Abstract
Endometriosis is an estrogen-dependent disease. The biologically active estrogen, estradiol, aggravates the pathological processes (e.g., inflammation and growth) and the symptoms (e.g., pain) associated with endometriosis. Abundant quantities of estradiol are available for endometriotic tissue via several mechanisms including local aromatase expression. The question remains, then, what mediates estradiol action. Because estrogen receptor (ER)β levels in endometriosis are >100 times higher than those in endometrial tissue, this review focuses on this nuclear receptor. Deficient methylation of the ERβ promoter results in pathological overexpression of ERβ in endometriotic stromal cells. High levels of ERβ suppress ERα expression. A severely high ERβ-to-ERα ratio in endometriotic stromal cells is associated with suppressed progesterone receptor and increased cyclo-oxygenase-2 levels contributing to progesterone resistance and inflammation. ERβ-selective estradiol antagonists may serve as novel therapeutics of endometriosis in the future.
Keywords: ER-β, nuclear receptor, estrogen, DNA methylation, epigenetic, promoter, ER-α, PR
Endometriosis is a chronic disease defined as the presence of endometrium-like tissue outside of the uterine cavity. Endometriosis is one of the most common causes of infertility and chronic pelvic pain and affects 1 in 10 women of reproductive age.1–3 Its incidence is estimated to be as high as 30% in patients with infertility and 45% in patients with chronic pelvic pain.3 Similar to other common chronic diseases, such as diabetes mellitus and asthma, endometriosis is possibly inherited in a polygenic manner and has a complex and multifactorial etiology.4 There is a sevenfold increase in the incidence of endometriosis in relatives of women with this disease compared with disease-free women.4,5 The most striking aspect of endometriosis is its dependence on estrogen for growth, similar to that seen in eutopic endometrium.6,7
Endometriosis causes infertility via impairing the tubal anatomy or function and also decreasing egg quality, embryo quality, and the rate of implantation. Assisted reproductive technologies constitute the first line of treatment of infertility associated with endometriosis.1 Endometriosis may cause pelvic pain via stimulating nerve endings in the ectopic peritoneal and eutopic endometrial tissue.8 Endometriosis-related pain has conventionally been treated with endocrine agents such as synthetic progestins, oral contraceptives, or gonadotropin-releasing hormone analogs.1 These treatments, which interrupt ovulation and ovarian estrogen production, are successful in only half of the patients treated, however.9–11 Treatment with aromatase inhibitors in combination with an ovulation suppressor has successfully been used in cases refractory to these conventional measures of pain management.12 Patients often develop resistance to repeated treatments with the same agent over a period of 6 months to 3 years.12 Because an average patient may need repeated treatments with various drugs during her reproductive lifespan, there is a clear need to identify novel molecular pathways that can be targeted with emerging therapeutic agents.
Here we discuss the mechanisms responsible for the strikingly high expression of estrogen receptor (ER)β and its targets in endometriosis. This low response rate is likely further reduced with repeated treatment attempts. New translational perspectives include treating endometriosis with new ERβ-selective compounds. Some consideration has been given to determining the effect of an ERβ ligand on endometriosis.13 No further clinical information, however, has come forth. ERβ-selective compounds may constitute a new class of drugs for the treatment of endometriosis-associated pain in the future.
Gene Expression in Endometrium and Endometriosis
We use the terms endometriotic tissue or endometriosis interchangeably in reference to the endometrium-like tissues that are present in the pelvic peritoneum or ovaries. However, the terms endometrial tissue or endometrium refer to uterine mucosa, which is appropriately located within the uterine cavity. A biological distinction is also made between endometrium from disease-free women versus women with endometriosis. Sampson proposed the most widely accepted mechanism for the development of endometriosis on pelvic peritoneal surfaces as the implantation of endometrial tissue on the peritoneum through retrograde menstruation. Because retrograde menstruation occurs in >90% of all women, endometriosis is believed to be caused by molecular defects that favor survival and establishment of endometrial tissue in menstrual debris on the peritoneum.14–16 Gene expression profiles characterized by microarray in the endometrium of women with or without endometriosis showed that a large number of genes were dysregulated.17,18 These findings suggested that the eutopic endometrium of women with endometriosis exhibit the pathology found within endometriotic tissue.17,19 This abnormal pattern of gene expression could also be traced to primary stromal cells isolated from endometriotic tissue, eutopic endometrium from endometriosis, and eutopic endometrium from disease-free women.7,20
