Abstract
As men age, serum testosterone (T) levels decline, whereas serum luteinizing hormone (LH) levels increase somewhat or remain unchanged. Age-related reductions in T levels may be associated with alterations in body composition; energy level; muscle strength; physical, sexual, and cognitive functions; and mood. The predominant contributor to the decline in serum T levels is the decreased ability of the aging testes to make T. As in humans, the Brown Norway rat demonstrates age-related reductions in serum T levels in the setting of unchanged or modestly increased serum LH levels. In this rat model, the ability of aged Leydig cells, the terminally differentiated T-producing cells of the testis, to produce T in response to LH stimulation is significantly diminished. This review begins with a discussion of what is known of the molecular mechanisms by which T synthesis declines with Leydig cell aging. It concludes with a brief history of T replacement therapy, current guidelines, controversies related to T replacement therapy in older men, and proposed future clinical directions.
Keywords: Leydig cells, hypogonadism
A number of well-designed longitudinal studies have shown that in most men there is a slow decline in serum total testosterone (T) levels with aging, even in the absence of disease (Figure) (Harman et al, 2001; Mohr et al, 2005). Disease, other comorbid conditions, and medications can exacerbate the decline. Decline in serum T levels has been shown to be associated with, and perhaps in some cases to contribute to, alterations in body composition, diminished energy, muscle strength, cognition, sexual function, and depressed mood (Matsumoto, 2002). Adult Leydig cells are terminally differentiated cells that produce T. The cells are regulated by luteinizing hormone (LH), which is synthesized and produced by the pituitary gland. Because T clearance from blood has been shown to slow with age (Coviello et al, 2006), the age-related reductions in serum levels of T suggest that less T is being made by the aging testes. Indeed, as discussed later, this has been shown to be the case in model systems. There are age-related alterations in the hypothalamic-pituitary regulatory system, including increased responsiveness to sex steroid negative feedback and alterations in gonadotropin-releasing hormone pulsatility and effects on the pituitary gland (Veldhuis et al, 1997). With declines in serum T levels in older men, serum LH levels rise modestly or do not change at all (Surampudi et al, 2012), suggesting that the reduced T levels are the not the result of reduced LH levels. Studies in men have shown an age-associated decrease in the ability of LH to stimulate testicular production of T (Veldhuis et al, 2012). Clinical studies related to declining T levels with age have been of 2 types: 1) epidemiologic studies of associations between T levels and various physiological conditions or disease states and 2) T replacement trials to evaluate efficacy and side effects of therapy in middle aged and older men.
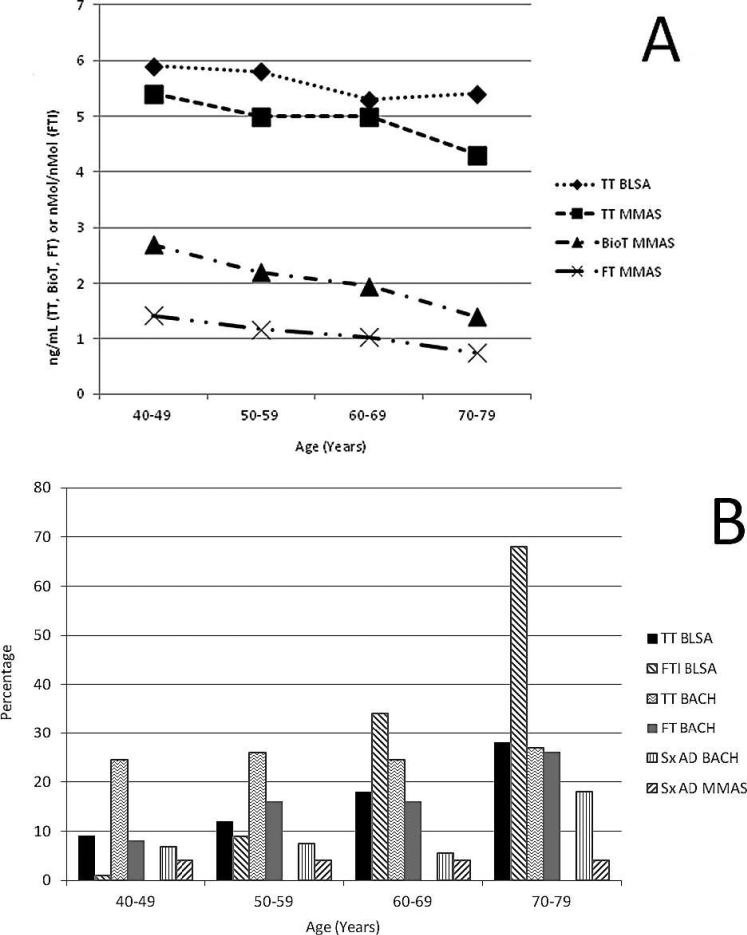
Figure.
