Abstract
Volatile anesthetics are used widely for achieving a state of unconsciousness, yet these agents are incompletely understood in their mechanisms of action and effects on neural development. There is mounting evidence that children exposed to anesthetic agents sustain lasting effects on learning and memory. The explanation for these behavioral changes remains elusive, although acute neuronal death after anesthesia is commonly believed to be a principal cause. Rodent models have shown that isoflurane exposure in newborns induces acute neuroapoptosis and long-term cognitive impairment. However, the assessment of predisposing factors is lacking. We investigated the role of sex by delivering isoflurane to postnatal day (P)7 male and female Sprague Dawley rats for 4 hours. Brain cell death was assessed 12 h later using FluoroJade C staining in the thalamus, CA1-3 regions of hippocampus, and dentate gyrus. Behavior was assessed separately using a series of object recognition tasks and a test of social memory beginning at P38. We found that isoflurane exposure significantly increased neuronal death in each brain region with no difference between sexes. Behavioral outcome was also equivalent in simple novel object recognition. However, only males were impaired in the recognition of objects in different locations and contexts. Males also exhibited deficient social memory while females were intact. The profound behavioral impairment in males relative to females, in spite of comparable cell death, suggests that males are more susceptible to long-term cognitive effects and this outcome may not be exclusively attributed to neuronal death.
Keywords: anesthetics, isoflurane, sex, toxicity, memory
Introduction
Anesthetic agents are instrumental in their ability to induce an unconscious state, devoid of pain and awareness, and millions of children undergo anesthesia each year as a routine part of their medical care. However, the safety of anesthesia in this population is a concern and prior retrospective studies have noted a correlation between anesthesia exposure and learning disabilities1,2. In addition, various animal models have shown that early anesthetic exposure results in significant long-term behavioral deficits 3-5.
The reason behind these behavioral changes remains unknown, although acute neuronal injury is widely perceived to be an underlying cause. Numerous animal experiments have documented neuronal death following exposure to volatile anesthetics 4,6,7. In addition, factors influencing cell death have been researched extensively, including duration of anesthesia and the specific anesthetic agent that is delivered 6,8,9. Various mechanisms have even been proposed to account for the process of anesthetic-induced neuroapoptosis 10,11. Nevertheless, the precise correlation between neuronal death and behavioral phenotype is undetermined, and although neuronal death is certainly alarming it has not been determined to be causative.
Because no adequate explanation exists, the final phenotype of behavior remains an indispensable tool for investigating anesthetic effects. Many questions, such as the types of memory that are affected and factors that predispose to cognitive dysfunction, are most aptly explored using behavioral models. Also, concerns of early anesthesia leading to autism-like or impaired social behavior have been raised and begun to be explored in animals 7. With mounting evidence of the detrimental effects of anesthetics on the developing brain, these questions become increasingly important to address.
Among potential risk factors, the role of sex has not been studied. Human gender differences in outcomes following traumatic and ischemic brain injury are well known 12,13 and are also seen in prematurity and neonatal stroke 14,15. Epidemiologic studies in humans investigating cognitive outcome after early anesthesia exposure suggest that males may be more susceptible to long-term effects 16-18, although methodological issues preclude us from identifying a true difference. We proposed to determine the influence of sex on two well-known outcomes – brain cell death and behavior.
Exposure to isoflurane, a common inhaled volatile anesthetic, has repeatedly been shown to cause acute neuronal death and persistent cognitive dysfunction in newborn male rodents 4,6, but it is unknown how females are affected. This is because most studies use only male rodents or do not separately assess male and female subjects. In rodent models, effects of anesthesia have been studied following exposure during a time of peak brain development and synaptogenesis, typically postnatal day 7 4,19-21. Cell death occurs acutely in the period immediately following anesthesia 4,8,21, and the thalamus and hippocampus are areas known to be susceptible to extensive neurodegeneration 4,7,21. Long-term behavior is separately assessed in adolescence or adulthood using a range of tasks 4,6,7,9.
In this study, we investigate sex-specific outcomes after anesthesia by assessing acute neurodegeneration after isoflurane exposure in the thalamus, CA1-3 regions of hippocampus, and dentate gyrus, as well as evaluating behavior with a series of object recognition and social memory tasks. In female rats, certain behavioral tests that use aversive stimuli or induce stress can be influenced by hormone cycling 22-24. To minimize these effects, we assessed behavior using spontaneous recognition tasks that rely on rodents' natural preference for novel stimuli. Moreover, the associative memory used in these tests has been shown to be sensitive to lesions in hippocampal and thalamic circuits 25-27.
Materials and Methods
Subjects
All experiments were conducted with approval from the Institutional Animal Care and Use Committee at the University of California, San Francisco. Sprague Dawley dams with litters containing male-only and female-only pups were obtained from Charles River Laboratories (Gilroy, CA). On postnatal day (P)7, animals were randomly assigned to control or treatment groups (Fig. 1). Following treatment, subjects were either killed and fixed for histology or cross fostered between dams. At P21, before reaching sexual maturity, each animal's sex was assessed and they were separated into groups by sex. Control and treatment animals were kept together in clean acrylic cages with bedding changed weekly and ad libitum access to food and water. Cages for both sexes were kept in the same room within the animal care facility with 12 h light-dark cycle and regulation of temperature (18 to 25° C) and humidity (45 to 65%). At P30, they were housed in pairs with one treatment and one control animal per cage. All behavioral testing occurred during the light cycle between 0800 and 1700 h. Animals were food restricted for tasks involving object recognition. Access to food was limited to the light cycle in order to increase activity and object exploration during the testing period.
Figure 1. Group assignment.

At postnatal day 7, male and female rats were randomized to control or isoflurane groups for separate experiments investigating neuronal death and behavior. Neuronal death was assessed 12 hours after anesthesia. Separate groups of animals underwent behavioral testing beginning at P38. The number of animals in each group (n) is also presented.