Estrogen Production in Endometriosis
Estradiol and progesterone are master regulators of endometrial tissue. Each steroid hormone is estimated to regulate expression of hundreds of genes during various phases of the menstrual cycle.21 Endometriotic and eutopic endometrial tissues respond to estradiol and progesterone with apparently similar histological changes, and both tissues contain immunoreactive estrogen and progesterone receptors (PRs). The eutopic endometrium predictably becomes atrophic in response to prolonged progestin therapy or oral contraceptives that contain progestins. Treatment with these agents, however, does not predictably suppress endometriotic tissue growth. Endometriotic tissue in ectopic locations, such as the peritoneum or ovary, is fundamentally different from eutopic endometrium within the uterus in terms of the production of cytokines and prostaglandins, estrogen biosynthesis and metabolism, and clinical response to progestins.11,22,23 There are substantial molecular differences with regard to progesterone response between normal endometrium and eutopic and ectopic tissues from women with endometriosis.17,24,25
Estradiol is the biologically active estrogen. It is produced in primarily three body sites in a woman with endometriosis.26 In all of these sites, expression of the enzyme aromatase is essential for estradiol production; additionally, several other steroidogenic proteins are expressed and complement aromatase activity for the production of estradiol (Fig. 1).26 The classical site for estrogen production is the ovary.26 The theca and granulosa cells of a preovulatory follicle convert cholesterol to estradiol that is actively secreted into the circulation in a cyclic fashion (Fig. 2).26 The second group of body sites is collectively referred to as the peripheral tissues, including bulky tissues such as fat, skin, and skeletal muscle, all of which express aromatase.26 In these peripheral tissues, circulating androstenedione is converted to estrone, which is further converted to estradiol. Peripheral tissues do not secrete estradiol in a classical sense, but because of their large quantity, they produce sufficient levels of estradiol to raise its blood levels, particularly in obese women.26 The third site for estradiol production is the endometriotic tissue itself (Fig. 1). The endometriotic stromal cell uniquely expresses the full complement of genes in the steroidogenic cascade, which is sufficient to convert cholesterol to estradiol.26
Figure 1.
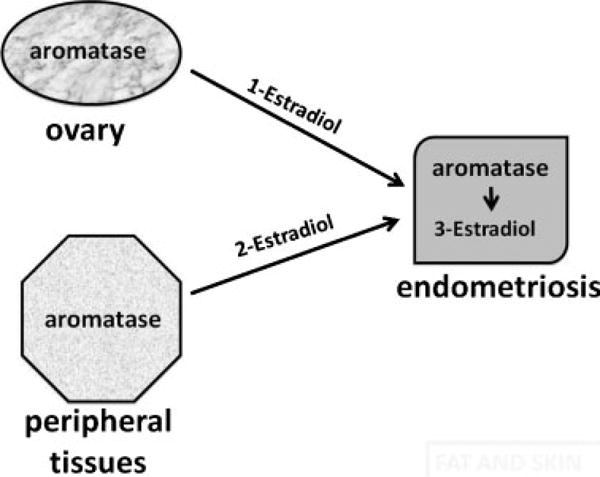
Estradiol production in endometriosis. Aromatase is encoded by a single gene and represents the rate-limiting step for estradiol biosynthesis. In a premenopausal woman with endometriosis, estradiol arises from three major tissue sites that express aromatase. (1) Aromatase is expressed under the influence of follicle-stimulating hormone and accounts for fluctuating serum estradiol levels. (2) Aromatase is also present in peripheral tissues such as the adipose tissue and is responsible for relatively small but clinically significant quantities of circulating estradiol levels. (3) Estradiol is produced locally in endometriosis per se via the presence of aromatase and other steroidogenic enzymes in this pathological tissue.
Figure 2.
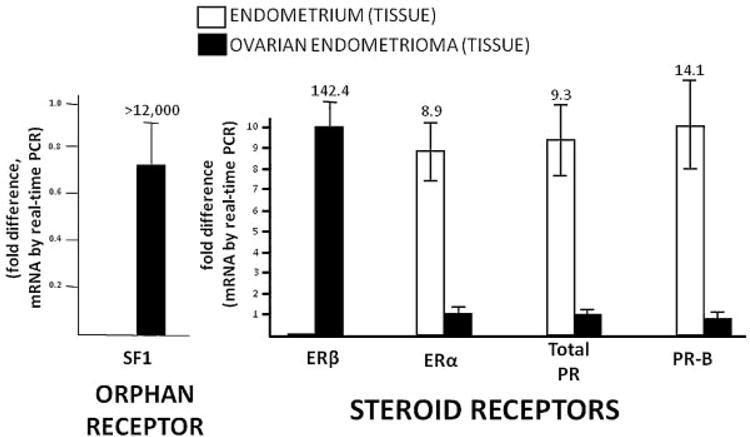
Nuclear receptor expression in endometriosis. A subfamily of nuclear receptors may act as transcription factors in a ligand-independent fashion. Steroidogenic factor (SF)1 belongs to the orphan nuclear receptor subfamily. Members of the steroid receptor subfamily, on the other hand, interact with ligands that activate their transcriptional activity at multiple gene promoters across the human genome. The estrogen receptors (ER)α and ERβ and the progesterone receptor (PR) are activated by their ligands, estradiol and progesterone, respectively. PR-B is the full-length variant of PR. These nuclear receptors are differentially expressed between endometrial and endometriotic tissues. The numbers on columns represent fold expression between the tissues. Endometrial tissues were obtained from five disease-free women; ovarian endometrioma walls were collected from a separate group of five women. Error bars represent standard error of mean. PCR, polymerase chain reaction.
