Abstract
In rodents, many exogenous and endogenous cannabinoids, such as anandamide (AEA) and 2-arachidonyl glycerol (2-AG), have been shown to play an important role in certain hippocampal memory processes. However, the mechanisms by which endogenous AEA regulate this processes are not well understood. Here the effects of AEA on long-term potentiation (LTP), hippocampal-dependent learning and memory tasks, pERK1/2, pCaMKIV, and pCREB signaling events in both cannabinoid receptor type 1 (CB1R) wild type (WT) and knockout (KO) mice were assessed following administration of URB597, an inhibitor of the fatty acid amide hydrolase (FAAH). Acute administration of URB597 enhanced AEA levels without affecting the levels of 2-AG or CB1R in the hippocampus and neocortex compared to vehicle. In hippocampal slices, URB597 impaired LTP in CB1R WT but not in KO littermates. URB597 impaired object recognition, spontaneous alternation and spatial memory in the Y-maze test in CB1R WT mice but not in KO mice. Furthermore, URB597 enhanced ERK phosphorylation in WT without affecting total ERK levels in WT or KO mice. URB597 impaired CaMKIV and CREB phosphorylation in WT but not in KO mice. CB1R KO mice have a lower pCaMKIV/CaMKIV ratio and higher pCREB/CREB ratio compared to WT littermates. Our results indicate that pharmacologically elevated AEA impair LTP, learning and memory and inhibit CaMKIV and CREB phosphorylation, via the activation of CB1Rs. Collectively, these findings also suggest that pharmacological elevation of AEA beyond normal concentrations is also detrimental for the underlying physiological responses.
Keywords: LTP, Anandamide, URB597, CB1 Null Mice, FAAH, MAPK, CREB, CaMKIV phosphorylation
INTRODUCTION
The main psychoactive component of marijuana (C. sativa), Δ9-tetrahydrocannabinol (Δ9-THC), is believed to produce disturbances in various aspects of learning and memory of humans (Chait and Pierri, 1992) and rodents (Lichtman and Martin, 1996). In fact, impairment of cognition and memory is perhaps the most persistent alteration induced by Δ9-THC and synthetic cannabinoids in rodents (Heyser et al., 1993; Lichtman et al., 1995). Most of the effects of Δ9-THC or synthetic cannabinoids are mediated through the cannabinoid receptor type 1 (CB1R) (Huestis et al., 2001; Monory et al., 2007). The CB1R is predominately expressed in the CNS, particularly in areas such as the hippocampus, basal ganglia, cortex, amygdala and cerebellum – areas linked to the behavioral effects of Δ9-THC (Egertova and Elphick, 2000; Herkenham et al., 1990; Herkenham et al., 1991). The CB1R is a G protein-coupled receptor (GPCR) that binds to the Gi/o class of G proteins and are primarily located on presynaptic terminals, a primary location to control neurotransmitter release (Katona et al., 2006; Nyiri et al., 2005). The ability of the CB1R to suppress neurotransmission allows both exogenous cannabinoids (such as Δ9-THC) and endogenous cannabinoids (ECs) to have a profound impact on neuronal communication, and thus learning and memory (Hampson and Deadwyler, 1999; Lichtman et al., 1995; Varvel et al., 2001). Although the detailed cellular mechanisms are not clear, it has been suggested that ECs such as anandamide (AEA) and 2-arachidonyl glycerol (2-AG) play an important role in certain memory processes (Castellano et al., 1997). Their presence in memory-relevant areas of the rodent brain (Di Marzo et al., 1994; Di Marzo and Petrosino, 2007), together with the localization of CB1Rs on various cell types in hippocampus and pre-frontal cortex (Egertova and Elphick, 2000; Herkenham et al., 1990; Herkenham et al., 1991), suggests that the ECs acting through CB1Rs could play a pivotal role in regulating learning and memory processes.
In addition, exogenous administration of AEA also produces memory deficits in operant conditioning (Mallet and Beninger, 1998), memory consolidation (Murillo-Rodriguez et al., 1998) and inhibitory avoidance (Barros et al., 2004). Because AEA is rapidly metabolized by fatty acid amide hydrolase (FAAH) in cells, others have used a metabolically stable analogue of AEA, R-meth-AEA, in memory related studies. Similar to AEA, R-meth-AEA also disrupts operant conditioning (Brodkin and Moerschbaecher, 1997), impairs ORT in rats (Kosiorek et al., 2003) and impairs learning and memory in the mouse water maze test (Varvel and Lichtman, 2002; Varvel et al., 2006). Recent studies using a pharmacological approach to modulate AEA levels by inhibition of FAAH by URB597 suggests that AEA impairs ORT (Busquets-Garcia et al., 2011) and hippocampal single cell firing and Delayed Non-Match to Sample in rats (Goonawardena et al., 2011). These studies together strongly implicate ECs in several forms of memory process. However, they do not exclude a role for transient receptor potential vanilloid (TRPV-1) in memory-related effects (Marsch et al., 2007) because R-meth-AEA and AEA can activate TRPV-1 in addition to CB1Rs in the hippocampus (Chavez et al., 2010).
Therefore, we hypothesized that acute administration of the FAAH inhibitor URB597, by decreasing AEA hydrolysis, would increase AEA tone and as a result impair LTP and several memory tasks in mice. The results demonstrate that the administration of URB597 increased brain tissue concentrations of AEA. URB597 impaired synaptic plasticity and performance on several learning and memory tasks, and altered intracellular signaling in CB1R WT mice but not in KO mice.
METHODS AND MATERIALS
Animals and Treatment
CB1R WT and KO mice (Subbanna et al., 2013) on C57BL/6J background were generated from heterozygous breeding colony at NKI and housed in groups under standard laboratory conditions (12 h light / 12 h dark cycle) with food and water available ad libitum. Animal care and handling procedures followed Institutional (NKI IACUC) and National Institutes of Health guidelines. The genotype of CB1R WT and KO mice was determined by polymerase chain reaction (PCR) of genomic DNA obtained from mouse tails as described before (Basavarajappa et al., 2003). Male mice were used throughout the study. For the URB597 ([3 fenilo (3-carbamoylphenyl)]-N-cyclohexylcarbamate) (Cayman, Ann Arbor, MI, USA) experiments, URB597 was dissolved in DMSO (less than 2%) followed by a 2-3 drops of Tween 80 and then volume was made up with sterile saline solution. The URB597 solution was administered (0-1.0 mg/kg) by IP injection at a volume of 10 ml/kg body weight. The selection of URB597 dose is based on its effects on various behavioral tests published before (Busquets-Garcia et al., 2011; Goonawardena et al., 2011; Seillier et al., 2010). The URB597 vehicle solution was injected as a control. Separate sets of animals were subjected to each behavioral study (three to four month old male mice, n=8/group). In some experiments, brains were processed for biochemical analyses, as described below. Five to 15 animals were used for each data point.
Measurement of AEA and 2-AG Levels by LC/MS
For AEA and 2-AG analysis, after the vehicle or URB597 administration (0-1mg/kg, 0-24h), male CB1R WT and KO mice were sacrificed by decapitation, hippocampus and cortex were dissected, flash frozen and stored at −80°C. AEA and 2-AG levels in hippocampal and cortical tissue extracts were measured by a LC–MS method using the isotopic dilution procedure as described previously (Subbanna et al., 2013). The standard curve was fitted with a quadratic equation with the curve encompassing a range of 0.5–50 ng for AEA, 50-2500 ng for 2-AG and was processed similarly with quality controls with brain tissue extracts.
