Abstract
Hippocampal (relational memory) and prefrontal cortex (PFC; working memory) impairments have been found in patients with schizophrenia (SP), possibly due to a dysfunctional connection between structures. Neuroanatomical studies that describe reduced fractional anisotropy (FA) in the uncinate fasciculus support this idea. The dysconnection hypothesis in SP was investigated by examining fronto-temporal anatomical connectivity (uncinate fasciculus FA) and PFC-hippocampal memory and their relationship with each other and everyday functioning. PFC-hippocampal memory was examined with two working-relational memory tasks: transverse patterning and virtual Morris water task. SP exhibited a performance deficit on both tasks and had lower FA in bilateral uncinate fasciculus than healthy volunteers. Lower fronto-temporal anatomical connectivity was related to lower working-relational memory performance, and both predicted worse everyday functioning.
Keywords: schizophrenia, hippocampus, prefrontal cortex, uncinate fasciculus, transverse patterning, and connectivity
The prefrontal cortex (PFC) and hippocampus are critical for different aspects of memory function, and together play a central role in the memory impairments exhibited in patients with schizophrenia (SP; Cirillo & Seidman, 2003; Goldman-Rakic, 1994; Saykin et al., 1991; Saykin et al., 1994). The temporal lobes, particularly the hippocampi, are theorized to play an important role in the formation of long-term memory traces that relate or combine stimuli to form unique representations (Cohen & Eichenbaum, 1993; Moses & Ryan, 2006; Rudy & Sutherland, 1995). The frontal lobes are also implicated in learning and memory. The PFC, specifically the dorsolateral region, is thought to be essential for working memory, the active maintenance and utilization of information kept in mind for a short period of time (Baddeley, 2003, 1986; Goldman-Rakic, 1994; Ragland, Yoon, Minzenberg, & Carter, 2007).
Investigations into the neurological deficits found in SP have focused on specific brain structures or systems. An alternative hypothesis is that these deficits may be due to a dysfunctional connection, or “dysconnection”, between the participating brain regions (Bullmore, Frangou, & Murray, 1997; Friston, 1999, 2002; Friston & Frith, 1995). In the present context, the dysconnection hypothesis implies that there may be abnormal functional integration between PFC and hippocampus. As a result, performance deficits are expected when coordinated activity between the dorsolateral PFC and hippocampus is required in a task that involves interplay between the working and relational memory systems. Deficits on such tasks (the transverse patterning (TP) task and the Virtual Morris Water Task (VMWT)) have been observed in SP by Hanlon and collaborators (Hanlon et al., 2011; Hanlon et al., 2006; Hanlon et al., 2005).
There are different theories on how and why dysconnection between brain regions may occur in SP. Stephan and colleagues (2006; 2009) propose that the main underlying mechanism for dysconnection is abnormal NMDA receptor-mediated synaptic plasticity. They also acknowledge that irregular wiring of association fibers may affect interregional functional integration, in addition to the effects of abnormal synaptic plasticity. Bullmore et al. (1997) alternatively propose a dysplastic net hypothesis, which integrates the dysconnection hypothesis and neurodevelopmental hypothesis in schizophrenia. They describe the dysconnection of neural networks as the result of dysplastic axonal projection formation occurring in the second half of gestation. Alternatively abnormal axonal connections could occur by different means, such as disrupted myelination of axons, or elimination of axons due to neuronal death (Spoletini et al., 2009; Walterfang, Velakoulis, Whitford, & Pantelis, 2011). Despite these diverging explanations for the etiology of this dysconnection, the dysconnection hypothesis consistently predicts impaired functional integration caused by a breakdown in normal neuronal interactions in SP (Bullmore et al., 1997; Ellison-Wright & Bullmore, 2009; Stephan et al., 2006; Stephan et al., 2009).
Neuroanatomical studies in SP provide the most striking evidence supporting abnormal fronto-temporal connectivity. Abnormalities in the uncinate fasciculus, the most prominent fiber tract containing direct frontal-temporal connections, have been reported in SP. This is seen as reduced fractional anisotropy (FA; Burns et al., 2003; Kawashima et al., 2009; Price et al., 2008; Spoletini et al., 2009; Sussmann et al., 2009; Szeszko et al., 2008; Voineskos et al., 2010), a scalar measure of the degree of directional movement of water molecules detected by diffusion tensor imaging (DTI), as well as in reduced hemisphere anisotropic asymmetry in FA (Kubicki et al., 2002; Park et al., 2004). A link has also been found between this anatomical fronto-temporal dysconnect and memory and executive dysfunction in SP. In patients, but not healthy volunteers (HV), lower FA in the uncinate fasciculus has been associated with reduced performance on declarative-episodic verbal memory (Nestor et al., 2004; Nestor et al., 2008), verbal learning memory (Szeszko et al., 2008), and executive functioning (Spoletini et al., 2009). These studies utilized traditional neuropsycholological tests that have been associated with particular cognitive abilities and particulur brain structures, such as the hippocampus or dorsolateral PFC. However, in order to specifically test the PFC-hippocampal dysconnection hypothesis, an appropriate task would be one that required the interaction of the dorsolateral PFC and hippocampus, one that required the integration of working and relational memory.
An example of such a task is the TP task. It has been shown to rely on both working and relational memory, activating both the PFC, including the dorsolateral region, and hippocampus. In the TP task, participants choose between novel stimuli presented in pairs, with the correct choice being a function of the specific pairing: stimulus A is correct when presented with stimulus B; stimulus B is correct when presented with C; stimulus C is correct when presented with A (Figure 1). The relationships between the stimuli in the TP task is similar to the childhood-game “Rock, Paper, Scissors”. The participant must discover, encode, maintain, and then utilize the distinct relationships among the stimuli to perform well at the task. The TP task relies on the hippocampus in both human studies (Moses, Ostreicher, Rosenbaum, & Ryan, 2008; Reed & Squire, 1999; Rickard & Grafman, 1998; Rickard, Verfaellie, & Grafman, 2006) and non-human animal lesion work (Alvarado & Bachevalier, 2005; Alvarado & Rudy, 1995a, 1995b; Alvarado, Wright, & Bachevalier, 2002; Driscoll, Howard, Prusky, Rudy, & Sutherland, 2005). Functional neuroimaging studies, including both functional magnetic resonance imaging (fMRI; Astur & Constable, 2004; Meltzer, Negishi, & Constable, 2008) and magnetoencephalography (MEG; Hanlon et al., 2011; Hanlon et al., 2005; Leirer et al., 2010; Moses et al., 2009), have also demonstrated that TP task performance activates PFC, including the dorsolateral region, in addition to hippocampus. The TP task utilizes the integration of working and relational memory and thus should require the interaction between the dorsolateral PFC and hippocampus.
Figure 1.
Upper panel: On each trial, two stimuli were presented simultaneously, one centered on the left side of the screen and one centered on the right. This illustrates the six possible stimulus arrangements that are presented randomly and one at a time to the participant for the nonverbal TP task. The participant was asked to choose which of the two stimuli was correct via a mouse button press. In this figure, an asterisk was placed under the correct stimulus for each pair. Lower panel: Stimuli and their relationship to one another for the nonverbal and verbal versions of the TP and elemental tasks. For the TP nonverbal and verbal task versions, stimulusAis correct when presented with stimulus B. Stimulus B is correct when presented with C. Stimulus C is correct when presented with A. For the Elemental nonverbal and verbal task versions, stimulus D is correct when presented with E. Stimulus E is correct when presented with F. Stimulus D is correct when presented with F. The gray arrows indicate the difference between the stimuli relations used in the TP task versions versus those used in the elemental task versions.
SP, when contrasted to HV, show abnormal activation of both hippocampus and PFC during TP task performance, as well as a TP task behavioral deficit (requiring more trials to learn and lower percent correct after training) (Hanlon et al., 2011; Hanlon et al., 2005). Using verbal and nonverbal stimuli versions of the TP task, HV show lateralized hippocampal activation, the left hippocampus activating during the verbal TP task and the right activating during the nonverbal TP task. SP exhibit less lateralized hippocampal activation than HV during the TP task versions (Hanlon et al., 2011; Hanlon et al., 2005). In addition, patients activate the left PFC (Brodmann's Areas 9, 10, 46) during both TP task versions, in contrast to HV activating the right PFC (Brodmann's Areas 9, 10, 46). Thus, HV show both hippocampal and PFC activation during this working-relational memory task, which is impaired in SP.
The hidden-platform version of the VMWT also requires the integration of working and relational memory (dorsolateral PFC-hippocampal network). In this version of the task, the participant must discover, encode, maintain, and then utilize the relationships between spatial cues in this virtual environment to navigate toward a platform hidden under the surface of the water to escape from the pool (allocentric spatial ability; Figure 2). This task has long been known to rely on the hippocampus (MWT in rats: Morris, Garrud, Rawlins, & O'Keefe, 1982; Sutherland, Kolb, & Whishaw, 1982; Sutherland, Whishaw, & Kolb, 1983) (VMWT in humans: Antonova et al., 2009; Astur, Taylor, Mamelak, Philpott, & Sutherland, 2002; Barkas, Henderson, Hamilton, Redhead, & Gray, 2010; Bartsch et al., 2010; Cornwell, Johnson, Holroyd, Carver, & Grillon, 2008; Goodrich-Hunsaker, Livingstone, Skelton, & Hopkins, 2010), and has also been shown to rely on frontal areas, including the dorsolateral region (MWT in rats: Kolb, Buhrmann, Mcdonald, & Sutherland, 1994; Kolb, Sutherland, & Whishaw, 1983; Sutherland et al., 1982) (VMWT in humans: Antonova et al., 2009; Folley, Astur, Jagannathan, Calhoun, & Pearlson, 2010). Importantly, SP have hippocampal activation and behavioral (i.e., traveled further and took longer to find the hidden platform) deficits during hidden-platform VMWT performance compared to HV (Folley et al., 2010; Hanlon et al., 2006).
