry. Neurogastroenterol. Motil. 2002;14:553–559. doi: 10.1046/j.1365-2982.2002.00352.x. [DOI] [PubMed] [Google Scholar]
- [56].Donnelly RF, Singh TRR, Tunney MM, Morrow DIJ, McCarron PA, O묺ahony C, Woolfson AD. Microneedle arrays allow lower microbial penetration than hypodermic needles in vitro. Pharm. Res. 2009;26:2513–2522. doi: 10.1007/s11095-009-9967-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Verweij-van Vught A, Namavar F, Sparrius M, Vel W, MacLaren D. Pathogenic synergy between Escherichia coli and Bacteroides fragilis: studies in an experimental mouse model. J. Med. Microbiol. 1985;19:325. doi: 10.1099/00222615-19-3-325. [DOI] [PubMed] [Google Scholar]
- [58].Bal SM, Caussin J, Pavel S, Bouwstra JA. In vivo assessment of safety of microneedle arrays in human skin. Eur. J. Pharm. Sci. 2008;35:193–202. doi: 10.1016/j.ejps.2008.06.016. [DOI] [PubMed] [Google Scholar]
- [59].Thakar R, Sultan A. Postpartum problems and the role of a perineal clinic, Perineal and Anal Sphincter Trauma. Springer; London: 2007. pp. 65–79. [Google Scholar]
- [60].Jacob R, Krishanan BS, Venkatesan T. Pharmacokinetics and pharmacodynamics of anesthetic drugs in pediatrics. Indian J. Anaesth. 2004;48:340–346. [Google Scholar]
- [61].Doyle D. Per rectum: a history of enemata. J. R. Coll. Physicians. Edinb. 2005;35:367–370. [PubMed] [Google Scholar]