(A) Total testosterone (TT, ng/mL), bioavailable testosterone (Bio T, ng/mL), and free testosterone (FT; ng/mL ×10), by age group, from either the Baltimore Longitudinal Study of Aging (BLSA; Harman et al, 2001) or the Massachusetts Male Aging Study (MMAS; Mohr et al, 2005). Data adapted. (B) Percentage of men, by age group, with levels of serum TT, FT, or a free testosterone index (FTI = TT/SHBG) that met the hormonal definition of androgen deficiency as defined by either the BLSA (Harman et al, 2001) or Boston Area Community Health survey (BACH; Araujo et al, 2007) and the percentage of men, by age group, with symptoms of androgen deficiency from the BACH (Sx AD BACH; Araujo et al, 2007) or the MMAS (Sx AD MMAS; Araujo et al, 2004). Data adapted. SHBG indicates sex hormone–binding globulin.
We begin this review article by discussing our understanding of the mechanisms responsible for reduced T levels and whether/how such reductions might be prevented or reversed. For this, we will focus on the rodent testis as a model for the human. We then will discuss the clinical effects/consequences of T replacement in aging men and issues surrounding replacement therapy. Please note that many of the references are to review articles rather than to the original research.
Steroidogenic Deficits in Aged Leydig Cells
Much of what we know of the mechanisms involved in age-related decline in serum T levels has been obtained through studies of rodents. In many rat strains, including Sprague-Dawley, hypothalamic-pituitary changes occur with aging and lead to decreased LH levels and thus reduced stimulation of the Leydig cells (Zirkin et al, 1993). In Brown Norway rats, however, as in men, T levels decrease with age in the face of unchanging or increasing LH levels (Wang et al, 1993; Chen et al, 1994, 2009). Because of this and other reasons, the Brown Norway rat has been studied in a number of laboratories as a model for the human. The testes of aged Brown Norway rats, when perfused with maximally stimulating LH, were shown to produce significantly less T than young rat testes (Zirkin et al, 1993). This suggested that the reduced serum T concentration in aged rats resulted from reduced T production by the Leydig cells. Indeed, Leydig cells isolated from aged rat testes and cultured with LH were found to produce less T than cells from the testes of young rats (Chen et al, 2009). Leydig cells in the adult testis rarely turn over, and the numbers of Leydig cells do not change with age. Rather, reduced Leydig cell T production results from the relative unresponsiveness of the aged cells to LH (Chen et al, 2009). Thus, the in vivo administration of exogenous LH to old Brown Norway rats and the in vitro short- or long-term culture of Leydig cells from aged rats with LH failed to raise the relatively low levels of T production by aged cells to the significantly higher levels of young cells (Chen et al, 2009).
These observations led to 2 major questions: 1) What is different about young and aged Leydig cells? and 2) By what mechanism(s) do these differences occur? Leydig cells synthesize steroid hormones from cholesterol through steps that include LH binding to its receptor, production of cAMP, cholesterol movement from intracellular stores into mitochondria, conversion of cholesterol to pregnenolone by the C27 cholesterol side-chain cleavage cytochrome P450 11A1 enzyme (CYP11A1) at the inner mitochondrial membrane (IMM), and enzymatic transformation of pregnenolone in the smooth endoplasmic reticulum (Payne and Hales, 2004). Cholesterol transport to the IMM is the rate-determining step in steroid biosynthesis, with 2 proteins, steroidogenic acute regulatory protein (STAR) and translocator protein (18-kd TSPO), playing major roles in this process (Midzak et al, 2011). It now is clear that aged Leydig cells have deficits in LH receptor number, cAMP production, STAR and TSPO cholesterol transport, and the steroidogenic enzyme amounts in the mitochondria and smooth endoplasmic reticulum (Chen et al, 2009).
Which of these deficits leads to reduced production of T? The amount of cAMP, produced in response to LH, is reduced in aged compared with young cells (Chen et al, 2004). Treatment of aged cells with dibutyryl cAMP for 3 days, however, resulted in the cells producing T at the levels of young cells. This suggests that relative insensitivity of aged cells to LH and thus reduced cAMP production might be responsible for age-related reductions in steroidogenesis by the aged cells. Evidence obtained to date suggests that reduced cAMP levels result from defects in the coupling of the LH receptor to adenylyl cyclase through Gs proteins, and not from deficits in the G protein or in adenylyl cyclase (Chen et al, 2009).
