Abstract
MicroRNAs (miRNAs) act as important epigenetic posttranscriptional regulators of gene expression. We aimed to gain more understanding of the complex gene expression regulation of endometrial receptivity by analyzing miRNA signatures of fertile human endometria. We set up to analyze miRNA signatures of receptive (LH + 7, n = 4) versus prereceptive (LH + 2, n = 5) endometrium from healthy fertile women. We found hsa-miR-30b and hsa-miR-30d to be significantly upregulated, and hsa-miR-494 and hsa-miR-923 to be downregulated in receptive endometrium. Three algorithms (miRanda, PicTar, and TargetScan) were used for target gene prediction. Functional analyses of the targets using Ingenuity Pathways Analysis and The Database for Annotation, Visualization and Integrated Discovery indicated roles in transcription, cell proliferation and apoptosis, and significant involvement in several relevant pathways, such as axon guidance, Wnt/β-catenin, ERK/MAPK, transforming growth factor β (TGF-β), p53 and leukocyte extravasation. Comparison of predicted miRNA target genes and our previous messenger RNA microarray data resulted in a list of 12 genes, including CAST, CFTR, FGFR2, and LIF that could serve as a panel of genes important for endometrial receptivity. In conclusion, we suggest that a subset of miRNAs and their target genes may play important roles in endometrial receptivity.
Keywords: endometrial receptivity, female infertility, gene expression, microarray, miRNA
Introduction
Receptive endometrium is a prerequisite for establishing and sustaining pregnancy. The development of endometrial receptivity is a complex process, as it is a spatially and temporally restricted phenomenon occurring in the secretory phase of the menstrual cycle known as the “window of implantation.”1 During this period, the endometrium acquires properties that permit the adhesion and invasion of an embryo. Derangements in endometrial maturation in the receptive phase have been proposed as important causes of infertility.2 Indeed, in assisted reproductive techniques, where good-quality embryos are transferred, impaired uterine receptivity is believed to be one of the major reasons for treatment failure.3–5
Molecular studies have extensively investigated the expression and regulation of different factors connected with uterine receptivity, with the list of possible genes involved in the establishment of receptive endometrium increasing exponentially. Nevertheless, the molecular mechanisms regulating the expression of these genes are poorly understood.
Given the dynamic nature of the endometrium, it is believed to be under epigenetic regulation.6 Indeed, several genes expressed in the endometrium have been identified as being epigenetically regulated.6 Epigenetic regulation means that protein production can be inhibited and cellular functions altered without changes in the DNA sequence itself.7 In recent years, it has been shown that small noncoding microRNAs (miRNAs) are important components of epigenetic regulators of gene regulatory networks,8,9 and several miRNAs have been recently identified in the human endometrium.10
MicroRNAs, RNAs of approximately 22 nucleotides in length,9 function as posttranscriptional regulators of gene expression by either degrading or translationally repressing target messenger RNAs (mRNAs).11,12 A single miRNA potentially regulates up to hundreds of mRNA targets, as recognition of a target mRNA depends mostly on a small seed region within the mature miRNA.13 To date, almost 1000 miRNAs have been identified and validated in humans,14 and the results of in silico analyses suggest that there may be thousands of potential miRNAs,15 targeting over 60% of mammalian genes.16 Thus, miRNAs orchestrate a large variety of cellular processes in humans, including the cyclic changes in the female reproductive tract.17
The involvement of miRNAs in the murine uterus at the time of embryo implantation was recently shown.18,19 In humans, miRNA expression profiles in isolated endometrial epithelial cells during the late proliferative phase and mid-secretory phase have been shown, and they suggest a new level of suppression of gene expression during epithelial cell proliferation in receptive endometrium.20 In a recent study on patients undergoing in vitro fertilization (IVF) in natural and stimulated cycles, miRNAs were proposed as novel biomarkers of human endometrial receptivity.5 In addition, in a study on mid-secretory endometria from women with repeated implantation failure, disease-specific miRNAs were identified that could be viewed as new candidates for the diagnosis and future treatment of embryo implantation failure.21
In the current study, we aimed to gain more understanding of the complex regulation of endometrial receptivity in healthy fertile women by analyzing miRNA expression profiles in prereceptive versus receptive human endometria.
Materials and Methods
Study Design and Tissue Collection
A total of 9 healthy fertile women (parity 1.5 ± 0.2), candidates for oocyte donation, were recruited for this study at the Instituto Valenciano de Infertilidad (IVI), Valencia, Spain. All women signed an informed consent document approved by the local ethics committee. The characteristics of the women are summarized in Table 1.
Table 1.
Characteristics of the Fertile Women Undergoing Endometrial Biopsy Sampling in the Prereceptive or Receptive Phase.a
| Nonreceptive (n = 5) | Receptive (n = 4) | |
|---|---|---|
| Age, years | 31.8 ± 3.8 | 30.5 ± 4.0 |
| BMI, kg/m2 | 23.5 ± 2.1 | 22.7 ± 2.3 |
| Cycle length, days | 28.4 ± 0.7 | 28.2 ± 0.5 |
| Menses duration, days | 4.0 ± 0.2 | 4.4 ± 0.6 |
| LH day | LH + 2 | LH + 7 |
Abbreviations: BMI, body mass index; LH day, day since the luteinizing hormone (LH) surge; SD, standard deviation.
a Results are expressed as mean ± SD.
Endometrial biopsy samples from these women were obtained from the anterior wall of the uterine cavity, without dilatation of the cervix, using a Pipelle catheter (Genetics, Namont-Achel, Belgium). Biopsy samples from 5 women were obtained from prereceptive, early secretory phase endometrium (LH + 2); and samples from receptive, mid-secretory phase endometrium (LH + 7) were obtained from 4 women. Detection of luteinizing hormone [LH] in the morning urine (Donacheck ovulación; Novalab Ibérica, S.A.L, Coslada, Madrid, Spain) was used to determine the day of the LH surge (day LH + 0). Histological evaluation of the samples showed normal maturation in relation to the cycle day, according to the criteria described by Noyes et al.22
Total RNA Isolation and miRNA Array Analysis
For miRNA array and real-time polymerase chain reaction (PCR) analysis, total RNA was extracted from the endometrial biopsy samples by the TRIzol method, and RNA quality was assessed by an Agilent Bioanalyzer, as described before.23 An RNA integrity value of >7.5 was considered acceptable.