Nuclear Receptor Expression in Endometriosis
Estrogen or progesterone action is mediated primarily by their nuclear receptors, abbreviated as ER and PR. The steroids estradiol and progesterone interact with their respective receptors and activate them to act as transcription factors. In contrast to ER and PR, another group of nuclear receptors, named orphan nuclear receptors, do not have any known ligands. Steroidogenic factor (SF)1 belongs to this latter group. Because of the roles of estrogen and progesterone in endometrium, we assessed the expression of these key nuclear receptors in endometriosis (Fig. 2).
Circumstantial and laboratory evidence strongly support the notion that estradiol is a key hormone for the growth and persistence of endometriotic tissue as well as inflammation and pain associated with it. Estradiol, which reaches endometriosis by circulation or is produced locally in endometriotic tissue, acts as a steroid hormone to regulate growth of endometriotic tissue. Estradiol enters cells and binds to the ER in estrogen-responsive cells. ER subtypes α and β are proteins with high affinity for estradiol and are encoded by separate genes. The classical human ERα was cloned in 1986, and a second estrogen receptor, ERβ, was cloned from rat prostate and human testis in 1996.27–29 Although both ERα and ERβ are present in the endometrium, ERα seems to be the primary mediator of the estrogenic action in this tissue.30
Despite its sensitivity to estrogen, endometriosis appears to contain a unique complement of steroid hormone receptors compared with that of its normal tissue counterpart, the eutopic endometrium. For example, several investigators reported markedly higher levels of ERβ and lower levels of ERα in human endometriotic tissues and primary stromal cells compared with eutopic endometrial tissues and cells.31,32 The levels of both isoforms of PR, particularly PR-B, are significantly lower in endometriosis compared with eutopic endometrium.6,33
The estradiol-receptor complex acts as a transcription factor that becomes associated with the promoters of estradiol-responsive genes via direct DNA binding or binding to other docking transcription factors at basal promoter regions.34 This interaction brings about ER-specific initiation of gene transcription, which promotes the synthesis of specific mRNAs and proteins.34 PR is one of many estradiol-responsive genes, and estradiol acts in eutopic endometrial tissues and stromal cells to promote endometrial responsiveness to progesterone.35 In contrast, PR mRNA and protein levels are not elevated in biopsied endometriotic tissues exposed to high estradiol levels during late proliferative phase or in endometriotic cells treated with estradiol, indicating that estradiol-induction PR expression in endometriosis is markedly blunted.33
In addition to ERα, ERβ, and PR, the orphan nuclear receptor SF1 is also differentially regulated in endometriosis versus eutopic endometrium (Fig. 2). SF1 is responsible for coordinately activating the full steroidogenic cascade of genes including aromatase. The protein products of this set of steroidogenic genes are capable of converting cholesterol to estradiol locally in endometriotic tissue.36 We recently used real-time polymerase chain reaction (RT-PCR) to compare tissue mRNA levels of these key nuclear receptors in endometriosis and eutopic endometrium (Fig. 2). Ovarian endometriotic tissue SF1 and ERβ mRNA levels were >12,000 times and 142 times higher than in endometrium, respectively. In contrast, ERα, PR, and PR-B levels were remarkably lower in endometriotic tissue (Fig. 2). The role of SF-1 in endometriosis has been reviewed elsewhere.37 This review covers primarily ERβ expression and action in endometriosis.
Primary Endometriotic Stromal Cells Mimic Tissue Levels of ERα, ERβ, and PR mRNA and Protein Levels
Differential expression of nuclear receptors in endometriotic versus endometrial tissues are preserved in stromal cells cultured from these tissues. Differential expression of steroid receptors in endometrial and endometriotic tissues and cells has been consistently reported.31,33 RT-PCR was used to quantify the mRNA levels of nuclear receptors in primary endometrial and endometriotic stromal cells. ERα mRNA levels were significantly lower (sevenfold) in endometriotic stromal cells compared with endometrial stromal cells. ERβ mRNA was strikingly higher (~34-fold) in endometriotic stromal cells, whereas it was much lower or nearly absent in endometrial stromal cells.38 The ratios of ERα to ERβ mRNA levels were, on average, 841 and 21 in endometrial and endometriotic stromal cells, respectively.38 Total PR and PR-B mRNA levels in endometriotic stromal cells were significantly lower than those in endometrial stromal cells.38 Protein levels of ERα and ERβ were significantly different in these two groups similar to the findings regarding mRNA levels.38 In conclusion, ERα, ERβ and PR levels were markedly different in endometrial versus endometriotic tissues or stromal cells derived from these tissues. Endometriotic stromal cells contained extraordinarily higher ERβ and significantly lower ERα and PR levels compared with endometrial stromal cells.