There also is evidence that cholesterol transport to the mitochondria, the rate-determining step in steroid biosynthesis, is compromised in aged Leydig cells (Liao et al, 1993). As indicated previously, both STAR and TSPO, 2 proteins that play particularly important roles in cholesterol transport to the IMM, are reduced in amount in aged Leydig cells. There is now good evidence that STAR binds and transfers free cholesterol to mitochondria and that TSPO is an outer mitochondrial membrane, cholesterol-binding protein that transfers cholesterol to the IMM for cleavage by CYP11A1 to pregnenolone (Midzak et al, 2010). Reductions in the activities of STAR and TSPO proteins, which occur as Leydig cells age (Chen et al, 2009), could have effects on steroidogenesis. In addition, the activities of the steroidogenic enzymes P450 side-chain cleavage, 3b– hydroxysteroid dehydrogenase, P450c17, and 17b–hydroxysteroid dehydrogenase are reduced in aged Leydig cells (Chen et al, 2009). Any of these defects might explain the reduced ability of aged Leydig cells to produce T in response to LH.
Mechanisms Responsible for Steroidogenic Deficits in Aged Leydig Cells
What causes inefficient coupling of the LH receptor to adenylyl cyclase in old Leydig cells, and what might cause altered cholesterol import? Reactive oxygen species (ROS) are produced in Leydig cells by both the mitochondrial electron transport chain and the P450 enzymes (Hanukoglu, 2006). A number of genes involved in free radical scavenging and repair of ROS-induced damage have been shown to become down-regulated with Leydig cell aging, and there are reduced activities of the antioxidants superoxide dismutase 1 and 2, glutathione peroxidase 1, and glutathione (Cao et al, 2004; Chen et al, 2009). There also are age-related increases in ROS production (Chen et al, 2009). These findings in Leydig cells and others in other steroid-producing cells suggest that redox imbalance/oxidative stress may play a central role in age-related deficits in steroid formation. Age-related increases in oxidative stress may damage cellular DNA, protein, and lipids. There also is the possibility of exogenous sources of ROS. Thus, macrophages, a major source of ROS, are present in the interstitial compartment of the testis (Haider, 2004).
Steroidogenesis itself is known to produce ROS. We reasoned that if steroidogenesis was a major source of damaging ROS, its long-term suppression might delay or prevent age-related reductions in formation of T (Chen and Zirkin, 1999). To test this, an LH-suppressive contraceptive dose of T was administered to rats from age 13 months to age 21 months, and then the exogenously administered T source was removed to allow LH to be produced and secreted. Suppression of steroidogenesis was found to prevent the age-related reduction in the ability of the Leydig cells to produce T that occurred in control rats. Additionally, long-term administration of the antioxidant vitamin E was found to delay age-related decreases in steroidogenesis, whereas long-term vitamin E deficiency had the opposite effect (Chen et al, 2005). Finally, experimental depletion of glutathione with buthionine sulfoximine reduced Leydig cell steroidogenic function both in vitro and in vivo, whereas the antioxidants vitamin E, N-tert-butyl-a-phenylnitrone, and 6-hydroxy-2,5,7, 8-tetramethylchroman-2-carboxylic acid prevented this (Chen et al, 2008). These studies, taken together, strongly suggest that reactive oxygen plays an important role in age-related reductions in Leydig cell T production.
In sum, the steroidogenic capacity of Leydig cells is reduced by approximately 50% with aging. There is evidence that ROS, derived from the mitochondrial electron transport chain, steroidogenesis, and/or macrophages, in some way affects cAMP production and cholesterol transport into the mitochondria by altering the redox environment of the aging Leydig cells. The change results in relative insensitivity to LH signaling and thus the reduced T levels that characterize aged Leydig cells.
T Treatment in Aging Men
Soon after its isolation and synthesis in 1935 and until the early 1950s, T was given to aging men for a variety of symptoms. These symptoms included irritability, fatigue, erectile problems, and low libido, and the symptom complex then was called the “male climacteric.” This was all before T could be measured in blood and therefore before there was any evidence that aging men might have low T levels or that there were specific androgen target organs in older men that might respond specifically to T therapy. Given the paucity of a scientific basis for the practice of T replacement for the aging man, it gradually fell out of favor, and little research in the area was accomplished for the next 3 decades. Methods to measure T in blood were developed in the 1960s and 1970s. With advances in quantitative hormonal evaluation, along with the development of other research techniques and a growing interest in delaying the aging process by the “baby boomer” generation, there was a resurgence of research in the 1980s in the area of T in aging men.