Figure 2.
VMWT environment. Upper panel: Participant's view while searching for the hidden platform. One of the four distal cues, the pool surface, and two pool walls are shown. Lower panel: An aerial view of the virtual environment, with the four vertical walls flattened. The circular pool is positioned in the center of the room. The hidden platform is shown as a black square within the northeast (upper right) quadrant of the pool. There are four walls with a cue placed on each.
The deficits seen in SP on the TP task and hidden-platform VMWT may at least in part be due to a dysconnection between dorsolateral PFC and hippocampal structures. To further explore this idea, in the present study we interrogated the relationship between fronto-temporal anatomical connectivity and performance on the TP task and VMWT. We proposed that SP would exhibit lower fronto-temporal anatomical connectivity (lower FA in the uncinate fasciculus) compared to HV. We hypothesized that abnormal anatomical connectivity in this PFC-hippocampal network underlies abnormal working and relational memory integration, resulting in lower performance on the TP task and the hidden-platform VMWT. Finally, because everyday functioning in SP is related to memory deficits associated with dorsolateral PFC and hippocampus (Sharma & Antonova, 2003), and should rely on relational and working memory integration, we hypothesized that it would be associated with both fronto-temporal anatomical connectivity and working-relational memory performance. Specifically, lower everyday functioning in patients will be related to lower FA in the uncinate fasciculus and to worse performance on the TP task and the VMWT. Also included were two control tasks, the elemental task and the visible-platform version of the VMWT. The elemental task involves similar pairings of the same type and number of individual stimuli as the TP task (Figure 1), and the visible-platform version of VMWT involves using the keyboard to navigate through the water toward a visible platform, instead of a hidden one. These tasks do not rely on working and relational memory integration. Thus, they will not show the same patient deficits, nor relationships with FA in the uncinate fasciculus or everyday functioning, seen for the TP task or hidden-platform version of the VMWT, providing specificity to results. The relationships between PFC-hippocampal anatomical connectivity and working-relational memory performance in SP and everyday functioning have not been investigated in SP prior to this study. Understanding these relationships could potentially lead to the identification of subgroups of SP, differing in degree of PFC-hippocampal connectivity and everyday functioning.
Methods
Participants
Forty-two participants were included in this study: 21 HV and 21 SP (6 females and 15 males in each group). These participants were enrolled as part of a larger project funded by the National Center for Research Resources (5P20RR021938) and the National Institute of General Medical Sciences (8 P20 GM103472) from the National Institutes of Health. Patients were recruited from the outpatient clinics at University of New Mexico (UNM) and the New Mexico VA Medical Health Care System (VAMHCS). HV were recruited from the same geographic location as the SP through community advertisements. Inclusion criteria for patients included diagnosis of schizophrenia, which was determined by the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV SCID-Clinician Version; First, Spitzer, Gibbon, & Williams, 1996). In addition, patients needed to be clinically stable. This was ascertained by the referring clinician with support from relevant psychiatric records that the patient has been clinically stable on the same dose of psychotropic medications for the previous 3 months. HV were excluded if they had any current or past Axis I disorder (assessed with SCID-NP). Inclusion criterion for both HV and SP included: 1) no current or past diagnosis of neurological disorder, history of head trauma (loss of consciousness > 5 min), or mental retardation; 3) no diagnosis of active substance dependence or abuse within the last 12 months (except for nicotine) and no past dependence on or any use in the past 12 months of PCP/Amphetamine/Cocaine; and 5) 18 to 60 years of age. All HV and SP signed informed consent.
All of the HV were right-handed, while two patients were left-handed. All patients with schizophrenia were outpatients who had been stable on either typical (3 on haloperidol and 1 on perphenazine) or atypical (3 on aripiprazole, 2 on olanzapine, 7 on risperidone, 1 on quetiapine, 2 on ziprasidone, and 5 on clozapine) antipsychotic medications for at least 3 months. SP and HV did not differ in sex, age (HV: M=33, SD=9.6; SP: M=38, SD=11.6; p=.182), and parental socioeconomic status of primary caregiver (HV: M=5.0, SD=1.4; SP: M=4.4, SD=1.9; p=.213). All participants’ structural MRIs were read as normal or normal variants by a board-certified neuroradiologist. This research was conducted after review and full approval of the Human Subjects Research Review Committee (HRRC) of the University of New Mexico Health Sciences Center.
Procedures
All procedures (clinical evaluations, task training, and DTI data collection) were performed in a period of approximately eight weeks, during which SP clinical stability and treatment remained unchanged.
Standardized clinical evaluations
Clinical assessments were administered by a trained rater on the same day as task performance. These assessments included the Positive and Negative Symptom Scale (PANSS) and the Clinical Global Impression (CGI) Scale. The UCSD Performance Based Skills Assessment (UPSA-2; Patterson, Goldman, McKibbin, Hughs, & Jeste, 2001) was included to evaluate everyday functioning in patients. The UPSA provides a performance based measure of everyday functioning based on realistic tasks: 1) medication management (understanding of when and amount of medications to take); 2) comprehension/planning (understanding of newspaper article and plan for outing); 3) financial skills (counting money, making change, and paying bills); 4) communication skills (telephone calls); 5) transportation (use of public transportation); and 6) household management (create a shopping list). The UPSA-2 total score ranges from 0 to 120, while the subscale score for each individual category ranges from 0 to 20. Higher scores indicate higher everyday functioning.
TP and elemental tasks
Participants were trained on all four tasks (verbal and nonverbal versions of the TP task and their elemental counterparts). The order of training on the tasks was randomized across participants. For each task, participants were trained to a criterion of 18 correct responses in a row or 200 trials if criterion was not met (Hanlon et al., 2011; Hanlon et al., 2005). Stimuli for the TP task and elemental task nonverbal versions consisted of three different abstract black and white pictures, unique in appearance for each of the tasks (see Figure 1). Stimuli for the TP task and elemental task verbal versions consisted of three pronounceable non-words for each task (see Figure 1). Presentation stimulus software version 13.1 (Neurobehavioral Systems) was used to present stimuli. On each trial, two stimuli were presented simultaneously, one centered on the left side of the screen and one centered on the right (see Figure 1). Within each task, each stimulus was balanced for left and right side presentation in a randomized order, and presentation of the different pairings was randomized. The participant was asked to choose which of the two stimuli was correct via a mouse button press. For the TP task versions, stimuli A, B, and C were used (Figure 1). A is correct when presented with B. B is correct when presented with C. C is correct when presented with A. There are three pairs of stimuli for each version (nonverbal vs. verbal) of the task. For the elemental task, like the TP task, there are three stimuli for the verbal and nonverbal versions of the task. The pairing and presentation of the elemental task was identical to the TP task except that the relationships between correct and incorrect stimuli were different. For the elemental task versions, stimulus D is correct when presented with E. Stimulus E is correct when presented with F. Stimulus D is correct when presented with F. For all of the tasks, each pair of stimuli was presented until the participant responded or until 3 s had passed. The response was followed by a feedback tone, high-pitched if correct and low-pitched if not correct. There was a randomly jittered 3 to 5 s inter-trial interval (ITI) from button press or end of the 3 s window until the next stimulus onset, during which a fixation cross appeared in the middle of the screen. Performance measures examined included the number of trials to reach criterion (18 correct in a row or 200 maximum trials) and percent correct during 60 trials (collected during magnetoencephalography (MEG: 5 HV and 3 SP) or functional magnetic resonance imaging (fMRI: 16 HV and 16 SP) after the completion of training. The presentation, task order, and instructions for the tasks were the same for training, MEG scan, and fMRI scan, except for the fMRI scan having a longer jittered ITI of 8 to 12 s to facilitate sampling of the hemodynamic response (Burock, Buckner, Woldorff, Rosen, & Dale, 1998). A detailed description of the MEG/fMRI results is beyond the scope of the present discussion. These functional neuroimaging data will be included in a future publication, as the data from each modality includes a slightly different sample of participants (i.e., not all patients were able to complete both scans), examining different hypotheses.
VMWT
Task procedures were those of Hamilton et al. (2003) and Hanlon et al. (2006). Participants were tested on two versions of the task. In both versions, navigation to a goal (platform) was measured with a computerized (virtual) version (NeuroInvestigations, Version 1.2) of the MWT (VMWT; Hamilton et al., 2003; Hamilton & Sutherland, 1999; Hanlon et al., 2006). The participant navigates in a virtual environment consisting of a room with a square floor-plan and a circular pool in the center (Figure 2). All four walls of the room are identical in appearance except for different landmarks flush with the wall to use as spatial cues (four landmarks in total; Figure 2). The field of view is 48 degrees, which allows participants to view one or two cues simultaneously on the display. The surface of the pool consists of an opaque, blue pattern that is tiled via anti-aliasing of the original images to reduce (if not eliminate) any grid-like pattern that could be detected. The pool contains a square platform, 1.75% of the pool area. For analysis the pool's area was divided into four quadrants (Figure 2).
