Abstract
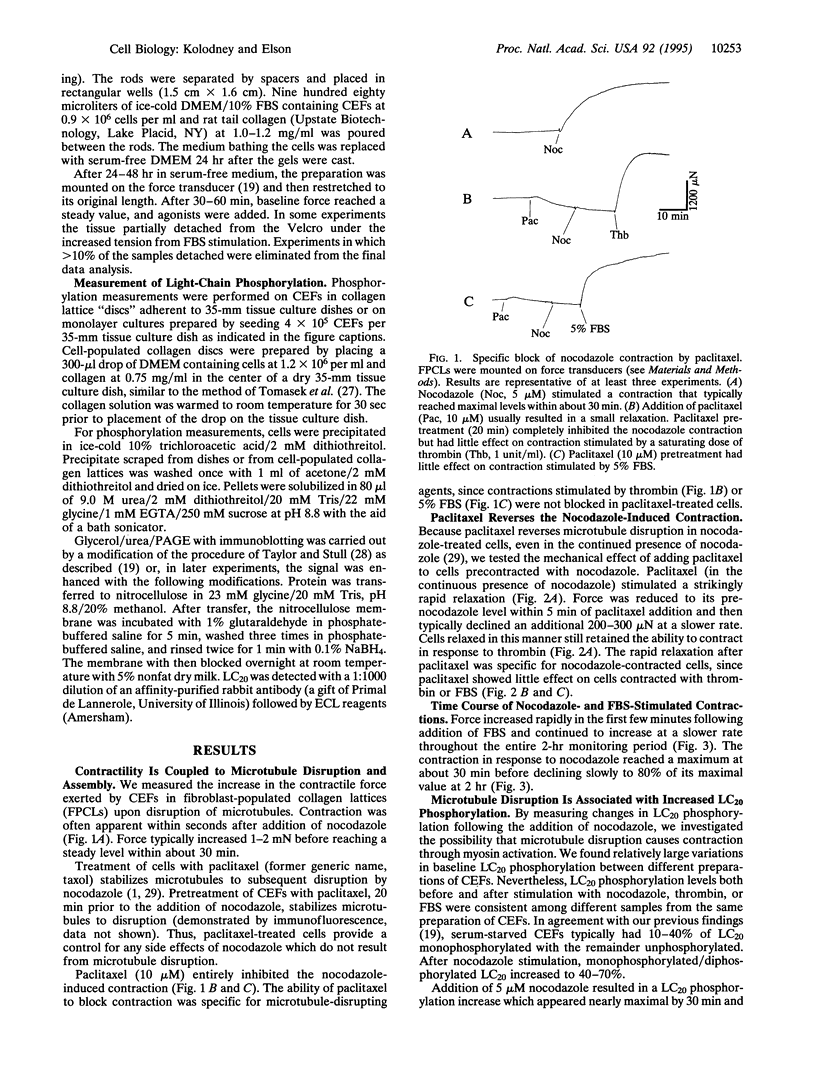
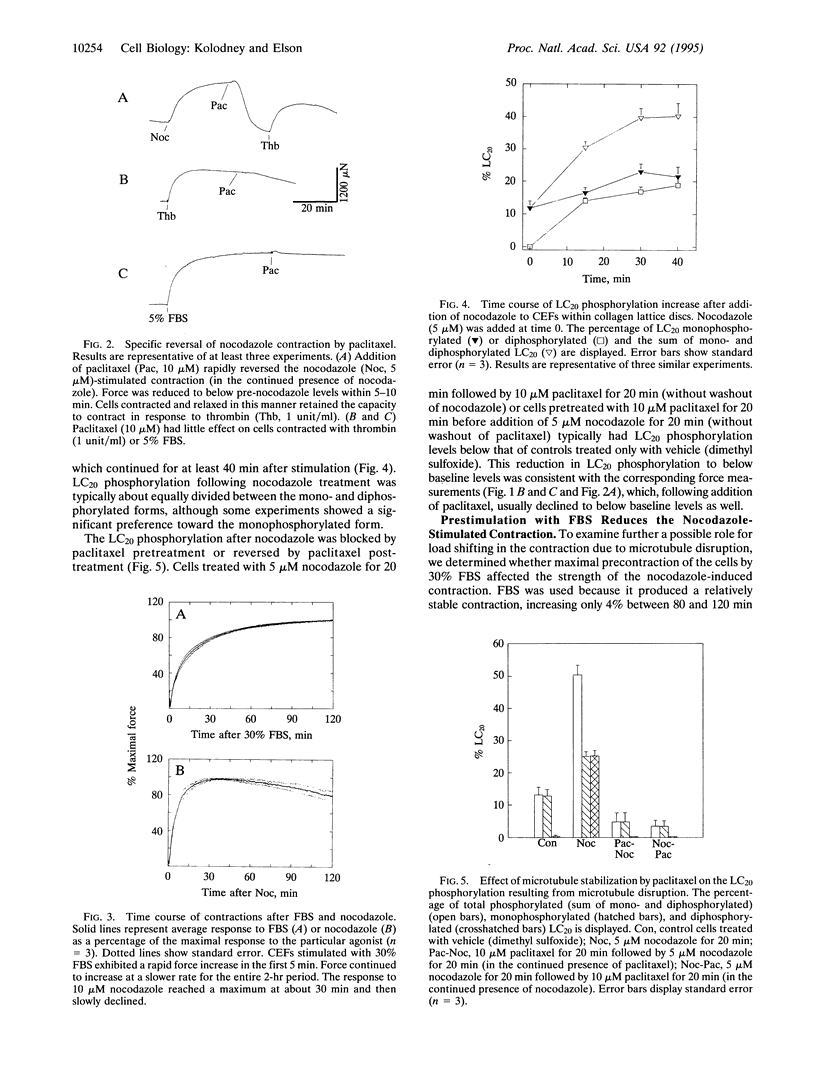
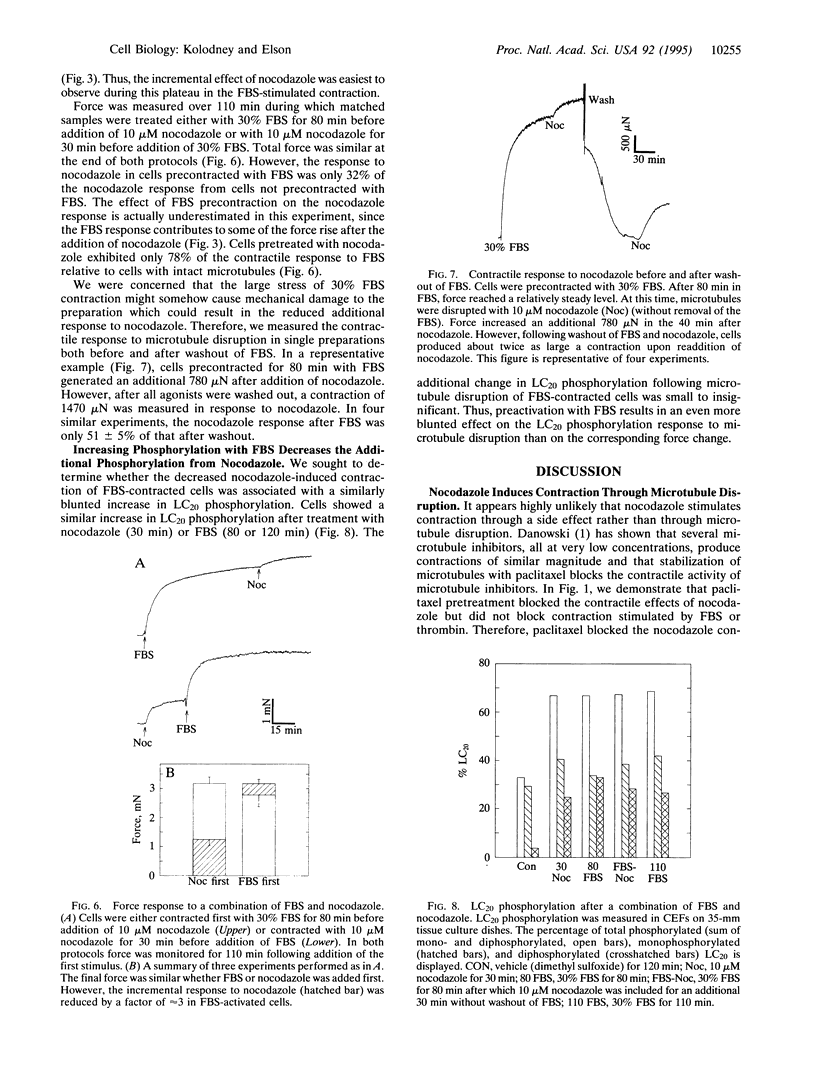
Microtubules have been proposed to function as rigid struts which oppose cellular contraction. Consistent with this hypothesis, microtubule disruption strengthens the contractile force exerted by many cell types. We have investigated alternative explanation for the mechanical effects of microtubule disruption: that microtubules modulate the mechanochemical activity of myosin by influencing phosphorylation of the myosin regulatory light chain (LC20). We measured the force produced by a population of fibroblasts within a collagen lattice attached to an isometric force transducer. Treatment of cells with nocodazole, an inhibitor of microtubule polymerization, stimulated an isometric contraction that reached its peak level within 30 min and was typically 30-45% of the force increase following maximal stimulation with 30% fetal bovine serum. The contraction following nocodazole treatment was associated with a 2- to 4-fold increase in LC20 phosphorylation. The increases in both force and LC20 phosphorylation, after addition of nocodazole, could be blocked or reversed by stabilizing the microtubules with paclitaxel (former generic name, taxol). Increasing force and LC20 phosphorylation by pretreatment with fetal bovine serum decreased the subsequent additional contraction upon microtubule disruption, a finding that appears inconsistent with a load-shifting mechanism. Our results suggest that phosphorylation of LC20 is a common mechanism for the contractions stimulated both by microtubule poisons and receptor-mediated agonists. The modulation of myosin activity by alterations in microtubule assembly may coordinate the physiological functions of these cytoskeletal components.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa T., Frieden C. Interaction of microtubule-associated proteins with actin filaments. Studies using the fluorescence-photobleaching recovery technique. J Biol Chem. 1984 Oct 10;259(19):11730–11734. [PubMed] [Google Scholar]