Anesthesia
Male and female subjects were separately anesthetized for a duration of four hours as we have previously described 28. Briefly, isoflurane was delivered into the anesthetic chamber, and gas concentrations were continuously monitored. The isoflurane concentration was initially set to 4% (time = 0 min) and subsequently maintained at 1 Minimum Alveolar Concentration (MAC, the concentration required to prevent movement in 50% of subjects in response to a painful stimulus, Fig. 2). Every 15 minutes after induction, a supramaximal pain stimulus was produced by applying an alligator clamp to each rat's tail. Movement was defined as any gross movement other than breathing, and the percent of animals that moved in response to tail-clamping was calculated. Isoflurane concentration was then adjusted to maintain 50% response to the stimulus. Control animals were treated identically without tail-clamping or administration of anesthetic. Animals in the anesthesia chamber were kept on a warming blanket and the temperature was measured every 15 minutes using infrared thermometer, and the position and heating were adjusted to maintain normothermia.
Figure 2.
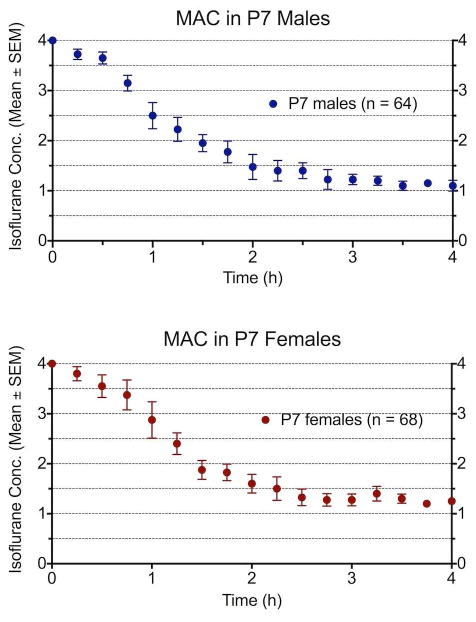
MAC and isoflurane concentration in subjects. In separate experiments, male and female P7 rats were anesthetized to determine MAC and compare the potency of isoflurane. These experiments provided a larger sample size to compare anesthetic effects, and comparison between sexes shows no difference in the concentration and MAC values. The subjects in this study underwent the same anesthetic model for experiments of neurodegeneration and behavior.
Histology
Brains from male and female treatment and control groups (n=10 per group) were assessed for acute neuronal death. Twelve hours after anesthesia, animals were anesthetized and transcardially perfused with cold 4% paraformaldehyde in phosphate-buffered saline and brains were removed, postfixed, and sunk in sucrose solution. They were then sliced into 60 micron-thick slices and every other slice was mounted and stained with FluoroJade C, a marker highly specific for neurodegeneration (FJC, 0.001%, Millipore, Billerica, MA). FJ-positive cells were counted using Nikon Eclipse 80i microscope under 20X magnification in each slice containing the structure of interest. Structures included in analysis were the anterodorsal (AD), anteroventral (AV), laterodorsal (LD), and anteromedial (AM) thalamic nuclei, as well as CA1-3 regions of the hippocampus and the dentate gyrus.
Because the sex of newborns rats is often ambiguous, genetic screening was used to confirm sex as described elsewhere 29. Briefly, DNA was isolated from tissue samples, and Sex-determining region Y (Sry, male-specific) and beta actin (autosomal) gene sequences were amplified by polymerase chain reaction (PCR) using Taq DNA polymerase (G-Biosciences, St. Louis, MO) and primers obtained from Eurofins MWG Operon (Huntsville, AL). After isolation of genomic DNA, PCR products were subjected to electrophoresis in 2% agarose gel, and males were identified by presence of two separate bands and females with a single band.
Object Recognition Tasks
Testing occurred similar to the paradigm used by others25,26. Male and female subjects were assessed using the same testing area and objects. Testing arenas and objects were wiped with 70% ethanol between subjects. Object recognition testing took place in two separate testing arenas, hereafter referred to as “contexts,” of identical size (61 cm square base, walls 50 cm high). The two were distinct in their appearance and texture to allow testing of context-specific memory. Context 1 had yellow walls and a base covered in wood-effect vinyl lining, while context 2 had black walls and a black plastic base. Visual cues were placed on three different walls within each context. Animals were introduced into the contexts facing the same direction and in the same location, and subjects were habituated to the contexts prior to testing. Each object was validated to avoid object bias. Investigation of an object was defined as sniffing or placing the nose within 1 cm of and oriented toward the object. Subjects were video recorded and reviewed by blinded observers to determine investigation times.
All subjects underwent the full series of testing in the order presented here with one trial per day. The subjects' order of testing also rotated each day so that the timing of behavioral testing was counterbalanced among subjects and groups. Testing began at postnatal day 38 (P38) with novel object recognition (Fig. 3). Subjects were assessed in their ability to recall a previously encountered object. A single trial was performed, and half of the subjects were tested in context 1 and the other half in context 2. During the “exposure”, the subject was placed into the context and explored two identical objects for four minutes. Following a two-minute delay, in the “test” phase, the animal was placed into the same context with one of the previous objects replaced with a novel object. The location (left or right) of the novel object within each context was counterbalanced among subjects. For each task, object investigation times during the initial exposure were compared, given possible confounding effects of varying investigation times on object recognition in the subsequent test phase.
Figure 3. Object recognition tasks.

A) Novel object recognition. Two identical objects are presented in the exposure phase, and one of these objects is replaced with a novel object (*) in the test phase. B) Object-place recognition. Two different objects are presented followed by two identical objects. In the test phase, one object (*) appears in a new location within the context. C) Object-context recognition. Two separate pairs of objects are presented in two different contexts so that each object is associated with a context. In the test phase, one object (*) appears within a context in which it has not been explored. D) Object-place-context recognition. Two different objects are presented in Exposure 1. These same objects are presented in a different context with their locations switched in Exposure 2. Thus, after two exposures, each object is seen both locations and contexts. In the test phase, two identical objects are presented in either context, so that one object (*) is presented in a novel configuration of place and context. There are four total configurations with two of these beginning in context 1 (Setup 1) and the other two in context 2 (Setup 2).
