Abstract
Sleep-disordered breathing (SDB) may be a treatable risk factor in patients with hypertrophic cardiomyopathy (HCM), the most common inherited cardiomyopathy. Evidence suggests a high prevalence of SDB in HCM. We summarize the pathophysiology of SDB as it relates to hypertension, coronary artery disease, atrial fibrillation, and sudden cardiac death in patients with HCM. The implications regarding the care of patients with HCM and SDB are discussed as well as the knowledge deficits needing further exploration.
Documented morbidity and mortality related to hypertrophic cardiomyopathy (HCM) possibly reach as far back as the ancient Greek messenger Pheidippides, who shouted “Niki!” (“Victory!”), collapsed, and died in 490 bc after running from the battlefield in Marathon to Athens.1 However, to our knowledge, the first modern pathologic and clinical reports of HCM were published only in the latter half of the 20th century.2 HCM is the most common inherited cardiomyopathy, which phenotypically affects one in 500 adults in the general population.3 At least 11 causative genes expressed primarily or exclusively in the heart have been identified, and these are believed to encode thick and thin myofilament proteins or contiguous Z-discs in the cardiac myocytes. Even though the exact disease mechanisms remain targets of ongoing investigation, the key processes at the cellular level involve increased myocyte size, accumulation of fibroblasts in the myocardium (collagen secretion leading to fibrosis), and malalignment of myocytes and sarcomeres. At the macroscopic level, the hearts of patients with HCM commonly manifest asymmetric ventricular septal hypertrophy leading to dynamic left ventricular outflow tract obstruction, systolic anterior motion of the mitral valve leading to mitral regurgitation, and increased stiffness of the ventricular myocardium leading to impaired diastolic filling. Occurrences of sudden cardiac death (SCD), exemplified by the tragic on-court death of the Boston Celtics and National Basketball Association all-star Reggie Lewis in 1993, represent an important, but small minority of patients with HCM. More typical is a gradual progression of symptoms, with eventual development of chronic heart failure.4-6 Importantly, many patients are relatively asymptomatic throughout their lives.4-6
Current Therapeutic Options
Even though the management of HCM should be tailored to the needs of each affected individual, both the 2003 and the 2011 guidelines list the three most prevalent symptomatic presentations to be2: (1) heart failure, which may be progressive and can lead to end-stage dysfunction; (2) atrial fibrillation (AF), which in patients with HCM carries a particularly unfavorable prognosis and often is itself associated with worsening heart failure; and (3) SCD, which strikes a small minority of patients with HCM for whom primary and secondary preventive placement of an implantable cardioverter-defibrillator (ICD) may play a crucial role.2,7 The treatment algorithm outlined in the guidelines integrates the use of medical therapy (β-blocking agents, verapamil, and disopyramide) as well as invasive measures (surgical myectomy, alcohol ablation, and placement of an ICD) when appropriate indications arise. Unfortunately, many heart failure symptoms progress despite the best available treatments; hence, novel therapeutic approaches are needed.
Identifying the Need
Clinical investigation of the role of sleep-disordered breathing (SDB) in patients with HCM is in a relatively early stage, hence, screening and treatment of SDB have not been incorporated into the routine management of HCM.2,8 Identifying potential links between SDB and HCM is important because SDB may contribute a uniquely reversible risk factor for HCM pathophysiology given that it has widely available and relatively benign treatment options that carry a favorable profile of side effects (weight loss, postural therapy, mandibular devices, and CPAP).9 Here, we explore some of the key pathophysiologic consequences of SDB, which may have direct relevance to patients with HCM, and review the emerging evidence suggesting associations between SDB and adverse outcomes in HCM.