Long-Term Potentiation (LTP)
Three month old male CB1R WT and KO mice (n=5/group/treatment) were sacrificed by cervical dislocation followed by decapitation. Hippocampi were quickly removed. Transverse hippocampal slices (400 μm) were cut and recorded according to standard procedures (Basavarajappa and Subbanna, 2014; Sadrian et al., 2012; Subbanna et al., 2013; Vitolo et al., 2009). Following cutting, hippocampal slices were transferred to a recording chamber where they were maintained at 29° C and perfused (1–3 ml/min) with artificial cerebrospinal fluid (ACSF) continuously bubbled with 95% O2 and 5% CO2. The ACSF composition in mM was: 124.0 NaCl, 4.4 KCl, 1.0 Na2HPO4, 25.0 NaHCO3, 2.0 CaCl2, 2.0 MgSO4, 10.0 glucose, osmolarity 290-300. Slices were permitted to recover for at least 90 min before recording. A concentric bipolar platinum-iridium stimulation electrode (FHC, Bowdoin, ME, USA) and a low-resistance glass recording microelectrode filled with ACSF solution (5 mΩ resistance) were placed in CA1 stratum radiatum to record the extracellular field excitatory postsynaptic potential (fEPSP). An input–output curve was used to set the baseline fEPSP at ≈35% of maximal slope. Baseline stimulation was delivered every minute (0.01-ms duration pulses) for 10 min before beginning the experiment to assure stability of the response. Responses were recorded for 2 h after and measured as fEPSP slope expressed as percentage of baseline. LTP was induced by using θ-burst stimulation (4 pulses at 100 Hz, with the bursts repeated at 5 Hz and each tetanus including three 10-burst trains separated by 15 s). Responses were recorded for 2 h after tetanization. The fEPSP slope was measured from the early rising phase of the recorded traces using Pclamp10 and Digitata 1322A acquisition system (Molecular Devices) as described (Vitolo et al., 2002). Data analysis was performed with ANOVA followed by post hoc correction. In some experiments, after 10 min baseline recording, hippocampal slices were perfused with URB597 (1.0 μM in DMSO) or vehicle (0.001% DMSO), for 30 min before inducing LTP with tetanic stimulation of the Schaeffer collateral pathway. The selection of URB597 concentration is based on previous electrophysiological studies (Basavarajappa et al., 2008; Caiati et al., 2012).
Novel Object Recognition Task
Novel object recognition memory (ORT), which is based on the natural tendency of rodents to explore a novel object more than a familiar one (Ennaceur and Delacour, 1988). In brief, Twenty-four hours after habituation, mice were treated with and without URB597 (30 min). After 30 min, novel object recognition task was evaluated as previously described (Subbanna et al., 2013). e1and e2 are measures of the total exploration time of both objects during T1 and T2 (1, 4 and 24 h retention), respectively. d2 was considered as measures of discrimination index between the novel and the familiar objects. d2 is a relative measure of discrimination which corrects the difference between exploring the familiar and the novel object for exploration activity (e2) and appears to be independent of the total exploration times (Sik et al., 2003). The times spent exploring each object during T1 and T2 were recorded manually with a personal computer.
Spontaneous Alternation Y Maze Task
Spontaneous alternation (Dember and Fowler, 1958) was tested as described previously (Holcomb et al., 1998). In brief, mice were treated with and without URB597, after 30 min, each mouse was placed in the center of the Y maze and was allowed to explore freely through the maze during an 8 min session. The sequence, time spent in each arm and total number of arms entered was recorded as described before (Basavarajappa and Subbanna, 2014). Arm entry was considered to be completed when the hind paws of the mouse had been completely placed in the arm. Percentage alternation is the number of triads containing entries into all three arms divided by the maximum possible alternations (the total number of arms entered minus 2) × 100.
Spatial Recognition Memory using the Y Maze
Spatial recognition memory (Dellu et al., 1992) was tested as described previously (Sarnyai et al., 2000). Briefly, mice were treated with and without URB597, after 30 min, mice were subjected to spatial recognition memory. The number of entries into, the time spent (dwell time) in each arm and the first choice of entry were registered manually from video recordings by an observer blind to the treatment or genotype of the mice. Discrimination ratio [Preference for the Novel arm over the familiar Other arm (Novel/Novel + Other)] for arm entries and dwell time of WT and KO mice treated with or without URB597 were calculated as described previously by us (Basavarajappa and Subbanna, 2014).
Electrophoresis and Immunoblotting
For Western blot analysis, 0, 30 and 60 min after the vehicle or URB597 administration, male mice were sacrificed by decapitation, hippocampus was dissected, flash frozen and stored at −80°C. Tissue homogenates from the hippocampus containing freshly added 1% protease inhibitor mixture (Roche, Indianapolis, IN, USA) and phosphatase inhibitors were centrifuged at 7700 g for 1 min, and the supernatant (total extract) was aspirated and stored at −80°C until use. The pellet (nuclear fraction) was then resuspended in a nuclear extraction buffer (Grabowski, 2005) and nuclear fraction was prepared as described before (Basavarajappa and Subbanna, 2014). The supernatant was used to prepare plasma membrane (PM) fractions as described before (Basavarajappa et al., 1998; Basavarajappa and Hungund, 1999; Basavarajappa et al., 2006; Subbanna et al., 2013). The nuclear and PM fractions were stored at −80°C until use. The samples were prepared in a sample buffer as previously described by our laboratory (Basavarajappa et al., 2008; Subbanna et al., 2013). The blots were incubated in primary antibody; anti-rabbit-CB1R (0.1μg/ml, Thermo Scientific, Waltham, MA), anti-mouse CaMKIV (Sc-55501, 1: 1000), anti-rabbit pCaMKIV (Sc-28443-R, 1: 1000) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-rabbit p44/42 MAPK (ERK1/2) (# 9102, 1:2000), anti-rabbit-phospho-p44/42 MAPK (# 9101, 1:1000), anti-mouse-β-actin (#3700, 1:1000, Cell Signaling Technology, Danvers, MA, USA), anti-mouse-pCREB (Ser133) (# 05-807, 1:1000) and anti-rabbit-CREB (# 04-218, 1:1000) (Millipore, Billerica, MA, USA) for 3 h at room temperature or overnight at 4°C and processed as previously described by our laboratory (Basavarajappa et al., 2008).
Statistical Analysis
Unless indicated otherwise, the experiments were performed using equal number of animals per treatment. All of the data are presented as the mean ± SEM. A statistical comparison of the data was performed by either a one-way analysis of variance ANOVA or a two-way ANOVA with Bonferroni’s post hoc test. In all of the comparisons, p < 0.05 was considered to indicate statistical significance. The statistical analyses were performed using the Prism software (GraphPad, San Diego, CA).