Using object recognition as the premise, the tasks were then made increasingly complex. By using different objects and varying the locations and contexts in which they were presented, subjects were assessed in their ability to associate an object with a particular location, context, or combination of location and context. The arrangement used to assess each of these associative memory tasks is presented in Fig 3.
In the final task of object-place-context recognition, control female subjects were identified as having increased object investigation during the exposure, thereby potentially conferring an advantage in subsequent object recognition. The following set of trials (Trials 3 and 4) were therefore performed while controlling for investigation times. Subjects were observed during the exposure with a goal of 15 seconds of investigation per object. Animals remained in the context for a minimum of two minutes and a maximum of five minutes to ensure adequate familiarization to the context. After the two-minute mark, if they reached the required investigation times, then they were removed. The test phase lasted four minutes and was recorded and later reviewed.
Social Behavior and Social Recognition
Social interaction and recognition were assessed using a discrimination paradigm. In the “exposure” phase, the subject was presented with a caged stimulus animal alongside an empty cage for five minutes. This arrangement evaluates social interaction by determining whether subjects appropriately spend more time investigating the social target 7. After a sixty-minute delay, the subject was presented simultaneously with the same “familiar” stimulus animal and a novel animal for three minutes. Social recognition is demonstrated by decreased investigation of the familiar target relative to the novel one.
Same-sex juvenile conspecifics were used as stimulus animals. Male and female pups five weeks of age were housed individually one week prior to testing. Investigation was defined as any direct contact with the subject's nose or paws, as well as sniffing toward any part of the juvenile including the tail if it extended outside of the cage. Investigation of the empty cage was defined as sniffing or placing the nose within 1 cm of and oriented toward the cage, and excluded using the cage as a support during rearing.
Statistical Analysis
Data were analyzed using Prism 6 Software for Mac OSX (GraphPad Software Inc., San Diego, CA). Data were assessed for normal distribution using the D'agonistino and Pearson test. Parametric tests were used for normally distributed data; otherwise, a nonparametric test was used. All comparisons used a two-tail test and a P value less than 0.05 was considered statistically significant.
Subjects were evaluated in their ability to recognize familiar stimuli, reflected by the relative time spent investigating two separate targets. For the final task (object-place-context recognition), times from Trials 1 and 2 were combined for analysis, and Trials 3 and 4 were assessed together. The ratio paired t-test was used to compare normally distributed data, and nonparametric data were analyzed with the Wilcoxon matched-pairs rank test. In addition, a “discrimination index” (DI) was calculated, representing the time spent investigating the novel target relative to the familiar target. To calculate DI, the time spent investigating the familiar target was subtracted from the time spent on the novel target, and this was divided by the total (eg. DI = (Novel-Familiar)/(Total Time)). DI provides a single value and therefore allows analysis by two-way ANOVA to compare effects of treatment or sex.
To identify and control for possible confounding effects of varying investigation times on subsequent object/animal recognition, the investigation times during the exposure phase were compared between the groups. These times were compared using one-way ANOVA for normally distributed data and Kruskal-Wallis test for nonparametric data. Bonferonni's post-test with multiple comparisons was used following one-way ANOVA, and Dunn's post-test was used with the Kruskal-Wallis test.
Two-way ANOVA was used to assess the effects of sex and treatment on neuronal death. Neuronal death for each brain region was compared using two-way ANOVA and Bonferroni post-test. The fold-increase in neuroapoptosis was determined for each structure by dividing the total FJ-positive cells of each treatment animal (n=20) by the average number of FJ-positive cells per structure for the whole control group (n=20).
Results
Brain cell death occurs similarly in males and females
There was increased neuronal death acutely in male and female treatment groups relative to the two control groups for each brain region with no significant effect of sex on the extent of cell death (Fig. 4A-F). Isoflurane resulted in markedly increased cell death in the anterodorsal thalamus (F(1,36) = 57.41, P < 0.0001), anteroventral thalamus (F(1,36) = 53.98, P < 0.0001), anteromedial thalamus(F(1,36) = 110.5, P < 0.0001), laterodorsal thalamus (F(1,36) = 92.56, P < 0.0001), hippocampus (F(1,36) = 38.92, P < 0.0001), and dentate gyrus (F(1,36) = 41.17, P < 0.0001). The fold-increase in cell death for each brain region is shown in Figure 4G.
Figure 4. Neuronal death results.

A to F) There is increased cell death in each brain region for both male and female animals immediately following anesthesia (n=10 per group). The extent of cell death in treatment males and females is similar. Representative images (FJC staining 12 h post-anesthesia) from male and female brains at 20X magnification are displayed alongside graphs comparing total FluoroJade-positive cells for each structure. G) The average fold-increases in neuronal death relative to controls are shown. The fold-increase is determined from pooled data of all anesthetized subjects relative to the average of the control subjects.
Behavior Group Assignment and Anesthesia
In separate series of experiments, survival and blood gas analyses (pH, pO2, pCO2) were performed and found to be the same in P7 male and female rats and similar to results reported previously9. In addition, we assessed MAC using pooled data from multiple rounds of anesthesia in separate male and female groups. There was no difference identified in the anesthetic requirement or MAC between sexes (Fig. 2). Therefore, at P7, groups of male and female animals were obtained from the vendor and assigned to control or treatment groups for behavioral experiments. Twelve pups were assigned to each male or female control group and eighteen pups to each male or female anesthesia group. Given the anticipated mortality, eighteen rats were anesthetized in each male and female group with the goal of yielding 11-12 treatment subjects per group. The mortality of 27% for all anesthetized subjects is consistent with prior results 6,9,20, although the difference in mortality for separately anesthetized groups yielded a greater number of treatment female rats. In addition, the sex of several pups was incorrectly assigned by the vendor, resulting in final group sizes of 8 male control, 16 female control, 8 male treatment, and 18 female treatment animals (Fig. 1).