Epigenetic Regulation of the ERβ Promoter is Responsible for Its High Levels in Endometriosis
Because of the extreme differential expression of ERβ between endometriotic and endometrial cells, we tested the hypothesis that alteration in DNA methylation is a mechanism responsible for severely increased ERβ mRNA levels in endometriotic cells.38 We identified a CpG island occupying the promoter region of the ERβ gene (Fig. 3). Bisulfite sequencing of this region showed significantly higher methylation in primary endometrial cells versus endometriotic cells. Treatment with a demethylating agent significantly increased ERβ mRNA levels in endometrial cells. The critical region that confers promoter activity also bears the identical CpG island (Fig. 3).38 The activity of the ERβ promoter was strongly inactivated by in vitro methylation. Therefore, methylation of a CpG island at the ERβ promoter region is a primary mechanism responsible for differential expression of ERβ in endometriosis and endometrium.38 Thus high ERβ mRNA and protein expression in endometriotic cells were mediated by an epigenetic defect involving hypomethylation of a CpG island occupying its promoter (Fig. 3).
Figure 3.
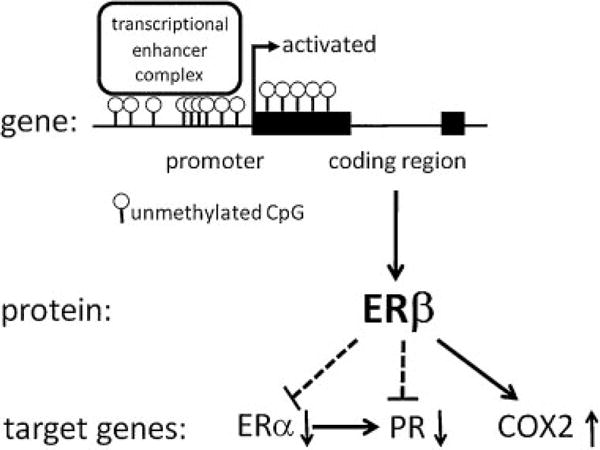
Epigenetic regulation of the estrogen receptor (ER)β gene and its downstream effects in endometriosis. In endometriotic stromal cells, a dense CpG island at ERβ promoter remains in an unmethylated state. This is associated with the presence of a transcriptional enhancer complex, which activates this promoter. In endometrial stromal cells, in contrast, this CpG island is heavily methylated and suppresses ERβ expression. In endometriotic cells, pathologically high ERβ levels suppress ERα and progesterone receptor (PR) (dotted lines) and stimulate cyclo-oxygenase (COX)2 expression (solid line).
ERβ Is Responsible for Low ERα Expression in Endometriotic Stromal Cells
ERα mRNA and protein levels are several fold lower in endometriotic tissue and stromal cells compared with endometrial tissue and stromal cells. ERα deficiency in endometriosis may be responsible for the failure of estradiol to induce PR expression, thus contributing to secondary PR deficiency and progesterone resistance in women with this disease. In vivo observations strongly suggest that estradiol induces ERα expression in mouse uterine tissue.39 It is quite likely that estradiol also plays a key role in regulating ERα expression in human endometrial stromal cells. However, strikingly high quantities of estradiol produced via local aromatase activity in addition to high ERβ levels in stromal cells of endometriosis may perturb this regulation and may suppress ERα expression.26,38 We tested the hypothesis whether ERβ is responsible for suppressing ERα promoter activity and mRNA and protein expression in endometriotic cells (Fig. 3). The human ERα gene is regulated via multiple promoters; the three major promoters are A, B, and C and are alternatively used in various tissues.40–42 Promoters A and B are located within the 2-kb region proximal to the translation start site, whereas promoter C lies some 101 kb upstream of this site.40,43
Primary endometriotic stromal cells in culture were utilized to determine the role of ERβ in estradiol-dependent regulation of the ERα gene.44 ERβ knockdown significantly increased ERα mRNA and protein levels in endometriotic stromal cells.44 Conversely, ERβ overexpression in endometrial stromal cells decreased ERα mRNA and protein levels. ERβ knockdown significantly decreased proliferation of endometriotic stromal cells.44 ERβ knockdown or overexpression most drastically altered ERα mRNA species arising from the far distal promoter C.
We screened the three ERα promoter regions using serial chromatin immunoprecipitation assays for binding of ERβ or ERα itself. Estradiol enhanced binding of both ERα and ERβ to a region containing a nonclassical activator protein (AP)-1 motif in promoter A in endometriotic cells. We investigated several regions of ERα promoter C, which lies some 101 kb upstream of the common splice junction. In the presence of estradiol, ERβ bound to a genomic region flanking an AP1 site upstream of promoter C and a region bearing a specificity protein (Sp)1 motif immediately downstream of promoter C in endometriotic stromal cells. In contrast, ERα bound to neither promoter C sequences. In summary, these findings collectively suggest that primarily ERα promoter C may mediate ERβ-mediated inhibition of ERα expression in endometriotic stromal cells (Fig. 3). A role of ERα promoter A could not be ruled out. Knockdown studies also suggested a positive role of ERβ on endometriotic stromal cell cycle progression and proliferation.