RESULTS
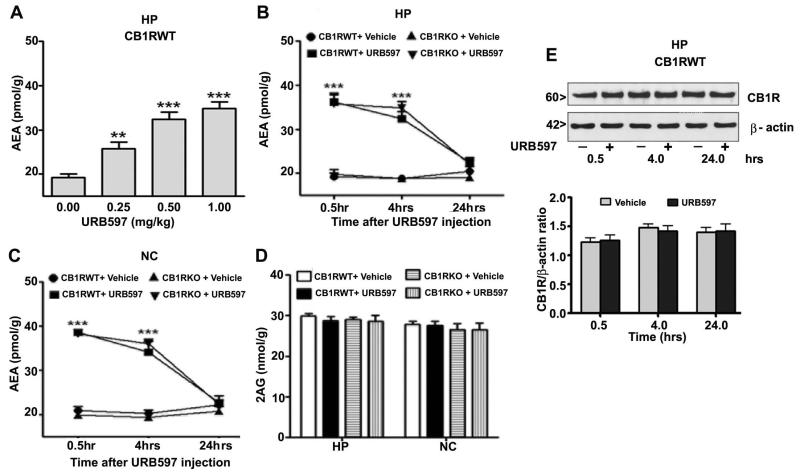
Pharmacological inhibition of FAAH enhances brain AEA levels without affecting the 2-AG levels
First, we determined the effect of various doses of acute administration of URB597 (30 min) on AEA levels in the hippocampus of CB1 receptor WT mice. AEA levels were enhanced by URB597 in a dose-dependent manner (Fig. 1A). We used optimum dose (0.5 mg/kg), which has been shown to block FAAH activity maximally (Kathuria et al., 2003), in all our subsequent experiments. We tested the effect of URB597 on the levels of AEA and 2-AG in CB1R WT and KO mice. The levels of AEA and 2-AG were evaluated using an LC/MS method 0.5 hr, 4 hrs and 24 hrs after URB597 administration. Results demonstrated that AEA levels were enhanced by URB597 in the hippocampus (F3, 84 = 77, p<0.001) and neocortex (F3, 84 = 94, p<0.001) (Fig. 1B-C) (Two-way ANOVA with Bonferroni’s post hoc tests) compared to vehicle at 0.5 hr and 4 hrs (p < 0.001) but not at 24 hrs in both WT and KO mice. However, no significant net change in the level of 2-AG (Fig. 1D) was observed. Therefore, we concluded that AEA levels are enhanced and maintained at least for 4 hrs after pharmacological inhibition of FAAH in mice. To further assess the elevation of endogenous AEA by URB597 on CB1 receptor protein levels in the hippocampus and neocortex, we determined the CB1R protein levels by Western blot analysis using a CB1R-specific antibody (Subbanna et al., 2013). Results suggest that elevation of AEA for up to 24 hrs has no significant influence on CB1 receptor proteins levels in the hippocampus (p > 0.05) or neocortex (data not shown) at 0.5 hr, 4 hrs and 24 hrs time points (Fig. 1E).
FIGURE 1.
Pharmacological inhibition of FAAH enhances AEA but not 2-AG levels in the hippocampus and cortex of CB1R WT and KO mice. (A-C) The hippocampal or cortex total extracts were subjected to LC-MS analysis for the AEA (A-C) and 2-AG (D) contents (30 min) (n = 10 mice/group) (**p < 0.05, ***p < 0.001vs. compared to respective vehicle or genotype). (E) The hippocampal PM fractions were subjected to Western blot and probed with CB1R specific Ab. Representative blot is shown for the hippocampal PM extracts (n = 6 mice/group). The error bars represent the SEM (two-way ANOVA with Bonferroni’s post hoc tests). HP, hippocampus; NC, neocortex.
Inhibition of FAAH by URB597 impairs LTP in CB1R WT mice but not in KO mice
We first determined the input/output (I/O) responses of fEPSPs and LTP of the fEPSP in the Schaffer collateral pathway of hippocampal slices (Fig. 2A) prepared from adult CB1R WT or KO animals that were exposed to vehicle or URB597 (1 μM) for 30 min. Increasing stimulus intensity evoked robust fEPSP I/O responses in all of the groups. The fEPSP I/O curve was not altered by vehicle or URB597 treatment (Fig. 2B) either in WT or KO slices. These findings suggest that neither vehicle nor URB597 significantly affects the fEPSP slope in pyramidal cells over the entire range of stimulation intensities. Prior to tetanic stimulations, a ten-minute baseline fEPSP response was established by stimulating at 60-s intervals with the stimulation intensity equivalent to ~35% of the maximum evoked response. Tetanic stimulation evoked a typical LTP of the fEPSP (Basavarajappa and Subbanna, 2014) in CB1R WT slices treated with vehicle (p< 0.001). These responses were stable over 140 min. However, tetanic stimulation evoked a significantly reduced fEPSP slope in slices (n = 10 slices/5 mice/group) treated with URB597 (solid line 1 μM for 30 min) (Fig. 2C) both induction (1 min) and maintenance (60 and 95 min). We next examined whether CB1R KO mice provides resistance to URB597-induced inhibition of LTP. Our results suggest that CB1R KO mice exhibited robust fEPSP I/O responses evoked by increasing stimulus intensity similar to WT mice. The fEPSP I/O curve was not altered by URB597 in the KO mice (Fig. 2D). These findings suggest that neither URB597 nor genetic deletion of CB1Rs significantly affects the fEPSPs slope in pyramidal cells over the entire range of stimulation intensities. Tetanic stimulation evoked an enhanced LTP in CB1R KO mice compared with WT mice at 1, 60 and 95 min and LTP was not reduced by URB597 treatment (Fig. 2E and F). Taken together, these findings [F3,108 = 83; p<0.001, two-way ANOVA with Bonferroni’s post hoc test] suggest that pharmacologically enhancing the levels of AEA in hippocampal slices impairs LTP through activation of CB1Rs.
FIGURE 2.
URB597 inhibits LTP in hippocampal slices from adult CB1R WT mice but not KO mice. (A) A schematic diagram showing the positions of the stimulating and recording electrodes in the CA1 region of the hippocampus. (B) A summary graph showing the field input/output relationships in WT slices. (C) Time course of the averaged fEPSP slopes in WT slices with and without URB597 (solid line 1 μM for 30 min). (D) A summary graph showing the field input/output relationships for KO slices with and without URB597. (E) Time course of the averaged fEPSP slopes in KO slices. Arrows denote the time of tetanic stimulation. (F) A combined plot of the averages of the fEPSP slopes for WT and KO slices with and without URB597. Each point is presented as the mean ± SEM (n= 5 mice/group; 10 slices/group) (Two-way ANOVA with Bonferroni’s post hoc tests), ***p < 0.001 vs. CB1WT + Vehicle; # p < 0.05 vs. CB1WT + Vehicle; $ p < 0.05 vs. CB1WT + URB597.
Pharmacologically enhanced AEA impairs learning and memory through CB1Rs
We used three different memory tasks, such as object recognition, spontaneous alternation and spatial recognition, to examine whether pharmacological elevation of AEA impairs these memory tasks. First, we investigated ORT task and the results demonstrated that there were no significant effect of URB597 treatment, in either CB1R WT or KO mice, on total exploration times at e1 (T1) or e2 (T2) (1 or 4 or 24 hrs retention) in the ORT task (Fig. 3A). URB597 treatment significantly impaired ORT performance both at 1 hr [F3,28 = 43; p<0.001] and 4 hrs retention [F3,28 = 29; p<0.001] (T2) in CB1R WT mice but not in KO mice (Fig. 3B and C). In addition, KO mice exhibited enhanced ORT performance at 1 hr, 4 hrs and 24 hrs retention (T2) compared to WT mice (p<0.01) (Fig. 3B-D). In addition, at 24 hrs retention, URB597 failed to impair ORT performance in both WT and KO mice (Fig. 3D). These data suggest that pharmacological elevation of AEA by acute administration of URB597 impairs short-term memory but not long-term memory in ORT task in a CB1R-dependent manner in mice.