Today, the possibility that an older adult man may have “low T” is openly discussed and marketed via a variety of media (eg, television, Internet, magazines). One can view television commercials (including during the Super Bowl) and magazine cartoons and advertisements about the “low T” condition, and there is an online “quiz” a man can take to see if he might have “low T.” Sales of T preparations have grown rapidly. Although exact numbers are difficult to obtain, it is estimated that T prescriptions increased more than 15-fold (from 122 000 to more than 1.8 million) between 1992 and 2002 and that there was a 29% increase between 2001 and 2002 alone (Liverman and Blazer, 2004). One might surmise that all this means is that clinicians now can accurately diagnose an older man as having low T levels, know how to best select treatment regimens and what to expect from the therapy, and are able to weigh the benefits and risks of that therapy for the individual. Unfortunately, this is not the case. Although the science of T therapy for the older adult male has come a long way over the past 30 years, there is a great deal more that needs to be learned to bring knowledge to the appropriate level to support good clinical practice.
The changing names for the aging-related T deficient “disease” state demonstrate just how fluctuating the ground is upon which this state is defined: male climacteric, male menopause, viropause, testopause, andropause, androgen deficiency in the aging male (ADAM), partial androgen deficiency of the aging male, testosterone deficiency syndrome, late-onset hypogonadism (LOH). Whatever name is used to describe the condition, there are many uncertainties as to what exactly T deficiency is and whether/when it should be treated. Because there is as yet no independent measurable marker of androgen activity in vivo, all guidelines and best practices emphasize that older men eligible for replacement therapy must have both “deficient” T levels and clinical signs/symptoms that might be related to that deficiency. There currently is no role for the use of T replacement therapy in older men for “prevention.”
Defining Clinically Relevant “Hypogonadism” in Aging Men
The first uncertainty in deciding which older men to consider for T replacement therapy is how to hormonally define T “deficiency,” including what component of T to measure and what cutoffs to use. Free T and albumin-bound T are referred to as the “bioavailable” T fraction, the portion widely believed to be readily available to leave the circulation and enter cells. Although there are data to support the idea that “bioavailable” T or free T fractions may reflect the clinical situation more accurately than total T levels, there have been no clinical trials that have indicated which method of T measurement best defines men who are T deficient or likely to respond to therapy. Selecting the T component to use to define hypogonadism in an older man is not inconsequential. Because of increases in sex hormone–binding globulin levels with age, bioavailable T levels decline to a greater extent than do total T levels (Figure), which means that using bioavailable T levels as the primary measure would lead to more older men being classified as being T deficient. Given that there are no strong data to support primary use of bioavailable or free T to define hypogonadism in the older man, and that the total T assay is the more reliable and least expensive of the T assays, the Endocrine Society's current best practice guidelines recommend that the initial T assessment for possible LOH should be total T level (Bhasin et al, 2010). Then, if a total T level is within a borderline low range, a repeat measure of total T should be accompanied by measurement of bioavailable or free T level.
The generally used T clinical assays currently lack accuracy, sensitivity, and precision. These clinical T assays have significant deficiencies, particularly in the low range, just where one would want them to work best for assisting with appropriate diagnoses and clinical management. A consensus conference was held in February 2010 to delineate the T assay issues, explore remedies, and come up with a list of actions deemed necessary to improve T testing (Rosner and Vesper, 2010). Work is in progress to improve technical aspects and standardization for the T assays. There also needs to be improvement in how the normal reference ranges are constructed. Even for the more straightforward total T assay, there is huge variation in the reference range for “normal.” The source for the plasma upon which “normal” is defined needs to be more consistently and appropriately defined.
Even given the problems with the current T assays and how the young adult male “normal” range values are obtained, deciding at what level of total T an older man is “low” enough to warrant a possible diagnosis of “hypogonadal” is another issue that has not yet been resolved. Unfortunately, using other hormonal evaluations, such as gonadotropin levels, to help make that decision is rarely helpful; as noted previously, the majority of older men, even those with quite low T levels, do not have significantly elevated LH levels. Given the current state of our knowledge, general “best practices” guidelines from organizations such as the Endocrine Society (Bhasin et al, 2010) suggest using values for total T that are below the normal range for young adult men as a starting place in assessing for LOH. It should be emphasized, that as in the original guidelines issued in 2006, the 2010 guideline recommendations for the definition of adult-onset hypogonadism and treatment are based on weak evidence.