Definitions
The current guidelines define HCM as a disease state characterized by unexplained left ventricular (LV) hypertrophy associated with nondilated ventricular chambers in the absence of another cardiac or systemic disease that itself would be capable of producing the magnitude of hypertrophy evident in a given patient.2 In clinical practice, HCM is usually recognized by asymmetric LV wall thickening (commonly ≥ 15 mm on echocardiography).2
SDB is defined according to the standard criteria set forth by the American Academy of Sleep Medicine.10-13 The most common type of SDB is OSA, and much of the pathophysiologic understanding of SDB relies on studies of OSA in patients without HCM. A further limitation is that many of the studies of sleep pathology in patients with HCM used overnight oximetry in the diagnosis of SDB. This technique is more readily available and affordable than polysomnography; however, oximetry alone provides only a limited characterization of SDB and cannot differentiate among the various types of sleep apnea.
Relevance of SDB Pathophysiology to HCM
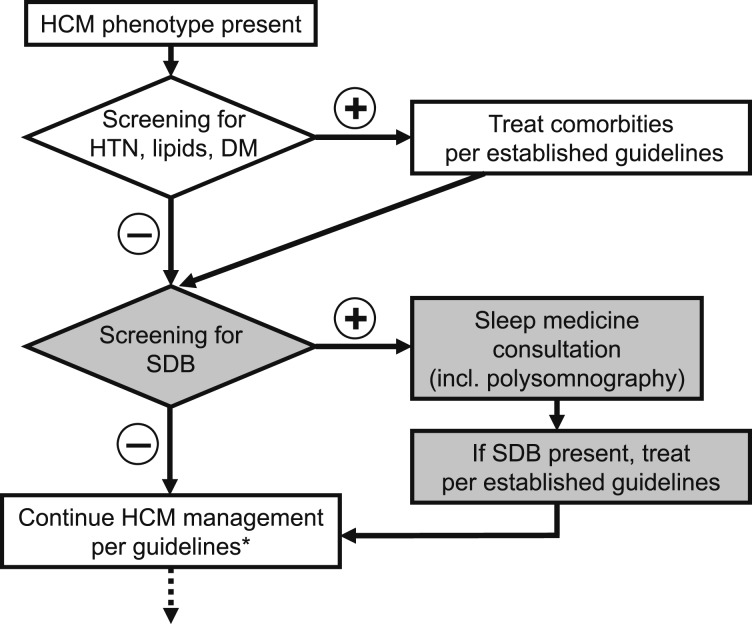
The current HCM guidelines support management algorithms beginning with the screening and treatment of comorbidities known to be particularly deleterious in patients with HCM (ie, hypertension, hyperlipidemia, diabetes mellitus, AF).2,7,14 An increasing body of evidence emerging from non-HCM study cohorts supports the notion that SDB may increase cardiovascular risk and that treatment of SDB may lead to improved outcomes.15 Given that patients with HCM are uniquely susceptible to the dangers of cardiovascular comorbidities, such as AF7 and coronary artery disease,14 it would be reasonable to consider whether their initial management should include routine screening for SDB and its effective treatment, if applicable (Fig 1). This section summarizes the key findings on the relationships between SDB and cardiac pathologies most relevant to patients with HCM; however, much of these data originated from non-HCM studies, and caution is advisable in their extrapolation to HCM. (For studies focusing on SDB in patients with HCM, see the Implications of Comorbid SDB in Patients With HCM section.)
Figure 1 .
– Suggested modification to the flowchart of the management of patients with phenotypic HCM (altered steps are highlighted in gray). *Current HCM guidelines. DM = diabetes mellitus; HCM = hypertrophic cardiomyopathy; HTN = hypertension; incl. = including; SDB = sleep-disordered breathing.
Hypertension
Hypertension in patients with HCM is believed to result in additive adverse consequences.16 Well-conducted prospective non-HCM studies link increasing BP and incident hypertension with the presence and severity of SDB, independent of confounding variables (especially age, sex, and obesity).17 The physiologic nocturnal decrease in BP is attenuated in patients with SDB, resulting in the so called nondipping BP pattern. Treatment of SDB leads to a relatively modest, but significant reduction in BP,18 and this conclusion is supported by several meta-analyses on the topic.19 Current guidelines on hypertension recommend treatment of SDB as a nonpharmacologic BP-lowering intervention.20
Elevated BP, especially at night,21 is an important cause of LV hypertrophy. OSA in and of itself has also been implicated in diastolic dysfunction22 and ventricular thickening.23 Whether and how OSA or OSA-induced hypertension modulate ventricular thickening in HCM remain to be determined.