The miRNA signature was analyzed by an Agilent Human miRNA Microarray, (V2), 8 × 15 K, which comprises 723 human and 76 human viral miRNA probes. In general, the Agilent protocol generates fluorescent miRNA from an initial amount of 100 ng of total RNA. The method involves the binding of molecules of cyanine 3-pCp at the 3′-ends of RNA molecules, with an efficiency above 90%. The first step of the protocol was a dephosphorylation reaction, taking 100 ng of total RNA, adding bovine intestinal alkaline phosphatase, and incubating at 37°C for 30 minutes in a circulating water bath. Next, dimethyl sulfoxide was added to 100% at 100°C to denature the RNAs. Ligation of the marked RNAs was performed by adding RNA ligases of phage T4 and Cy3-pCp, incubating for 2 hours at 16°C, and drying completely in a vacuum centrifuge for 3 hours at 45°C. After that, the samples were resuspended in 18 μL of water, next a blocking agent and hybridization buffer were added, and the samples were incubated for 5 minutes at 100°C before being transferred to ice for another 5 minutes. The samples were then loaded onto the microarray and incubated for 20 hours at 55°C, rotating at 20 rpm. Finally, the microarrays were washed in 3 steps of 5 minutes and scanned on an Axon 4100A scanner (Molecular Devices, Sunnyvale, California).
Array Data Analysis
Preprocessing
GenePix Pro 6.0 software (Molecular Devices) was used to analyze the images obtained after scanning the microarrays, as described in one of our previous studies.23
Differential miRNA expression
Data analyses were performed using the R-statistical software system (Free Software Foundation, Boston, Massachusetts; http://www.r-project.org/). The data were first normalized using the “variance stabilization and calibration for microarray data” procedure provided in the VSN Bioconductor package (http://www.bioconductor.org/packages/devel/bioc/html/vsn.html). The miRNA expression profiles were determined by comparing the prereceptive and receptive groups (2 × 2 comparisons) by means of the rank product nonparametric test in the Bioconductor RankProd package (http://www.bioconductor.org/packages/devel/bioc/html/RankProd.html). Two criteria were used to define the miRNAs with altered abundance among the different sample sets: an absolute fold change (Fc) of >2.0 and a proportion of false positives (PFP) of <0.05. The latter is used to control errors in multiple tests, where it effectively controls the accumulation of false-positives relative to the total number of positive results.24
Sample clustering
In order to validate the above miRNA selection with a nonparametric method, a hierarchical clustering was performed. For that we used Pearson correlation distance and complete linkage, with TM4 microarray software suite (www.tm4.org/). Samples with similar trends in their miRNA expression profiles tend to cluster close together in the heat map.
Functional analyses of array results
Functional analysis of differentially regulated miRNAs was explored using Ingenuity Pathways Analysis ([IPA] Ingenuity Systems, Redwood City, CA, USA). This platform provides a literature-curated analysis of networks, biological processes, and canonical pathways concerning miRNA and target gene data. An association between predicted miRNA gene targets and biologically relevant pathways identified by way of this analysis was deemed statistically significant at a P value of <.05.
In Silico Analysis of miRNA Target Genes
For computational prediction of miRNA target genes, we used 3 different algorithms: miRanda (August 2010 release, http://www.microrna.org), PicTar (http://pictar.mdc-berlin.de/),25 and TargetScan 5.1 (April 2009 release, http://www.targetscan.org).26 On the basis of these 3 algorithms, we created a list of common target genes for each miRNA in order to narrow down the gene list of thousands of targets. These common targets were further annotated by functional annotation tools at IPA software and the Database for Annotation, Visualization and Integrated Discovery (DAVID).27 The DAVID is a gene set-based algorithm that detects functionally related genes in lists of genes ordered according to differential expression. The DAVID can search blocks of functionally related genes according to different criteria such as the gene ontology (GO) terms “biological process,” “cellular component,” and “molecular function.” Values of P were adjusted by way of false discovery rate (FDR) correction,28 based on the number of GO categories that were tested; an FDR of 5% was considered statistically significant.
Additionally, we compared the obtained list of predicted target genes with our previous mRNA microarray data from the same group of women, LH + 7 versus LH + 2,29 with parameters of fold change ≥2 and P < .05. Our focus was on up-/downregulated genes that corresponded, respectively, to down-/upregulated miRNAs. The genes common to these 2 distinct lists of differentially regulated genes were further annotated by the functional annotation tools, IPA and DAVID.
The miRNA Array Validation by Real-Time PCR
Quantitative real-time PCR was used as a validation tool for confirming the miRNA expression results obtained from microarray analysis. The microarrays were validated by predesigned and custom-made TaqMan MicroRNA assays (Applied Biosystems, Inc., Foster City, CA, USA). These predesigned assays are available for the majority of content found in the miRBase miRNA sequence repository. They are ideal for targeted quantification, screening, and validation of miRNA profiling results. The TaqMan MicroRNA assays we used were
4427975 000602 UGUAAACAUCCUACACUCAGCU hsa-miR-30b,
4427975 000420 UGUAAACAUCCCCGACUGGAAG hsa-miR-30d,
4427975 001041 UGAAACAUACACGGGAAACCUCUU hsa-miR-494, and
4427975 002153 GUCAGCGGAGGAAAAGAAACU hsa-miR-923.
First, reverse transcription was performed using the miRNA-specific RT primer contained in the TaqMan MicroRNA assay. Next, a qualitative-PCR validation protocol was performed as follows: each reaction mixture (20 µL) contained 2.5 µL of each sample, 1 µL of 20 × TaqMan miRNA assay reagent (containing preformulated forward/reverse primer and MGB probe), 10 µL of TaqMan 2 × Universal PCR Master Mix No AmpErase UNG, and 6.5 µL of PCR nuclease-free water. Each sample was analyzed in duplicate for each miRNA and a negative control without sample was run with every assay to assess the overall specificity. Roche LightCycler platform was used for amplification of the product (Roche, Göttingen, Germany). The amplification program consisted of 1 cycle of 10 minutes at 95°C for activation of the AmpliTaq Gold Enzyme, followed by 40 cycles of 95°C for 15 seconds, and 60°C for 60 seconds. A calibration curve was included in each experiment with 5 serial dilutions and the obtained products were analyzed using the provided software (Roche Molecular Biochemicals LightCycler Software v3.5). SNORD96 miScript PCR control was used for normalization of miRNAs according to the manufacturer’s protocol (Qiagen, Venlo, Netherlands). Melting curves of PCR reactions were monitored to ensure a single PCR product and no primer dimer. Analysis of gene expression differences between the study groups was carried out by the Mann-Whitney U test. A value of P < .05 was considered statistically significant.