FIGURE 3.
Acute administration of URB597 impairs novel object recognition in adult mice. (A) The level of exploration at el and e2 (1 or 4 or 24 hrs), and the time spent exploring the two objects at T1 and T2 (at 1, 4 and 24 hrs) in WT or KO mice treated with vehicle or URB597. (B-D) Discrimination indices (d2) obtained from the WT or KO mice treated with vehicle or URB597 at 1 hr (B), 4 hrs (C) and 24 hrs (D) retention intervals. One-way ANOVA with Bonferroni’s post hoc test; ***p < 0.001 vs. CB1WT + Vehicle; # p < 0.05 vs. CB1WT + Vehicle; $ p < 0.05 vs. CB1WT + URB597; @ p < 0.001 vs. CB1WT + Vehicle.
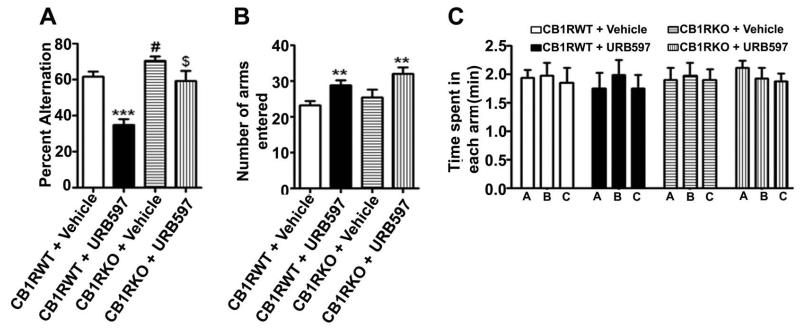
In our next behavioral test, animals treated with URB597 were tested for spontaneous alternation in the Y maze. CB1R WT and KO mice were treated with URB597 30 min before the test. Consistent with the ORT performance, KO mice exhibited significantly enhanced spontaneous alternation behavior compared to WT mice. Analysis by one-way ANOVA revealed that URB597 treatment significantly reduced spontaneous alternation performance in WT mice [F3,28 = 28, p<0.001] (Fig. 4A). Importantly, URB597 treatment failed to induce a spatial working memory deficit in the Y maze test in KO mice. Furthermore, URB597 significantly enhanced (p<0.01) exploratory activity, as assessed by the number of arm entries during Y-maze testing in both WT and KO mice (Fig. 4B). However, the amount of time spent in each arm was not altered by URB597 or vehicle in both WT and KO mice (Fig. 4C). Together, these findings suggest that pharmacological elevation of AEA due to inhibition of FAAH by acute administration of URB597 enhanced exploratory activity in CB1R independent manner but impaired the spontaneous alteration task in a CB1R-dependent manner in mice.
FIGURE 4.
Acute administration of URB597 impairs spontaneous alternation performance in adult mice. (A) Spatial working memory of CB1R WT and KO mice treated with or without URB597. *** p < 0.001, # p < 0.05 versus CB1WT + V; @ p < 0.05 versus CB1WT + V; $ p < 0.05 versus CB1WT + URB597). (B) The number of arm entries for WT and KO mice (** p < 0.001 versus CB1WT + V) (** p < 0.001 versus CB1KO + V). (C) The time spent in each arm for WT and KO mice with or without URB597 treatment. Each point is the mean ± SEM (n= 8 mice/group). One-way ANOVA with Bonferroni’s post hoc test.
In our next behavioral test, we used spatial recognition memory using a Y-maze. WT mice more frequently entered into and spent more time in the novel, previously unvisited arm of the maze. KO mice showed an enhanced preference toward the novel arm (p<0.001) and spent more time in the novel arm (p<0.001) compared to WT mice (Fig. 5A and B). Although all WT and KO mice selected the novel arm as the first choice, URB597-treated WT animals had a decreased preference for the novel arm as the first choice (Fig. 5C). URB597 treatment significantly impaired the ability of WT mice to enter more frequently into [F3,28 = 70, p<0.001] and spend more time in [F3,28 = 22, p<0.001] the novel, previously unvisited arm of the maze. In contrast, URB597 treatment failed to alter KO mice preference toward the novel arm or the time spent in the novel arm. Together, these findings suggest that enhanced levels of endogenous AEA impair spatial recognition memory in a CB1R-dependent manner in mice.
FIGURE 5.
URB597 administration impairs spatial memory performance as measured by Y maze. (A-B) Discrimination ratio for arm entries (A) and dwell time (time in the arm) (B) of WT and KO mice treated with or without URB597, 1 h after the first encounter with the partially opened maze. The dashed line indicates chance performance (0.5). (C) The percentage of animals selecting the novel arm as the first choice is shown for WT and KO mice treated with or without URB597, 1 h after the first encounter with the partially opened maze. Each point is the mean ± SEM (n= 8 mice/group). One-way ANOVA with Bonferroni’s post hoc test; ***p < 0.001 vs. CB1WT + Vehicle; # p < 0.05 vs. CB1WT + Vehicle; $ p < 0.05 vs. CB1WT + URB597; @ p < 0.001 vs. CB1WT + Vehicle.
URB597 enhances ERK phosphorylation and impairs CaMKIV and CREB phosphorylation in CB1R WT but not in KO mice
To elucidate the downstream intracellular pathways involved in the effects of endogenous AEA, we studied the involvement of pERK, pCaMKIV and pCREB, key regulators of synaptic plasticity and learning and memory (Martin et al., 2000). It is entirely unknown how endogenous elevation of AEA regulates these signaling molecules in parallel with LTP and learning and memory in animals. First, we assessed pERK and the total amount of ERK protein. We found that ERK phosphorylation was significantly enhanced at 60 min and total ERK was not altered by URB597 treatment in the hippocampus of WT mice compared to KO mice. Total ERK levels did not differ between WT and KO mice (Fig. 6A). Next, we assessed pCaMKIV and the total amount of CaMKIV protein. We found that in WT mice, CaMKIV phosphorylation was significantly (p<0.001) inhibited at 30 and 60 min after URB597 treatment. We found lowered pCaMKIV/CaMKIV ratio in KO mice compared to WT mice [Fig. 6B (i)]. However, total CaMKIV protein levels were not significantly different in CB1R KO mice compared to WT mice. URB597 failed to alter total CaMKIV levels either in WT or in KO mice. Next, we determined pCREB and CREB protein levels. We found that in WT mice, CREB phosphorylation was significantly (p<0.001) inhibited at 30 and 60 min after URB597 treatment [Fig. 6B (ii)]. We found increased pCREB/CREB ratio in KO mice compared to WT mice and total CREB protein levels were not significantly different between KO and WT mice. URB597 failed to alter total CREB levels either in WT or in KO mice. Collectively, our data suggest that pharmacological elevation of AEA specifically enhances ERK phosphorylation and impairs CaMKIV and CREB phosphorylation via CB1Rs.
FIGURE 6.
Effect of acute administration of URB597 on pERK, pCaMKIV and pCREB levels in the hippocampus. (A) The levels of pERK1/2 and ERK1/2 in hippocampal total extracts. Representative blots are shown for the hippocampal total extracts (n = 6 mice/group). **p < 0.01 vs. CB1WT + Vehicle (0 min). (B) The levels of pCaMKIV, pCREB, CaMKIV and CREB. Representative blots are shown for the hippocampal nuclear extracts (n = 6 mice/group). Student’s t-test was used for statistical analysis. The error bars represent SEM. ***p < 0.001 vs. CB1WT + Vehicle (0 min); **p < 0.01 vs. CB1WT + Vehicle (0 min); *p < 0.05 vs. CB1WT + Vehicle (0 min).