Object discrimination remains intact in both sexes
All groups were able to distinguish familiar and novel objects evidenced by greater investigation of the novel object (control male P = 0.0151, treatment male P = 0.023, control female P < 0.0001, treatment female P < 0.0001, ratio paired t-test of familiar vs. novel object, Fig. 5A). Two-way ANOVA of the Discrimination Index (DI), which represents the time spent investigating the novel target relative to the familiar one, demonstrated no effect of treatment (F(1,45) = 0.0005, P = 0.98) or sex (F(1,45) = 0.002, P = 0.96, Fig. 5B). There was no difference among groups in time spent investigating the objects during the initial exposure (P = 0.29, Kruskal-Wallis test).
Figure 5.
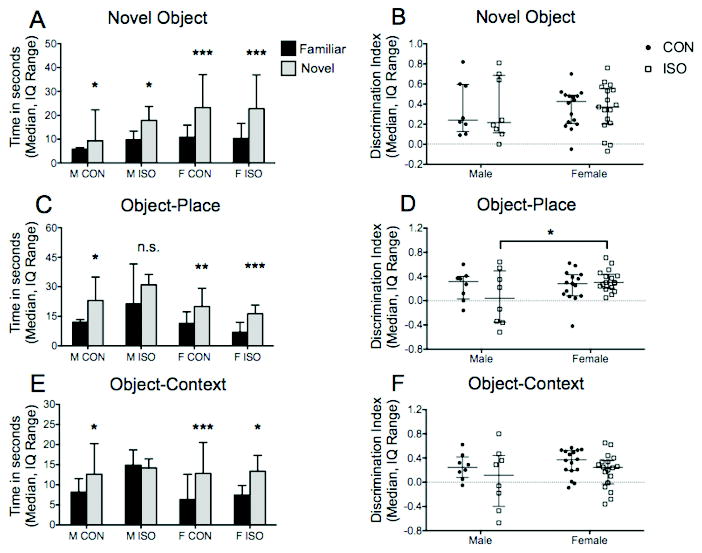
A) Novel Object Recognition In the task, subjects are tested in their ability to recognize a familiar object independent of location or context. All groups were able to recognize and distinguish the two objects reflected by increased investigation of the novel object. B) The discrimination index (DI), representing the time spent investigating the novel object relative to the familiar one, was similar among the groups. C) Object-Place Recognition. This task requires the subject to identify an object and its previous location within the context. Treatment males were the only subjects unable to recognize the object and its spatial location. D) Two-way ANOVA with Bonferroni post-test identified the female treatment DI as significantly higher than male treatment DI. E) Object-Context Recognition. Subjects are tested in their ability to recognize an object and the context in which it appeared. All but the male treatment group successfully recognized an object with its associated context. F) The male treatment DI was lower than the female treatment DI but did not reach statistical significance. * P < 0.05, ** P < 0.01, *** P < 0.001, n.s. = not significant
Males are impaired in object-place, object-context, and object-place-context recognition while females are unaffected
All groups but the male treatment group were able to recognize an object and its spatial location. Subjects, except male treatment animals, preferentially explored the object occupying a novel location (control male P = 0.027, treatment male P = 0.73, control female P = 0.0028, treatment female P < 0.0001, ratio paired t-test of familiar vs. novel location, Fig. 5C). Two-way ANOVA of DI did not demonstrate a significant effect of treatment (F(1,44) = 0.49, P = 0.48) or sex (F(1,44) = 2.88, P = 0.09), although further investigation comparing treatments groups by sex revealed that the female treatment DI was significantly higher than the male treatment DI (P = 0.041, Bonferroni post-test, Fig. 5D). This difference occurred despite increased object exploration by the male treatment group during the initial exposure (P = 0.0011, Kruskal-Wallis test; male treatment vs. female treatment, P = 0.02, Dunn's post-test).
Male treatment subjects were also impaired in object-context recognition (P = 0.69, ratio paired t-test of familiar vs. out-of-context, Fig. 5E). All other groups spent more time investigating the out-of-context object (control male P = 0.015, control female P < 0.0001, treatment female P = 0.012, ratio paired t-test of familiar vs. out-of-context object, Fig. 5E). DI was unaffected by treatment or sex (F(1,46) = 3.2, P = 0.08; F(1,46) = 1.09, P = 0.3; two-way ANOVA). Investigation times of the initial exposures were combined for analysis, and one-way ANOVA revealed no difference among groups (P = 0.25).
The male treatment group was the only one impaired in object-place-context recognition, as well. The investigation times for Trials 1 and 2 were combined for analysis, and all groups but the male treatment group spent more time investigating the “displaced” object (control male P = 0.0034, treatment male P = 0.20, control female P < 0.0001, treatment female P = 0.013; ratio paired t-test of familiar vs. displaced object, Fig. 6A). Comparison of DI by two-way ANOVA found a significant interaction between treatment and sex (F(1,46) = 8.9, P = 0.004), and further evaluation identified the male treatment DI as significantly lower than female treatment DI (P = 0.01, Bonferonni post-test, Fig. 6B). In this task, the female control group spent more time investigating the objects during the exposure than the male treatment group (P = 0.041, one-way ANOVA, Bonferonni post-test).
Figure 6. Object-Place-Context Recognition.

A) In the final test of object recognition, animals were evaluated in their memory of an object, its spatial position, and the context in which it appeared. Only the male treatment group was unable to recognize the object with its associated location and context. B) Two-way ANOVA identified a significant interaction of treatment and sex, and the treatment female DI was greater than the treatment male DI. C) A second set of trials was performed for the task because of differences in investigation time between groups during initial exposure, possibly conferring an advantage to females in subsequent recognition. Even when controlling for investigation time during the exposure, female treatment animals demonstrated successful object recognition while male treatment subjects were again impaired. D) In trials 3 and 4, the female treatment DI was again higher than male treatment DI but did not reach statistical significance.