Coronary Artery Disease
Coronary atherosclerosis potentiates a negative prognosis in HCM.2,24 Among patients without HCM, myocardial ischemia and infarction are independently associated with SDB, and patients with untreated SDB experience an increased incidence of adverse outcomes connected with coronary artery disease (all-cause and cardiac mortality)15 as well as an increased rate of nocturnal myocardial infarction.25 SDB is highly prevalent in patients with ischemic heart disease and often remains undiagnosed, even after admission for myocardial infarction.26
Atrial Fibrillation
The occurrence of AF in patients with HCM often portends a rapid progression in heart failure symptoms, and the available therapeutic options for maintaining sinus rhythm in HCM are far from optimal.7,27 In non-HCM studies, SDB has been strongly and independently associated with the incidence of AF.28 Prospective data from patients after cardioversion29 and after pulmonary vein isolation30 show that AF recurs more often in those with SDB than in those without and suggest that if patients with SDB tolerate effective treatment with CPAP, the risk of AF recurrence is reduced. Among a number of potential mechanisms,31 data from patients without HCM suggest that increased atrial size may contribute to the greater preponderance of AF in patients with SDB.16,32 The association of SDB and AF remains significant after accounting for BMI, neck circumference, hypertension, and diabetes mellitus.28
Sudden Cardiac Death
Optimal risk stratification for ICD placement plays an important role in HCM management, and the current criteria identifying patients in need of primary prevention are undergoing continual evidence-based revisions.2,33 A significant proportion of patients with HCM suffer sudden death or its equivalent during the night.32 Non-HCM data focusing on the diurnal variation of SCD show that patients free of SDB have the greatest likelihood of sudden death between 6 and 11 am; in striking contrast, more than one-half of SCDs in patients with SDB occur during the night (10:00 pm-6:00 am).15,34 Other data in patients with ICDs show that device firing for ventricular arrhythmias occurs more commonly at night in patients with OSA.35 If SDB can play a role in nocturnal ventricular arrhythmias and sudden death in patients without HCM, it is conceivable that patients with HCM could also be similarly affected, with SDB potentiating the risk of sleep-related cardiac events in these patients. Further studies on this possible association would be clinically relevant.
Implications of Comorbid SDB in Patients With HCM
Studies in patients with HCM have encountered two inherent obstacles. First, the relatively low population prevalence of HCM poses a challenge to subject recruitment and may lead to insufficient power and referral bias. The second obstacle lies in the wide spectrum of HCM phenotypes, which complicates systematic classification, analysis, and generalizability of results. Therefore, it is not surprising that there is a paucity of large randomized controlled studies. On the other hand, a number of reports have suggested a compelling case for SDB as a highly prevalent, potentially harmful, and uniquely treatable comorbidity in patients with HCM.