- Broschat K. O., Stidwill R. P., Burgess D. R. Phosphorylation controls brush border motility by regulating myosin structure and association with the cytoskeleton. Cell. 1983 Dec;35(2 Pt 1):561–571. doi: 10.1016/0092-8674(83)90190-3. [DOI] [PubMed] [Google Scholar]
- Buxbaum R. E., Heidemann S. R. A thermodynamic model for force integration and microtubule assembly during axonal elongation. J Theor Biol. 1988 Oct 7;134(3):379–390. doi: 10.1016/s0022-5193(88)80068-7. [DOI] [PubMed] [Google Scholar]
- Cande W. Z., Ezzell R. M. Evidence for regulation of lamellipodial and tail contraction of glycerinated chicken embryonic fibroblasts by myosin light chain kinase. Cell Motil Cytoskeleton. 1986;6(6):640–648. doi: 10.1002/cm.970060612. [DOI] [PubMed] [Google Scholar]
- Craig R., Smith R., Kendrick-Jones J. Light-chain phosphorylation controls the conformation of vertebrate non-muscle and smooth muscle myosin molecules. 1983 Mar 31-Apr 6Nature. 302(5907):436–439. doi: 10.1038/302436a0. [DOI] [PubMed] [Google Scholar]
- Danowski B. A. Fibroblast contractility and actin organization are stimulated by microtubule inhibitors. J Cell Sci. 1989 Jun;93(Pt 2):255–266. doi: 10.1242/jcs.93.2.255. [DOI] [PubMed] [Google Scholar]
- De Brabander M., Geuens G., Nuydens R., Willebrords R., De Mey J. Taxol induces the assembly of free microtubules in living cells and blocks the organizing capacity of the centrosomes and kinetochores. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5608–5612. doi: 10.1073/pnas.78.9.5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennerll T. J., Joshi H. C., Steel V. L., Buxbaum R. E., Heidemann S. R. Tension and compression in the cytoskeleton of PC-12 neurites. II: Quantitative measurements. J Cell Biol. 1988 Aug;107(2):665–674. doi: 10.1083/jcb.107.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugina V. B., Svitkina T. M., Vasiliev J. M., Gelfand I. M. Special type of morphological reorganization induced by phorbol ester: reversible partition of cell into motile and stable domains. