Abstract
BACKGROUND:
Approximately 10% of patients with rheumatoid arthritis (RA) have interstitial lung disease (ILD), and one-third have subclinical ILD on chest CT scan. In this study, we aimed to further characterize functional decrements in a spectrum of RA-associated ILD.
METHODS:
All subjects were enrolled in the Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Study (BRASS). The presence of interstitial lung abnormalities (ILAs) on clinically indicated chest CT scans was determined using a previously validated sequential reading method. Univariate and multivariate analyses were used to assess the association between degree of ILAs and physiologic, functional, and demographic variables of interest.
RESULTS:
Of 1,145 BRASS subjects, 91 subjects (8%) were included in this study. Twelve had radiologically severe ILAs, 34 had ILAs, and 38 had no ILAs on CT scan. Subjects with radiologically severe ILAs were older (P = .0037), had increased respiratory symptoms (cough, P = .027; dyspnea, P = .010), and more severe RA disease (rheumatoid factor, P = .018; total swollen joints, P = .046) compared with subjects with no ILAs. Participants also had a trend toward having an increased smoking history (P = .16) and having lower FVC % predicted (77% vs 94%, P = .097) and diffusion capacity of carbon monoxide % predicted (52% vs 77%, P = .068). Similar but attenuated increases in respiratory symptoms, functional decrements, and RA disease severity were observed in subjects with ILAs compared with those with no ILAs.
CONCLUSIONS:
We have shown that patients with RA have varying degrees of ILAs that are associated with a spectrum of functional and physiologic decrements. Our findings suggest that improved risk stratification and detection of ILAs will provide a therapeutic window that could improve RA-ILD outcomes.
Rheumatoid arthritis (RA) is a destructive, systemic, inflammatory disorder1 that currently affects approximately 3 million adults in the United States, or 1% of the population.2,3 A major portion of disease burden and excess mortality is due to extraarticular manifestations,4 such as interstitial lung disease (ILD). Median survival following a diagnosis of RA-associated ILD (RA-ILD) is 2.6 years,5 and the risk of death for individuals with RA-ILD is three times higher than in patients with RA and without ILD. Although studies have demonstrated that overall mortality rates for RA are declining, death from RA-ILD has increased significantly.3 Overall, these statistics highlight the need for strategies that improve risk stratification, detection, and timely interventions for patients with RA and ILD.
Approximately 10% of the RA population has ILD,3 with an additional notably high percentage of subclinical RA-ILD demonstrated on high-resolution CT (HRCT) scans.6 In one study, 33% of patients with RA without dyspnea or cough had interstitial lung abnormalities (ILAs) on chest CT scans.7 Importantly, radiologic progression has been observed in 34% to 57% of subjects with subclinical RA-ILD after a mean follow-up of 1.5 to 2 years,7,8 suggesting that progression of asymptomatic radiologic changes could lead to the development of symptomatic RA-ILD. Taken together, these findings strongly suggest that subclinical RA-ILD is prevalent and that a subset of patients will experience progression of lung disease over time.
Currently, there is limited understanding surrounding the clinical and physiologic characteristics of ILD in the RA population, and even less is known about individuals with subclinical RA-ILD or the natural history of progressive subclinical disease. In this study, we aimed to further characterize clinical characteristics and functional decrements in patients affected with a spectrum of RA-ILD.
Materials and Methods
Study Design
Protocols for participant enrollment and data collection in the Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Study (BRASS) have been described previously.9,10 The BRASS registry is a large, single-center, prospective, observational cohort of 1,145 subjects established in 2003. Patients aged ≥ 18 years with a diagnosis of RA1 were recruited from rheumatology practices. Information was collected on > 1,000 variables including demographics, RA disease activity, and comorbidities. Further details of the BRASS study are available online (www.BRASSstudy.org) and in e-Appendix 1 (415.4KB, pdf) . This project was granted institutional review board approval by the Partners Human Research Committee at the Brigham and Women’s Hospital (protocol number 2010-P-002840/1).
HRCT Scanning Analysis
BRASS subjects with chest CT scans performed for clinical indications prior to 2010 were included in this study; the scan performed in closest temporal proximity with a BRASS visit was chosen for review. All CT scans were evaluated for the presence of ILAs (no ILAs, indeterminate, ILAs, radiologically severe ILAs) using a sequential reading method as previously described (e-Appendix 1 (415.4KB, pdf) ).11,12 No pathology was available for definitive diagnosis.