Possible PR Suppression in the Presence of High ERβ-to-ERα Ratio in Endometriosis
ERα mediates estradiol induction of PR in malignant breast epithelial cells. Thus PR has been viewed as a classical ERα target gene.45 It has been reported that two distinct estradiol-regulated promoters generate transcripts encoding the two functionally different human PR isoforms, PR-A and PR-B.46 Previous studies have demonstrated that maximal PR mRNA and protein levels are reached after human breast cancer cells have been exposed to estradiol for 3 days.47–49 Two major transcriptional start sites have been identified. The upstream transcription start site gives rise to a full-length mRNA species that encodes the PR-B protein. Another mRNA species with a further downstream transcription initiation site gives rise to the truncated PR-A form. Despite the fact that both proposed PR promoter sequences are estradiol responsive, neither contains a classical palindromic estrogen response element sequence.50 Several nonclassical regulatory elements (e.g., AP-1, Sp1) in the human PR promoter have been reported. These sites have been shown to bind ERα and on one occasion ERβ.35,50–58 Intriguingly, more recent studies suggest that distal regions of the PR gene, as far as 311 kb upstream of the PR-B transcription start site, participate in the dynamic regulation of this gene and that the coordinated action of proximal and distal PR gene regions allows cells to respond to changes in estradiol levels.45
Our unpublished observations suggest that a reduction in the ERα-to-ERβ ratio in endometrial stromal cells diminishes estradiol induction of PR expression. Thus we speculate that a critical level of ERα may be necessary for estradiol-dependent induction of PR in endometrial stromal cells. The occupancy of the PR promoter regions with varying ratios of ERα to ERβ may be critical in determining the effect of estradiol on PR expression. A profoundly low ERα-to-ERβ ratio in endometriotic stromal cells may be responsible for a shift from estradiol stimulation to failure of induction of PR expression in endometriotic stromal cells (Fig. 3).
Is ERβ Responsible for High Cox2 Expression in Endometriotic Stromal Cells?
ERβ is the only estrogen receptor subtype expressed in primary placental villus endothelial cells.59,60 In these cells, ERβ is essential for maintaining cyclo-oxygenase (COX)2 mRNA and protein levels.59,60 In vitro, this effect of ERβ seems to be independent of the availability of estradiol.59,60 Because COX2 expression is also severely higher in endometriotic versus endometrial tissues and cells, it is tempting to speculate that ERβ is at least in part responsible for high COX2 levels and prostaglandin biosynthesis in endometriosis (Fig. 3).7,26
Summary
High estrogen production is a consistently observed endocrine feature of endometriosis. Expression of steroid receptors and other nuclear receptors are strikingly different between endometriotic and eutopic endometrial tissues. Among these nuclear receptors, ERβ expression is maybe >100 times higher in endometriotic tissue than in endometrium. Defective DNA methylation and other accompanying epigenetic mechanisms may be responsible for strikingly high ERβ expression in endometriosis. ERβ suppresses ERα expression and results in strikingly high ERβ-to-ERα ratios in endometriotic cells. We speculate that a strikingly lower ERα-to-ERβ ratio in endometriotic stromal cells may cause a shift from estradiol stimulation to inhibition of PR expression in endometriotic stromal cells under in vivo circumstances (Fig. 3). This proposed mechanism may explain severely deficient PR-B in endometriotic stromal cells, which contributes to progesterone resistance in women with endometriosis. ERβ overexpression in endometriosis possibly has other broad effects important in the pathology of endometriosis. It is likely that ERβ simulates prostaglandin production in endometriotic tissues and cells via inducing COX2 expression. Thus ERβ represents a key therapeutic target for endometriosis-associated pain. ERβ-selective compounds that antagonize estradiol in endometriotic stromal cells may be the future therapeutics of endometriosis.
Acknowledgments
This manuscript was supported by a grant from the NICHD (R37-HD37691, to SEB).