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4122–4125. doi: 10.1073/pnas.84.12.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson E. L. Cellular mechanics as an indicator of cytoskeletal structure and function. Annu Rev Biophys Biophys Chem. 1988;17:397–430. doi: 10.1146/annurev.bb.17.060188.002145. [DOI] [PubMed] [Google Scholar]
- Giuliano K. A., Kolega J., DeBiasio R. L., Taylor D. L. Myosin II phosphorylation and the dynamics of stress fibers in serum-deprived and stimulated fibroblasts. Mol Biol Cell. 1992 Sep;3(9):1037–1048. doi: 10.1091/mbc.3.9.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen G. G., Bulinski J. C. Selective stabilization of microtubules oriented toward the direction of cell migration. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5946–5950. doi: 10.1073/pnas.85.16.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber D. E. Cellular tensegrity: defining new rules of biological design that govern the cytoskeleton. J Cell Sci. 1993 Mar;104(Pt 3):613–627. doi: 10.1242/jcs.104.3.613. [DOI] [PubMed] [Google Scholar]
- Ishikawa R., Kagami O., Hayashi C., Kohama K. Characterization of smooth muscle caldesmon as a microtubule-associated protein. Cell Motil Cytoskeleton. 1992;23(4):244–251. doi: 10.1002/cm.970230404. [DOI] [PubMed] [Google Scholar]
- Jay P. Y., Pham P. A., Wong S. A., Elson E. L. A mechanical function of myosin II in cell motility. J Cell Sci. 1995 Jan;108(Pt 1):387–393. doi: 10.1242/jcs.108.1.387. [DOI] [PubMed] [Google Scholar]
- Kolodney M. S., Elson E. L. Correlation of myosin light chain phosphorylation with isometric contraction of fibroblasts. J Biol Chem. 1993 Nov 15;268(32):23850–23855. [PubMed] [Google Scholar]