Pulmonary Function Testing
Pulmonary function tests (PFTs) performed for clinical indications and in closest temporal proximity to a BRASS visit were selected. For consistency, prebronchodilator values were used for the analysis as not all patients had a trial of bronchodilators. Reference values were derived from the third National Health and Nutrition Examination Survey in the United States.13
6-Min Walk Tests
A subset of BRASS subjects had a standardized 6-min walk test performed in accordance with American Thoracic Society guidelines.14 These data were included in the BRASS database and included in our analysis.
Respiratory Questionnaires
At 6-month intervals, each patient received a questionnaire in the mail that included a section on respiratory symptoms. The questionnaire in closest proximity to the HRCT scan was used for this study. The question used for the study was “Please fill in the response next to any of the following symptoms that you have experienced in the PAST MONTH: Chest, Lungs, Heart: Coughing. Shortness of breath.” A filled-in square was considered a positive response.
Statistical Analysis
Univariate analyses were conducted with Fisher exact tests (for binary and categorical variables), and two-tailed t tests or Wilcoxon rank-sum tests (for continuous variables) where appropriate. For multivariate analyses, unadjusted and adjusted linear and logistic regression models were used to assess the strength of the association between RA-ILD and variables of interest. In all models, subjects indeterminate for ILAs (n = 50) were excluded. All analyses were performed using Statistical Analysis Software, version 9.2 (SAS Institute Inc). P values < .05 were considered statistically significant.
Results
Of 1,145 BRASS subjects, 188 subjects (16%) had clinically indicated chest CT scans performed as part of routine care or due to a change in symptoms, such as hypoxia or increased dyspnea (Fig 1). Subjects were excluded if radiologic changes suggesting concurrent illness (ie, pleural effusions or pneumonia) limited the CT scan interpretation (n = 47) (e-Table 1 (415.4KB, pdf) ). The remaining 141 subjects were scored for ILAs, as discussed later in this section. Ten individuals (29%) with subclinical ILD and five individuals (13%) with no evidence of ILAs had CT scans done for chronic respiratory symptoms, including cough, shortness of breath, or dyspnea on exertion. Other clinical indications for CT scans included lung nodule follow-up, cancer staging and follow-up, acute infections, and evaluation for possible vascular or cardiac pathology (e-Table 2 (415.4KB, pdf) ).
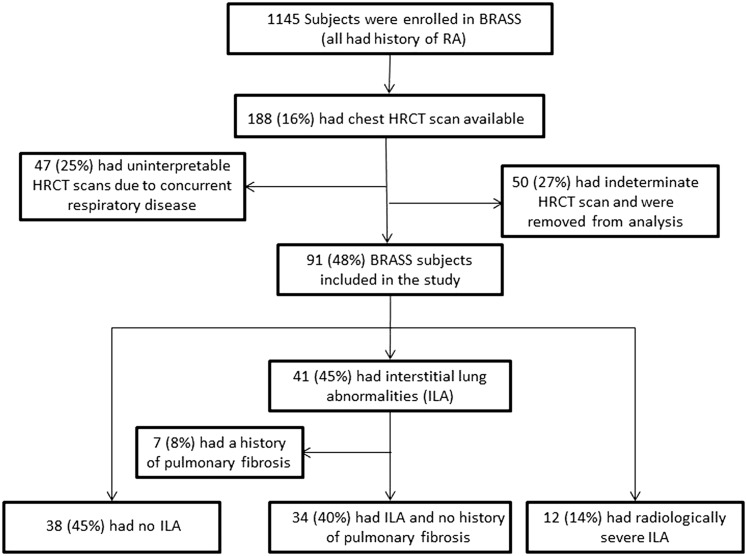
Figure 1 .
– A flow diagram of study enrollment divides participants into three groups according to presence and subtype of subclinical ILAs. BRASS = Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Study; HRCT = high resolution CT; RA = rheumatoid arthritis.