Results
Differential miRNA Expression in Receptive Versus Prereceptive Endometrium
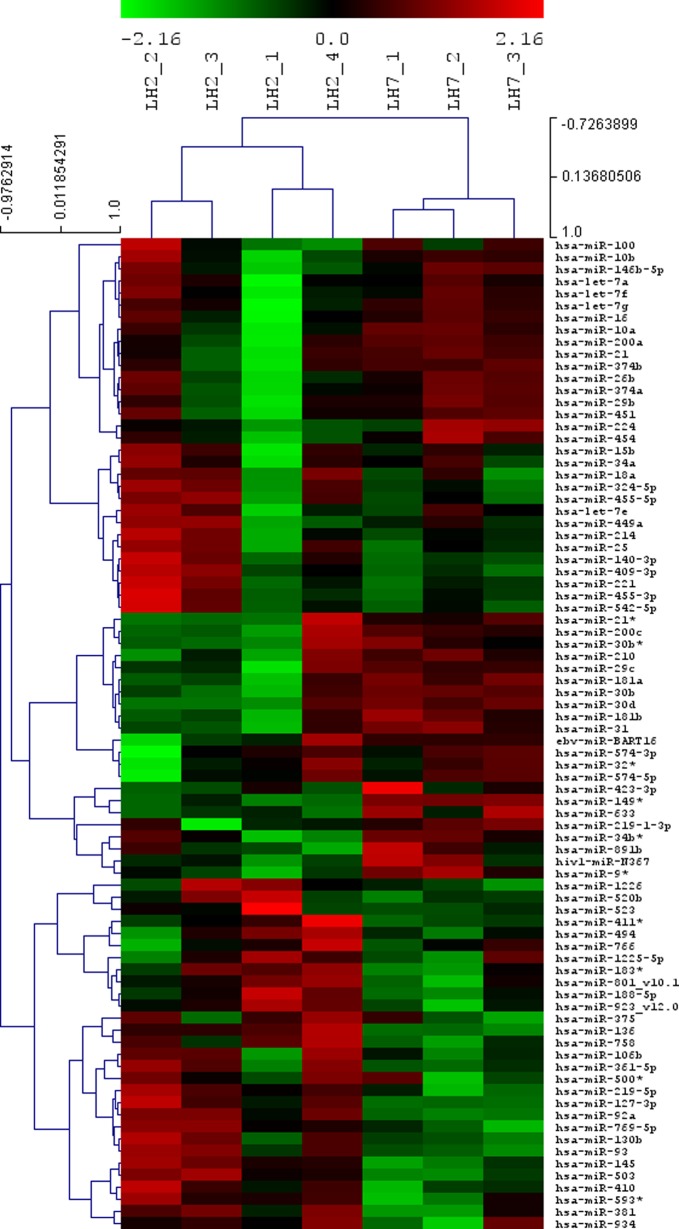
Our primary microarray data are available in the public database Gene Expression Omnibus repository (accession number GSE34435). Two samples did not pass the stringent quality control and thus the study results are obtained from 4 prereceptive phase endometrium and 3 receptive phase endometrium samples. Hierarchical clustering was applied to the miRNA array expression profiles, resulting in 2 distinct groups: prereceptive and receptive endometrium (Figure 1).
Figure 1.
Heat map and cluster dendrograms of microRNA (miRNA) clusters differentially expressed between prereceptive (luteinizing hormone [LH] + 2) and receptive (LH + 7) endometrium. Upregulated miRNAs are marked in red and downregulated in green. The figure was done with the VSN normalized values of the 82 miRNAs with a RankProd raw P value <.05.
In total, we identified 8 miRNAs that were differentially expressed in receptive versus prereceptive endometrium (Table 2). However, after correction for multiple tests, we found 4 miRNAs to be significantly differentially expressed between the groups: hsa-miR-30b, hsa-miR-30d, hsa-miR-494, and hsa-miR-923_v12.0.
Table 2.
Differentially Expressed miRNAs in Receptive Versus Prereceptive Endometrium.
| miRNA | Fca | P Valueb | PFP |
|---|---|---|---|
| hsa-miR-30b | 4.23 | <.001 | <0.001c |
| hsa-miR-30d | 3.29 | <.001 | 0.015c |
| hsa-let-7g | 2.44 | .001 | 0.166 |
| hsa-miR-10a | 2.18 | .001 | 0.130 |
| hsa-miR-26b | 2.16 | .001 | 0.150 |
| hsa-miR-21 | 2.12 | .002 | 0.248 |
| hsa-miR-494 | −2.15 | <.001 | <0.001c |
| hsa-miR-923_v12.0 | −4.27 | <.001 | <0.001c |
a An absolute fold change (Fc) of >2.0.
b A significant P value <.05.
c A significant P value <.05 after proportion of false positives (PFP) correction.
We then investigated the possible roles of these differentially expressed miRNAs using the Ingenuity Pathways Knowledge Base. The software allowed us to identify a network where genes SEPT7, CRMP1, SLC44A1, HES1, FXR2, and TNF144B were interacting with genes MIR30B and MIR30D (Supplementary Figure 1). Ingenuity pathway analysis of differentially expressed miRNAs in relation to function and disease identified their involvement in carcinoma, specifically breast carcinoma, and schizophrenia (P < .003).
Functional Annotation of the Predicted Gene Targets
Using miRanda, PicTar, and TargetScan search algorithms for differentially regulated miRNA target gene prediction, a list of 434 target genes common to all 3 was obtained (Supplementary Table 1). The miRanda algorithm alone predicted thousands of targets for each miRNA, except for miR-923_v12.0. This high number of predicted target genes was obtained by a new regression method, mirSVR (support vector regression) scoring, which is used in miRanda for predicting the likelihood of target mRNA regulation from sequence and structural features in miRNA/mRNA predicted target sites.30 For miR-30b and miR-30d, miRanda and PicTar algorithms predicted very similar targets but as the TargetScan algorithm predicted only 14 possible targets for miR-30d, the final list of common genes is not similar. miR-923_v12.0 appears to be a fragment of 28S ribosomal RNA (rRNA) and, therefore, it was removed from the miRanda and PicTar databases, although it exists in the TargetScan database. Nevertheless, miR-923_v12.0 was excluded from our further analyses. Figure 2 demonstrates the number of target genes for each miRNA. Five targets were similar to miR-30b (upregulated miRNA) and miR-494 (downregulated miRNA). Although, most miRNAs are believed to negatively regulate expression of their target genes, this theory has been recently challenged.31,32
Figure 2.