DISCUSSION
This study provides an important insight into the role of endogenous AEA in regulating hippocampal synaptic plasticity, as it affects performance in a short-term memory tasks, and demonstrates that pharmacologically elevated AEA specifically inhibits both LTP in hippocampal slices and learning and memory in mice through CB1Rs. Consistent with our previous observations (Basavarajappa et al., 2008) elevation of endogenous AEA by URB597 for up to 24 hrs has no significant effect on CB1R protein levels. Although both AEA and 2-AG are known to regulate synaptic neurotransmission mainly through presynaptic CB1Rs (Piomelli, 2003), 2-AG seems to be relatively specific for CB1Rs, whereas AEA is known to bind to both CB1 and TRPV1 receptors (Di Marzo and De Petrocellis, 2010). At inhibitory synapses, 2-AG has been proposed to mediate long-term depression (LTD) based on studies that used specific inhibitors of 2-AG synthesis, uptake or degradation (Heifets and Castillo, 2009; Kano et al., 2009). However, exogenous AEA has been shown to inhibit LTP induction in the Schaffer collateral-CA1 field complex, and this inhibition is prevented by SR141617A, a CB1R antagonist (Terranova et al., 1995). Induction of LTP is blocked in hippocampal slices by acute application of Δ9-THC (Nowicky et al., 1987), 2-AG (Stella et al., 1997) or synthetic cannabinoid receptor agonists, such as HU-210 (Collins et al., 1995) and WIN-55,212-2 (Paton et al., 1998; Terranova et al., 1995). Additionally, hippocampal LTP is enhanced in CB1R KO mice (Bohme et al., 2000; Subbanna et al., 2013), whereas the CB1R antagonists SR141716 and AM251 facilitate LTP (Slanina et al., 2005). In our previous studies, URB597 treatment suppressed miniature excitatory postsynaptic currents (mEPSCs) in hippocampal neurons and potentiated acute ethanol-induced suppression of mEPSCs, and these effects were reversed by SR141617 (Basavarajappa and Arancio, 2008; Basavarajappa et al., 2008). In a recent study, AEA also produced a decrease in mEPSCs in the presence of URB597 (1 μM) in midbrain periaquedutal grey slices (Kawahara et al., 2011). Together, our data strongly suggest that the action of AEA on CB1Rs is responsible for impaired LTP because acute blockade of AEA degradation by URB597 did not affect LTP in CB1R KO mice, suggesting that URB597-induced LTP deficits are mediated via AEA/CB1Rs.
The use of the various memory tests, such as the ORT, spontaneous alternation test and spatial recognition test, allowed for assessment of cognitive performance after pharmacological elevation of AEA by URB597 in both CB1R WT and KO mice. Neuropsychological assessment of amnesic patients and experiments in laboratory animals suggest that the functional integrity of the temporal lobe, including the hippocampal formation and perirhinal cortex, are essential for processing this type of information (Ennaceur and Delacour, 1988; Logothetis and Sheinberg, 1996). Moreover, the role of the EC system in recognition memory processing has not yet been examined in detail. We found a selective short-term (1-4 hrs) but not long-term (24 hrs) deficit in novel object recognition performance after acute administration of URB597 in CB1R WT mice but not in CB1R KO, suggesting that the effects are CB1R mediated. The lack of URB597 effect on ORT at 24 hrs retention is may be due to normalization of AEA to basal levels and mice failed to recall the learning event. This would constitute drug state dependent learning and warrant future experiments to address the AEA role in memory consolidation and recall events. The present findings are congruent with previous reports of impairments in ORT resulting from acute injections of various cannabinoids (Kosiorek et al., 2003) including EC cellular uptake inhibitor, VDM-11 (Clarke et al., 2008) and URB597 (Busquets-Garcia et al., 2011). In addition, we also found that CB1R KO mice exhibited enhanced performance in the ORT compared to WT littermates, suggesting that genetic deletion of CB1R enhances memory. Our results are consistent with the enhanced memory observed either in CB1R KO mice (Reibaud et al., 1999; Subbanna et al., 2013) or with CB1R blockade in rats (Terranova et al., 1996).
In agreement with earlier reports (Lichtman, 2000; Lichtman et al., 1995), our findings demonstrate that pharmacological elevation of AEA also impairs spontaneous alternation performance through CB1Rs. Interestingly, pharmacologically elevated AEA also impairs spatial memory performance and is dependent on the availability of CB1Rs. Moreover, mice lacking CB1Rs performed better at spatial memory tasks compared to WT littermates. Similarly, Δ9-THC has been shown to produce impairments in spatial learning in rodents (Da and Takahashi, 2002; Robinson et al., 2007; Varvel et al., 2005; Varvel et al., 2001). Taken together, our findings suggest that an acute administration of URB597, which enhances levels of AEA in hippocampus and cortex, can cause deficits in spatial learning in mice. In addition, URB597 treated mice performed well below 50% chance in Y-maze suggesting the intervention of neophobia and aversion. Although further research is warranted, these findings seems to be consistent with the other study in which stimulating endogenous cannabinoid signaling by blocking FAAH enhances acquisition of aversively reinforced spatial memory tasks (Varvel et al., 2007).
Our findings are consistent with the recent data in which pharmacological elevation of AEA by URB597 or an AEA uptake inhibitor (AM404) produced impairments in the Delayed Non-Match to Sample performance task in Long-Evans rats (Goonawardena et al., 2011). Additionally, our findings are in agreement with other behavioral data in which exogenous R-methanandamide impaired working memory in the water maze test (Varvel et al., 2006; Varvel and Lichtman, 2002) or learning an operant conditioning task (Brodkin and Moerschbaecher, 1997). The observed potent effect of URB597 on LTP and several learning and memory tasks is directly related to its inhibitory action on FAAH, preventing the breakdown of not only AEA but also other ECs, such as palmitylethanolamide (PEA) and oleoylethanolamide (OEA), that are also substrates of FAAH (Mazzola et al., 2009; Wise et al., 2008). PEA and OEA are also known to be co-synthesized with AEA and can potentiate AEA responses through the activation of other cannabinoid-sensitive receptors, such as GPR119 (G-protein coupled), TRPV-1 and PPARα (Peroxisome-Proliferator-Activated-Receptor-alpha) receptors (Hansen, 2010; Ho et al., 2008; Ning et al., 2008). However, the possibility of acting through other receptors is unlikely to exist in these studies because URB597 failed to induce LTP, learning and memory impairments in CB1R KO mice. Collectively these observations suggest that a transient increase in the levels of endogenous AEA in the hippocampus and cortex, by inhibiting its degradation, could impair several memory tasks in a CB1R-dependent manner in mice. Future investigation on the role of chronic long-lasting elevation of AEA on several aspects of synaptic plasticity as well as learning and memory will add to the understanding of the physiological significance of CB1 signaling and its role in cognition-related disorders.