References
- 1.Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362(25):2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–1799. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 3.Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24(2):235–258. doi: 10.1016/s0889-8545(05)70302-8. [DOI] [PubMed] [Google Scholar]
- 4.Simpson JL, Elias S, Malinak LR, Buttram VC., Jr Heritable aspects of endometriosis. I. Genetic studies. Am J Obstet Gynecol. 1980;137(3):327–331. doi: 10.1016/0002-9378(80)90917-5. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy SMH, Mardon H, Barlow D. Familial endometriosis. J Assist Reprod Genet. 1995;12(1):32–34. doi: 10.1007/BF02214126. [DOI] [PubMed] [Google Scholar]
- 6.Bulun SE, Cheng YH, Yin P, et al. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006;248(1–2):94–103. doi: 10.1016/j.mce.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 7.Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–279. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 8.Schulke L, Berbic M, Manconi F, Tokushige N, Markham R, Fraser IS. Dendritic cell populations in the eutopic and ectopic endometrium of women with endometriosis. Hum Reprod. 2009;24(7):1695–1703. doi: 10.1093/humrep/dep071. [DOI] [PubMed] [Google Scholar]
- 9.Vercellini P, Trespidi L, Colombo A, Vendola N, Marchini M, Crosignani PG. A gonadotropin-releasing hormone agonist versus a low-dose oral contraceptive for pelvic pain associated with endometriosis. Fertil Steril. 1993;60(1):75–79. [PubMed] [Google Scholar]
- 10.Vercellini P, Trespidi L, De Giorgi O, Cortesi I, Parazzini F, Crosignani PG. Endometriosis and pelvic pain: relation to disease stage and localization. Fertil Steril. 1996;65(2):299–304. [PubMed] [Google Scholar]
- 11.Vercellini P, Cortesi I, Crosignani PG. Progestins for symptomatic endometriosis: a critical analysis of the evidence. Fertil Steril. 1997;68(3):393–401. doi: 10.1016/s0015-0282(97)00193-3. [DOI] [PubMed] [Google Scholar]
- 12.Abushahin F, Goldman KN, Barbieri E, Milad M, Rademaker A, Bulun SE. Aromatase inhibition for refractory endometriosis-related chronic pelvic pain. Fertil Steril. 2011;96(4):939–942. doi: 10.1016/j.fertnstert.2011.07.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris HA. Estrogen receptor-beta: recent lessons from in vivo studies. Mol Endocrinol. 2007;21(1):1–13. doi: 10.1210/me.2005-0459. [DOI] [PubMed] [Google Scholar]
- 14.Halme J, Becker S, Hammond MG, Raj MHG, Raj S. Increased activation of pelvic macrophages in infertile women with mild endometriosis. Am J Obstet Gynecol. 1983;145(3):333–337. doi: 10.1016/0002-9378(83)90720-2. [DOI] [PubMed] [Google Scholar]
- 15.Halme J, White C, Kauma S, Estes J, Haskill S. Peritoneal macrophages from patients with endometriosis release growth factor activity in vitro. J Clin Endocrinol Metab. 1988;66(5):1044–1049. doi: 10.1210/jcem-66-5-1044. [DOI] [PubMed] [Google Scholar]
- 16.Dmowski WP, Steele RW, Baker GF. Deficient cellular immunity in endometriosis. Am J Obstet Gynecol. 1981;141(4):377–383. doi: 10.1016/0002-9378(81)90598-6. [DOI] [PubMed] [Google Scholar]
- 17.Kao LC, Germeyer A, Tulac S, et al. Expression profiling of endo-metrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144(7):2870–2881. doi: 10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- 18.Burney RO, Talbi S, Hamilton AE, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148(8):3814–3826. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- 19.Osteen KG, Bruner-Tran KL, Eisenberg E. Reduced progesterone action during endometrial maturation: a potential risk factor for the development of endometriosis. Fertil Steril. 2005;83(3):529–537. doi: 10.1016/j.fertnstert.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 20.Noble LS, Takayama K, Zeitoun KM, et al. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab. 1997;82(2):600–606. doi: 10.1210/jcem.82.2.3783. [DOI] [PubMed] [Google Scholar]
- 21.Kao LC, Tulac S, Lobo S, et al. Global gene profiling in human endometrium during the window of implantation. Endocrinology. 2002;143(6):2119–2138. doi: 10.1210/endo.143.6.8885. [DOI] [PubMed] [Google Scholar]
- 22.Hornung D, Ryan IP, Chao VA, Vigne JL, Schriock ED, Taylor RN. Immunolocalization and regulation of the chemokine RANTES in human endometrial and endometriosis tissues and cells. J Clin Endocrinol Metab. 1997;82(5):1621–1628. doi: 10.1210/jcem.82.5.3919. [DOI] [PubMed] [Google Scholar]
- 23.Zeitoun KM, Bulun SE. Aromatase: a key molecule in the pathophysiology of endometriosis and a therapeutic target. Fertil Steril. 1999;72(6):961–969. doi: 10.1016/s0015-0282(99)00393-3. [DOI] [PubMed] [Google Scholar]