Because increased object exploration during the exposure leads to deeper encoding of memory, we repeated the experiment while controlling for investigation time. Times were combined for analysis in trials 3 and 4, and once again the male treatment group was unable to distinguish between familiar and novel configurations of object, place, and context (control male P = 0.048, treatment male P = 0.1239, control female P = 0.0002, treatment female P = 0.001, ratio paired t-test of familiar vs. displaced object, Fig. 6C). The female treatment DI was higher than male treatment DI although this did not reach statistical significance (F(1,46) = 3.3, P = 0.07; F(1,46) = 0.69, P = 0.41; two-way ANOVA, Fig. 6D). As expected, no difference was found between groups for the exposure phase (one-way ANOVA, P = 0.43).
Both sexes display normal social investigatory behavior but only males have impaired social memory
All animals demonstrated normal social investigatory behavior and spent much more time with the social target than the empty cage (all P < 0.0001, ratio paired t-test of social target vs. empty cage, Fig. 7A). There was no difference in total time spent investigating the stimulus animal among the four groups (P = 0.39, one-way ANOVA), reflecting equal motivation and interest regardless of treatment or sex.
Figure 7.

A) Social Interaction. In the exposure, the subjects were simultaneously presented with a caged same-sex juvenile and an inanimate object, and all subjects displayed normal social behavior and spent more time with the social target. B) Social Recognition. Animals were tested in their ability to recognize a previously encountered juvenile using a discrimination model. In the test phase, subjects were presented simultaneously with a familiar and novel juvenile. All groups except the male treatment group were able to recognize the familiar animal, evidenced by the decreased investigation of the familiar relative to the novel animal. C) Discrimination Index. Two-way ANOVA identified the treatment female DI as significantly higher than the treatment male DI. * P < 0.05, ** P < 0.01, *** P < 0.001
Treatment males were the only subjects with deficient social memory and could not distinguish familiar and novel stimulus animals following a one-hour delay (P = 0.32, ratio paired t-test of familiar vs. novel, Fig. 7B). All other groups preferentially explored the novel stimulus animal (control male P = 0.037, control female P = 0.0202, treatment female P < 0.0001, ratio paired t-test of familiar vs. novel, Fig. 7B). The male treatment DI was also significantly lower than the female treatment DI (F(1,46) = 6.5, P = 0.04; two-way ANOVA, Bonferonni post-test, Fig. 7C).
Discussion
The major finding of this study is that while neuronal death occurs similarly in the hippocampus and thalamic nuclei of both sexes immediately following isoflurane exposure, there is a profound difference in behavioral outcomes. Males are impaired in multiple tasks that rely on allocentric cues, association of contextual details, and social recognition. Anesthetized females, meanwhile, were unaffected in these tasks. This suggests that males and females follow distinct paths of neural and cognitive development after an early anesthetic-mediated effect on the brain. Both male and female rats exhibit extensive neuronal death, yet drastic behavioral impairment is manifested only in male subjects. This observation not only identifies a worse behavioral outcome in males but also challenges the theory that neuronal death has a causative relationship.
The lack of studies investigating sex differences after anesthesia exposure may be due, in part, to intrinsic difficulties faced when comparing subjects with species and sex-specific differences in memory. In rats, females generally outperform males in tasks of object recognition, while males are better at tasks of spatial learning and memory 30. Studies in mice are variable with some reporting either no difference or male superiority in object recognition 31,32, while others find females to be better in object discrimination 33,34. An investigation in Long-Evans rats shows that age and sex could influence social recognition ability, although estrous cycle appeared to have no effect 35. The differences we find between treatment animals of each sex may be attributed partially to innate variability in learning and memory; however, this alone likely does not account for the significant disparity in behavior. By using a series of tasks with increasing difficulty we are able to make comparisons along a spectrum, and it is doubtful that memory in females is robust enough to completely mask an insult to the developing brain, while males are unable to distinguish between targets in any task relying on associative or social memory.
Male impairment in memory may be related to early anesthetic effects on the developing brain. Associative memory involves learning relationships among distinct elements and is necessary for understanding the significance of combinations of cues 36,37. Hippocampal and thalamic lesions in rats affect associative memory and cause impaired learning of spatial relationships between elements 37,38 and object-place recognition 27. Social recognition relies on the interaction of multiple brain regions and hormones, including oxytocin and vasopressin 39-41. The lateral septum contains high numbers of vasopressin receptors 42 and has reciprocal connections to higher order brain regions including the thalamus and hippocampus 43,44. Anesthesia exposure early in life causing brain cell death, which is particularly profound in the thalamus 4,6,7,21, may potentially contribute to the behavioral deficits we observed. Unlike Satomoto et al, we did not observe abnormal social behavior, and both male and female treatment subjects displayed the same social interaction as controls; therefore, our findings do not support a correlation between anesthesia and autism-like behavior, and the impairment in social recognition is more likely a consequence of memory encoding than innate social behavior.
Importantly, females suffer a similar extent of cell death yet do not display behavioral deficits. A possible explanation is that females have improved recovery following neurotoxic effects of volatile anesthetics, especially since many studies find protective effects of hormones that are expressed predominantly in females 45-47. Brain-derived neurotrophic factor (BDNF) is a secreted protein involved in the survival, growth, and development of neurons and proper synapse formation 48,49, and estrogen has been shown to increase the expression of BDNF 10,50-52. Anesthetics not only cause cell death but have been shown to result in significant neuroinflammation 53, in addition to changes in cell signaling 54, stem cell proliferation 55, and synapse formation 56,57; these effects could be mitigated by protective properties of estrogen and progesterone 45-47,58,59. Due to hormonal differences, females may be more resilient in their ability to recover and form proper connections after anesthesia-induced effects on neural development. This might explain why females in our study were unaltered while males were profoundly impaired in tests of both associative memory and social recognition.
The timing of isoflurane exposure in the rats at postnatal day 7 occurs during a period of peak neural development and synaptogenesis, which overlaps with a corresponding stage of development in humans in the late 3rd trimester through the first several months of life depending on the specific brain region of interest60. The subjects in our study were tested during adolescence, which is when most retrospective human trials identify a cognitive deficit in children after anesthesia1,2. Thus, the timing of anesthetic exposure and subsequent cognitive outcomes are consistent between species, which suggests that similar processes of neural development may be affected in both. Still, rodents are simply a model, and it is important to understand the differences in the overall complexity and timing of development between species when interpreting the experimental findings in animals and how they apply to humans.