Investigation of specific signaling pathways mediating the endogenous AEA may provide clues for candidate cellular mechanisms involved in synaptic plasticity and learning and memory deficits. Our findings of impaired CaMKIV and CREB phosphorylation by URB597 treatment are consistent with previous data in which Δ9-THC significantly reduced CREB phosphorylation (Rubino and Parolaro, 2008) and another calmodulin kinase related molecule such as CaMKII phosphorylation in a CB1R-dependent manner (Rubino et al., 2007). Interestingly, our data show that URB597 inhibition of CaMKIV and CREB phosphorylation was absent in mice lacking CB1Rs. These findings suggest that endogenous AEA affect cellular events and hippocampal function by CB1R-dependent mechanisms. Activation of CB1R signaling is associated with activation of mitogen-activated protein kinase (MAPK) activity in cultured cells (Bouaboula et al., 1995; Daigle et al., 2008), hippocampal slices (Derkinderen et al., 2003) and embryonic rat cortices (Berghuis et al., 2007). Although in vivo studies of the intracellular signaling events coupling MAPK activation to the binding of CB1Rs by Δ9-THC or other cannabinoids are limited (Rubino et al., 2007), several studies using cell lines suggest both up- and down regulation of MAPKs by Δ9-THC (De Petrocellis et al., 1998; Galve-Roperh et al., 2000). In a recent study, synthetic cannabinoid enhanced ERK1/2 phosphorylation in HEK293 cells stably expressing CB1Rs (Atwood et al., 2010). In our in vivo studies, URB597 enhanced ERK1/2 phosphorylation at longer time points in the hippocampus of CB1R WT but not in KO mice. In general, the CaMKIV mediated phosphorylation of CREB at Ser133 is essential to the transcriptional activation of CREB/CRE-mediated-signaling pathway (Bito et al., 1996) and have been thought to play a central role in memory consolidation and LTP (Martin et al., 2000). To our knowledge, this is the first study to suggest that CB1R-mediated inhibition of CaMKIV and CREB phosphorylation play an important role in the neural correlate of endogenous AEA on LTP and memory impairing effects. Future studies addressing the involvement of other specific signaling pathways in the action of endogenous AEA could provide additional mechanistic leads on the hippocampus function.
In conclusion, this is the first study that reports neuronal correlation between LTP, learning and memory deficits with that of CaMKIV and CREB phosphorylation deficits following enhancement of hippocampus and cortex AEA levels via the blockade of its breakdown. These findings suggest that the elevation of AEA beyond normal concentrations is detrimental for the underlying physiological responses in the brain.
Acknowledgements
This work in part was supported by NIH/NIAAA grant AA019443 (BSB).
Footnotes
Disclosure
The authors declare no conflict of interest.
REFERENCES
- Atwood BK, Huffman J, Straiker A, Mackie K. JWH018, a common constituent of ‘Spice’ herbal blends, is a potent and efficacious cannabinoid CB receptor agonist. Br J Pharmacol. 2010;160(3):585–93. doi: 10.1111/j.1476-5381.2009.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros DM, Carlis V, Maidana M, Silva ES, Baisch AL, Ramirez MR, Izquierdo I. Interactions between anandamide-induced anterograde amnesia and post-training memory modulatory systems. Brain Res. 2004;1016(1):66–71. doi: 10.1016/j.brainres.2004.04.067. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Arancio O. Synaptic Plasticity: Emerging Role for Endocannabinoid system. In: Kaiser TF, Peters FJ, editors. Synaptic Plasticity: New Research. Nova Science Publishers, Inc.; NY, USA: 2008. pp. pp77–112. [Google Scholar]
- Basavarajappa BS, Cooper TB, Hungund BL. Chronic ethanol administration down-regulates cannabinoid receptors in mouse brain synaptic plasma membrane. Brain. Res. 1998;793:212–218. doi: 10.1016/s0006-8993(98)00175-9. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Hungund BL. Down-regulation of cannabinoid receptor agonist-stimulated [35S] GTPgS binding in synaptic plasma membrane from chronic ethanol exposed mouse. Brain Res. 1999;815:89–97. doi: 10.1016/s0006-8993(98)01072-5. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Ninan I, Arancio O. Acute Ethanol Suppresses Glutamatergic Neurotransmission through Endocannabinoids in Hippocampal Neurons. J Neurochem. 2008;107(4):1001–1013. doi: 10.1111/j.1471-4159.2008.05685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Chronic ethanol inhibits the anandamide transport and increases extracellular anandamide levels in cerebellar granule neurons. Eur J Pharmacol. 2003;466:73–83. doi: 10.1016/s0014-2999(03)01557-7. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Subbanna S. CB1 Receptor-Mediated Signaling Underlies the Hippocampal Synaptic, Learning and Memory Deficits Following Treatment with JWH-081, a New Component of Spice/K2 Preparations. Hippocampus. 2014;24(2):178–188. doi: 10.1002/hipo.22213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavarajappa BS, Yalamanchili R, Cravatt BF, Cooper TB, Hungund BL. Increased ethanol consumption and preference and decreased ethanol sensitivity in female FAAH knockout mice. Neuropharmacology. 2006;50:834–844. doi: 10.1016/j.neuropharm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urban GM, Monory K, Marsicano G, Matteoli M, Canty A. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science. 2007;316(5828):1212–6. doi: 10.1126/science.1137406. others. [DOI] [PubMed] [Google Scholar]
- Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87(7):1203–14. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- Bohme GA, Laville M, Ledent C, Parmentier M, Imperato A. Enhanced long-term potentiation in mice lacking cannabinoid CB1 receptors. Neuroscience. 2000;95:5–7. doi: 10.1016/s0306-4522(99)00483-2. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Poinot-Chazel C, Bourrie B, Canat X, Calandra B, Rinaldi-Carmona M, Le Fur G, Casellas P. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochem J. 1995;312(pt 2):637–641. doi: 10.1042/bj3120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquets-Garcia A, Puighermanal E, Pastor A, de la Torre R, Maldonado R, Ozaita A. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biol Psychiatry. 2011;70(5):479–86. doi: 10.1016/j.biopsych.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Caiati MD, Sivakumaran S, Lanore F, Mulle C, Richard E, Verrier D, Marsicano G, Miles R, Cherubini E. Developmental regulation of CB1-mediated spike-time dependent depression at immature mossy fiber-CA3 synapses. Sci Rep. 2012;2:285. doi: 10.1038/srep00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano C, Cabib S, Palmisano A, Di Marzo V, Puglisi-Allegra S. The effects of anandamide on memory consolidation in mice involve both D1 and D2 dopamine receptors. Behav Pharmacol. 1997;8(8):707–12. doi: 10.1097/00008877-199712000-00005. [DOI] [PubMed] [Google Scholar]
- Chait LD, Pierri J. Effects of smoked marijuana on human performance. In: Murphy L, Bartke A, editors. Marijuana/Cannabinoids: Neurobiology and Neurophysiology. Boca Raton, FL: 1992. pp. 387–423. a critical review. [Google Scholar]