- 24.Osteen KG, Bruner-Tran KL, Keller NR, Eisenberg E. Progesterone-mediated endometrial maturation limits matrix metalloproteinase (MMP) expression in an inflammatory-like environment: a regulatory system altered in endometriosis. Ann N Y Acad Sci. 2002;955:37–47. doi: 10.1111/j.1749-6632.2002.tb02764.x. discussion 86–88, 396–406. [DOI] [PubMed] [Google Scholar]
- 25.Zeitoun K, Takayama K, Sasano H, et al. Deficient 17beta-hydroxysteroid dehydrogenase type 2 expression in endometriosis: failure to metabolize 17beta-estradiol. J Clin Endocrinol Metab. 1998;83(12):4474–4480. doi: 10.1210/jcem.83.12.5301. [DOI] [PubMed] [Google Scholar]
- 26.Bulun SE, Lin Z, Imir G, et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57(3):359–383. doi: 10.1124/pr.57.3.6. [DOI] [PubMed] [Google Scholar]
- 27.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93(12):5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392(1):49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- 29.Green S, Walter P, Kumar V, et al. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 30.Hewitt SC, Harrell JC, Korach KS. Lessons in estrogen biology from knockout and transgenic animals. Annu Rev Physiol. 2005;67:285–308. doi: 10.1146/annurev.physiol.67.040403.115914. [DOI] [PubMed] [Google Scholar]
- 31.Brandenberger AW, Lebovic DI, Tee MK, et al. Oestrogen receptor (ER)-alpha and ER-beta isoforms in normal endometrial and endometriosis-derived stromal cells. Mol Hum Reprod. 1999;5(7):651–655. doi: 10.1093/molehr/5.7.651. [DOI] [PubMed] [Google Scholar]
- 32.Fujimoto J, Hirose R, Sakaguchi H, Tamaya T. Expression of oestrogen receptor-alpha and -beta in ovarian endometriomata. Mol Hum Reprod. 1999;5(8):742–747. doi: 10.1093/molehr/5.8.742. [DOI] [PubMed] [Google Scholar]
- 33.Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85(8):2897–2902. doi: 10.1210/jcem.85.8.6739. [DOI] [PubMed] [Google Scholar]
- 34.Lin Z, Reierstad S, Huang CC, Bulun SE. Novel estrogen receptor-alpha binding sites and estradiol target genes identified by chromatin immunoprecipitation cloning in breast cancer. Cancer Res. 2007;67(10):5017–5024. doi: 10.1158/0008-5472.CAN-06-3696. [DOI] [PubMed] [Google Scholar]
- 35.Schultz JR, Petz LN, Nardulli AM. Cell- and ligand-specific regulation of promoters containing activator protein-1 and Sp1 sites by estrogen receptors alpha and beta. J Biol Chem. 2005;280(1):347–354. doi: 10.1074/jbc.M407879200. [DOI] [PubMed] [Google Scholar]
- 36.Attar E, Tokunaga H, Imir G, et al. Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. J Clin Endocrinol Metab. 2009;94(2):623–631. doi: 10.1210/jc.2008-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bulun SE, Utsunomiya H, Lin Z, et al. Steroidogenic factor-1 and endometriosis. Mol Cell Endocrinol. 2009;300(1–2):104–108. doi: 10.1016/j.mce.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Xue Q, Lin Z, Cheng YH, et al. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol Reprod. 2007;77(4):681–687. doi: 10.1095/biolreprod.107.061804. [DOI] [PubMed] [Google Scholar]
- 39.Bergman MD, Schachter BS, Karelus K, Combatsiaris EP, Garcia T, Nelson JF. Up-regulation of the uterine estrogen receptor and its messenger ribonucleic acid during the mouse estrous cycle: the role of estradiol. Endocrinology. 1992;130(4):1923–1930. doi: 10.1210/endo.130.4.1547720. [DOI] [PubMed] [Google Scholar]
- 40.Grandien K. Determination of transcription start sites in the human estrogen receptor gene and identification of a novel, tissue-specific, estrogen receptor-mRNA isoform. Mol Cell Endocrinol. 1996;116(2):207–212. doi: 10.1016/0303-7207(95)03716-0. [DOI] [PubMed] [Google Scholar]
- 41.Donaghue C, Westley BR, May FE. Selective promoter usage of the human estrogen receptor-alpha gene and its regulation by estrogen. Mol Endocrinol. 1999;13(11):1934–1950. doi: 10.1210/mend.13.11.0366. [DOI] [PubMed] [Google Scholar]
- 42.Grandien K, Bäckdahl M, Ljunggren O, Gustafsson JA, Berkenstam A. Estrogen target tissue determines alternative promoter utilization of the human estrogen receptor gene in osteoblasts and tumor cell lines. Endocrinology. 1995;136(5):2223–2229. doi: 10.1210/endo.136.5.7720671. [DOI] [PubMed] [Google Scholar]
- 43.Grandien KF, Berkenstam A, Nilsson S, Gustafsson JA. Localization of DNase I hypersensitive sites in the human oestrogen receptor gene correlates with the transcriptional activity of two differentially used promoters. J Mol Endocrinol. 1993;10(3):269–277. doi: 10.1677/jme.0.0100269. [DOI] [PubMed] [Google Scholar]