For LOH, what has driven both the field of clinical research and the marketing and sales of T has been the emphasis on symptoms and signs that point to the possibility that the older man has T deficiency. Part of this issue is the underlying hypothesis by clinical researchers, and the assumption by the pharmaceutical marketing and sales groups, that the symptoms attributed to T deficiency will improve significantly with T replacement. Much of the clinical research over the past 20 to 30 years has been to try to define the phenotype of the older man who might benefit from T therapy. Although our knowledge about the clinical response to T therapy in older men has increased greatly during this time, no clear definitive phenotype has arisen. T has significant effects on a large number of organ systems. Symptoms possibly related to low T levels include decreased energy and motivation, depressed mood, poor concentration, reduced muscle mass and strength, increased body fat, diminished work performance, osteopenia (leading to fractures and skeletal pain), and decreased libido and erectile dysfunction. These symptoms are not specific to hypogonadism, however, and so other medical or psychological problems that increase in prevalence with age could also be contributors. None of the current screening questionnaires for hypogonadism in older men (eg, ADAM, Massachusetts Male Aging Study, Aging Males Survey scales) has high enough specificity to help either with clinical diagnosis or monitoring response to therapy. Work continues on trying to develop a better screening tool.
Many older men with T levels that are in the young adult hypogonadal range are relatively asymptomatic, making the prevalence of symptomatic T deficiency in the older age group lower than that based on serum T levels alone (Figure; Araujo et al, 2004, 2007; Wu et al, 2010). On the other hand, some symptoms in older men, such as sexual dysfunction and fatigue, are much more prevalent in those men with low T levels (Wu et al, 2010). In addition, many of the T target-organ symptoms in truly hypogonadal young adult men are significantly improved when these young men are treated with T. Assuming that the androgen target tissues of older men are still responsive to T, then improving their T deficiency also might improve their symptoms. Early trials of T therapy in older men, directed at treatment of symptoms, were suggestive of possible benefit of T therapy in a number of realms, but changes with therapy were small and inconsistent across trials. Contributing to study inconsistencies were variations in the T levels used to define the treatment population, method of T replacement, and serum levels of T obtained; how clinical outcomes were evaluated; and length of treatment trials.
In 2004, the Institute of Medicine highlighted the lack of data to support substantial benefits from T therapy in older men with low to low-normal T levels and noted a need for more research to better define both the benefits of therapy and the population in which these benefits occur (Liverman and Blazer, 2004). Eight years later, although there has been progress made, overall there still is no strong convincing evidence that T replacement therapy in the older man has significant health benefits or avoids harm for many older men with low T levels. Most directive data come from meta-analyses. From population studies (Wu et al, 2010) and direct clinical studies (Kelleher et al, 2004), there appears to be variability by target organ in threshold concentration of T responsiveness so that the T dose requirement for improvement of one specific androgen target organ is not going to be the same for all target organs. This needs to be taken into account when studies of T replacement therapy are designed or interpreted.
Some of the clinical outcomes that are being targeted by T replacement studies in older men also are ones in which multiple factors other than T are implicated. This makes it more challenging to have definitive outcome studies. T replacement targeted at sarcopenia and decline in physical function status is a good example. This is an area in which the anabolic actions of T might be expected to have major impact in the older hypogonadal man. Most studies of T replacement in older men have demonstrated an increase in skeletal muscle mass with T therapy. At replacement doses in the normal range, muscle of older men responds similarly to that of young men (Bhasin et al, 2005). In terms of improvement in muscle strength with T therapy, a meta-analysis (Ottenbacher et al, 2006) reported a modest increase in overall muscle strength, but the magnitude of the improvement was largely from one trial. Subsequent trials (Kenny et al, 2010; Srinivas-Shankar et al, 2010) have not altered the overall finding that there are small increases in muscle strength in some older men receiving T therapy. In terms of improvement in the most meaningful of clinical outcomes, daily physical function, the data generated so far are not impressive in showing a major impact of T therapy. Only a few T replacement trials have shown improvement in any measure of physical function, and improvement occurred only in certain subgroups (Srinivas-Shankar et al, 2010) in some of these trials. The theme seems to be that T therapy improves muscle mass and, to a lesser extent, voluntary muscle strength, but this does not seem to reliably translate into functional improvements. This result is probably not surprising because function is not just related to strength but relies on neuromuscular connections, behavioral and cognitive input, and adaptation. As suggested by a few small studies, T therapy in conjunction with functional training may prove more beneficial. Results from larger studies of this dual therapy should soon be forthcoming.
In terms of effects of T therapy on other androgen target organs in older men, a number of studies have shown increases in bone mineral density with therapy (Amory et al, 2004). Data suggest that the effects of T on bone are predominantly dependent on aromatization to estradiol (Khosla et al, 2008). Although estradiol effects on bone in women lead to improvement in osteoporosis and decrease in fracture risk, T therapy has not been studied in a large enough sample of older men to evaluate effect on fracture risk. In terms of effects of T replacement therapy on depression, quality of life, and cognition in older men, data are inconsistent, with at least as many negative trials as positive ones. The results of T therapy trials in older men for outcomes such as sexual dysfunction and metabolic syndrome are discussed elsewhere in this series.