- Chavez AE, Chiu CQ, Castillo PE. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat Neurosci. 2010;13(12):1511–8. doi: 10.1038/nn.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JR, Rossato JI, Monteiro S, Bevilaqua LR, Izquierdo I, Cammarota M. Posttraining activation of CB1 cannabinoid receptors in the CA1 region of the dorsal hippocampus impairs object recognition long-term memory. Neurobiol Learn Mem. 2008;90:374–81. doi: 10.1016/j.nlm.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Collins DR, Pertwee RG, Davies SN. Prevention by the cannabinoid antagonist, SR141716A, of cannabinoid-mediated blockade of long-term potentiation in the rat hippocampal slice. Br J Pharmacol. 1995;115(6):869–70. doi: 10.1111/j.1476-5381.1995.tb15889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da S, Takahashi RN. SR 141716A prevents delta 9-tetrahydrocannabinol-induced spatial learning deficit in a Morris-type water maze in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(2):321–5. doi: 10.1016/s0278-5846(01)00275-5. [DOI] [PubMed] [Google Scholar]
- Daigle TL, Kearn CS, Mackie K. Rapid CB1 cannabinoid receptor desensitization defines the time course of ERK1/2 MAP kinase signaling. Neuropharmacology. 2008;54(1):36–44. doi: 10.1016/j.neuropharm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petrocellis L, Melck D, Palmisano A, Bisogno T, Laezza C, Bifulco M, Di Marzo V. The endogenous cannabinoid anandamide inhibits human breast cancer cell proliferation. Proc Natl Acad Sci U S A. 1998;95(14):8375–80. doi: 10.1073/pnas.95.14.8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellu F, Mayo W, Cherkaoui J, Le Moal M, Simon H. A two-trial memory task with automated recording: study in young and aged rats. Brain Res. 1992;588(1):132–9. doi: 10.1016/0006-8993(92)91352-f. [DOI] [PubMed] [Google Scholar]
- Dember WN, Fowler H. Spontaneous alternation behavior. Psychol Bull. 1958;55(6):412–28. doi: 10.1037/h0045446. [DOI] [PubMed] [Google Scholar]
- Derkinderen P, Valjent E, Toutant M, Corvol JC, Enslen H, Ledent C, Trzaskos J, Caboche J, Girault JA. Regulation of extracellular signal-regulated kinase by cannabinoids in hippocampus. J Neurosci. 2003;23(6):2371–82. doi: 10.1523/JNEUROSCI.23-06-02371.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L. Endocannabinoids as regulators of transient receptor potential (TRP) channels: A further opportunity to develop new endocannabinoid-based therapeutic drugs. Curr Med Chem. 2010;17(14):1430–49. doi: 10.2174/092986710790980078. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Petrosino S. Endocannabinoids and the regulation of their levels in health and disease. Curr Opin Lipidol. 2007;18(2):129–40. doi: 10.1097/MOL.0b013e32803dbdec. [DOI] [PubMed] [Google Scholar]
- Egertova M, Elphick MR. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB. J Comp Neurol. 2000;422:159–71. doi: 10.1002/(sici)1096-9861(20000626)422:2<159::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31(1):47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Galve-Roperh I, Sanchez C, Cortes ML, Gomez del Pulgar T, Izquierdo M, Guzman M. Anti-tumoral action of cannabinoids: involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat Med. 2000;6(3):313–9. doi: 10.1038/73171. [DOI] [PubMed] [Google Scholar]
- Goonawardena AV, Sesay J, Sexton CA, Riedel G, Hampson RE. Pharmacological elevation of anandamide impairs short-term memory by altering the neurophysiology in the hippocampus. Neuropharmacology. 2011;61(5-6):1016–25. doi: 10.1016/j.neuropharm.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski PJ. Splicing-active nuclear extracts from rat brain. Methods. 2005;37(4):323–30. doi: 10.1016/j.ymeth.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Deadwyler SA. Cannabinoids, hippocampal function and memory. Life Sci. 1999;65(6-7):715–23. doi: 10.1016/s0024-3205(99)00294-5. [DOI] [PubMed] [Google Scholar]
- Hansen HS. Palmitoylethanolamide and other anandamide congeners. Proposed role in the diseased brain. Exp Neurol. 2010;224(1):48–55. doi: 10.1016/j.expneurol.2010.03.022. [DOI] [PubMed] [Google Scholar]
- Heifets BD, Castillo PE. Endocannabinoid signaling and long-term synaptic plasticity. Annu Rev Physiol. 2009;71:283–306. doi: 10.1146/annurev.physiol.010908.163149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, A.B. L, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. U .S .A. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Cost BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;16:8057–8066. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser CJ, Hampson RE, Deadwyler SA. Effects of delta-9-tetrahydrocannabinol on delayed match to sample performance in rats: alterations in short-term memory associated with changes in task specific firing of hippocampal cells. J Pharmacol Exp Ther. 1993;264(1):294–307. [PubMed] [Google Scholar]
- Ho WS, Barrett DA, Randall MD. ‘Entourage’ effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. Br J Pharmacol. 2008;155(6):837–46. doi: 10.1038/bjp.2008.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med. 1998;4(1):97–100. doi: 10.1038/nm0198-097. others. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, Frank RA. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry. 2001;58:322–8. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89(1):309–80. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9(1):76–81. doi: 10.1038/nm803. others. [DOI] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26(21):5628–37. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara H, Drew GM, Christie MJ, Vaughan CW. Inhibition of fatty acid amide hydrolase unmasks CB1 receptor and TRPV1 channel-mediated modulation of glutamatergic synaptic transmission in midbrain periaqueductal grey. Br J Pharmacol. 2011;163(6):1214–22. doi: 10.1111/j.1476-5381.2010.01157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosiorek P, Hryniewicz A, Bialuk I, Zawadzka A, Winnicka MM. Cannabinoids alter recognition memory in rats. Pol J Pharmacol. 2003;55(5):903–10. [PubMed] [Google Scholar]
- Lichtman AH. SR 141716A enhances spatial memory as assessed in a radial-arm maze task in rats. Eur J Pharmacol. 2000;404(1-2):175–9. doi: 10.1016/s0014-2999(00)00615-4. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Dimen KR, Martin BR. Systemic or intrahippocampal cannabinoid administration impairs spatial memory in rats. Psychopharmacology (Berl) 1995;119(3):282–90. doi: 10.1007/BF02246292. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. Delta 9-tetrahydrocannabinol impairs spatial memory through a cannabinoid receptor mechanism. Psychopharmacology (Berl) 1996;126(2):125–31. doi: 10.1007/BF02246347. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Sheinberg DL. Visual object recognition. Annu Rev Neurosci. 1996;19:577–621. doi: 10.1146/annurev.ne.19.030196.003045. [DOI] [PubMed] [Google Scholar]
- Mallet PE, Beninger RJ. The cannabinoid CB1 receptor antagonist SR141716A attenuates the memory impairment produced by delta9-tetrahydrocannabinol or anandamide. Psychopharmacology (Berl) 1998;140(1):11–9. doi: 10.1007/s002130050733. [DOI] [PubMed] [Google Scholar]