From the 141 RA chest CT scans interpreted, 12 patients (9%) had radiologically severe ILAs, 41 had ILA (29%), and 38 had no evidence of ILAs (26%) (Fig 2). Fifty subjects (35%) had indeterminate CT scans and were excluded from the primary analyses except where indicated. Subjects with ILAs on CT scan who had a documented history of pulmonary fibrosis by their physician (n = 7) were also excluded from the primary analyses. Additional analyses including these seven individuals are given in e-Figure 1 (415.4KB, pdf) and e-Table 3 (415.4KB, pdf) , which include those with ILAs and a history of pulmonary fibrosis in the radiologically severe ILA group, and in e-Figure 2 (415.4KB, pdf) and e-Table 4 (415.4KB, pdf) , which include those with ILAs and a history of pulmonary fibrosis in the ILA group.
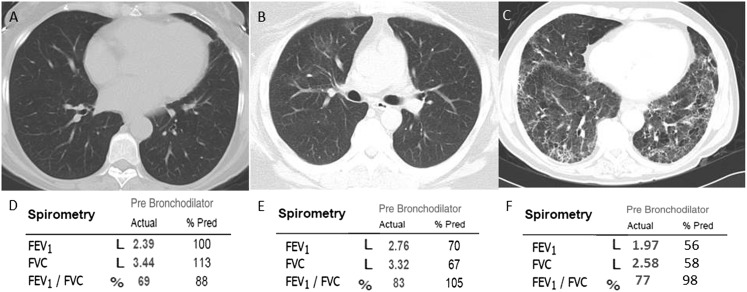
Figure 2 .
– A-C, Chest CT images from three representative subjects. D-F, Pulmonary function test results obtained in three representative individuals. A, D, Images from an individual without evidence of ILAs and normal spirometry. B, E, Images from an individual with evidence of ILAs and spirometry suggestive of a mild restrictive ventilatory defect. C, F, Images from an individual with evidence of radiologically severe ILAs and spirometry suggestive of a moderate restrictive ventilatory defect. % Pred = % predicted. See Figure 1 legend for expansion of other abbreviation.
Characteristics of Subjects With Radiologically Severe ILAs
Characteristics of individuals with radiologically severe ILAs as compared with those without ILAs are presented in Table 1. As expected, individuals with radiologically severe ILAs were older, had higher rheumatoid factor (RF) levels, increased levels of anti-cyclic citrullinated peptide (CCP) antibodies, and greater RA disease severity as measured by the Multi-Dimensional Health Assessment Questionnaire (MDHAQ) score and total swollen joint count. Subjects demonstrated trends toward more likely being male (P = .082) and having an increased smoking history (P = .16). Subjects with radiologically severe ILAs also had a significantly increased incidence of cough, dyspnea, and a trend toward lower FEV1 % predicted (P = .086), FVC % predicted (P = .097), and diffusion capacity of the lung for carbon monoxide (Dlco) % predicted (P = .068). A statistically significant decline in FEV1 % predicted and FVC % predicted was observed with the addition of the seven individuals with ILAs and a history of pulmonary fibrosis (e-Table 3 (415.4KB, pdf) ). Multivariable analyses adjusting for age and smoking history demonstrated attenuated but consistent associations in respiratory symptom and functional variables. Of note, there was no difference between use of tumor necrosis factor-α (TNF-α) inhibitors or methotrexate in subjects with and without radiologically severe ILAs (Table 1).
TABLE 1 .