Participants were able to navigate forward, and to turn left and right using the keyboard. The participant's position in the pool was collected in Cartesian coordinates in software every 100 ms. When the platform location was discovered, a bell sounded, and a verbal message saying, “Platform found” appeared on the screen. When the duration of the trial exceeded 60 s without the platform location being found, an aversive tone sounded, the platform became visible, and a verbal message saying, “The platform is visible” appeared on the screen.
In the first version of this task participants learned to navigate to a platform hidden under the surface of the water. The participants were instructed to navigate to the hidden platform as quickly as possible. Each participant received 28 training trials in seven blocks of four trials. The four trials in each block began at different starting locations. The hidden platform was in a fixed position over trials. Starting positions were chosen according to a pseudorandom sequence. If the platform was found within 60 s, the participant remained on the platform for 5 s, during which time they could rotate and view the environment but could not leave the platform. The display was then removed, and a 2 s intertrial interval followed. Latency to find the platform and path length were recorded on these training trials.
After the hidden platform trials, a probe trial was presented in which the platform was removed. Participants, uninformed about platform removal, navigated for 45 s trying to locate the platform. The starting location for the probe trial was selected pseudorandomly from the two starting locations furthest from the learned platform location (S (bottom) and W (left) starting points in Figure 2). Percentage of the 45 s swim spent in the quadrant that housed the platform during training (northeast or upper right quadrant in Figure 2) was the dependent measure for the probe trial.
The probe trial was followed by a visible platform version of the VMWT. Participants navigated to a visible platform that was in same location that the hidden platform had occupied on previous trials. Participants completed eight trials; two from each of the four starting location in a pseudorandom sequence. Latency (maximum trial length 60 s) and path length to find the platform were recorded.
DTI data collection and analysis
Fronto-temporal anatomical connectivity was assessed using DTI measures of FA in the uncinate fasciculus. DTI data was collected along the AC/PC line, throughout the whole brain, with FOV = 256 × 256 mm, 128 × 128 matrix, 72 slices with a slice thickness of 2 mm (isotropic 2 mm resolution), NEX = 1, TE = 84 ms and TR = 9000 ms. A multiple-channel radiofrequency (RF) coil was used, with GRAPPA (X2), 30 gradient directions with b = 800 s/mm2. The b = 0 experiment was repeated five times (Jones, Horsfield, & Simmons, 1999), and equally inter-spread between the 30 gradient directions. The total imaging time was approximately 6 min. This experiment was repeated twice to increase the signal to noise ratio (SNR). The data from the two DTI experiments, each with a dimension of 128 × 128 × 72 × 35 was concatenated together to form one larger data set of dimension 128 × 128 × 72 × 70. All b = 0 images were registered to the first b = 0 image with a 6 degrees-of-freedom transformation. This was followed by registering the b = 800 s/mm2 image to the b = 0 image immediately before it by an affine 12 degrees-of-freedom transformation. The two transformations were multiplied and then one transformation applied to the b = 800 s/mm2 image to align it to the first b = 0 image. This resulted in all images being registered to the first b = 0 image. Flirt/FSL was used for all registration steps.
Analysis consisted of the following steps: 1) quality check, any gradient directions with excessive motion or vibration artifacts were identified and removed; 2) motion and eddy current correction; 3) the gradient directions were corrected for any image rotation done during the previous motion correction step; 4) calculation of diffusion tensor and scalar measures such as FA, mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD); 5) spatial normalization by FNIRT/FSL to MNI space; and 6) apply tract based spatial statistics (TBSS/FSL) method to calculate participant specific skeletons (Smith et al., 2006). The TBSS method does not find tracts from one region to another based on eigenvector properties, and is not a tractography method. It is a robust method of doing voxel based comparisons by restricting the analysis to white matter skeletons (Smith et al., 2006). In this analysis, the skeleton was further restricted to uncinate fasciculus as defined by the JHU atlas (Wakana, Jiang, Nagae-Poetscher, van Zijl, & Mori, 2004; see top row of Figure 3 for the uncinate fasciculus skeleton). Group differences were evaluated by comparing group mean values for FA, MD, AD, and RD over the uncinate fasciculus skeleton.
Figure 3.
The top row shows the TBSS analysis. It illustrates the uncinate fasciculus skeleton mask (in green color) in three slices (MNI z coordinate indicated) shown over the mean FA image for all participants. Group differences were examined using the mean FA calculated over this uncinate fasciculus skeleton. The bottom row shows the nonparametric randomise/FSL test, run with 5,000 permutations. The voxels (in red color) denote significant group differences (p = .05), SP exhibiting lower FA bilaterally. Multiple comparisons were accounted for with the TFCE approach. Radiological convention is used.
In order to confirm the results found by calculating the mean FA over the uncinate fasciculus skeleton, voxel-by-voxel differences over the same region were examined. A non-parametric randomise/FSL test (number of permutations = 5000) was performed for the uncinate fasciculus skeleton mask, with multiple comparisons accounted by the threshold-free cluster approach (TFCE; (Smith & Nichols, 2009)).
Regression analyses
Hierarchical linear regressions were conducted to evaluate the contribution of fronto-temporal anatomical connectivity (FA in the uncinate fasciculus) to behavioral performance on working-relational memory tasks (TP task and VMWT) and control tasks (elemental task) and how diagnostic group affects these relationships. A strong correlation between right- and left-hemisphere uncinate fasciculus FA (r = .905, p < .001) suggests that their shared variance would result in similar relationships with behavioral measures. Thus, to reduce the amount of regressions performed, a factor score based on bilateral FA values was created using principal component analysis. This FA factor score was then used in the hierarchical regression analyses. Task performance was the dependent variable (DVs; Regression 1: nonverbal TP task, number of trials to criterion; Regression 2: verbal TP task, trials to criterion; Regression 3: nonverbal elemental task, trials to criterion; Regression 4: verbal elemental task, trials to criterion; Regression 5; VMWT, percent time spent in the correct quadrant during the probe trial). Each hierarchical regression included the following independent variables (IVs) placed in the linear model in the following order: (1) Group; (2) FA in the uncinate fasciculus (bilateral factor score); and (3) their interaction (Group x FA). These analyses determined the variance (R2) of the DV accounted for by Group, as well as the incremental variance or change in R2 (Δ R2) contributed by FA in the uncinate fasciculus, and their interaction term.
Three linear regressions were done to evaluate the relationship between everyday functioning (DV: UPSA-2 total score) in SP and FA in the uncinate fasciculus (FA factor score) (1), performance on working-relational memory tasks (2), and performance on control tasks (3). The relationship between positive and negative symptoms with FA in the uncinate fasciculus in SP is not clear as some report a relationship with positive symptoms (Cheung et al., 2011; Skelly et al., 2008), others with negative symptoms (Szeszko et al., 2008), and others not finding a relationship (McIntosh et al., 2008; Price et al., 2008). Therefore, the same three linear regressions performed with everyday functioning were conducted with positive (DV: PANSS Positive Symptoms Score) and negative (DV: PANSS Negative Symptoms Score) symptoms.
Results
Clinical Findings
Recruited patients were mildly to moderately ill, with a mean CGI severity score of 3.52 (SD = .75) and a PANSS Positive Symptoms mean score of 15 (SD = 5), Negative Symptoms of 14 (SD = 4), General of 24 (SD = 8), and Total of 52 (SD = 12). The UPSA-2 mean subscale scores for each category based on 20 patients (one patient's data was not collected) are as follows: 1) medication management: M = 16, SD = 5; 2) comprehension / planning: M = 13, SD = 3; 3) financial: M = 18, SD = 2; 4) communication: M = 16, SD = 2; 5) transportation: M = 15, SD = 3; 6) household: M = 16, SD = 3; and 7) total score: M = 94, SD = 13.
Behavioral Findings
Two patients (1 female and 1 male) withdrew from the study after training on the TP and elemental tasks. Thus, their data is missing for the entire VMWT, and the TP and elemental tasks after training. Fortunately, their training and DTI data could still be analyzed. Also, one patient's data was not collected for the nonverbal elemental task, during and after training, due to exceeding the time allocated for training.
TP and elemental tasks
As predicted, SP performed worse (required more trials to learn and had lower percent correct after training) on both versions of the TP task, verbal and nonverbal, than did HV. A repeated-measures analysis of variance (ANOVA) using Version (verbal and nonverbal) and Task (TP and elemental) as within-participant variables and Group (SP and HV) as the between-participants factor was performed for each of the behavioral measures (number of trials to meet criterion and percent correct after training). Examining the number of trials to reach criterion, there were significant effects for Group, F(1,39) = 21.66, p < .001 and Task, F(1,39) = 60.36, p < .001, with a trend toward a Group × Task interaction, F(1,39) = 3.50, p = .069 (see Figure 4). Thus, patients required more trials during training than HV for both versions of the TP and elemental tasks (no Version, Version × Group, Version × Task, or Version × Task × Group effects) and this tended to be worse for the TP task versions than the elemental task versions. Importantly, this trend was significant when examining percent correct for the first 60 trials after training. Groups differed on performance, depending on the task and the version (Group: F(1,37) = 9.81, p = .003; Task: F(1,37) = 55.79, p < .001; Task × Group: F(1,37) = 14.35, p = .001; Task × Group × Version: F(1,37) = 4.68, p = .037). Exploring these significant effects further, it was found that patients performed worse (lower percent correct) than HV on both the verbal TP task (F(1,39) = 17.74, p < .001) and nonverbal TP task versions (F(1,39) = 7.36, p = .010) , but did not show a performance decrement on the verbal or nonverbal elemental tasks (see Figure 4). Therefore, as predicted, SP required more trials to learn the verbal and nonverbal versions of the TP task and performed worse on them after training than HV.