- Marsch R, Foeller E, Rammes G, Bunck M, Kossl M, Holsboer F, Zieglgansberger W, Landgraf R, Lutz B, Wotjak CT. Reduced anxiety, conditioned fear, and hippocampal long-term potentiation in transient receptor potential vanilloid type 1 receptor-deficient mice. J Neurosci. 2007;27(4):832–9. doi: 10.1523/JNEUROSCI.3303-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Mazzola C, Medalie J, Scherma M, Panlilio LV, Solinas M, Tanda G, Drago F, Cadet JL, Goldberg SR, Yasar S. Fatty acid amide hydrolase (FAAH) inhibition enhances memory acquisition through activation of PPAR-alpha nuclear receptors. Learn Mem. 2009;16(5):332–7. doi: 10.1101/lm.1145209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monory K, Blaudzun H, Massa F, Kaiser N, Lemberger T, Schutz G, Wotjak CT, Lutz B, Marsicano G. Genetic dissection of behavioural and autonomic effects of Delta(9)-tetrahydrocannabinol in mice. PLoS Biol. 2007;5(10):e269. doi: 10.1371/journal.pbio.0050269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murillo-Rodriguez E, Sanchez-Alavez M, Navarro L, Martinez-Gonzalez D, Drucker-Colin R, Prospero-Garcia O. Anandamide modulates sleep and memory in rats. Brain Res. 1998;812(1-2):270–4. doi: 10.1016/s0006-8993(98)00969-x. [DOI] [PubMed] [Google Scholar]
- Ning Y, O’Neill K, Lan H, Pang L, Shan LX, Hawes BE, Hedrick JA. Endogenous and synthetic agonists of GPR119 differ in signalling pathways and their effects on insulin secretion in MIN6c4 insulinoma cells. Br J Pharmacol. 2008;155(7):1056–65. doi: 10.1038/bjp.2008.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicky AV, Teyler TJ, Vardaris RM. The modulation of long-term potentiation by delta-9-tetrahydrocannabinol in the rat hippocampus, in vitro. Brain Res Bull. 1987;19(6):663–72. doi: 10.1016/0361-9230(87)90052-9. [DOI] [PubMed] [Google Scholar]
- Nyiri G, Cserep C, Szabadits E, Mackie K, Freund TF. CB1 cannabinoid receptors are enriched in the perisynaptic annulus and on preterminal segments of hippocampal GABAergic axons. Neuroscience. 2005;136(3):811–22. doi: 10.1016/j.neuroscience.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Paton GS, Pertwee RG, Davies SN. Correlation between cannabinoid mediated effects on paired pulse depression and induction of long term potentiation in the rat hippocampal slice. Neuropharmacology. 1998;37(9):1123–30. doi: 10.1016/s0028-3908(98)00096-3. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–84. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Reibaud M, Obinu MC, Ledent C, Parmentier M, Bohme GA, Imperato A. Enhancement of memory in cannabinoid CB1 receptor knock-out mice. Eur J Pharmacol. 1999;379:R1–2. doi: 10.1016/s0014-2999(99)00496-3. [DOI] [PubMed] [Google Scholar]
- Robinson L, Goonawardena AV, Pertwee RG, Hampson RE, Riedel G. The synthetic cannabinoid HU210 induces spatial memory deficits and suppresses hippocampal firing rate in rats. Br J Pharmacol. 2007;151(5):688–700. doi: 10.1038/sj.bjp.0707273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Parolaro D. Long lasting consequences of cannabis exposure in adolescence. Mol Cell Endocrinol. 2008;286(1-2 Suppl 1):S108–13. doi: 10.1016/j.mce.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Rubino T, Sala M, Vigano D, Braida D, Castiglioni C, Limonta V, Guidali C, Realini N, Parolaro D. Cellular mechanisms underlying the anxiolytic effect of low doses of peripheral Delta9-tetrahydrocannabinol in rats. Neuropsychopharmacology. 2007;32(9):2036–45. doi: 10.1038/sj.npp.1301330. [DOI] [PubMed] [Google Scholar]
- Sadrian B, Subbanna S, Wilson DA, Basavarajappa BS, Saito M. Lithium prevents long-term neural and behavioral pathology induced by early alcohol exposure. Neuroscience. 2012;206:122–135. doi: 10.1016/j.neuroscience.2011.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnyai Z, Sibille EL, Pavlides C, Fenster RJ, McEwen BS, Toth M. Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin(1A) receptors. Proc Natl Acad Sci U S A. 2000;97(26):14731–6. doi: 10.1073/pnas.97.26.14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seillier A, Advani T, Cassano T, Hensler JG, Giuffrida A. Inhibition of fatty-acid amide hydrolase and CB1 receptor antagonism differentially affect behavioural responses in normal and PCP-treated rats. Int J Neuropsychopharmacol. 2010;13(3):373–86. doi: 10.1017/S146114570999023X. [DOI] [PubMed] [Google Scholar]
- Sik A, van Nieuwehuyzen P, Prickaerts J, Blokland A. Performance of different mouse strains in an object recognition task. Behav Brain Res. 2003;147(1-2):49–54. doi: 10.1016/s0166-4328(03)00117-7. [DOI] [PubMed] [Google Scholar]
- Slanina KA, Roberto M, Schweitzer P. Endocannabinoids restrict hippocampal long-term potentiation via CB1. Neuropharmacology. 2005;49(5):660–8. doi: 10.1016/j.neuropharm.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Subbanna S, Shivakumar M, Psychoyos D, Xie S, Basavarajappa BS. Anandamide-CB1 Receptor Signaling Contributes to Postnatal Ethanol-Induced Neonatal Neurodegeneration, Adult Synaptic and Memory Deficits. Journal of neuoscience. 2013;33(15):6350–6366. doi: 10.1523/JNEUROSCI.3786-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova JP, Michaud JC, Le Fur G, Soubrie P. Inhibition of long-term potentiation in rat hippocampal slices by anandamide and WIN55212-2: reversal by SR141716 A, a selective antagonist of CB1 cannabinoid receptors. Naunyn Schmiedebergs Arch Pharmacol. 1995;352(5):576–9. doi: 10.1007/BF00169393. [DOI] [PubMed] [Google Scholar]
- Terranova JP, Storme JJ, Lafon N, Perio A, Rinaldi-Carmona M, Le Fur G, Soubrie P. Improvement of memory in rodents by the selective CB1 cannabinoid receptor antagonist, SR 141716. Psychopharmacology (Berl) 1996;126(2):165–72. doi: 10.1007/BF02246352. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Anum E, Niyuhire F, Wise LE, Lichtman AH. Delta(9)-THC-induced cognitive deficits in mice are reversed by the GABA(A) antagonist bicuculline. Psychopharmacology (Berl) 2005;178(2-3):317–27. doi: 10.1007/s00213-004-1988-2. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Cravatt BF, Engram AE, Lichtman AH. Fatty acid amide hydrolase (−/−) mice exhibit an increased sensitivity to the disruptive effects of anandamide or oleamide in a working memory water maze task. J Pharmacol Exp Ther. 2006;317(1):251–7. doi: 10.1124/jpet.105.095059. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Hamm RJ, Martin BR, Lichtman AH. Differential effects of delta 9-THC on spatial reference and working memory in mice. Psychopharmacology (Berl) 2001;157(2):142–50. doi: 10.1007/s002130100780. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Lichtman AH. Evaluation of CB1 receptor knockout mice in the Morris water maze. J Pharmacol Exp Ther. 2002;301(3):915–24. doi: 10.1124/jpet.301.3.915. [DOI] [PubMed] [Google Scholar]
- Vitolo O, Gong B, Cao Z, Ishii H, Jaracz S, Nakanishi K, Arancio O, Dzyuba SV, Lefort R, Shelanski M. Protection against beta-amyloid induced abnormal synaptic function and cell death by Ginkgolide J. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitolo OV, Sant’Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M. Amyloid beta -peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proc Natl Acad Sci U S A. 2002;99(20):13217–21. doi: 10.1073/pnas.172504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise LE, Cannavacciulo R, Cravatt BF, Martin BF, Lichtman AH. Evaluation of fatty acid amides in the carrageenan-induced paw edema model. Neuropharmacology. 2008;54(1):181–8. doi: 10.1016/j.neuropharm.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]