] Baseline Characteristics of BRASS Subjects Stratified by ILAs, Excluding Subjects Indeterminate for ILAs (n = 50) or Unable to Assess (n = 47)
| Variablea | No ILAs (n = 38, 76%) | Radiologically Severe ILAs (n = 12, 24%) | P Value |
| Demographic parameters | |||
| Age, y | 53 ± 11 | 65 ± 7 | .0037 |
| Female sex | 34 (89) | 8 (67) | .082 |
| White race | 36 (95) | 11 (92) | 1.00 |
| BMI | 28 ± 7 | 27 ± 4 | .92 |
| Pack-y of smoking | 9 ± 17 | 19 ± 22 | .16 |
| Ever smoker | 15 (39) | 7 (58) | .32 |
| Past medical history | |||
| Asthma | 2 (5) | 0 (0) | 1.00 |
| COPD | 0 (0) | 4 (33) | .0021 |
| Bronchiolitis obliterans | 0 (0) | 1 (8) | .24 |
| Bronchiectasis | 0 (0) | 4 (33) | .0021 |
| RA parameters | |||
| RF level | 90 ± 165 | 415 ± 731 | .018 |
| Anti-CCP antibody positive | 16 (43) | 9 (82) | .039 |
| HLA-DR1 shared epitope | 0.74 ± 0.75 | 0.89 ± 0.78 | .62 |
| DAS28-CRP3 score | 3.52 ± 1.49 | 4.36 ± 1.96 | .23 |
| MDHAQ score | 0.59 ± 0.48 | 0.89 ± 0.45 | .041 |
| Total swollen joints | 6 ± 7 | 11 ± 8 | .046 |
| Medication use | |||
| TNF-α inhibitor use | 23 (61) | 10 (83) | .18 |
| Methotrexate use | 30 (79) | 10 (83) | 1.00 |
| Respiratory parameters | |||
| Cough | 8 (21) | 7 (58) | .027 |
| Dyspnea | 8 (21) | 8 (67) | .010 |
| Spirometric parametersb | n = 14 | n = 9 | |
| FEV1 % predicted | 92 ± 18 | 78 ± 18 | .086 |
| FVC % predicted | 94 ± 18 | 77 ± 18 | .097 |
| FEV1/FVC % predicted | 94 ± 11 | 103 ± 10 | .086 |
| n = 8 | n = 6 | ||
| TLC % predicted | 86 ± 16 | 79 ± 32 | .24 |
| n = 8 | n = 7 | ||
| Dlco % predicted | 77 ± 18 | 52 ± 24 | .068 |
| Exercise capacity | n = 5 | n = 3 | |
| 6-min walk distance | 1,715 ± 206 | 1,451 ± 281 | .40 |
| Systolic BP | 118 ± 22 | 113 ± 16 | .89 |
| Heart rate | 76 ± 7 | 81 ± 29 | 1.00 |
| Resting oxygen saturation | 98 ± 2 | 95 ± 3 | .22 |
Data given as mean ± SD or No. (%). BRASS = Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Study; CCP = cyclic citrullinated peptide; DAS28-CRP3 = Disease Activity Score-28-C-reactive protein (three variable); Dlco = diffusion capacity of the lung for carbon monoxide; ILA = interstitial lung abnormality; MDHAQ = Multi-Dimensional Health Assessment Questionnaire; RA = rheumatoid arthritis; RF = rheumatoid factor; TLC = total lung capacity; TNF-α = tumor necrosis factor-α.
Data missing: BMI (n = 2), pack-y of smoking (n = 8), RF level (n = 4), anti-CCP antibody level (n = 2), HLA-DR1 shared epitope (n = 18), DAS28 score (n = 1), MDHAQ score (n = 3), total swollen joints (n = 1).
Prebronchodilator pulmonary function measurements presented.
Characteristics of Subjects With ILAs
Characteristics of individuals with ILAs as compared with no ILAs are presented in Table 2. After excluding subjects with RA who had a history of pulmonary fibrosis, those with ILAs were significantly older, had an increased smoking history, and had higher levels of RF and anti-CCP antibodies. Subjects with ILAs also had an increased incidence of cough and dyspnea, as well as significantly lower FEV1 % predicted and FVC % predicted. Interestingly, the addition of the seven individuals with ILAs and a history of pulmonary fibrosis to the ILA group did not significantly change the results (e-Table 4 (415.4KB, pdf) ). Multivariable analyses adjusting for age and smoking history demonstrated attenuated but consistent associations in respiratory symptom and functional variables. As with subjects with radiographically severe ILAs, there was no difference between use of TNF-α inhibitors or methotrexate in subjects with and without ILAs (Table 2).
TABLE 2 .