Prevalence
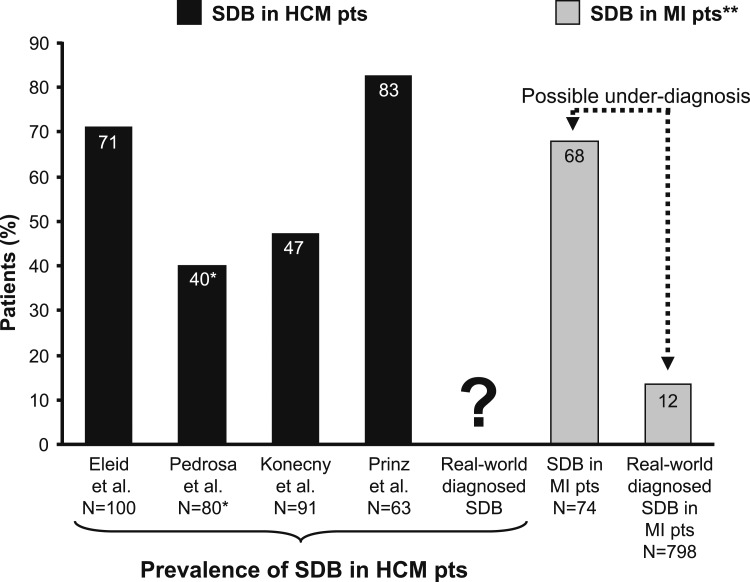
The prevalence of SDB in patients with HCM, averaged from the four available studies, is about 62% (Fig 2). An important caveat is that most of these studies used overnight oximetry as opposed to complete polysomnography in the diagnosis of SDB. The wide range of 40% to 83% is partly due to varying definitions of SDB in these studies.10,27,36,37 Although this may seem extraordinarily high, a similarly common prevalence of SDB has been reported in patients with ischemic cardiomyopathy.38 Indeed, patients after myocardial infarction, who in prospective studies also manifest a very high SDB prevalence, may often be significantly underdiagnosed in the real-world setting (Fig 2).26 Further studies are needed to define how many patients with HCM have undiagnosed SDB and to estimate which subgroup of patients with HCM could benefit from routine sleep apnea screening (Fig 1).
Figure 2 .
– SDB in patients with HCM10,27,36,37 approaches a similar prevalence as that reported for SDB in patients with ischemic cardiomyopathy.26 SDB in patients with MI often is underdiagnosed (the 12% of documented SDB in the real-world sample of these patients is in striking contrast with the prevalence of 68% when such patients undergo routine screening for SDB as part of a study setting).26 Whether SDB is underdiagnosed in patients with HCM has not been studied. SDB is defined as an oxygen desaturation index ≥ 5 or apnea-hypopnea index ≥ 5. *In this study, SDB was defined as an apnea-hypopnea index of ≥ 15. **Overnight attended polysomnography was used in this study to detect sleep apnea. MI = myocardial infarction; pts = patients. See Figure 1 legend for expansion of other abbreviations. (Reprinted with permission from the Mayo Foundation for Medical Education and Research. All rights reserved.)
Symptoms
A greater proportion of patients with HCM and SDB admit to functional impairment as measured on the New York Heart Association scale compared with those without SDB.36 Anecdotal reports suggest that heart failure symptoms in patients with HCM may improve with successful treatment of severe SDB.39 Studies currently under way are assessing whether objective measures of functional impairment (peak oxygen consumption calculated during standardized exercise testing) are independent of the many confounding factors, particularly obesity and increased age.40 Such data are particularly clinically relevant in HCM given that in many patients with HCM, exercise limitations can go unnoticed until exposed by a provocative exercise study with objective measurement of physical performance.41 Impaired quality of sleep, which patients with HCM commonly report, can also contribute to a decreased quality of life as recorded by the Minnesota Living with Heart Failure Questionnaire.42 Whether these sleep symptoms are secondary to SDB and, hence, potentially remediable with SDB treatment, remains to be determined.