Figure 4.
SP and HV performance on the TP and elemental task versions for (upper panel) number of trials to reach criterion (18 consecutive trials in a row correct or 200 trials) and (lower panel) mean percent correct for the first 60 trials after training. Error bars indicate SE. Patients took more trials to reach criterion (upper panel) and performed worse after training (lower panel) compared to HV for the verbal and nonverbal TP task. For the control elemental (EL) task versions, patients required more trials to learn the task (upper panel). Importantly, once the EL task versions were learned, there was no group difference seen for performance (lower panel).
VMWT hidden-platform version
Over the 28 trials of training, SP (M = 47%, SD = 15) spent less time in the correct quadrant than did HV (M = 60%, SD = 8; F(1,39) = 12.45, p = .001). Patients took longer than HV to locate the hidden platform (Group: F(1,38) = 11.57, p = .002). Latency decreased linearly with practice for participants generally (linear Block: F(1,38) = 50.84, p < .001), but not differentially by group (Group × linear Block interaction). Importantly, patients were slower to reach the platform overall than HV, and were also slower after sufficient practice locating the platform (Block 7: F(1,39) = 9.42, p = .004).
Figure 5 shows total path length as a function of group and block. Similar to escape latency, there was an overall group difference in path length to locate the hidden platform (Group: F(1,38) = 7.00, p = .012), with patients having greater path length. Path length decreased with practice for participants generally (linear Block: F(1,38) = 13.96, p = .001), but not differentially by group (Group × linear Block). Patients took a longer path than did HV to locate the hidden platform overall and in the last block of trials (Block 7: F(1,39) = 8.70, p = .005). In summary, patients were slower and took a longer path than HV during the VMWT hidden-platform version.
Figure 5.
Upper panel: Patients traveled farther over the seven blocks of trials (four trials per block) to locate the VMWT hidden platform than HV. Importantly, there was no group difference in path length traveled to locate the visible platform (two blocks of four trials). Path length is expressed as the ratio of the total path length to the diameter of the pool. Bars indicate SE. Note: SE bars are not apparent around means for the visible version of the task because the values were very small. Lower panel: The pseudocolor circles show average composite dwell time for each group during the probe trial. Prior to platform removal for the probe trial, it was located in the northeast (upper right) quadrant. Areas in yellow depict regions occupied for a relatively high percentage of the time, whereas areas in red were occupied a relatively low percentage of the time. HV spent more time in the correct quadrant than SP.
VMWT probe trial
Figure 5 (lower panel) shows that patients spent less time in the correct quadrant of the pool during the probe trial than did HV, F(1,39) = 16.92, p < .000. Importantly, there was no difference between patients and HV in mean path length during the probe trial, F(1,39) = 1.35, p = .252, verifying that both groups were searching for the platform and that patients were engaged in the task.
VMWT visible-platform version
Escape latency and path length to locate the visible platform did not significantly decrease with practice for participants generally (linear Block), nor differentially by group (Group × linear Block). Importantly, patients did not take significantly more time or path length to locate the visible platform. In addition, there was no Group difference in percent time spent in the correct quadrant (HV: M = 57%, SD = 3; SP: M = 56%, SD = 5). Therefore, patients did not show detectable behavioral impairments on the visible-platform variant of the VMWT.
DTI Findings
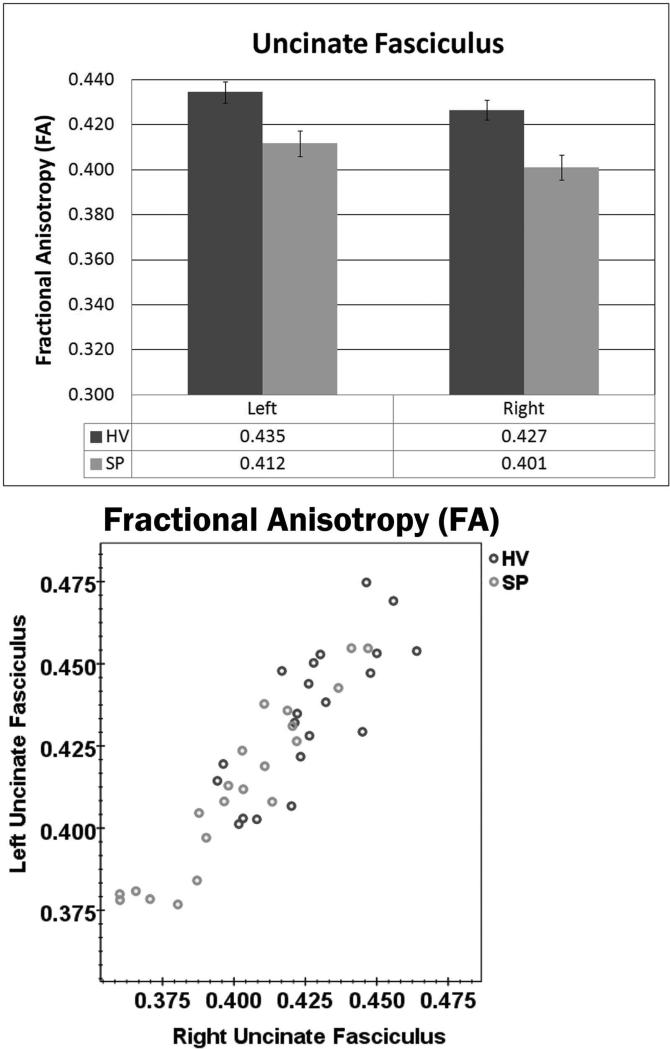
Since age-related deficits have been reported for uncinate fasciculus FA in SP (Rosenberger et al., 2008), age was used as a covariate in the DTI analyses. When examining FA and MD in the uncinate fasciculus, significant Hemisphere and Group main effects were found for FA (Figure 6), but not MD. Overall, FA in the uncinate fasciculus was found to be lower in the right hemisphere than the left (F(1,39) = 5.96, p = .019) and lower in SP compared to HV (F(1,39) = 9.62, p = .004). There were no interactions between Group, Age, and Hemisphere for either FA or MD. Figure 6 shows the distribution of FA values for all participants and the strong correlation that was found between right- and left-hemisphere uncinate fasciculus FA (r = .905, p < .001). To follow up on the FA group difference, AD and RD were examined. There were no significant effects found.
Figure 6.
Upper panel: SP showed lower FA in the uncinate fasciculus compared to HV in the left and right hemispheres. Overall, FA in the uncinate fasciculus was found to be lower in the right hemisphere than the left. Lower panel: This scatter plot illustrates the distribution of FA values for all participants and the strong correlation that was found between rightand left-hemisphere uncinate fasciculus FA (r = .905, p < .001).
In order to confirm the above results, voxel-by-voxel differences over the same region were examined. A non-parametric randomise/FSL test was performed for the uncinate fasciculus skeleton mask. The regions with significant group differences are shown (in red voxels) in the bottom row of Figure 3 at p-value = 0.05, with multiple comparisons accounted by the TFCE approach (Smith & Nichols, 2009). Again, SP showed lower FA in bilateral uncinate fasciculus.
Regression Analyses
The top of Table 1 illustrates the results of hierarchical linear regressions examining the TP task versions and FA in the uncinate fasciculus (bilateral factor score). These analyses were done to determine how much fronto-temporal anatomical connectivity contributes to performance on the TP task versions and whether groups differ in this relationship. Results showed that Group accounts for a significant amount of the performance variance for both versions of the TP task (15% for nonverbal TP and 28% for verbal TP; see Table 1). Focusing on the change in R2 for FA in the uncinate fasciculus, results show that it accounts for a significant amount of the variance in performance on verbal and nonverbal TP task versions. FA accounts for 10% of the variance for the nonverbal TP task and 19% of the variance for the verbal TP task, above the variance accounted for by Group. This is the case for both SP and HV, since the change in R2 for the interaction term was not significant for either of the TP task regressions. The β shows a negative relationship: regardless of Group, a higher number of trials to learn the TP task (lower performance) is related to lower FA in the uncinate fasciculus (lower fronto-temporal anatomical connectivity).
Table 1.