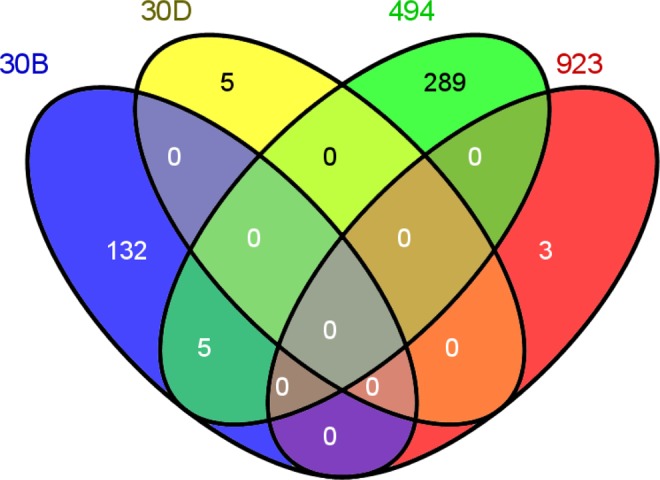
Venn diagram of the predicted target genes of miR-30b, miR-30d, miR-494, and miR-923. PicTar, miRanda, and TargetScan algorithms were used to create the common list of targets. Venn diagrams were obtained using the Venny interactive tool (http://bioinfogp.cnb.csic.es/tools/venny/index.html).
We then analyzed biological function of the predicted target genes in GO terms. The predicted target genes were involved in various biological processes such as regulation of transcription (24.4% of common target genes), RNA metabolic processes (18.3%), biosynthetic processes (14.8%), cell proliferation (9%), apoptosis (8.8%) and regulation of gene expression (6.5%), and various molecular functions such as DNA binding (20.9% of target genes), transcription regulator/factor activity (16.7%/11.8%), and other functions (Table 3). A significant number of predicted target gene functions were located in an intracellular organelle lumen (14.4%), mainly the nuclear lumen (13.9%; Table 3).
Table 3.
Biological Function Analysis in Gene Ontology Terms of Predicted Gene Targets of Differentially Regulated miRNAs in Receptive Versus Prereceptive Endometrium Using the Database for Annotation, Visualization and Integrated Discovery (DAVID).a
| Term | Genes, n | % | FDR |
|---|---|---|---|
| Biological processes | |||
| Regulation of transcription | 105 | 24.4 | 0.002 |
| Regulation of RNA metabolic process | 79 | 18.3 | 0.005 |
| Regulation of biosynthetic process | 64 | 14.8 | 2.05 |
| Regulation of cell proliferation | 39 | 9.0 | 0.28 |
| Regulation of apoptosis | 38 | 8.8 | 0.86 |
| Positive regulation of gene expression | 28 | 6.5 | 4.2 |
| Negative regulation of gene expression | 26 | 6.0 | 2.6 |
| Chromatin organization | 21 | 4.9 | 3.6 |
| Cellular components | |||
| Intracellular organelle lumen | 62 | 14.4 | 0.85 |
| Nuclear lumen | 56 | 13.9 | 0.13 |
| Nucleoplasm | 34 | 7.9 | 4.6 |
| Neuron projection | 21 | 4.9 | 0.16 |
| Perinuclear region of cytoplasm | 16 | 3.7 | 3.5 |
| Molecular functions | |||
| DNA binding | 90 | 20.9 | 0.015 |
| Transcription regulator activity | 72 | 16.7 | <0.01 |
| Transcription factor activity | 51 | 11.8 | <0.01 |
| Sequence-specific DNA binding | 31 | 7.2 | 0.39 |
| Transcription factor binding | 27 | 6.3 | 0.68 |
| Enzyme binding | 25 | 5.8 | 4.2 |
| Transcription cofactor activity | 20 | 4.6 | 2.7 |
a A false discovery rate (FDR) of 5% was considered statistically significant.
When investigating the biological pathways active in receptive endometrium, we found that the miRNAs, through their target genes, can influence axon guidance, Wnt signaling, ERK/MAPK signaling, transforming growth factor β (TGF-β) signaling, p53 signaling, leukocyte extravasation signaling, and several other processes (Table 4).
Table 4.
Pathway Analysis of Predicted Gene Targets of Differentially Regulated miRNAs in Receptive Versus Prereceptive Endometrium Using the Database for Annotation, Visualization and Integrated Discovery (DAVID) and Ingenuity Pathways Analysis (IPA).a
| Pathways | Genes, n | P Value |
|---|---|---|
| KEGG (DAVID) | ||
| Axon guidance | 12 | .03 |
| Wnt signaling pathway | 11 | .04 |
| Ingenuity canonical pathways | ||
| Breast cancer regulation | 13 | <.001 |
| Synaptic long-term depression | 10 | .002 |
| Axon guidance signaling | 19 | .002 |
| Phospholipase C signaling | 13 | .005 |
| Wnt/β-catenin signaling | 10 | .009 |
| ERK/MAPK signaling | 10 | .01 |
| Cell cycle regulation | 4 | .01 |
| DNA methylation and transcriptional repression signaling | 3 | .01 |
| TGF-β signaling | 6 | .01 |
| Protein kinase A signaling | 14 | .02 |
| Corticotrophin-releasing hormone signaling | 7 | .02 |
| p53 signaling | 6 | .03 |
| Actin cytoskeleton signaling | 10 | .04 |
| Leukocyte extravasation signaling | 9 | .04 |
| Chemokine signaling | 5 | .04 |
| Dopamine receptor signaling | 5 | .04 |
a A corrected P value < .05 was considered statistically significant.