There are certain other limitations to consider when interpreting our results. While a deficit was not identified in the female treatment subjects, this does not necessarily mean that females were entirely unaffected. A behavioral deficit might be revealed by increasing the difficulty of the tasks (for instance, prolonging delay between exposure and memory retrieval). Other factors that may play a role in the cognitive outcomes of male and female subjects in the study include possible differences between sexes in their response to food restriction, activity patterns during the day, as well as hormone cycling, which might influence behavioral findings and should be kept in mind. Also, a comprehensive analysis of neuronal death was not undertaken, and it is possible that other brain regions show a difference. The hippocampus and thalamus were chosen, however, because of their underlying role in the investigated behavior. Finally, there are inherent limitations in anesthetizing P7 animals that expose them to physiologic changes, notably that of hypercarbia 6. Although physiologic parameters are difficult to reliably monitor or control in P7 rodents, blood gas analyses (pH, pO2, pCO2) can be measured and reveal no difference between males and females, and hypercarbia alone does not predispose subjects to memory deficits 6.
The mechanisms by which anesthetics alter long-term behavior remain unknown. Within the nervous system, processes of brain cell death, changes in synapses, and alteration of stem cell function have been implicated in behavioral deficits. To date, no studies have conclusively tied behavior to a neuro-anatomic or neuro-chemical change. The results of this study suggest that brain cell death alone is not sufficient to account for the cognitive dysfunction since both sexes had equivalent cell death. Future studies exploring sex-specific outcomes will help elucidate these effects and lead to a better understanding of the roles of sex and hormones on brain development and protection.
Highlights.
Isoflurane exposure in infancy induces equivalent neurodegeneration in male and female rats
Male rats anesthetized as newborns suffer long-term problems with contextual learning and memory
Female rats did not exhibit long-term cognitive impairment after anesthesia
Male and female rats have distinct cognitive outcomes in spite of similar neuronal death
Acknowledgments
Funding provided by grant award GM086511 to JWS from National institutes of Health, Bethesda, MD. UCSF Department of Anesthesia and Perioperative Care Hamilton Award to JWS. The authors declare no competing financial interests.
Funding and Disclosure: This work was supported by NIH Grant Award GM086511 and UCSF Department of Anesthesia and Perioperative Care Hamilton Award to Dr. Jeffrey Sall.
Footnotes
Conflict of Interest: The authors have no conflicts of interest to report.
Author Contributions: JS and BL designed the project, analyzed data, and wrote the first draft of the manuscript. JC, EK, KP, and JL designed and analyzed data from cell death and behavioral experiments. All authors contributed to the final draft of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, Sprung J, Weaver AL, Schroeder DR, Warner DO. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128:e1053–61. doi: 10.1542/peds.2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gentry KR, Steele LM, Sedensky MM, Morgan PG. Early developmental exposure to volatile anesthetics causes behavioral defects in Caenorhabditis elegans. Anesth Analg. 2013;116:185–9. doi: 10.1213/ANE.0b013e31826d37c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–82. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Zhang X, Dissen GA, Creeley CE, Olney JW. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–41. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stratmann G, May LD, Sall JW, Alvi RS, Bell JS, Ormerod BK, Rau V, Hilton JF, Dai R, Lee MT, Visrodia KH, Ku B, Zusmer EJ, Guggenheim J, Firouzian A. Effect of hypercarbia and isoflurane on brain cell death and neurocognitive dysfunction in 7-day-old rats. Anesthesiology. 2009;110:849–61. doi: 10.1097/ALN.0b013e31819c7140. [DOI] [PubMed] [Google Scholar]
- 7.Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, Imaki J. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–37. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- 8.Istaphanous GK, Howard J, Nan X, Hughes EA, McCann JC, McAuliffe JJ, Danzer SC, Loepke AW. Comparison of the neuroapoptotic properties of equipotent anesthetic concentrations of desflurane, isoflurane, or sevoflurane in neonatal mice. Anesthesiology. 2011;114:578–87. doi: 10.1097/ALN.0b013e3182084a70. [DOI] [PubMed] [Google Scholar]
- 9.Ramage TM, Chang FL, Shih J, Alvi RS, Quitoriano GR, Rau V, Barbour KC, Elphick SA, Kong CL, Tantoco NK, Ben-Tzur D, Kang H, McCreery MS, Huang P, Park A, Uy J, Rossi MJ, Zhao C, Di Geronimo RT, Stratmann G, Sall JW. Distinct long-term neurocognitive outcomes after equipotent sevoflurane or isoflurane anaesthesia in immature rats. Br J Anaesth. 2013 doi: 10.1093/bja/aet103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu LX, Yon JH, Carter LB, Jevtovic-Todorovic V. General anesthesia activates BDNF-dependent neuroapoptosis in the developing rat brain. Apoptosis. 2006;11:1603–15. doi: 10.1007/s10495-006-8762-3. [DOI] [PubMed] [Google Scholar]
- 11.Head BP, Patel HH, Niesman IR, Drummond JC, Roth DM, Patel PM. Inhibition of p75 neurotrophin receptor attenuates isoflurane-mediated neuronal apoptosis in the neonatal central nervous system. Anesthesiology. 2009;110:813–25. doi: 10.1097/ALN.0b013e31819b602b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roof RL, Hall ED. Gender differences in acute CNS trauma and stroke: neuroprotective effects of estrogen and progesterone. J Neurotrauma. 2000;17:367–88. doi: 10.1089/neu.2000.17.367. [DOI] [PubMed] [Google Scholar]