Hierarchical Linear Regression analyses with Performance on Nonverbal and Verbal TP, Elemental, and hidden-platform VMWT as the Dependent Variables and Group, FA in Uncinate Fasciculus (bilateral FA factor score), and their Interaction as the Independent Variables.
| R2 | F | p | β | ||
|---|---|---|---|---|---|
| DV | 1. Nonverbal TP - Trials to Criterion | ||||
| IVs | Group | 0.153 | 7.24 | 0.010 | 0.391 |
| R Change for FA (bilateral factor score) | 0.104 | 5.46 | 0.025 | −0.368 | |
| R Change for Interaction of Group and FA | 0.001 | 0.05 | 0.824 | 0.032 | |
| DV | 2. Verbal TP – Trials to Criterion | ||||
| IVs | Group | 0.278 | 15.38 | 0.000 | 0.527 |
| R2 Change for FA (bilateral factor score) | 0.192 | 14.08 | 0.001 | −0.500 | |
| R2 Change for Interaction of Group and FA | 0.019 | 1.39 | 0.247 | −0.141 | |
| DV | 3. Nonverbal EL Trials to Criterion | ||||
| IVs | Group | 0.088 | 3.75 | 0.060 | 0.296 |
| R2 Change for FA (bilateral factor score) | 0.001 | 0.06 | 0.807 | −0.043 | |
| R2 Change for Interaction of Group and FA | 0.009 | 0.37 | 0.545 | −0.099 | |
| DV | 4. Verbal EL – Trials to Criterion | ||||
| IVs | Group | 0.264 | 14.35 | 0.001 | 0.514 |
| R2 Change for FA (bilateral factor score) | 0.000 | 0.00 | 0.972 | 0.006 | |
| R2 Change for Interaction of Group and FA | 0.000 | 0.01 | 0.919 | −0.015 | |
| DV | 5. Probe Trial – % Time in Correct Quadrant | ||||
| IVs | Group | 0.308 | 16.92 | 0.000 | −0.555 |
| R2 Change for FA (bilateral factor score) | 0.142 | 9.57 | 0.004 | 0.428 | |
| R2 Change for Interaction of Group and FA | 0.030 | 2.09 | 0.157 | 0.179 |
Table 1 also illustrates these same analyses for the elemental task versions. In strong contrast to the above TP task results, FA in the uncinate fasciculus did not account for a significant amount of the elemental task performance variance. The Group × FA interaction terms also did not reach significance for either elemental task version. The only significant effects were of Group for the verbal elemental task. Patients required more trials to learn the verbal elemental task than HV, and performance on both versions of the elemental task was not related to fronto-temporal anatomical connectivity.
At the bottom of Table 1, regression results are displayed for the VMWT using performance on the probe trial (percent of time spent in the correct quadrant) as the DV. Results were similar to that seen for the TP task. Results showed that Group accounts for a significant amount of the performance variance during the probe trial (31%). Focusing on the change in R2, FA in the uncinate fasciculus accounts for a significant amount of the variance in probe trial performance (14%), above the variance accounted for by Group. Since the change in R2 for the interaction term was not significant, this is the case for both SP and HV. The β shows a positive relationship, so regardless of Group, the less time spent in the correct quadrant during the probe trial (lower performance), the less FA in the uncinate fasciculus (lower fronto-temporal anatomical connectivity).
The top of Table 2 shows the results for the linear regression analyses examining everyday functioning (measured with the UPSA-2) in patients and 1) FA in the uncinate fasciculus; 2) working-relational memory performance measures (nonverbal and verbal TP task versions’ trials to reach criterion and mean path length over trials to locate the hidden platform for the VMWT); and 3) control measures (nonverbal and verbal elemental task versions’ trials to reach criterion and mean path length over trials to locate the visible platform for the VMWT). For the first linear regression, FA in the uncinate fasciculus accounts for a significant amount of variance (24%) in UPSA-2 total score. The β is positive for this relationship, indicating that the lower the UPSA score (lower everyday functioning), the less FA in the uncinate fasciculus (lower fronto-temporal anatomical connectivity). For the second regression, three measures of working-relational memory performance from the TP task and VMWT were in the model together. Overall, these measures accounted for 53% of the variance of the UPSA score (p = .012). Due to the significance of this result, a follow-up analysis was done in which each of the three working-relational memory performance measures were regressed separately on UPSA score. Each of the three measures accounted for a significant amount of the UPSA variance. In addition, all three had negative βs, indicating that lower everyday functioning was related to lower working-relational memory performance (lower PFC-hippocampal memory integration). In contrast, this relationship was not significant for the control task measures. When all three control measures were added into the regression model together, they only accounted for 4% of the UPSA score variance, as opposed to the 53% seen with the working-relational memory performance measures. Thus, overall both fronto-temporal anatomical (uncinate fasciculus FA) and performance (TP task and VMWT) measures accounted for a significant amount of the variance in everyday functioning (UPSA score). In patients, lower fronto-temporal white-matter connectivity was related to lower everyday functioning.
Table 2.
Linear Regression Analyses in SP with UPSA Total Score, PANSS Negative Symptoms Score, and PANSS Positive Symptoms Score as the Dependent Variables and FA in Uncinate Fasciculus (bilateral FA factor score) and Performance Measures on Working-Relational Memory Tasks and Control Tasks as Independent Variables.
| R2 | F | p | β | ||
|---|---|---|---|---|---|
| DV | 1. UPSA Total Score | ||||
| IV | FA (bilateral factor score) | 0.242 | 5.75 | 0.028 | 0.492 |
| DV | 2. UPSA Total Score | ||||
| IVs | Working-Relational Memory Measures Together | 0.533 | 5.32 | 0.012 | |
| #1: Nonverbal TP – Trials to Reach Criterion | 0.234 | 5.50 | 0.031 | −0.484 | |
| #2: Verbal TP – Trials to Reach Criterion | 0.265 | 6.48 | 0.020 | −0.514 | |
| #3: VMWT Hidden Platform – Path Length | 0.420 | 11.58 | 0.004 | −0.648 | |
| DV | 3. UPSA Total Score | ||||
| IVs | Control Measures Together | 0.039 | 0.17 | 0.912 | |
| #1: Nonverbal EL – Trials to Reach Criterion | |||||
| #2: Verbal EL – Trials to Reach Criterion | |||||
| #3: VMWT Visible Platform – Path Length | |||||
| DV | 1. PANSS Negative Symptoms Score | ||||
| IV | FA (bilateral factor score) | 0.062 | 1.27 | 0.275 | |
| DV | 2. PANSS Negative Symptoms Score | ||||
| IVs | Working-Relational Memory Measures Together | 0.251 | 1.68 | 0.215 | |
| DV | 3. PANSS Negative Symptoms Score | ||||
| IVs | Control Measures Together | 0.185 | 1.06 | 0.398 | |
| DV | 1. PANSS Positive Symptoms Score | ||||
| IV | FA (bilateral factor score) | 0.133 | 2.91 | 0.104 | |
| DV | 2. PANSS Positive Symptoms Score | ||||
| IVs | Working-Relational Memory Measures Together | 0.188 | 1.16 | 0.359 | |
| DV | 3. PANSS Positive Symptoms Score | ||||
| IVs | Control Measures Together | 0.171 | 0.96 | 0.437 |
The bottom of Table 2 shows the results for the linear regression analyses examining negative and positive symptoms (measured with the PANSS) in patients and 1) FA in the uncinate fasciculus; 2) working-relational memory performance measures; and 3) control measures. There were no significant relationships found.
Discussion
This study directly tested the dysconnection hypothesis in SP by examining fronto-temporal anatomical connectivity (FA in the uncinate fasciculus) and dorsolateral PFC-hippocampal memory integration (working-relational memory performance) and their relationship with each other and everyday functioning in schizophrenia. SP showed a performance deficit on two working-relational memory tasks relative to HV. SP required more trials to learn the TP task than did HV and exhibited a lower level of performance after training, with no decrement after training on an elemental control task. These performance results replicate those found in Hanlon et al. (2011; 2005). Consistent with Hanlon et al. (2006), SP required more time and traveled farther to find a hidden platform on the VMWT than did HV. Further, patients searched less time in the correct quadrant during a probe trial. These results support the notion of a dorsolateral PFC-hippocampal memory integration deficit in SP.
SP were found to have lower fronto-temporal anatomical connectivity, as indexed by lower FA in the right and left uncinate fasciculus compared to HV. This replicates previous research (Kawashima et al., 2009; Szeszko et al., 2008). As a measure of the degree of directionally coherent movement of water molecules, reduced FA in the uncinate fasciculus of SP implies a reduction in white matter integrity. Also, right-hemisphere FA was lower than left for both groups. Kubicki et al. (2002) found this same pattern in HV, but not in SP. However, Kubicki et al. (2002) also found only the left-hemisphere FA to be decreased, in contrast to the present results of a bilateral decrease. This difference between studies is seen by others as well, with some finding a lateralized left-hemisphere decrease in uncinate fasciculus FA (Burns et al., 2003; Kubicki et al., 2002; Voineskos et al., 2010) and others finding a bilateral reduction (Kawashima et al., 2009; Szeszko et al., 2008). MD is a measurement of total water displacement in each direction, averaged for the three directions in space. SP did not show a difference from HV for MD in the uncinate fasciculus, as was reported by Kawashima et al. (2009).
A unique and important finding in this study was the relationship found between fronto-temporal anatomical connectivity and working-relational memory performance. We found that FA in the uncinate fasciculus shared a significant amount of variance with performance measures from both the TP task and VMWT, beyond that shared with group. Thus, regardless if a participant was a HV or a SP, lower fronto-temporal anatomical connectivity (less FA) was related to lower working-relational memory performance as measured by performance on the TP task and VMWT (Table 1). In addition, lower fronto-temporal anatomical connectivity and lower working-relational memory performance predicted worse everyday functioning in SP (Table 2).