Functional Annotation of the Genes Common to the Predicted Targets and mRNA Microarray Data
Twelve genes were differentially regulated in LH + 7 versus LH + 2 endometria and were in both gene lists; in our common miRNA gene targets (434 genes) and in our previous gene expression microarray data (972 genes; Table 5). This short list of genes did not reflect any significant biological function when using DAVID analysis. The IPA analysis demonstrated involvement of these genes in various aspects such as tissue development (7 genes, P = .004), implantation (2 genes, P = .004), differentiation of cells (6 genes, P = .005), including differentiation of epithelial and endothelial cells (3 genes, P = .005), cancer (9 genes, P = .005), immunological functions (8 genes, P = .005), inflammatory responses (8 genes, P = .006), communication of cells (3 genes, P = .006), steroid synthesis (3 genes, P = .006), migration of cells (5 genes, P = .01), including leukocyte migration (4 genes, P = .007), and angiogenesis (3 genes, P = .01). The pathways identified in IPA analysis involved vitamin B5 and coenzyme A biosynthesis (P = .01), β-alanine metabolism (P = .03), semaphorin signaling in neurons (P = .04), ERK5 signaling (P = .04), and PXR/RXR activation (P = .05).
Table 5.
List of Genes Common to In Silico Prediction of miRNA Targets and Gene Expression Microarray Data (LH + 7 Versus LH + 2).a
| Gene Symbol | Gene Name | Biological Function | miRNA | mRNA Expression | EDB |
|---|---|---|---|---|---|
| CAST | Calpastatin | Endogenous calpain inhibitor | −miR-494 | +2.06 | + |
| CFTR | Cystic fibrosis transmembrane conductance regulator | Functions as chloride channel and controls regulation of other transport pathways | −miR-494 | +22 | + |
| DPYSL2 | Dihydropyrimidinase-like 2 | Axon guidance, signal transduction | −miR-494 | +2.03 | |
| F11R | F11 receptor | Role in cell adhesion, tight junction, leukocyte transendothelial migration | −miR-494 | +2.04 | |
| FGFR2 | Fibroblast growth factor receptor 2 | MAPK signaling pathway | −miR-494 | +5.57 | + |
| LIF | Leukemia inhibitory factor | Cytokine, JAK-STAT signaling pathway | −miR-494 | +36.62 | + |
| MTF1 | Metal-regulatory transcription factor 1 | Transcription factor | −miR-494 | +3.93 | |
| NPAS2 | Neuronal PAS domain protein 2 | Transcription factor | −miR-494 | +2.95 | |
| P4HA2 | Prolyl 4-hydroxylase alpha polypeptide II | Arginine and proline metabolism | +miR-30b | −2.05 | + |
| PPARGC1A | Peroxisome proliferator-activated receptor gamma coactivator 1 alpha | Transcriptional coactivator | −miR-494 | +4.46 | |
| TACC2 | Transforming acidic coiled-coil containing protein 2 | Tumorigenesis | −miR-494 | +3.18 | + |
| RAB40B | RAB40B member RAS oncogene family | Regulating secretory vesicles | −miR-494 | +3.61 |
Abbreviation: miRNA, microRNA.
a Down-(up-) regulated miRNA and up-(down-) regulated mRNA are indicated. EDB—gene is reported in the Endometrial Data Base (www.endometrialdatabase.com).
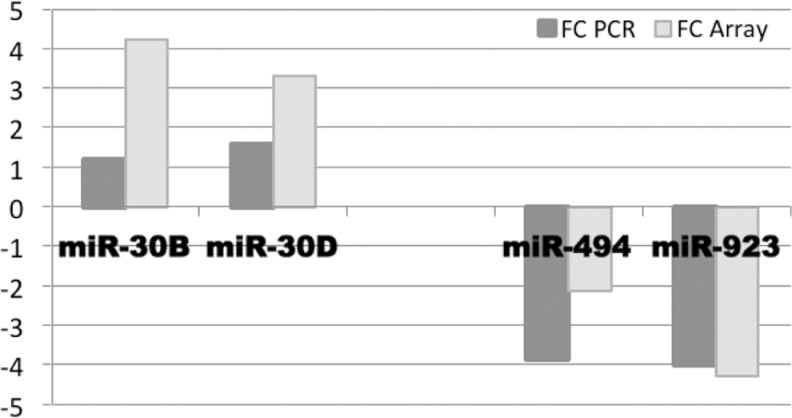
The miRNA Microarray Validation
In order to confirm the results obtained via miRNA microarray technology, expression analysis of all 4 significantly regulated miRNAs in receptive endometrium was carried out by means of real-time PCR. The results obtained were in accordance: miR-30b and miR-30d were significantly upregulated, while miR-494 and miR-923 were significantly downregulated in receptive endometrium versus the prereceptive state (P values < .05; Figure 3).
Figure 3.
MicroRNA (miRNA) array validation by real-time polymerase chain reaction (PCR). Expression levels of miR-30b, miR-30d, miR-494, and miR-923 in receptive versus prereceptive endometrium in miRNA array analysis in comparison with real-time PCR analysis are presented.
Discussion
In the present study, we analyzed miRNA expression patterns in normal fertile endometria at prereceptive and receptive phases of the cycle and identified 4 miRNAs that are specifically regulated in receptive endometria at the time of implantation.
To the best of our knowledge, this is the first study in which the miRNA signature in fertile, nondiseased endometria at the day of embryo implantation has been analyzed. Although previous studies have focused on miRNAs in connection with endometrial receptivity, our study design has been different, as we studied fertile nonstimulated endometria collected at the same time (LH + 7). The endometrial gene expression changes throughout the cycle days and, therefore, the timing of the biopsy collection is considered to be critical.33,34 The few studies published have concerned miRNA expression patterns in isolated epithelial cells from mid-secretory endometria (cycle days 19-23),20 in early mid-secretory endometria and their isolated stromal and glandular epithelial cells,35 in mid-secretory endometria from women with repeated implantation failure (cycle days 20-24),21 in mid-secretory nonstimulated endometria (LH + 7) from infertile women undergoing IVF treatment,5 and in receptive endometria from IVF stimulated women with tubal or male factor infertility (6 days after oocyte retrieval).36 Nevertheless, all these studies demonstrate the involvement of miRNAs in endometrial remodelling in the receptive phase. Understanding the regulation of endometrial receptivity in fertile healthy women will provide essential knowledge as regard establishing a consensus of opinion concerning receptive endometrium and its dysregulation and how to treat women with reproductive failure resulting from a loss of endometrial receptivity.