- Kolodney M. S., Wysolmerski R. B. Isometric contraction by fibroblasts and endothelial cells in tissue culture: a quantitative study. J Cell Biol. 1992 Apr;117(1):73–82. doi: 10.1083/jcb.117.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb N. J., Fernandez A., Conti M. A., Adelstein R., Glass D. B., Welch W. J., Feramisco J. R. Regulation of actin microfilament integrity in living nonmuscle cells by the cAMP-dependent protein kinase and the myosin light chain kinase. J Cell Biol. 1988 Jun;106(6):1955–1971. doi: 10.1083/jcb.106.6.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiber D., Jasper J. R., Alousi A. A., Martin J., Bernstein D., Insel P. A. Alteration in Gs-mediated signal transduction in S49 lymphoma cells treated with inhibitors of microtubules. J Biol Chem. 1993 Feb 25;268(6):3833–3837. [PubMed] [Google Scholar]
- Mabuchi I., Okuno M. The effect of myosin antibody on the division of starfish blastomeres. J Cell Biol. 1977 Jul;74(1):251–263. doi: 10.1083/jcb.74.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madreperla S. A., Adler R. Opposing microtubule- and actin-dependent forces in the development and maintenance of structural polarity in retinal photoreceptors. Dev Biol. 1989 Jan;131(1):149–160. doi: 10.1016/s0012-1606(89)80046-6. [DOI] [PubMed] [Google Scholar]
- Okuhara K., Murofushi H., Sakai H. Binding of kinesin to stress fibers in fibroblasts under condition of microtubule depolymerization. Cell Motil Cytoskeleton. 1989;12(2):71–77. doi: 10.1002/cm.970120202. [DOI] [PubMed] [Google Scholar]
- Rappaport R. Cytokinesis in animal cells. Int Rev Cytol. 1971;31:169–213. doi: 10.1016/s0074-7696(08)60059-5. [DOI] [PubMed] [Google Scholar]
- Rappaport R., Rappaport B. N. Establishment of cleavage furrows by the mitotic spindle. J Exp Zool. 1974 Aug;189(2):189–196. doi: 10.1002/jez.1401890206. [DOI] [PubMed] [Google Scholar]
- Sattilaro R. F., Dentler W. L., LeCluyse E. L. Microtubule-associated proteins (MAPs) and the organization of actin filaments in vitro. J Cell Biol. 1981 Aug;90(2):467–473. doi: 10.1083/jcb.90.