Both the TP task and the hidden-platform version of VMWT utilize relational memory, forming and relying on the relations between unique combinations of stimuli, and working memory, maintaining and using these relationships for a short time period. To employ newly formed relationships between stimuli in working memory, the dorsolateral PFC must access these relational representations, most likely from the structure that formed them, the hippocampus. Thus, the integration and use of novel relationships between distinct elements in working memory should involve interactions between the dorsolateral PFC and hippocampus. In support of this notion, the acquisition of the TP task and VMWT relationships activate both PFC, including dorsolateral regions, and hippocampus (TP task: Astur & Constable, 2004; Hanlon et al., 2011; Hanlon et al., 2005; Leirer et al., 2010; Meltzer et al., 2008; Moses et al., 2009) (VMWT: Antonova et al., 2009; Folley et al., 2010), and there is some preliminary evidence for PFC and hippocampal interaction during TP task performance (Meltzer, Fonzo, & Constable, 2009). In addition, lateral PFC may be increasingly involved in relational tasks, such as the TP task, once the relationships are well formed and must be maintained (Doeller, Opitz, Krick, Mecklinger, & Reith, 2005). The current findings of correlations between behavioral deficits on tasks that rely on working-relational memory integration and a measure of fronto-temporal anatomical connectivity lend support to the idea of abnormal interaction between dorsolateral PFC and hippocampus in schizophrenia. Therefore, this data support the fronto-temporal dysconnection (anatomical and functional) hypothesis in schizophrenia.
Importantly, SP did not show a behavioral decrement on either control task, the visible-platform version of the VMWT and the elemental task. For the elemental task versions, SP did require more trials to learn. However, they were able to learn the task, performing both versions above 90% accuracy after training. This task involves similar pairings of the same type and number of individual stimuli as the TP task, however, the pairings do not require discovery and use of relations between the individual stimuli. Similarly, the visible-platform version of VMWT does not require relational memory, as the participant does not have to use the relationship between stimuli on the walls to locate the platform, but only needs the ability to use the keyboard to navigate through the water toward a visual goal to escape the pool. Thus, the elemental task and the visible-platform VMWT are particularly valuable control tasks for studying the interaction between dorsolateral PFC and hippocampus, as both may require some working memory, although neither requires relational memory. The interaction between dorsolateral PFC and hippocampus is not needed for successful performance on either control task. Therefore, it is not surprising that fronto-temporal anatomical connectivity (FA in the uncinate fasciculus) was not associated with performance on either version of the elemental task, as it was with the TP task. In addition, using the same performance measure from the elemental (trials to reach criterion on both nonverbal and verbal versions) and visible-platform version of VMWT (path length) as were used for the TP task and the hidden-platform version, there was no association found with everyday functioning (Table 2). Overall, in contrast to findings with the PFC-hippocampal dependent tasks, SP did not show a behavioral deficit on the control tasks once learned, nor were these tasks associated with anatomical connectivity or everyday functioning.
A novel and exciting finding in this study is that both working-relational memory performance and anatomical connectivity were associated with everyday functioning in SP. Even in light of adequate control of psychiatric symptoms, SP still display poor everyday functioning, seen socially (Nanko & Moridaira, 1993), occupationally (Lehman et al., 2002), and in the ability to live independently (Scott, 1993). Everyday functioning in SP has been found to be related to memory deficits, most consistently with declarative, episodic, and working memory (Sharma & Antonova, 2003). We believe that memory dysfunction, particularly the integration of working and relational memory as elucidated with the TP task and the VMWT, is a key component of this reduced functioning capacity in SP. Being able to function socially and occupationally, and to live independently, all require the ability to learn new and remember old relationships (relational memory), and use this information in an effective manner (working memory). Therefore, productive daily functioning requires the integration of working and relational memory (i.e., normal dorsolateral PFC and hippocampal connectivity). Our results support this idea, finding that working-relational memory performance (dorosolateral PFC-hippocampal memory integration) accounts for a significant amount of variance in everyday functioning (UPSA). Fronto-temporal anatomical connectivity may underlie this relationship, since FA in the uncinate fasciculus accounted for a significant amount of variance for both working-relational memory performance and everyday functioning. It should be noted, that this relationship does not preclude other brain networks underlying everyday functioning in SP as well.
Future studies examining the specificity of the present findings should test the extent to which the relationships found with the TP task and VMWT, which rely on the integration of working and relational memory, are also seen with tasks that rely only on relational memory or only on working memory, and if so, how these relationships compare. Another test of the specificity of the present findings would be the inclusion of another network, task and white matter tract that has been found to be abnormal in schizophrenia and may also be related to everyday functioning. Future work should also include a larger sample size. To keep the ratio of predictors to sample size lower we have restricted the number of regressions performed by creating a factor score based on bilateral uncinate fasciculus FA values. A larger sample size would allow for FA hemisphere comparisons. Finally, we would like to note that a decrease in FA does not have a specific cause and can be caused by multiple factors, including axonal degradation or demyelination. Magnetization transfer ratio is another complimentary parameter that is sensitive to myelination and can help in future work to understand the mechanism of FA changes in SP (Kubicki et al., 2005). In conclusion, our results support a fronto-temporal dysconnection (anatomical and functional) in schizophrenia, which is associated with lower everyday functioning. Understanding these relationships could potentially lead to the identification of subgroups of SP, differing in degree of PFC-hippocampal connectivity and everyday functioning. Knowing where the patient lies on a continuum of functioning and network connectivity may help identify specific treatment strategies, such as cognitive remediation, that would be most successful.
Acknowledgments
This research was supported by an award from the National Center for Research Resources (5P20RR021938) and the National Institute of General Medical Sciences (8 P20 GM103472) from the National Institutes of Health. The authors wish to thank Vince Calhoun, Christopher Abbott, Jose Canive, Tara Biehl, Heather Hawk, Clinton Pyeatt, Anthony Lee, Nick Lemke, Aaron Baca, Kris Daza, and Rozella Zaleski.
Contributor Information
Faith M. Hanlon, The Mind Research Network, Department of Psychology, University of New Mexico, and Department of Psychiatry, University of New Mexico Health Sciences Center
Jon M. Houck, The Mind Research Network and Department of Psychology, University of New Mexico
Stefan D. Klimaj, The Mind Research Network
Arvind Caprihan, The Mind Research Network.
Andrew R. Mayer, The Mind Research Network and Department of Neurology, University of New Mexico Health Sciences Center
Michael P. Weisend, The Mind Research Network and Department of Psychology, University of New Mexico
Juan R. Bustillo, Department of Psychiatry, University of New Mexico Health Sciences Center
Derek A. Hamilton, Department of Psychology, University of New Mexico
Claudia D. Tesche, Department of Psychology, University of New Mexico.