] Baseline Characteristics of BRASS Subjects Without a History of Pulmonary Fibrosis, Stratified by ILAs, Excluding Subjects Indeterminate for ILAs (n = 50) or Unable to Assess (n = 47)
| Variablea | No ILAs (n = 38, 53%) | ILAs (n = 34, 47%) | P Value |
| Demographic parameters | |||
| Age, y | 53 ± 11 | 68 ± 10 | < .0001 |
| Female sex | 34 (89) | 27 (79) | .33 |
| White race | 36 (95) | 29 (88) | .41 |
| BMI | 28 ± 7 | 28 ± 5 | .6817 |
| Pack-y of smoking | 9 ± 17 | 23 ± 36 | .031 |
| Ever smoker | 15 (39) | 21 (62) | .098 |
| Past medical history | |||
| Asthma | 2 (5) | 1 (3) | 1.00 |
| COPD | 0 (0) | 2 (6) | .22 |
| Bronchiolitis obliterans | 0 (0) | 1 (3) | .47 |
| Bronchiectasis | 0 (0) | 2 (6) | .22 |
| RA parameters | |||
| RF level | 90 ± 165 | 319 ± 638 | .013 |
| Anti-CCP antibody positive | 16 (43) | 28 (88) | .00049 |
| HLA-DR1 shared epitope | 0.74 ± 0.75 | 0.86 ± 0.83 | .65 |
| DAS28-CRP3 score | 3.52 ± 1.49 | 4.10 ± 1.62 | .16 |
| MDHAQ score | 0.59 ± 0.48 | 0.82 ± 0.55 | .11 |
| Total swollen joints | 6 ± 7 | 7 ± 6 | .21 |
| Medication use | |||
| TNF-α inhibitor use | 23 (61) | 19 (56) | .81 |
| Methotrexate use | 30 (79) | 5 (15) | .55 |
| Respiratory parameters | |||
| Cough | 8 (21) | 15 (44) | .045 |
| Dyspnea | 8 (21) | 16 (47) | .025 |
| Spirometric parametersb | n = 14 | n = 18 | |
| FEV1 % predicted | 92 ± 18 | 69 ± 27 | .0048 |
| FVC % predicted | 94 ± 18 | 75 ± 21 | .0093 |
| FEV1/FVC % predicted | 94 ± 11 | 87 ± 16 | .30 |
| n = 8 | n = 7 | ||
| TLC % predicted | 86 ± 16 | 78 ± 15 | .34 |
| n = 8 | n = 7 | ||
| Dlco % predicted | 77 ± 18 | 69 ± 13 | .40 |
| Exercise capacity | n = 5 | n = 3 | |
| 6-min walk distance | 1,715 ± 206 | 1,382 ± 388 | .40 |
| Systolic BP | 118 ± 22 | 117 ± 22 | 1.00 |
| Heart rate | 76 ± 7 | 74 ± 10 | 1.00 |
| Resting oxygen saturation | 98 ± 2 | 95 ± 6 | .66 |
Data given as mean ± SD or No. (%). See Table 1 legend for expansion of abbreviations.
Data missing: BMI (n = 2), pack-y of smoking (n = 10), race (n = 1), RF level (n = 5), anti-CCP antibody level (n = 3), HLA-DR1 shared epitope (n = 27), DAS28-CRP3 score (n = 13, MDHAQ score (n = 6), total swollen joints (n = 1).
Prebronchodilator pulmonary function measurements presented.
A comparison of subjects in all study groups, including a combined group of ILAs and radiologically severe ILAs, is presented in Table 3. A comparison that includes subjects with indeterminant CT scan abnormalities is presented in e-Table 5 (415.4KB, pdf) . Interestingly, individuals with indeterminant CT scan abnormalities had an intermediate phenotype for some variables (pack-years of smoking, RF and anti-CCP levels, Disease Activity Score-28-C-reactive protein with three variables and MDHAQ scores, and respiratory symptoms) and were similar to ILAs and radiologically severe ILAs for age and most physiologic and functional variables.
TABLE 3 .