Atrial Arrhythmias
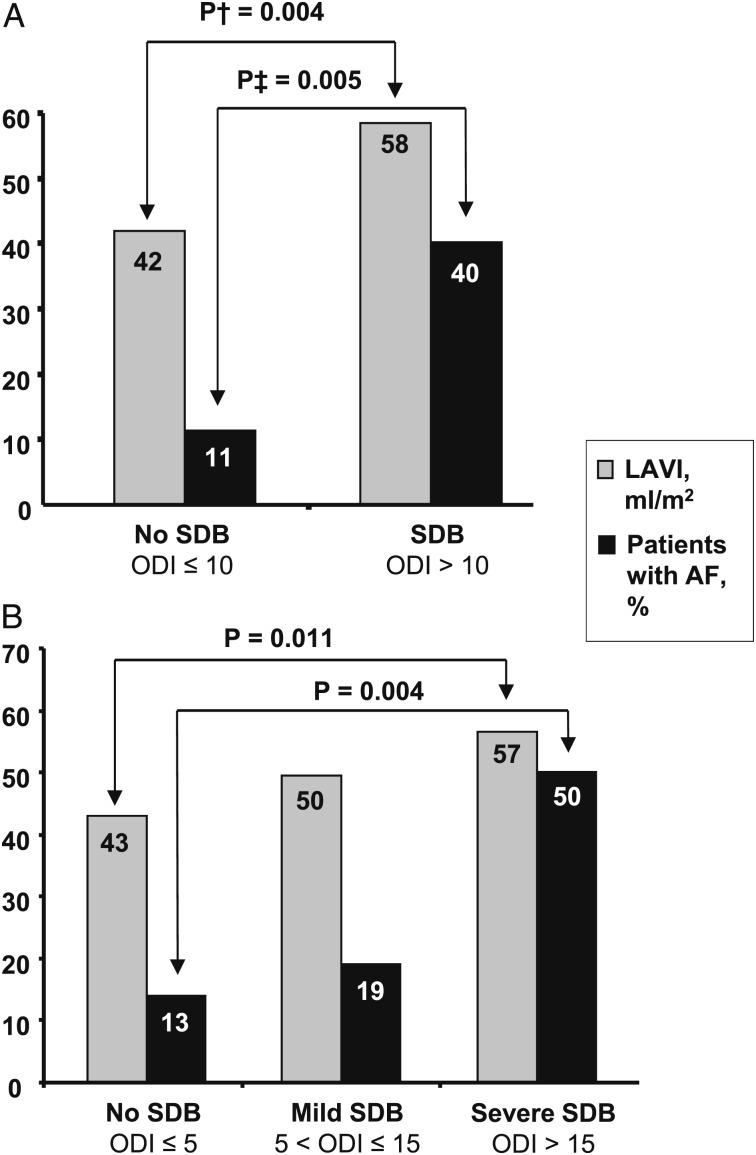
Several studies provide evidence for an independent association between AF and SDB in patients with HCM.27,37 These data also support the notion that increasing SDB severity relates to a high prevalence of AF in HCM and that SDB is associated with the most important risk factor for AF: increased left atrial size (Fig 3).27 SDB-induced alterations to the autonomic nervous system (including changes in sympathetic-parasympathetic balance and other more complex modulations through the intrinsic and extrinsic cardiac ganglia) likely play a contributory role in this association, and epidemiologic studies exploring these pathophysiologic links are under way.43 Promising evidence from canine studies suggests that radiofrequency ablation of the ganglionated plexi adjacent to the right-side pulmonary veins reduces the susceptibility to AF during acute apneic episodes.44
Figure 3 .
– The presence and severity of SDB is independently associated with a greater prevalence of atrial fibrillation and with an increased LAVI as measured by transthoracic echocardiography. A and B, Analysis according to various cutoffs for ODI. †P value adjusted for age, sex, mitral valve regurgitation, ratio of mitral velocity to early diastolic velocity of the mitral annulus, and left ventricular outflow tract pressure gradient. ‡P value adjusted for age, sex, BMI, hypertension, diabetes mellitus, and history of coronary artery disease. AF = atrial fibrillation; LAVI = left atrial volume index; ODI = oxygen desaturation index. See Figure 1 legend for expansion of other abbreviation. (Adapted with permission from Konecny et al.27)
Ventricular Arrhythmias
Whether the existence of SDB can be related to the occurrence of ventricular arrhythmia or SCD experienced by patients with HCM remains unclear, but termination of recurrent ventricular tachycardias in patients without HCM after initiation of effective SDB treatment has been reported.45
Knowledge Deficits in Comorbid SDB and HCM
Epidemiology of SDB in HCM
Delineation of possible associations among the various types of SDB (obstructive, central, Pickwickian) and cardiovascular pathology (hypertension, extent of hypertrophy, proarrhythmic potential, cardiovascular peak performance) could help identify the optimal screening and treatment strategies in HCM. Even though OSA likely represents by far the most common SDB in HCM, with 75% of patients with HCM and SDB reported to have OSA,10 additional studies are needed to confirm these findings in a larger cohort with reduced selection and referral bias.