References
- Alvarado MC, Bachevalier J. Selective neurotoxic damage to the hippocampal formation impairs performance of the transverse patterning and location memory tasks in rhesus macaques. Hippocampus. 2005;15(1):118–131. doi: 10.1002/hipo.20037. doi:10.1002/hipo.20037. [DOI] [PubMed] [Google Scholar]
- Alvarado MC, Rudy JW. A comparison of kainic acid plus colchicine and ibotenic acid-induced hippocampal formation damage on four configural tasks in rats. Behavioral Neuroscience. 1995a;109(6):1052–1062. doi: 10.1037//0735-7044.109.6.1052. doi:10.1037/0735-7044.109.6.1052. [DOI] [PubMed] [Google Scholar]
- Alvarado MC, Rudy JW. Rats with damage to the hippocampal-formation are impaired on the transverse-patterning problem but not on elemental discriminations. Behavioral Neuroscience. 1995b;109(2):204–211. doi: 10.1037//0735-7044.109.2.204. doi:10.1037/0735-7044.109.2.204. [DOI] [PubMed] [Google Scholar]
- Alvarado MC, Wright AA, Bachevalier J. Object and spatial relational memory in adult rhesus monkeys is impaired by neonatal lesions of the hippocampal formation but not the amygdaloid complex. Hippocampus. 2002;12(4):421–433. doi: 10.1002/hipo.1115. doi:10.1002/hipo.1115. [DOI] [PubMed] [Google Scholar]
- Antonova E, Parslow D, Brammer M, Dawson GR, Jackson SH, Morris RG. Age-related neural activity during allocentric spatial memory. Memory. 2009;17(2):125–143. doi: 10.1080/09658210802077348. doi:10.1080/09658210802077348. [DOI] [PubMed] [Google Scholar]
- Astur RS, Constable RT. Hippocampal dampening during a relational memory task. Behavioral Neuroscience. 2004;118(4):667–675. doi: 10.1037/0735-7044.118.4.667. doi:10.1037/0735-7044.118.4.667. [DOI] [PubMed] [Google Scholar]
- Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behavioural Brain Research. 2002;132(1):77–84. doi: 10.1016/s0166-4328(01)00399-0. doi:10.1016/S0166-4328(01)00399-0. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: Looking back and looking forward. Nature Reviews Neuroscience. 2003;4(10):829–839. doi: 10.1038/nrn1201. doi:10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working Memory. Oxford University Press; London: 1986. [Google Scholar]
- Barkas LJ, Henderson JL, Hamilton DA, Redhead ES, Gray WP. Selective temporal resections and spatial memory impairment: Cue dependent lateralization effects. Behavioural Brain Research. 2010;208(2):535–544. doi: 10.1016/j.bbr.2009.12.035. doi:10.1016/j.bbr.2009.12.035. [DOI] [PubMed] [Google Scholar]
- Bartsch T, Schonfeld R, Muller FJ, Alfke K, Leplow B, Aldenhoff J, Koch JM. Focal lesions of human hippocampal CA1 neurons in transient global amnesia impair place memory. Science. 2010;328(5984):1412–1415. doi: 10.1126/science.1188160. doi:10.1126/science.1188160. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Frangou S, Murray RM. The dysplastic net hypothesis: An integration of developmental and dysconnectivity theories of schizophrenia. Schizophrenia Research. 1997;28(2-3):143–156. doi: 10.1016/s0920-9964(97)00114-x. doi:10.1016/S0920-9964(97)00114-X. [DOI] [PubMed] [Google Scholar]
- Burns J, Job D, Bastin ME, Whalley H, MacGillivray T, Johnstone EC, Lawrie SM. Structural disconnectivity in schizophrenia: A diffusion tensor magnetic resonance imaging study. British Journal of Psychiatry. 2003;182:439–443. doi:10.1192/bjp.182.5.439. [PubMed] [Google Scholar]
- Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. Neuroreport. 1998;9(16):3735–3739. doi: 10.1097/00001756-199811160-00030. doi:10.1097/00001756-199811160-00030. [DOI] [PubMed] [Google Scholar]
- Cheung V, Chiu CP, Law CW, Cheung C, Hui CL, Chan KK, Chen E. Positive symptoms and white matter microstructure in never-medicated first episode schizophrenia. Psychological Medicine. 2011;41(8):1709–1719. doi: 10.1017/S003329171000156X. doi:10.1017/S003329171000156X. [DOI] [PubMed] [Google Scholar]
- Cirillo MA, Seidman LJ. Verbal declarative memory dysfunction in schizophrenia: From clinical assessment to genetics and brain mechanisms. Neuropsychology Review. 2003;13(2):43–77. doi: 10.1023/a:1023870821631. doi:10.1023/A:1023870821631. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, Amnesia, and the Hippocampal System. MIT Press; Cambridge, MA: 1993. [Google Scholar]
- Cornwell BR, Johnson LL, Holroyd T, Carver FW, Grillon C. Human hippocampal and parahippocampal theta during goal-directed spatial navigation predicts performance on a virtual Morris water maze. The Journal of Neuroscience. 2008;28(23):5983–5990. doi: 10.1523/JNEUROSCI.5001-07.2008. doi:10.1523/JNEUROSCI.5001-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeller CF, Opitz B, Krick CM, Mecklinger A, Reith W. Prefrontal-hippocampal dynamics involved in learning regularities across episodes. Cerebral Cortex. 2005;15(8):1123–1133. doi: 10.1093/cercor/bhh211. doi:10.1093/cercor/bhh211. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Howard SR, Prusky GT, Rudy JW, Sutherland RJ. Seahorse wins all races: Hippocampus participates in both linear and non-linear visual discrimination learning. Behavioural Brain Research. 2005;164(1):29–35. doi: 10.1016/j.bbr.2005.05.006. doi:10.1016/j.bbr.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophrenia Research. 2009;108(1-3):3–10. doi: 10.1016/j.schres.2008.11.021. doi:10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, editors. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID), Clinician Version. American Psychiatric Press; Washington, D.C.: 1996. [Google Scholar]
- Folley BS, Astur R, Jagannathan K, Calhoun VD, Pearlson GD. Anomalous neural circuit function in schizophrenia during a virtual Morris water task. Neuroimage. 2010;49(4):3373–3384. doi: 10.1016/j.neuroimage.2009.11.034. doi:10.1016/j.neuroimage.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Schizophrenia and the disconnection hypothesis. Acta Psychiatrica Scandinavica. 1999;99(S395):68–79. doi: 10.1111/j.1600-0447.1999.tb05985.x. doi:10.1111/j.1600-0447.1999.tb05985.x. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Dysfunctional connectivity in schizophrenia. World Psychiatry. 2002;1(2):66–71. [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: A disconnection syndrome. Clinical Neuroscience. 1995;3(2):89–97. [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. Journal of Neuropsychiatry and Clinical Neurosciences. 1994;6(4):348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Livingstone SA, Skelton RW, Hopkins RO. Spatial deficits in a virtual water maze in amnesic participants with hippocampal damage. Hippocampus. 2010;20(4):481–491. doi: 10.1002/hipo.20651. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD. Children with Fetal Alcohol Syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task. Behavioural Brain Research. 2003;143(1):85–94. doi: 10.1016/s0166-4328(03)00028-7. doi:10.1016/S0166-4328(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Hamilton DA, Sutherland RJ. Blocking in human place learning: Evidence from virtual navigation. Psychobiology. 1999;27(4):453–461. [Google Scholar]
- Hanlon FM, Houck JM, Pyeatt CJ, Lundy SL, Euler MJ, Weisend MP, Tesche CD. Bilateral hippocampal dysfunction in schizophrenia. NeuroImage. 2011;58(4):1158–1168. doi: 10.1016/j.neuroimage.2011.06.091. doi:10.1016/j.neuroimage.2011.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon FM, Weisend MP, Hamilton DA, Jones AP, Thoma RJ, Huang MX, Canive JM. Impairment on the hippocampal-dependent virtual Morris water task in schizophrenia. Schizophrenia Research. 2006;87(1-3):67–80. doi: 10.1016/j.schres.2006.05.021. doi:10.1016/j.schres.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Hanlon FM, Weisend MP, Yeo RA, Huang MX, Lee RR, Thoma RJ, Canive JM. A specific test of hippocampal deficit in schizophrenia. Behavioral Neuroscience. 2005;119(4):863–875. doi: 10.1037/0735-7044.119.4.863. doi:10.1037/0735-7044.119.4.863. [DOI] [PubMed] [Google Scholar]
- Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magnetic Resonance in Medicine. 1999;42(3):515–525. doi:10.1002/(SICI)1522-2594(199909)42:3<515::AIDMRM14>3.0.CO;2-Q. [PubMed] [Google Scholar]
- Kawashima T, Nakamura M, Bouix S, Kubicki M, Salisbury DF, Westin CF, Shenton ME. Uncinate fasciculus abnormalities in recent onset schizophrenia and affective psychosis: A diffusion tensor imaging study. Schizophrenia Research. 2009;110(1-3):119–126. doi: 10.1016/j.schres.2009.01.014. doi:10.1016/j.schres.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Buhrmann K, Mcdonald R, Sutherland RJ. Dissociation of the Medial Prefrontal, Posterior Parietal, and Posterior Temporal Cortex for Spatial Navigation and Recognition Memory in the Rat. Cerebral Cortex. 1994;4(6):664–680. doi: 10.1093/cercor/4.6.664. doi:10.1093/cercor/4.6.664. [DOI] [PubMed] [Google Scholar]
- Kolb B, Sutherland RJ, Whishaw IQ. A comparison of the contributions of the frontal and parietal association cortex to spatial localization in rats. Behavioral Neuroscience. 1983;97(1):13–27. doi: 10.1037//0735-7044.97.1.13. doi:10.1037/0735-7044.97.1.13. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Park H, Westin CF, Nestor PG, Mulkern RV, Maier SE, Shenton ME. DTI and MTR abnormalities in schizophrenia: Analysis of white matter integrity. Neuroimage. 2005;26:1109–1118. doi: 10.1016/j.neuroimage.2005.03.026. doi:10.1016/j.neuroimage.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki M, Westin CF, Maier SE, Frumin M, Nestor PG, Salisbury DF, Shenton ME. Uncinate fasciculus findings in schizophrenia: A magnetic resonance diffusion tensor imaging study. American Journal of Psychiatry. 2002;159(5):813–820. doi: 10.1176/appi.ajp.159.5.813. doi:10.1176/appi.ajp.159.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman AF, Goldberg R, Dixon LB, McNary S, Postrado L, Hackman A, McDonnell K. Improving employment outcomes for persons with severe mental illnesses. Archives of General Psychiatry. 2002;59(2):165–172. doi: 10.1001/archpsyc.59.2.165. doi:10.1001/archpsyc.59.2.165. [DOI] [PubMed] [Google Scholar]