We found hsa-miR-30b and hsa-miR-30d to be upregulated, while hsa-miR-494 and hsa-miR-923 were downregulated in receptive endometria. As hsa-miR-923 appears to be a fragment of 28S rRNA, it has been removed from the majority of the databases and was not further discussed in our study. The IPA analysis highlighted the involvement of hsa-miR-30b, hsa-miR-30d, and hsa-miR-494 in carcinoma and schizophrenia. Interestingly, similarities between receptive endometrium and carcinogenesis and schizophrenia have been reported before, where common pathways are shown to be activated.37,38 Ingenuity pathway analysis revealed a network in which MIR30B and MIR30D genes interact with gene targets SEPT7, CRMP1, SLC44A1, HES1, FXR2, and TNF144B. Again we see the link to carcinogenesis and/or neural development, as all these genes are involved in these processes.39–43 The roles of hsa-miR-30b and 30d in enhancing metastasis have recently been shown,44 and a role of hsa-miR-494 in cardioprotective effects against ischemia/reperfusion-induced injury has been suggested.45 These studies are the first in which the functions of hsa-miR-30b, hsa-miR-30d, and hsa-miR-494 have been examined, and the roles of these miRNAs in receptive endometrium are yet to be determined.
Our results are in accordance with those of the previous studies where hsa-miR-30b and hsa-miR-30d were upregulated in nonstimulated LH + 7 versus LH + 2 endometria in infertile women.5 In addition, Kuokkanen et al detected MIR30B and MIR30D upregulation in mid-secretory endometrial epithelial cells when compared with epithelial cells from the late proliferative phase.20 In line with this, hsa-miR-30d has been reported to be downregulated in decidualized endometrial stromal cells versus nondecidualized cells.46 The importance of hsa-miR-30b in normal receptive endometrium is also indicated in a study by Li et al, where this miRNA was upregulated in IVF-treated women with normal serum progesterone levels versus women with elevated progesterone concentrations on the day of human chorionic gonadotropin administration.36 Further, in normal early mid-secretory endometrium, hsa-miR-30b and hsa-miR-30d have been found to be upregulated, while hsa-miR-494 was downregulated when compared with endometriosis samples.35 All these data, together with our results, highlight the roles of hsa-miR-30b, hsa-miR-30d, and hsa-miR-494 in preparation of the endometrium into its receptive state.
We identified the predicted target genes to be mainly involved in regulation of transcription and gene expression, cell proliferation, and apoptosis. All these molecular functions, both up- and downregulation of these processes, are involved in the cyclic remodelling of the endometrium, including endometrial maturation to the receptive state.17,47–49 Pathway analyses revealed several signaling pathways that were regulated by the miRNAs in receptive endometrium, such as Wnt signaling, axon guidance signaling, ERK/MAPK signaling, TGF-β signaling, p53 signaling and leukocyte extravasation signaling, plus cell cycle regulation, and other processes. Remarkably, all these have been reported to be highly involved in the development of receptive endometrium for successful implantation.23,47,49–60 Interestingly, a previous study of miRNAs in mid-secretory endometrium also revealed the Wnt signaling pathway, the p53 signaling pathway, and cell cycle regulation to be involved in endometrial receptivity.21
Surprisingly, there were only 12 genes common to the predicted miRNA targets and the gene expression data obtained in our previous study29: CAST, CFTR, DPYSL2, F11R, FGFR2, LIF, MTF1, NPAS2, P4HA2, PPARGC1A, TACC2, and RAB40B. Little overlap between miRNA targets and mRNA expression has also been reported in the previous studies.36,61,62 In addition to the previously mentioned complexity of regulation, one explanation for this could be that the miRNA targets are tissue specific.61 Another reason could be that as miRNAs are posttranscriptional regulators (translation modulation or regulation of mRNA degradation), they do not directly control mRNA expression.61,62 Furthermore, we have to recognize the putative nature of the miRNA-mRNA relationship, which is currently based on computational biology rather than experimental validation. Nevertheless, our list of 12 genes may represent a panel of genes that have important roles in endometrial receptivity. This list includes several genes involved in implantation, epithelial and endothelial cell differentiation, angiogenesis, migration and communication of cells, immunological and inflammatory responses, and in relevant canonical pathways concerning metabolism of carbohydrates and fatty acids (B5 and coenzyme A biosynthesis, β-alanine metabolism), axon guidance (morphogenesis and homeostasis, semaphorin signaling), cell proliferation and differentiation (ERK5 signaling), and detoxification (PXR/RXR activation). Interestingly, we identified within the list the gene commonly believed to be important in endometrial receptivity for leukemia inhibitory factor (LIF).
Although statistically valid, the weakness of our study is the small number of women recruited. However, all previous studies of this nature have involved similar numbers of endometrial samples.5,20,21,35,36 Taking into consideration the small sample size, we applied stringent data analysis, which might have led to us overlooking some miRNAs and/or gene targets. Additionally, our study approach enabled us to profile only a fraction of human miRNAs because microarray technology is limited to the probes on the array. Next-generation sequencing should enable profiling of miRNAs at a high level of sensitivity, which has been successfully performed in infertile women at the time of implantation,5 but so far not on fertile women.
In conclusion, our results demonstrate that both miRNAs and the molecular pathways they target are differentially expressed in prereceptive and receptive normal endometria. We suggest that a subset of miRNAs, namely hsa-miR-30b, hsa-miR-30d, and hsa-miR-494, plays an important role in gene reprogramming at the time of endometrial receptivity. Additionally, we identified a subset of miRNA target genes that could have an important role in endometrial receptivity. The miRNAs involved could serve as novel biomarkers of fertile receptive endometrium in future diagnostic and therapeutic approaches in reproductive medicine.
Supplementary Material
Supplementary Material
Acknowledgments
We are grateful to all the participants in this study.