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott C. W., Caputo C. B., Salama A. I. Properties of a microtubule-associated cofactor-independent protein kinase from pig brain. Biochem J. 1989 Oct 1;263(1):207–214. doi: 10.1042/bj2630207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden S. C., Pollard T. D. Phosphorylation of microtubule-associated proteins regulates their interaction with actin filaments. J Biol Chem. 1983 Jun 10;258(11):7064–7071. [PubMed] [Google Scholar]
- Serrano L., Hernández M. A., Díaz-Nido J., Avila J. Association of casein kinase II with microtubules. Exp Cell Res. 1989 Mar;181(1):263–272. doi: 10.1016/0014-4827(89)90200-0. [DOI] [PubMed] [Google Scholar]
- Shinohara-Gotoh Y., Nishida E., Hoshi M., Sakai H. Activation of microtubule-associated protein kinase by microtubule disruption in quiescent rat 3Y1 cells. Exp Cell Res. 1991 Mar;193(1):161–166. doi: 10.1016/0014-4827(91)90551-5. [DOI] [PubMed] [Google Scholar]
- Taylor D. A., Stull J. T. Calcium dependence of myosin light chain phosphorylation in smooth muscle cells. J Biol Chem. 1988 Oct 5;263(28):14456–14462. [PubMed] [Google Scholar]
- Tomasek J. J., Haaksma C. J., Eddy R. J., Vaughan M. B. Fibroblast contraction occurs on release of tension in attached collagen lattices: dependency on an organized actin cytoskeleton and serum. Anat Rec. 1992 Mar;232(3):359–368. doi: 10.1002/ar.1092320305. [DOI] [PubMed] [Google Scholar]
- Tsutsui H., Ishihara K., Cooper G., 4th Cytoskeletal role in the contractile dysfunction of hypertrophied myocardium. Science. 1993 Apr 30;260(5108):682–687. doi: 10.1126/science.8097594. [DOI] [PubMed] [Google Scholar]
- Vallee R. B., DiBartolomeis M. J., Theurkauf W. E. A protein kinase bound to the projection portion of MAP 2 (microtubule-associated protein 2). J Cell Biol. 1981 Sep;90(3):568–576. doi: 10.1083/jcb.90.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysolmerski R. B., Lagunoff D. Involvement of myosin light-chain kinase in endothelial cell retraction. Proc Natl Acad Sci U S A. 1990 Jan;87(1):16–20. doi: 10.1073/pnas.87.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]