- Leirer VM, Wienbruch C, Paul-Jordanov I, Kolassa S, Elbert T, Kolassa IT. Hippocampal activity during the transverse patterning task declines with cognitive competence but not with age. BMC Neuroscience. 2010;11(1):113. doi: 10.1186/1471-2202-11-113. doi:10.1186/1471-2202-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AM, Muñoz Maniega S, Lymer GK, McKirdy J, Hall J, Sussmann JE, Lawrie SM. White matter tractography in bipolar disorder and schizophrenia. Biological Psychiatry. 2008;64(12):1088–1092. doi: 10.1016/j.biopsych.2008.07.026. doi:10.1016/j.biopsych.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Meltzer JA, Fonzo GA, Constable RT. Transverse patterning dissociates human EEG theta power and hippocampal BOLD activation. Psychophysiology. 2009;46(1):153–162. doi: 10.1111/j.1469-8986.2008.00719.x. doi:10.1111/j.1469-8986.2008.00719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer JA, Negishi M, Constable RT. Biphasic hemodynamic responses influence deactivation and may mask activation in block-design fMRI paradigms. Human Brain Mapping. 2008;29(4):385–399. doi: 10.1002/hbm.20391. doi:10.1002/hbm.20391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi: 10.1038/297681a0. doi:10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Moses SN, Ostreicher ML, Rosenbaum RS, Ryan JD. Successful transverse patterning in amnesia using semantic knowledge. Hippocampus. 2008;18(2):121–124. doi: 10.1002/hipo.20378. doi:10.1002/hipo.20378. [DOI] [PubMed] [Google Scholar]
- Moses SN, Ryan JD. A comparison and evaluation of the predictions of relational and conjunctive accounts of hippocampal function. Hippocampus. 2006;16(1):43–65. doi: 10.1002/hipo.20131. doi:10.1002/hipo.20131. [DOI] [PubMed] [Google Scholar]
- Moses SN, Ryan JD, Bardouille T, Kovacevic N, Hanlon FM, McIntosh AR. Semantic information alters neural activation during transverse patterning performance. Neuroimage. 2009;46(3):863–873. doi: 10.1016/j.neuroimage.2009.02.042. doi:10.1016/j.neuroimage.2009.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanko S, Moridaira J. Reproductive rates in schizophrenic outpatients. Acta Psychiatrica Scandinavica. 1993;87(6):400–404. doi: 10.1111/j.1600-0447.1993.tb03395.x. doi:10.1111/j.1600-0447.1993.tb03395.x. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Gurrera RJ, Niznikiewicz M, Frumin M, McCarley RW, Shenton ME. Neuropsychological correlates of diffusion tensor imaging in schizophrenia. Neuropsychology. 2004;18(4):629–637. doi: 10.1037/0894-4105.18.4.629. doi:10.1037/0894-4105.18.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Niznikiewicz M, Gurrera RJ, McCarley RW, Shenton ME. Neuropsychological disturbance in schizophrenia: A diffusion tensor imaging study. Neuropsychology. 2008;22(2):246–254. doi: 10.1037/0894-4105.22.2.246. doi:10.1037/0894-4105.22.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HJ, Westin CF, Kubicki M, Maier SE, Niznikiewicz M, Baer A, Shenton ME. White matter hemisphere asymmetries in healthy subjects and in schizophrenia: A diffusion tensor MRI study. Neuroimage. 2004;23(1):213–223. doi: 10.1016/j.neuroimage.2004.04.036. doi:10.1016/j.neuroimage.2004.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson TL, Goldman S, McKibbin CL, Hughs T, Jeste DV. UCSD Performance-Based Skills Assessment: Development of a new measure of everyday functioning for severely mentally ill adults. Schizophrenia Bulletin. 2001;27(2):235–245. doi: 10.1093/oxfordjournals.schbul.a006870. doi:10.1093/oxfordjournals.schbul.a006870. [DOI] [PubMed] [Google Scholar]
- Price G, Cercignani M, Parker GJM, Altmann DR, Barnes TRE, Barker GJ, Ron MA. White matter tracts in first-episode psychosis: A DTI tractography study of the uncinate fasciculus. Neuroimage. 2008;39(3):949–955. doi: 10.1016/j.neuroimage.2007.09.012. doi:10.1016/j.neuroimage.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Yoon J, Minzenberg MJ, Carter CS. Neuroimaging of cognitive disability in schizophrenia: Search for a pathophysiological mechanism. International Review of Psychiatry. 2007;19(4):419–429. doi: 10.1080/09540260701486365. doi:10.1080/09540260701486365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JM, Squire LR. Impaired transverse patterning in human amnesia is a special case of impaired memory for two-choice discrimination tasks. Behavioral Neuroscience. 1999;113(1):3–9. doi: 10.1037//0735-7044.113.1.3. doi:10.1037/0735-7044.113.1.3. [DOI] [PubMed] [Google Scholar]
- Rickard TC, Grafman J. Losing their configural mind: Amnesic patients fail on transverse patterning. Journal of Cognitive Neuroscience. 1998;10(4):509–524. doi: 10.1162/089892998562915. doi:10.1162/089892998562915. [DOI] [PubMed] [Google Scholar]
- Rickard TC, Verfaellie M, Grafman J. Transverse patterning and human amnesia. Journal of Cognitive Neuroscience. 2006;18(10):1723–1733. doi: 10.1162/jocn.2006.18.10.1723. doi:10.1162/jocn.2006.18.10.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger G, Kubicki M, Nestor PG, Connor E, Bushell GB, Markant D, Shenton ME. Age-related deficits in fronto-temporal connections in schizophrenia: A diffusion tensor imaging study. Schizophrenia Research. 2008;102(1-3):181–188. doi: 10.1016/j.schres.2008.04.019. doi:10.1016/j.schres.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, Sutherland RJ. Configural association theory and the hippocampal formation: An appraisal and reconfiguration. Hippocampus. 1995;5(5):375–389. doi: 10.1002/hipo.450050502. doi:10.1002/hipo.450050502. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, Stafiniak P. Neuropsychological function in schizophrenia: Selective impairment in memory and learning. Archives of General Psychiatry. 1991;48(7):618–624. doi: 10.1001/archpsyc.1991.01810310036007. doi:10.1001/archpsyc.1991.01810310036007. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Shtasel DL, Gur RE, Kester DB, Mozley LH, Stafiniak P, Gur RC. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Archives of General Psychiatry. 1994;51(2):124–131. doi: 10.1001/archpsyc.1994.03950020048005. doi:10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- Scott J. Homelessness and mental-illness. British Journal of Psychiatry. 1993;162:314–324. doi: 10.1192/bjp.162.3.314. doi:10.1192/bjp.162.3.314. [DOI] [PubMed] [Google Scholar]
- Sharma T, Antonova L. Cognitive function in schizophrenia: Deficits, functional consequences, and future treatment. Psychiatric Clinics of North America. 2003;26(1):25–40. doi: 10.1016/s0193-953x(02)00084-9. doi:10.1016/S0193-953X(02)00084-9. [DOI] [PubMed] [Google Scholar]
- Skelly LR, Calhoun V, Meda SA, Kim J, Mathalon DH, Pearlson GD. Diffusion tensor imaging in schizophrenia: Relationship to symptoms. Schizophrenia Research. 2008;98(1-3):157–162. doi: 10.1016/j.schres.2007.10.009. doi:10.1016/j.schres.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Behrens TE. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. doi:10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. doi:10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Spoletini I, Cherubini A, Di Paola M, Banfi G, Rüsch N, Martinotti G, Spalletta G. Reduced fronto-temporal connectivity is associated with frontal gray matter density reduction and neuropsychological deficit in schizophrenia. Schizophrenia Research. 2009;108(1-3):57–68. doi: 10.1016/j.schres.2008.11.011. doi:10.1016/j.schres.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biological Psychiatry. 2006;59(10):929–939. doi: 10.1016/j.biopsych.2005.10.005. doi:10.1016/j.biopsych.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: From abnormal synaptic plasticity to failures of self-monitoring. Schizophrenia Bulletin. 2009;35(3):509–527. doi: 10.1093/schbul/sbn176. doi:10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussmann JE, Lymer GKS, McKirdy J, Moorhead TWJ, Maniega SM, Job D, McIntosh AM. White matter abnormalities in bipolar disorder and schizophrenia detected using diffusion tensor magnetic resonance imaging. Bipolar Disorders. 2009;11(1):11–18. doi: 10.1111/j.1399-5618.2008.00646.x. doi:10.1111/j.1399-5618.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Kolb B, Whishaw IQ. Spatial mapping: Definitive disruption by hippocampal or medial frontal cortical damage in the rat. Neuroscience Letters. 1982;31(3):271–276. doi: 10.1016/0304-3940(82)90032-5. doi:10.1016/0304-3940(82)90032-5. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Whishaw IQ, Kolb B. A behavioral-analysis of spatial localization following electrolytic, kainate-induced or colchicine-induced damage to the hippocampal formation in the rat. Behavioural Brain Research. 1983;7(2):133–153. doi: 10.1016/0166-4328(83)90188-2. doi:10.1016/0166-4328(83)90188-2. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Robinson DG, Ashtari M, Vogel J, Betensky J, Sevy S, Bilder RM. Clinical and neuropsychological correlates of white matter abnormalities in recent onset schizophrenia. Neuropsychopharmacology. 2008;33(5):976–984. doi: 10.1038/sj.npp.1301480. doi:10.1038/sj.npp.1301480. [DOI] [PubMed] [Google Scholar]
- Voineskos AN, Lobaugh NJ, Bouix S, Rajji TK, Miranda D, Kennedy JL, Shenton ME. Diffusion tensor tractography findings in schizophrenia across the adult lifespan. Brain. 2010;133:1494–1504. doi: 10.1093/brain/awq040. doi:10.1093/brain/awq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230(1):77–87. doi: 10.1148/radiol.2301021640. doi:10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Walterfang M, Velakoulis D, Whitford TJ, Pantelis C. Understanding aberrant white matter development in schizophrenia: An avenue for therapy? Expert Review of Neurotherapeutics. 2011;11(7):971–987. doi: 10.1586/ern.11.76. doi:10.1586/ern.11.76. [DOI] [PubMed] [Google Scholar]