Footnotes
Authors’ Notes: Study design, S.A., A.S.-E., J.A.H., A.S.; collection and assembly of data, J.A.M.-C., M.R.-A.; data analysis, S.A., F.J.E., J.A.M.-C.; data interpretation, S.A., J.A.H.; and manuscript preparation, S.A., J.A.M.-C., F.J.E., M.R.-A., A.S.-E., J.A.H., A.S. The online data supplements are available at http:/rsx.sagepub.com/supplemental.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: the European Science Foundation (ESF) for the activity ‘Frontiers of Functional Genomics’, Uppsala University, the Family Planning Foundation, Uppsala, the Spanish Ministry of Science and Innovation (SAF2008-04349), Spanish Ministry of Education (grant no. SB2010-0025), Junta de Andalucía (BIO-302), the Estonian Ministry of Education and Science (core grant SF0180044s09), and by Enterprise Estonia (grant no. EU30200).
References
- 1. Harper MJ. The implantation window. Baillieres Clin Obstet Gynaecol. 1992;6(2):351–371. [DOI] [PubMed] [Google Scholar]
- 2. Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22(6):1506–1512. [DOI] [PubMed] [Google Scholar]
- 3. Edwards RG. Clinical approaches to increasing uterine receptivity during human implantation. Hum Reprod. 1995;10(suppl 2):60–66. [DOI] [PubMed] [Google Scholar]
- 4. Macklon NS, Stouffer RL, Giudice LC, Fauser BC. The science behind 25 years of ovarian stimulation for in vitro fertilization. Endocr Rev. 2006;27(2):170–207. [DOI] [PubMed] [Google Scholar]
- 5. Sha AG, Liu JL, Jiang XM, et al. Genome-wide identification of micro-ribonucleic acids associated with human endometrial receptivity in natural and stimulated cycles by deep sequencing. Fertil Steril. 2011;96(1):150–155 e155. [DOI] [PubMed] [Google Scholar]
- 6. Munro SK, Farquhar CM, Mitchell MD, Ponnampalam AP. Epigenetic regulation of endometrium during the menstrual cycle. Mol Hum Reprod. 2010;16(5):297–310. [DOI] [PubMed] [Google Scholar]
- 7. Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429(6990):457–463. [DOI] [PubMed] [Google Scholar]
- 8. He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5(7):522–531. [DOI] [PubMed] [Google Scholar]
- 9. Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10(2):94–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Creighton CJ, Benham AL, Zhu H, et al. Discovery of novel microRNAs in female reproductive tract using next generation sequencing. PLoS One. 2010;5(3):e9637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125(6):1111–1124. [DOI] [PubMed] [Google Scholar]
- 12. Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136(4):642–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lim LP, Lau NC, Garrett-Engele P, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433(7027):769–773. [DOI] [PubMed] [Google Scholar]
- 14. Griffiths-Jones S. miRBase: microRNA sequences and annotation. Curr Protoc Bioinformatics. John Wiley & Sons, Inc, Hoboken, NJ, USA: 2010;Chapter 12:Unit 12.9.1-10. [DOI] [PubMed] [Google Scholar]
- 15. Miranda KC, Huynh T, Tay Y, et al. A pattern-based method for the identification of MicroRNA binding sites and their corresponding heteroduplexes. Cell. 2006;126(6):1203–1217. [DOI] [PubMed] [Google Scholar]
- 16. Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19(1):92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lam EW, Shah K, Brosens JJ. The role of microRNAs and FOXO transcription factors in cycling endometrium and cancer. J Endocrinol. 2012;212(1):13–25. [DOI] [PubMed] [Google Scholar]
- 18. Hu SJ, Ren G, Liu JL, et al. MicroRNA expression and regulation in mouse uterus during embryo implantation. J Biol Chem. 2008;283(34):23473–23484. [DOI] [PubMed] [Google Scholar]
- 19. Chakrabarty A, Tranguch S, Daikoku T, Jensen K, Furneaux H, Dey SK. MicroRNA regulation of cyclooxygenase-2 during embryo implantation. Proc Natl Acad Sci U S A. 2007;104(38):15144–15149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuokkanen S, Chen B, Ojalvo L, Benard L, Santoro N, Pollard JW. Genomic profiling of microRNAs and messenger RNAs reveals hormonal regulation in microRNA expression in human endometrium. Biol Reprod. 2010;82(4):791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Revel A, Achache H, Stevens J, Smith Y, Reich R. MicroRNAs are associated with human embryo implantation defects. Hum Reprod. 2011;26(10):2830–2840. [DOI] [PubMed] [Google Scholar]
- 22. Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975;122(2):262–263. [DOI] [PubMed] [Google Scholar]
- 23. Altmäe S, Martinez-Conejero JA, Salumets A, Simon C, Horcajadas JA, Stavreus-Evers A. Endometrial gene expression analysis at the time of embryo implantation in women with unexplained infertility. Mol Hum Reprod. 2010;16(3):178–187. [DOI] [PubMed] [Google Scholar]
- 24. Fernando RL, Nettleton D, Southey BR, Dekkers JC, Rothschild MF, Soller M. Controlling the proportion of false positives in multiple dependent tests. Genetics. 2004;166(1):611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krek A, Grun D, Poy MN, et al. Combinatorial microRNA target predictions. Nat Genet. 2005;37(5):495–500. [DOI] [PubMed] [Google Scholar]
- 26. Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. [DOI] [PubMed] [Google Scholar]
- 27. Dennis G, Jr, Sherman BT, Hosack DA, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4(5):P3. [PubMed] [Google Scholar]
- 28. Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125(1-2):279–284. [DOI] [PubMed] [Google Scholar]
- 29. Riesewijk A, Martin J, van Os R, et al. Gene expression profiling of human endometrial receptivity on days LH+2 versus LH+7 by microarray technology. Mol Hum Reprod. 2003;9(5):253–264. [DOI] [PubMed] [Google Scholar]
- 30. Betel D, Koppal A, Agius P, Sander C, Leslie C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol. 2010;11(8):R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318(5858):1931–1934. [DOI] [PubMed] [Google Scholar]
- 32. Place RF, Li LC, Pookot D, Noonan EJ, Dahiya R. MicroRNA-373 induces expression of genes with complementary promoter sequences. Proc Natl Acad Sci U S A. 2008;105(5):1608–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haouzi D, Mahmoud K, Fourar M, et al. Identification of new biomarkers of human endometrial receptivity in the natural cycle. Hum Reprod. 2009;24(1):198–205. [DOI] [PubMed] [Google Scholar]