- 13.Ottochian M, Salim A, Berry C, Chan LS, Wilson MT, Margulies DR. Severe traumatic brain injury: is there a gender difference in mortality? Am J Surg. 2009;197:155–8. doi: 10.1016/j.amjsurg.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Kesler SR, Ment LR, Vohr B, Pajot SK, Schneider KC, Katz KH, Ebbitt TB, Duncan CC, Makuch RW, Reiss AL. Volumetric analysis of regional cerebral development in preterm children. Pediatr Neurol. 2004;31:318–25. doi: 10.1016/j.pediatrneurol.2004.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayoral SR, Omar G, Penn AA. Sex differences in a hypoxia model of preterm brain damage. Pediatr Res. 2009;66:248–53. doi: 10.1203/PDR.0b013e3181b1bc34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiMaggio C, Sun LS, Li G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg. 2011;113:1143–51. doi: 10.1213/ANE.0b013e3182147f42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen TG, Pedersen JK, Henneberg SW, Pedersen DA, Murray JC, Morton NS, Christensen K. Academic performance in adolescence after inguinal hernia repair in infancy: a nationwide cohort study. Anesthesiology. 2011;114:1076–85. doi: 10.1097/ALN.0b013e31820e77a0. [DOI] [PubMed] [Google Scholar]
- 18.Jevtovic-Todorovic V, Absalom AR, Blomgren K, Brambrink A, Crosby G, Culley DJ, Fiskum G, Giffard RG, Herold KF, Loepke AW, Ma D, Orser BA, Planel E, Slikker W, Jr, Soriano SG, Stratmann G, Vutskits L, Xie Z, Hemmings HC., Jr Anaesthetic neurotoxicity and neuroplasticity: an expert group report and statement based on the BJA Salzburg Seminar. Br J Anaesth. 2013;111:143–51. doi: 10.1093/bja/aet177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olney JW, Ishimaru MJ, Bittigau P, Ikonomidou C. Ethanol-induced apoptotic neurodegeneration in the developing brain. Apoptosis. 2000;5:515–21. doi: 10.1023/a:1009685428847. [DOI] [PubMed] [Google Scholar]
- 20.Stratmann G, Sall JW, May LD, Bell JS, Magnusson KR, Rau V, Visrodia KH, Alvi RS, Ku B, Lee MT, Dai R. Isoflurane differentially affects neurogenesis and long-term neurocognitive function in 60-day-old and 7-day-old rats. Anesthesiology. 2009;110:834–48. doi: 10.1097/ALN.0b013e31819c463d. [DOI] [PubMed] [Google Scholar]
- 21.Shih J, May LD, Gonzalez HE, Lee EW, Alvi RS, Sall JW, Rau V, Bickler PE, Lalchandani GR, Yusupova M, Woodward E, Kang H, Wilk AJ, Carlston CM, Mendoza MV, Guggenheim JN, Schaefer M, Rowe AM, Stratmann G. Delayed environmental enrichment reverses sevoflurane-induced memory impairment in rats. Anesthesiology. 2012;116:586–602. doi: 10.1097/ALN.0b013e318247564d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boscolo A, Ori C, Bennett J, Wiltgen B, Jevtovic-Todorovic V. Mitochondrial protectant pramipexole prevents sex-specific long-term cognitive impairment from early anaesthesia exposure in rats. Br J Anaesth. 2013 doi: 10.1093/bja/aet073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simpson J, Kelly JP. An investigation of whether there are sex differences in certain behavioural and neurochemical parameters in the rat. Behav Brain Res. 2012;229:289–300. doi: 10.1016/j.bbr.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 24.Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav. 2001;74:435–40. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- 25.Eacott MJ, Norman G. Integrated memory for object, place, and context in rats: a possible model of episodic-like memory? J Neurosci. 2004;24:1948–53. doi: 10.1523/JNEUROSCI.2975-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langston RF, Wood ER. Associative recognition and the hippocampus: differential effects of hippocampal lesions on object-place, object-context and object-place-context memory. Hippocampus. 2010;20:1139–53. doi: 10.1002/hipo.20714. [DOI] [PubMed] [Google Scholar]
- 27.Cross L, Brown MW, Aggleton JP, Warburton EC. The medial dorsal thalamic nucleus and the medial prefrontal cortex of the rat function together to support associative recognition and recency but not item recognition. Learn Mem. 2012;20:41–50. doi: 10.1101/lm.028266.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stratmann G, Sall JW, Eger EI, 2nd, Laster MJ, Bell JS, May LD, Eilers H, Krause M, Heusen F, Gonzalez HE. Increasing the duration of isoflurane anesthesia decreases the minimum alveolar anesthetic concentration in 7-day-old but not in 60-day-old rats. Anesth Analg. 2009;109:801–6. doi: 10.1213/ane.0b013e3181aff364. [DOI] [PubMed] [Google Scholar]
- 29.Miyajima A, Sunouchi M, Mitsunaga K, Yamakoshi Y, Nakazawa K, Usami M. Sexing of postimplantation rat embryos in stored two-dimensional electrophoresis (2-DE) samples by polymerase chain reaction (PCR) of an Sry sequence. J Toxicol Sci. 2009;34:681–5. doi: 10.2131/jts.34.681. [DOI] [PubMed] [Google Scholar]
- 30.Saucier DM, Shultz SR, Keller AJ, Cook CM, Binsted G. Sex differences in object location memory and spatial navigation in Long-Evans rats. Anim Cogn. 2008;11:129–37. doi: 10.1007/s10071-007-0096-1. [DOI] [PubMed] [Google Scholar]
- 31.Frick KM, Gresack JE. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav Neurosci. 2003;117:1283–91. doi: 10.1037/0735-7044.117.6.1283. [DOI] [PubMed] [Google Scholar]
- 32.Benice TS, Rizk A, Kohama S, Pfankuch T, Raber J. Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience. 2006;137:413–23. doi: 10.1016/j.neuroscience.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 33.Bettis T, Jacobs LF. Sex differences in object recognition are modulated by object similarity. Behav Brain Res. 2012;233:288–92. doi: 10.1016/j.bbr.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 34.Bettis TJ, Jacobs LF. Sex-specific strategies in spatial orientation in C57BL/6J mice. Behav Processes. 2009;82:249–55. doi: 10.1016/j.beproc.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Markham JA, Juraska JM. Social recognition memory: influence of age, sex, and ovarian hormonal status. Physiol Behav. 2007;92:881–8. doi: 10.1016/j.physbeh.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aggleton JP, Sanderson DJ, Pearce JM. Structural learning and the hippocampus. Hippocampus. 2007;17:723–34. doi: 10.1002/hipo.20323. [DOI] [PubMed] [Google Scholar]