- 44.Trukhacheva E, Lin Z, Reierstad S, Cheng YH, Milad M, Bulun SE. Estrogen receptor (ER) beta regulates ERalpha expression in stromal cells derived from ovarian endometriosis. J Clin Endocrinol Metab. 2009;94(2):615–622. doi: 10.1210/jc.2008-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonéy-Montoya J, Ziegler YS, Curtis CD, Montoya JA, Nardulli AM. Long-range transcriptional control of progesterone receptor gene expression. Mol Endocrinol. 2010;24(2):346–358. doi: 10.1210/me.2009-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kastner P, Krust A, Turcotte B, et al. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9(5):1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nardulli AM, Greene GL, O’Malley BW, Katzenellenbogen BS. Regulation of progesterone receptor messenger ribonucleic acid and protein levels in MCF-7 cells by estradiol: analysis of estrogen’s effect on progesterone receptor synthesis and degradation. Endocrinology. 1988;122(3):935–944. doi: 10.1210/endo-122-3-935. [DOI] [PubMed] [Google Scholar]
- 48.Wei LL, Krett NL, Francis MD, et al. Multiple human progesterone receptor messenger ribonucleic acids and their autoregulation by progestin agonists and antagonists in breast cancer cells. Mol Endocrinol. 1988;2(1):62–72. doi: 10.1210/mend-2-1-62. [DOI] [PubMed] [Google Scholar]
- 49.Read LD, Snider CE, Miller JS, Greene GL, Katzenellenbogen BS. Ligand-modulated regulation of progesterone receptor messenger ribonucleic acid and protein in human breast cancer cell lines. Mol Endocrinol. 1988;2(3):263–271. doi: 10.1210/mend-2-3-263. [DOI] [PubMed] [Google Scholar]
- 50.Petz LN, Ziegler YS, Schultz JR, Nardulli AM. Fos and Jun inhibit estrogen-induced transcription of the human progesterone receptor gene through an activator protein-1 site. Mol Endocrinol. 2004;18(3):521–532. doi: 10.1210/me.2003-0105. [DOI] [PubMed] [Google Scholar]
- 51.Matthews J, Wihlén B, Tujague M, Wan J, Ström A, Gustafsson JA. Estrogen receptor (ER) beta modulates ERalpha-mediated transcriptional activation by altering the recruitment of c-Fos and c-Jun to estrogen-responsive promoters. Mol Endocrinol. 2006;20(3):534–543. doi: 10.1210/me.2005-0140. [DOI] [PubMed] [Google Scholar]
- 52.Petz LN, Ziegler YS, Schultz JR, Kim H, Kemper JK, Nardulli AM. Differential regulation of the human progesterone receptor gene through an estrogen response element half site and Sp1 sites. J Steroid Biochem Mol Biol. 2004;88(2):113–122. doi: 10.1016/j.jsbmb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 53.Petz LN, Nardulli AM. Sp1 binding sites and an estrogen response element half-site are involved in regulation of the human progesterone receptor A promoter. Mol Endocrinol. 2000;14(7):972–985. doi: 10.1210/mend.14.7.0493. [DOI] [PubMed] [Google Scholar]
- 54.Petz LN, Ziegler YS, Loven MA, Nardulli AM. Estrogen receptor alpha and activating protein-1 mediate estrogen responsiveness of the progesterone receptor gene in MCF-7 breast cancer cells. Endocrinology. 2002;143(12):4583–4591. doi: 10.1210/en.2002-220369. [DOI] [PubMed] [Google Scholar]
- 55.Schultz JR, Petz LN, Nardulli AM. Estrogen receptor alpha and Sp1 regulate progesterone receptor gene expression. Mol Cell Endocrinol. 2003;201(1–2):165–175. doi: 10.1016/s0303-7207(02)00415-x. [DOI] [PubMed] [Google Scholar]
- 56.Savouret JF, Bailly A, Misrahi M, et al. Characterization of the hormone responsive element involved in the regulation of the progesterone receptor gene. EMBO J. 1991;10(7):1875–1883. doi: 10.1002/j.1460-2075.1991.tb07713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Montano MM, Kraus WL, Katzenellenbogen BS. Identification of a novel transferable cis element in the promoter of an estrogen-responsive gene that modulates sensitivity to hormone and anti-hormone. Mol Endocrinol. 1997;11(3):330–341. doi: 10.1210/mend.11.3.9899. [DOI] [PubMed] [Google Scholar]
- 58.Scott RE, Wu-Peng XS, Yen PM, Chin WW, Pfaff DW. Interactions of estrogen- and thyroid hormone receptors on a progesterone receptor estrogen response element (ERE) sequence: a comparison with the vitellogenin A2 consensus ERE. Mol Endocrinol. 1997;11(11):1581–1592. doi: 10.1210/mend.11.11.0003. [DOI] [PubMed] [Google Scholar]
- 59.Su EJ, Lin ZH, Zeine R, et al. Estrogen receptor-beta mediates cyclooxygenase-2 expression and vascular prostanoid levels in human placental villous endothelial cells. Am J Obstet Gynecol. 2009;200(4):427-e1–e8. doi: 10.1016/j.ajog.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 60.Su EJ, Ernst L, Abdallah N, et al. Estrogen receptor-β and fetoplacental endothelial prostanoid biosynthesis: a link to clinically demonstrated fetal growth restriction. J Clin Endocrinol Metab. 2011;96(10):E1558–E1567. doi: 10.1210/jc.2011-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]