The Need for a Randomized Trial of SDB Treatment in HCM
Treatment of sleep apnea, whether by weight loss, postural therapy, mandibular devices, or CPAP, is accompanied by relatively few side effects in otherwise healthy patients, but data on patients with HCM are lacking. How SDB treatment would affect outcomes of patients with HCM needs to be carefully considered and requires experimental validation. On the one hand, there are anecdotal reports of symptomatic improvement in patients started on CPAP, but on the other hand, the positive intrathoracic pressure exerted by CPAP theoretically could result in worsening hemodynamics in some patients. Definitive evidence of an improvement in outcomes elicited by screening and treating SDB in patients with HCM requires a well-designed randomized trial. Powering such a trial would require a relatively large cohort of subjects and, hence, a close coordination of centers specializing in HCM care. Given the potential of SDB treatment to reduce symptoms, improve quality of life and clinical outcomes, and improve the economics of care in patients with HCM, we propose that a coordinated effort leading to a randomized trial be undertaken in the near future.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Somers has served as a consultant for Neu Pro; Respircardia, Inc; Sorin Inc; Price Waterhouse; and ResMed and has received grant support from Philips-Respironics Foundation. Dr Konecny has reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
ABBREVIATIONS
- AF
atrial fibrillation
- HCM
hypertrophic cardiomyopathy
- ICD
implantable cardioverter-defibrillator
- LV
left ventricular
- SCD
sudden cardiac death
- SDB
sleep-disordered breathing
Footnotes
FUNDING/SUPPORT: This study was supported by the Mayo Clinic Foundation; the National Institutes of Health [Grant HL65176]; the European Regional Development Fund [Grant CZ.1.05/1.1.00/02.0123]; and the Internal Grant Agency, Ministry of Health, Czech Republic [Grants NT11401-5/2011 and CZ.1.07/2.3.00/20.0022].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Athens Marathon. Apostolos Greek Tours, Inc, website. http://www.athensmarathon.com/marathon/history.php. Accessed September 1, 2013.
- 2.Gersh BJ, Maron BJ, Bonow RO, et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Practice; American Association for Thoracic Surgery; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Failure Society of America; Heart Rhythm Society; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 2011;142(6):e153-e203 [DOI] [PubMed] [Google Scholar]
- 3.Nishimura RA, Holmes DR., Jr Clinical practice. Hypertrophic obstructive cardiomyopathy. N Engl J Med. 2004;350(13):1320-1327 [DOI] [PubMed] [Google Scholar]
- 4.Frank S, Braunwald E. Idiopathic hypertrophic subaortic stenosis. Clinical analysis of 126 patients with emphasis on the natural history. Circulation. 1968;37(5):759-788 [DOI] [PubMed] [Google Scholar]
- 5.Maron BJ, Bonow RO, Cannon RO, III, Leon MB, Epstein SE. Hypertrophic cardiomyopathy. Interrelations of clinical manifestations, pathophysiology, and therapy (2). N Engl J Med. 1987;316(14):844-852 [DOI] [PubMed] [Google Scholar]
- 6.Maron BJ, Bonow RO, Cannon RO, III, Leon MB, Epstein SE. Hypertrophic cardiomyopathy. Interrelations of clinical manifestations, pathophysiology, and therapy (1). N Engl J Med. 1987;316(13):780-789 [DOI] [PubMed] [Google Scholar]
- 7.Olivotto I, Cecchi F, Casey SA, Dolara A, Traverse JH, Maron BJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation. 2001;104(21):2517-2524 [DOI] [PubMed] [Google Scholar]