Obviously, even if T was a wonder drug in terms of its clinical benefits, one would still need to balance any possible benefit with the potential risks. Although there were many potential risks to evaluate in the earlier T replacement trials, many of these have been shown with time to either not be of concern (eg, lipids, liver function abnormalities, aggressive mood) or to be easily monitored and controlled (eg, increased hematopoiesis). What has remained as risk concerns are primarily those involving the cardiovascular (CV) system and prostate.
Some epidemiologic studies have reported association between higher T levels and lower rates of all-cause and CV mortality in older men (Ohlsson et al, 2011). A recently published observational study of older male veterans with low T levels reported that T treatment was associated with decreased mortality compared with no T treatment (Shores et al, 2012). In one study, T replacement in older men with chronic heart failure led to an increase in exercise capacity (Caminiti et al, 2009). A meta-analysis of T replacement trials suggested that T therapy was not a risk for CV disease (Haddad et al, 2007), but at least one trial since that review was stopped prematurely because of excess “cardiovascular-related” events (Basaria et al, 2010). It should be noted, however, that men in this recent study had more baseline CV disease and CV risk factors than did participants in other T replacement studies, some of the reported CV-related events possibly were not truly CV events (such as peripheral edema without any mention of congestive heart failure symptoms), and the serum T levels achieved during the replacement were higher than those in most T replacement studies. However, this trial does seem to suggest that one needs to carefully define and monitor the population receiving T.
In terms of the prostate, there has not been any direct evidence to date that T replacement therapy in men with T deficiency will promote prostate problems, particularly prostate cancer (Tenover, 2007; Fernandez-Balsells et al, 2010). The real caveat here is the limited length of observation and the small number of men observed. It is estimated that a T replacement clinical trial would need to enroll a minimum of 6000 men, who would need to be followed for at least 5 years, to have adequate power to appropriately assess prostate risk. Such a large clinical trial is unlikely to be undertaken, at least in the near future. As a surrogate, there will need to be continued monitoring of men receiving T therapy and meta-analyses updates of trial findings. In addition, progress in knowledge of the pathophysiology and development of prostate cancer should continue to evolve and may assist with clinical decisions. It may be, for example, that most older men who are candidates for T therapy have baseline T levels already above the prostate threshold, so that administering additional T will have little effect.
What about T replacement methods? Early in T replacement trials in the United States, injectable esters administered every week to every 3 weeks, or implantable pellets changed every 3 months, were the only viable methods for T replacement; oral T undecanoate dosed several times a day was available outside the United States. Over time, new modalities have been developed, including daily use scrotal patch, transdermal patch, transdermal gels, and the twice-daily buccal patch. More recently developed have been injectable T undecanoate (dosed every 12–14 weeks) and the matrix patch (dosed every other day). Selective androgen receptor modulators also are in development. Although none of these methods are ideal and all have non–hormone-related side effects, we have come a long way in improving T replacement methods.
Future Clinical Directions
Areas for future research and expansion of knowledge in male hormone replacement therapy in the older man are many. Among the priorities are 1) improvement in reliability and precision of T assays; 2) national standards used to determine normal ranges for young adult men; 3) carefully constructed clinical studies in both middle-aged and older men to delineate the serum T thresholds for the various clinical target organs of interest; 4) continued research to better define the phenotype of the older man most likely to benefit from T therapy and to continue to evaluate adjunctive therapies that may lead to more benefit than T therapy alone; 5) research toward discovery of an in vivo marker for evaluating androgenicity that can be measured easily in humans; 6) development of a safe, effective, and hopefully not too expensive selective androgen receptor modulator and/or an oral pill that can raise serum T levels to therapeutic levels; and 7) eventually a large male health initiative study to evaluate the long-term benefits as well as the CV and prostate risks of T therapy for older men.