- 34. Mirkin S, Arslan M, Churikov D, et al. In search of candidate genes critically expressed in the human endometrium during the window of implantation. Hum Reprod. 2005;20(8):2104–2117. [DOI] [PubMed] [Google Scholar]
- 35. Pan Q, Luo X, Toloubeydokhti T, Chegini N. The expression profile of micro-RNA in endometrium and endometriosis and the influence of ovarian steroids on their expression. Mol Hum Reprod. 2007;13(11):797–806. [DOI] [PubMed] [Google Scholar]
- 36. Li R, Qiao J, Wang L, et al. MicroRNA array and microarray evaluation of endometrial receptivity in patients with high serum progesterone levels on the day of hCG administration. Reprod Biol Endocrinol. 2011;9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. von Rango U, Classen-Linke I, Krusche CA, Beier HM. The receptive endometrium is characterized by apoptosis in the glands. Hum Reprod. 1998;13(11):3177–3189. [DOI] [PubMed] [Google Scholar]
- 38. Zhang D, Lei C, Zhang W. Up-regulated monoamine oxidase in the mouse uterus during the peri-implantation period. Arch Gynecol Obstet. 2011;284(4):861–866. [DOI] [PubMed] [Google Scholar]
- 39. Igci YZ, Arslan A, Akarsu E, et al. Differential expression of a set of genes in follicular and classic variants of papillary thyroid carcinoma. Endocr Pathol. 2011;22(2):86–96. [DOI] [PubMed] [Google Scholar]
- 40. Pan SH, Chao YC, Hung PF, et al. The ability of LCRMP-1 to promote cancer invasion by enhancing filopodia formation is antagonized by CRMP-1. J Clin Invest. 2011;121(8):3189–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Michel V, Bakovic M. The solute carrier 44A1 is a mitochondrial protein and mediates choline transport. FASEB J. 2009;23(8):2749–2758. [DOI] [PubMed] [Google Scholar]
- 42. Liu J, Lu WG, Ye F, et al. Hes1/Hes5 gene inhibits differentiation via down-regulating Hash1 and promotes proliferation in cervical carcinoma cells. Int J Gynecol Cancer. 2010;20(7):1109–1116. [DOI] [PubMed] [Google Scholar]
- 43. Coffee RL, Jr, Tessier CR, Woodruff EA, 3rd, Broadie K. Fragile X mental retardation protein has a unique, evolutionarily conserved neuronal function not shared with FXR1P or FXR2P. Dis Model Mech. 2010;3(7-8):471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gaziel-Sovran A, Segura MF, Di Micco R, et al. miR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis. Cancer Cell. 2011;20(1):104–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang X, Zhang X, Ren XP, et al. MicroRNA-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardiac injury. Circulation. 2010;122(13):1308–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Qian K, Hu L, Chen H, et al. Hsa-miR-222 is involved in differentiation of endometrial stromal cells in vitro. Endocrinology. 2009;150(10):4734–4743. [DOI] [PubMed] [Google Scholar]
- 47. Aghajanova L, Hamilton AE, Giudice LC. Uterine receptivity to human embryonic implantation: histology, biomarkers, and transcriptomics. Semin Cell Dev Biol. 2008;19(2):204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Driak D, Dvorska M, Svandova I, et al. Changes in expression of some apoptotic markers in different types of human endometrium. Folia Biol (Praha). 2011;57(3):104–111. [DOI] [PubMed] [Google Scholar]
- 49. Altmäe S, Reimand J, Hovatta O, et al. Research resource: interactome of human embryo implantation: identification of gene expression pathways, regulation, and integrated regulatory networks. Mol Endocrinol. 2012;26(1):203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dickinson RE, Duncan WC. The SLIT-ROBO pathway: a regulator of cell function with implications for the reproductive system. Reproduction. 2010;139(4):697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen Q, Zhang Y, Lu J, et al. Embryo-uterine cross-talk during implantation: the role of Wnt signaling. Mol Hum Reprod. 2009;15(4):215–221. [DOI] [PubMed] [Google Scholar]
- 52. Koler M, Achache H, Tsafrir A, Smith Y, Revel A, Reich R. Disrupted gene pattern in patients with repeated in vitro fertilization (IVF) failure. Hum Reprod. 2009;24(10):2541–2548. [DOI] [PubMed] [Google Scholar]
- 53. Liu Y, Kodithuwakku SP, Ng PY, et al. Excessive ovarian stimulation up-regulates the Wnt-signaling molecule DKK1 in human endometrium and may affect implantation: an in vitro co-culture study. Hum Reprod. 2010;25(2):479–490. [DOI] [PubMed] [Google Scholar]
- 54. Kao LC, Tulac S, Lobo S, et al. Global gene profiling in human endometrium during the window of implantation. Endocrinology. 2002;143(6):2119–2138. [DOI] [PubMed] [Google Scholar]
- 55. Horcajadas JA, Pellicer A, Simon C. Wide genomic analysis of human endometrial receptivity: new times, new opportunities. Hum Reprod Update. 2007;13(1):77–86. [DOI] [PubMed] [Google Scholar]
- 56. Hu W, Feng Z, Teresky AK, Levine AJ. p53 regulates maternal reproduction through LIF. Nature. 2007;450(7170):721–724. [DOI] [PubMed] [Google Scholar]
- 57. Feng Z, Zhang C, Kang HJ, et al. Regulation of female reproduction by p53 and its family members. FASEB J. 2011;25(7):2245–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Massuto DA, Kneese EC, Johnson GA, et al. Transforming growth factor beta (TGFB) signaling is activated during porcine implantation: proposed role for latency-associated peptide interactions with integrins at the conceptus-maternal interface. Reproduction. 2010;139(2):465–478. [DOI] [PubMed] [Google Scholar]
- 59. Genbacev OD, Prakobphol A, Foulk RA, et al. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science. 2003;299(5605):405–408. [DOI] [PubMed] [Google Scholar]
- 60. Dominguez F, Yanez-Mo M, Sanchez-Madrid F, Simon C. Embryonic implantation and leukocyte transendothelial migration: different processes with similar players? FASEB J. 2005;19(9):1056–1060. [DOI] [PubMed] [Google Scholar]
- 61. Dahiya N, Sherman-Baust CA, Wang TL, et al. MicroRNA expression and identification of putative miRNA targets in ovarian cancer. PLoS One. 2008;3(6):e2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Boren T, Xiong Y, Hakam A, et al. MicroRNAs and their target messenger RNAs associated with endometrial carcinogenesis. Gynecol Oncol. 2008;110(2):206–215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.