- 8.Maron BJ, McKenna WJ, Danielson GK, et al. ; Task Force on Clinical Expert Consensus Documents. American College of Cardiology; Committee for Practice Guidelines. European Society of Cardiology. American College of Cardiology/European Society of Cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol. 2003;42(9):1687-1713 [DOI] [PubMed] [Google Scholar]
- 9.Ludka O, Konecny T, Somers V. Sleep apnea, cardiac arrhythmias, and sudden death. Tex Heart Inst J. 2011;38(4):340-343 [PMC free article] [PubMed] [Google Scholar]
- 10.Berry RB, Budhiraja R, Gottlieb DJ, et al. ; American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597-619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pack AI. Advances in sleep-disordered breathing. Am J Respir Crit Care Med. 2006;173(1):7-15 [DOI] [PubMed] [Google Scholar]
- 12.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667-689 [PubMed] [Google Scholar]
- 13.Gersh BJ, Maron BJ, Bonow RO, et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Failure Society of America; Heart Rhythm Society; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg. 2011;142(6):1303-1338 [DOI] [PubMed] [Google Scholar]
- 14.Sorajja P, Ommen SR, Nishimura RA, Gersh BJ, Berger PB, Tajik AJ. Adverse prognosis of patients with hypertrophic cardiomyopathy who have epicardial coronary artery disease. Circulation. 2003;108(19):2342-2348 [DOI] [PubMed] [Google Scholar]
- 15.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation scientific statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol. 2008;52(8):686-717 [DOI] [PubMed] [Google Scholar]
- 16.Aslam F, Haque A, Foody J, Shirani J. The frequency and functional impact of overlapping hypertension on hypertrophic cardiomyopathy: a single-center experience. J Clin Hypertens (Greenwich). 2010;12(4):240-245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konecny T, Kara T, Somers VK. Obstructive sleep apnea and hypertension: an update. Hypertension. 2014;63(2):203-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbé F, Durán-Cantolla J, Capote F, et al. ; Spanish Sleep and Breathing Group. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181(7):718-726 [DOI] [PubMed] [Google Scholar]
- 19.Montesi SB, Edwards BA, Malhotra A, Bakker JP. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med. 2012;8(5):587-596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chobanian AV, Bakris GL, Black HR, et al. ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572 [DOI] [PubMed] [Google Scholar]
- 21.Verdecchia P, Schillaci G, Borgioni C, et al. Gender, day-night blood pressure changes, and left ventricular mass in essential hypertension. Dippers and peakers. Am J Hypertens. 1995;8(2):193-196 [DOI] [PubMed] [Google Scholar]
- 22.Usui Y, Takata Y, Inoue Y, et al. Severe obstructive sleep apnea impairs left ventricular diastolic function in non-obese men. Sleep Med. 2013;14(2):155-159 [DOI] [PubMed] [Google Scholar]
- 23.Chami HA, Devereux RB, Gottdiener JS, et al. Left ventricular morphology and systolic function in sleep-disordered breathing: the Sleep Heart Health Study. Circulation. 2008;117(20):2599-2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakamura T, Chin K, Hosokawa R, et al. Corrected QT dispersion and cardiac sympathetic function in patients with obstructive sleep apnea-hypopnea syndrome. Chest. 2004;125(6):2107-2114 [DOI] [PubMed] [Google Scholar]
- 25.Kuniyoshi FH, Garcia-Touchard A, Gami AS, et al. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol. 2008;52(5):343-346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konecny T, Kuniyoshi FH, Orban M, et al. Under-diagnosis of sleep apnea in patients after acute myocardial infarction. J Am Coll Cardiol. 2010;56(9):742-743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konecny T, Brady PA, Orban M, et al. Interactions between sleep disordered breathing and atrial fibrillation in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2010;105(11):1597-1602 [DOI] [PubMed] [Google Scholar]