- 37.Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learn Mem. 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piterkin P, Cole E, Cossette MP, Gaskin S, Mumby DG. A limited role for the hippocampus in the modulation of novel-object preference by contextual cues. Learn Mem. 2008;15:785–91. doi: 10.1101/lm.1035508. [DOI] [PubMed] [Google Scholar]
- 39.Bluthe RM, Schoenen J, Dantzer R. Androgen-dependent vasopressinergic neurons are involved in social recognition in rats. Brain Res. 1990;519:150–7. doi: 10.1016/0006-8993(90)90073-k. [DOI] [PubMed] [Google Scholar]
- 40.Landgraf R, Gerstberger R, Montkowski A, Probst JC, Wotjak CT, Holsboer F, Engelmann M. V1 vasopressin receptor antisense oligodeoxynucleotide into septum reduces vasopressin binding, social discrimination abilities, and anxiety-related behavior in rats. J Neurosci. 1995;15:4250–8. doi: 10.1523/JNEUROSCI.15-06-04250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lukas M, Bredewold R, Landgraf R, Neumann ID, Veenema AH. Early life stress impairs social recognition due to a blunted response of vasopressin release within the septum of adult male rats. Psychoneuroendocrinology. 2011;36:843–53. doi: 10.1016/j.psyneuen.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 42.De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273:307–17. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- 43.Caffe AR, van Leeuwen FW, Luiten PG. Vasopressin cells in the medial amygdala of the rat project to the lateral septum and ventral hippocampus. J Comp Neurol. 1987;261:237–52. doi: 10.1002/cne.902610206. [DOI] [PubMed] [Google Scholar]
- 44.Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25:1565–74. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 45.Ishihara Y, Kawami T, Ishida A, Yamazaki T. Allopregnanolone-mediated protective effects of progesterone on tributyltin-induced neuronal injury in rat hippocampal slices. J Steroid Biochem Mol Biol. 2013;135:1–6. doi: 10.1016/j.jsbmb.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 46.Brinton RD, Tran J, Proffitt P, Montoya M. 17 beta-Estradiol enhances the outgrowth and survival of neocortical neurons in culture. Neurochem Res. 1997;22:1339–51. doi: 10.1023/a:1022015005508. [DOI] [PubMed] [Google Scholar]
- 47.Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 2008;11:334–43. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- 48.Bath KG, Schilit A, Lee FS. Stress effects on BDNF expression: effects of age, sex, and form of stress. Neuroscience. 2013;239:149–56. doi: 10.1016/j.neuroscience.2013.01.074. [DOI] [PubMed] [Google Scholar]
- 49.Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–37. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- 50.Sato K, Akaishi T, Matsuki N, Ohno Y, Nakazawa K. beta-Estradiol induces synaptogenesis in the hippocampus by enhancing brain-derived neurotrophic factor release from dentate gyrus granule cells. Brain Res. 2007;1150:108–20. doi: 10.1016/j.brainres.2007.02.093. [DOI] [PubMed] [Google Scholar]
- 51.Blurton-Jones M, Tuszynski MH. Estradiol-induced modulation of estrogen receptor-beta and GABA within the adult neocortex: a potential transsynaptic mechanism for estrogen modulation of BDNF. J Comp Neurol. 2006;499:603–12. doi: 10.1002/cne.21122. [DOI] [PubMed] [Google Scholar]
- 52.Begliuomini S, Casarosa E, Pluchino N, Lenzi E, Centofanti M, Freschi L, Pieri M, Genazzani AD, Luisi S, Genazzani AR. Influence of endogenous and exogenous sex hormones on plasma brain-derived neurotrophic factor. Hum Reprod. 2007;22:995–1002. doi: 10.1093/humrep/del479. [DOI] [PubMed] [Google Scholar]
- 53.Shen X, Dong Y, Xu Z, Wang H, Miao C, Soriano SG, Sun D, Baxter MG, Zhang Y, Xie Z. Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology. 2013;118:502–15. doi: 10.1097/ALN.0b013e3182834d77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Masaki E, Kawamura M, Kato F. Attenuation of gap-junction-mediated signaling facilitated anesthetic effect of sevoflurane in the central nervous system of rats. Anesth Analg. 2004;98:647–52. doi: 10.1213/01.ane.0000103259.72635.72. table of contents. [DOI] [PubMed] [Google Scholar]
- 55.Sall JW, Stratmann G, Leong J, McKleroy W, Mason D, Shenoy S, Pleasure SJ, Bickler PE. Isoflurane inhibits growth but does not cause cell death in hippocampal neural precursor cells grown in culture. Anesthesiology. 2009;110:826–33. doi: 10.1097/ALN.0b013e31819b62e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lunardi N, Ori C, Erisir A, Jevtovic-Todorovic V. General anesthesia causes long-lasting disturbances in the ultrastructural properties of developing synapses in young rats. Neurotox Res. 2010;17:179–88. doi: 10.1007/s12640-009-9088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Briner A, Nikonenko I, De Roo M, Dayer A, Muller D, Vutskits L. Developmental Stage-dependent persistent impact of propofol anesthesia on dendritic spines in the rat medial prefrontal cortex. Anesthesiology. 2011;115:282–93. doi: 10.1097/ALN.0b013e318221fbbd. [DOI] [PubMed] [Google Scholar]
- 58.Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18:2550–9. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwarz JM, McCarthy MM. Steroid-induced sexual differentiation of the developing brain: multiple pathways, one goal. J Neurochem. 2008;105:1561–72. doi: 10.1111/j.1471-4159.2008.05384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–33. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]