Footnotes
Contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- Amory JK, Watts NB, Easley KA, Sutton PR, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab. 2004;89:503–510. doi: 10.1210/jc.2003-031110. [DOI] [PubMed] [Google Scholar]
- Araujo AB, Esche GR, Kupelian V, O'Donnell AB, Travison TG, Williams RE, Clark RV, McKinlay JB. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92:4241–4247. doi: 10.1210/jc.2007-1245. [DOI] [PubMed] [Google Scholar]
- Araujo AB, O'Donnell AB, Brambilla DJ, Simpson WB, Longcope C, Matsumoto AM, McKinlay JB. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging study. J Clin Endocrinol Metab. 2004;89:5920–5926. doi: 10.1210/jc.2003-031719. [DOI] [PubMed] [Google Scholar]
- Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, et al. Adverse effects associated with testosterone administration. N Engl J Med. 2010;363:109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM. Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, Yarasheski KE, Sinha-Hikim I, Dzekov C, Dzekov J, Magliano L, Storer TW. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on skeletal muscle. J Clin Endocrinol Metab. 2005;90:678–688. doi: 10.1210/jc.2004-1184. [DOI] [PubMed] [Google Scholar]
- Caminiti G, Volterrani M, Iellamo F, Marazzi G, Massaro R, Miceli M, Mammi C, Piepoli M, Fini M, Rosano GM. Effect of long-acting testosterone treatment on functional exercise capacity, skeletal muscle performance, insulin resistance, and baroreflex sensitivity in elderly patients with chronic heart failure. J Am Coll Cardiol. 2009;54:919–927. doi: 10.1016/j.jacc.2009.04.078. [DOI] [PubMed] [Google Scholar]
- Cao L, Leers-Sucheta S, Azhar S. Aging alters the functional expression of enzymatic and non-enzymatic anti-oxidant defense systems in testicular rat Leydig cells. J Steroid Biochem Mol Biol. 2004;88:61–67. doi: 10.1016/j.jsbmb.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Chen H, Ge RS, Zirkin BR. Leydig cells: from stem cells to aging. Mol Cell Endocrinol. 2009;306:9–16. doi: 10.1016/j.mce.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Hardy MP, Huhtaniemi I, Zirkin BR. Age-related decreased Leydig cell testosterone production in the Brown Norway rat. J Androl. 1994;15:551–557. [PubMed] [Google Scholar]
- Chen H, Liu J, Luo L, Baig MU, Kim JM, Zirkin BR. Vitamin E, aging and Leydig cell steroidogenesis. Exp Gerontol. 2005;40:728–736. doi: 10.1016/j.exger.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Chen H, Liu J, Luo L, Zirkin BR. Dibutyryl cyclic adenosine monophosphate restores the ability of aged Leydig cells to produce testosterone at the high levels characteristic of young cells. Endocrinology. 2004;145:4441–4446. doi: 10.1210/en.2004-0639. [DOI] [PubMed] [Google Scholar]
- Chen H, Pechenino AS, Liu J, Beattie MC, Brown TR, Zirkin BR. Effect of glutathione depletion on Leydig cell steroidogenesis in young and old Brown Norway rats. Endocrinology. 2008;149:2612–2619. doi: 10.1210/en.2007-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zirkin BR. Long term suppression of Leydig cell steroidogenesis prevents Leydig cell aging. Proc Natl Acad Sci U S A. 1999;96:14877–14881. doi: 10.1073/pnas.96.26.14877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coviello AD, Lakshman K, Mazer NA, Bhasin S. Differences in the apparent metabolic clearance rate of testosterone in young and older men with gonadotropin suppression receiving graded doses of testosterone. J Clin Endocrinol Metab. 2006;91:4669–4675. doi: 10.1210/jc.2006-0822. [DOI] [PubMed] [Google Scholar]
- Fernández-Balsells MM, Murad MH, Lane M, Lampropulos JF, Albuquerque F, Mullan RJ, Agrwal N, Elamin MB, Gallegos-Orozco JF, Wang AT, Erwin PJ, Bhasin S, Montori VM. Clinical review 1: adverse effects of testosterone in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95:2560–2575. doi: 10.1210/jc.2009-2575. [DOI] [PubMed] [Google Scholar]
- Haddad RM, Kennedy CC, Caples SM, Tracz MJ, Boloña ER, Sideras K, Uraga MV, Erwin PJ, Montori VM. Testosterone and cardiovascular risk in men: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc. 2007;82:29–39. doi: 10.4065/82.1.29. [DOI] [PubMed] [Google Scholar]
- Haider SG. Cell biology of Leydig cells in the testis. Int Rev Cytol. 2004;233:181–241. doi: 10.1016/S0074-7696(04)33005-6. [DOI] [PubMed] [Google Scholar]
- Hanukoglu I. Antioxidant protective mechanisms against reactive oxygen species (ROS) generated by mitochondrial P450 systems in steroidogenic cells. Drug Metab Rev. 2006;38:171–196. doi: 10.1080/03602530600570040. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total free testosterone levels in healthy men. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Kelleher S, Conway AJ, Handelsman DJ. Blood testosterone threshold for androgen deficiency symptoms. J Clin Endocinol Metab. 2004;89:3813–3817. doi: 10.1210/jc.2004-0143. [DOI] [PubMed] [Google Scholar]