- 28.Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004;110(4):364-367 [DOI] [PubMed] [Google Scholar]
- 29.Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107(20):2589-2594 [DOI] [PubMed] [Google Scholar]
- 30.Fein AS, Shvilkin A, Shah D, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol. 2013;62(4):300-305 [DOI] [PubMed] [Google Scholar]
- 31.Caples SM, Somers VK. Sleep-disordered breathing and atrial fibrillation. Prog Cardiovasc Dis. 2009;51(5):411-415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maron BJ, Semsarian C, Shen WK, et al. Circadian patterns in the occurrence of malignant ventricular tachyarrhythmias triggering defibrillator interventions in patients with hypertrophic cardiomyopathy. Heart Rhythm. 2009;6(5):599-602 [DOI] [PubMed] [Google Scholar]
- 33.Maron BJ, Spirito P, Shen WK, et al. Implantable cardioverter-defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. JAMA. 2007;298(4):405-412 [DOI] [PubMed] [Google Scholar]
- 34.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352(12):1206-1214 [DOI] [PubMed] [Google Scholar]
- 35.Serizawa N, Yumino D, Kajimoto K, et al. Impact of sleep-disordered breathing on life-threatening ventricular arrhythmia in heart failure patients with implantable cardioverter-defibrillator. Am J Cardiol. 2008;102(8):1064-1068 [DOI] [PubMed] [Google Scholar]
- 36.Eleid MF, Konecny T, Orban M, et al. High prevalence of abnormal nocturnal oximetry in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2009;54(19):1805-1809 [DOI] [PubMed] [Google Scholar]
- 37.Pedrosa RP, Drager LF, Genta PR, et al. Obstructive sleep apnea is common and independently associated with atrial fibrillation in patients with hypertrophic cardiomyopathy. Chest. 2010;137(5):1078-1084 [DOI] [PubMed] [Google Scholar]
- 38.Glantz H, Thunström E, Herlitz J, et al. Occurrence and predictors of obstructive sleep apnea in a revascularized coronary artery disease cohort. Ann Am Thorac Soc. 2013;10(4):350-356 [DOI] [PubMed] [Google Scholar]
- 39.Sengupta PP, Sorajja D, Eleid MF, et al. Hypertrophic obstructive cardiomyopathy and sleep-disordered breathing: an unfavorable combination. Nat Clin Pract Cardiovasc Med. 2009;6(1):14-15 [DOI] [PubMed] [Google Scholar]
- 40.Konecny T, Ludka O, Orban M, et al. Worsening sleep disordered breathing is associated with lower peak oxygen consumption in patients with hypertrophic cardiomyopathy [abstract]. Circulation. 2010;122:A14449 [Google Scholar]
- 41.Sharma S, Firoozi S, McKenna WJ. Value of exercise testing in assessing clinical state and prognosis in hypertrophic cardiomyopathy. Cardiol Rev. 2001;9(2):70-76 [DOI] [PubMed] [Google Scholar]
- 42.Pedrosa RP, Lima SG, Drager LF, et al. Sleep quality and quality of life in patients with hypertrophic cardiomyopathy. Cardiology. 2010;117(3):200-206 [DOI] [PubMed] [Google Scholar]
- 43.Konecny T, Covassin N, Park JY, et al. Mechanisms of increased heart rate with sleep disordered breathing in hypertrophic cardiomyopathy [abstract]. Heart Rhythm. 2013;10(5):S456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghias M, Scherlag BJ, Lu Z, et al. The role of ganglionated plexi in apnea-related atrial fibrillation. J Am Coll Cardiol. 2009;54(22):2075-2083 [DOI] [PubMed] [Google Scholar]
- 45.Shimada YJ, Sato K, Hanon S, Schweitzer P. Termination of recurrent ventricular tachycardia by continuous positive airway pressure ventilation. Ann Noninvasive Electrocardiol. 2009;14(4):404-406 [DOI] [PMC free article] [PubMed] [Google Scholar]