- Kenny AM, Kleppinger A, Annis K, Rathier M, Browner B, Judge JO, McGee D. Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels, low bone mass, and physical frailty. J Am Geriatr Soc. 2010;58:1134–1143. doi: 10.1111/j.1532-5415.2010.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocrin Rev. 2008;29:441–464. doi: 10.1210/er.2008-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C, Reaven E, Azhar S. Age-related decline in the steroidogenic capacity of isolated rat Leydig cells: a defect in cholesterol mobilization and processing. J Steroid Biochem Mol Biol. 1993;46:39–47. doi: 10.1016/0960-0760(93)90207-d. [DOI] [PubMed] [Google Scholar]
- Liverman CT, Blazer DG, editors. Testosterone and Aging: Clinical Research Directions. National Academies Press; Washington, DC: 2004. [PubMed] [Google Scholar]
- Matsumoto AM. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol A Biol Sci Med Sci. 2002;57:M76–M99. doi: 10.1093/gerona/57.2.m76. [DOI] [PubMed] [Google Scholar]
- Midzak A, Rone M, Aghazadeh Y, Culty M, Papadopoulos V. Mitochondrial protein import and the genesis of steroidogenic mitochondria. Mol Cell Endocrinol. 2011;336:70–79. doi: 10.1016/j.mce.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr BA, Guay AT, O'Donnell AB, McKinlay JB. Normal, bound and nonbound testosterone levels in normally ageing men: results from the Massachusetts Male Ageing study. Clin Endocrinol. 2005;62:64–73. doi: 10.1111/j.1365-2265.2004.02174.x. [DOI] [PubMed] [Google Scholar]
- Ohlsson C, Barrett-Connor E, Bhasin S, Orwoll E, Labrie F, Karlsson MK, Ljunggren O, Vandenput L, Mellström D, Tivesten A. High serum testosterone is associated with reduced risk of cardiovascular events in elderly men. The MrOS (Osteoporotic Fractures in Men) study in Sweden. J Am Coll Cardiol. 2011;58:1674–1681. doi: 10.1016/j.jacc.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Ottenbacher KJ, Ottenbacher ME, Ottenbacher AJ, Acha AA, Ostir GV. Androgen treatment and muscle strength in elderly men: a meta-analysis. J Am Geriatr Soc. 2006;54:1666–1673. doi: 10.1111/j.1532-5415.2006.00938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- Rosner W, Vesper H. Toward excellence in testosterone testing: a consensus statement. J Clin Endocrinol Metab. 2010;95:4542–4548. doi: 10.1210/jc.2010-1314. [DOI] [PubMed] [Google Scholar]
- Shores MM, Smith NL, Forsberg CW, Anawalt BD, Matsumoto AM. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab. 2012;97:2050–2058. doi: 10.1210/jc.2011-2591. [DOI] [PubMed] [Google Scholar]
- Srinivas-Shankar U, Roberta SA, Connolly MJ, O'Connell MD, Adams JE, Oldham JA, Wu FC. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2010;95:639–650. doi: 10.1210/jc.2009-1251. [DOI] [PubMed] [Google Scholar]
- Surampudi PN, Wang C, Swerdloff R. Hypogonadism in the aging male diagnosis, potential benefits, and risks of testosterone replacement therapy. Int J Endocrinol. 2012;2012:625434. doi: 10.1155/2012/625434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover JL. Testosterone replacement therapy and the prostate. Curr Sex Health Rep. 2007;4:79–82. [Google Scholar]
- Veldhuis JD, Iranmanesh A, Samojlik E, Urban RJ. Differential sex steroid negative feedback regulation of pulsatile follicle-stimulating hormone secretion in healthy older men: deconvolution analysis and steady-state sex-steroid hormone infusions in frequently sampled healthy older individuals. J Clin Endocrinol Metab. 1997;82:1248–1254. doi: 10.1210/jcem.82.4.3869. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Liu PT, Keenan DM, Takahashi PY. Older men exhibit reduced efficacy of and heightened potency downregulation by intravenous pulses of recombinant human LH: a study in 92 healthy men. Am J Physiol Endocrinol Metab. 2012;302:E117–E122. doi: 10.1152/ajpendo.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Leung A, Sinha-Hikim AP. Reproductive aging in the male Brown-Norway rat: a model for the human. Endocrinology. 1993;133:2773–2781. doi: 10.1210/endo.133.6.8243304. [DOI] [PubMed] [Google Scholar]
- Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, O'Neill TW, Bartfai G, Casanueva FF, Forti G, Giwercman A, Han TS, Kula K, Lean ME, Pendleton N, Punab M, Boonen S, Vanderschueren D, Labrie F, Huhtaniemi IT. EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363:123–135. doi: 10.1056/NEJMoa0911101. [DOI] [PubMed] [Google Scholar]
- Zirkin BR, Santulli R, Strandberg JD, Wright WW, Ewing LL. Testicular steroidogenesis in the aging Brown Norway rat. J Androl. 1993;14:118–123. [PubMed] [Google Scholar]