] Characteristics of BRASS Subjects Stratified by ILAs, Excluding Subjects Indeterminate (n = 50) or Unable to Assess (n = 47)
| Variable | No ILAs (n = 38) | ILAs (n = 34) | Radiologically Severe ILAs (n = 12) | ILAs and Radiologically Severe ILAs (n = 46) |
| Demographic parameters | ||||
| Age, y | 53 ± 11 | 68 ± 10a | 65 ± 7a | 67 ± 9a |
| Pack-y of smokingb | 9 ± 17 | 23 ± 36a | 19 ± 22 | 22 ± 33a |
| Respiratory parameters | ||||
| Cough | 8 (21) | 15 (44)a | 7 (58)a | 22 (48)a |
| Dyspnea | 8 (21) | 16 (47)a | 8 (67)a | 24 (52)a |
| Spirometric parametersc | n = 14 | n = 18 | n = 9 | n = 27 |
| FEV1 % predicted | 92 ± 18 | 69 ± 27a | 78 ± 18d | 72 ± 24a |
| FVC % predicted | 94 ± 18 | 75 ± 21a | 77 ± 18d | 76 ± 20a |
| n = 8 | n = 7 | n = 6 | n = 13 | |
| TLC % predicted | 86 ± 16 | 78 ± 15 | 79 ± 32 | 78 ± 23 |
| n = 8 | n = 7 | n = 7 | n = 14 | |
| Dlco % predicted | 77 ± 18 | 69 ± 13 | 52 ± 24d | 60 ± 21 |
| Exercise capacity | n = 5 | n = 3 | n = 3 | n = 6 |
| 6-min walk distance | 1,715 ± 206 | 1,382 ± 388 | 1,451 ± 281 | 1,416 ± 305 |
Data given as mean ± SD or No. (%). See Table 1 legend for expansion of abbreviations.
P ≤ .05 when compared with No ILA group.
Data missing: pack-years of smoking (n = 12).
Prebronchodilator pulmonary function measurements presented.
P ≤ .10.
Discussion
Our study demonstrates that patients with RA who also have ILAs or radiologically severe ILAs have a spectrum of disease severity that is associated with smoking, severity of RA, and physiologic and functional decrements of varying degrees. Individuals with ILAs had increased cough and dyspnea, and decreased PFT results and 6-min walk distance (6MWD). Individuals with radiologically severe ILAs had similar but more severe increases in respiratory symptoms, as well as functional decrements of a similar magnitude to ILAs. These results outline the clinical and physiologic characteristics of a spectrum of RA-ILD, and suggest that individuals with ILAs may have more severe functional impairments than previously expected, even when not recognized by either the subject or physician. The unrecognized increase in respiratory symptoms and functional decrements noted in patients with RA and ILAs in our study suggest that this population may have subclinical RA-ILD; the radiologically severe ILA group represents clinically significant RA-ILD, as all these subjects had CT scans done for follow-up of pulmonary fibrosis and almost all indicated they had a previous diagnosis of pulmonary fibrosis on their baseline BRASS physician questionnaire.
Our previous research demonstrated that ILAs are frequently associated with physiologic and functional abnormalities in at-risk populations, such as smokers12,15 and individuals with RA.7 We have shown that smokers with ILAs, even when unrecognized by the patient or physician, may already exhibit increased respiratory symptoms and have reductions in lung volumes,12,16,17 Dlco,7,18‐20 and 6MWD.12,18 We have also demonstrated that individuals with subclinical RA-ILD have impaired gas exchange demonstrated by a lower Dlco % predicted, but PFTs were insensitive methods to detect subclinical RA-ILD.7 In this article, we demonstrate not only that respiratory symptoms, PFT results, and 6MWD may be abnormal in patients with RA and ILAs, but that some of these decrements are of a similar magnitude to those seen in RA-ILD. Taken together, these findings suggest that some patients with RA and ILAs may already have increased respiratory symptoms, physiologic decrements, and a reduced exercise capacity. This, coupled with the expectation that a subset of these individuals with subclinical disease may progress to established disease,7 demonstrates a spectrum of RA-ILD and may lead to an improved understanding of the natural history of RA-ILD.
In general, physiologic and functional decrements have not been well characterized in the RA-ILD population, although some small retrospective studies have shown that RA-ILD may be associated with a restrictive deficit and decreased Dlco.21,22 It has been suggested that RA-ILD may be compared with idiopathic pulmonary fibrosis (IPF), as they have similar radiologic and pathologic findings.22‐27 It has been well established that IPF is more prevalent in older men with a history of smoking, which is similar to the demographics of individuals in our study with RA-ILD. IPF is also associated with increased dyspnea and decreased PFT and 6MWD results, which is consistent with our findings.28 This study further suggests that ILD in RA has a similar physiologic profile to IPF and, importantly, begins to define the magnitude of the physiologic and functional decrements specifically associated with RA-ILD.
Smoking has been shown to be a risk factor for fibrotic lung diseases in general,24,29‐32 as well as RA-ILD.7,33 It has also been demonstrated that a significantly higher percentage of subjects with subclinical ILD are current or former smokers.17,18,34‐36 The high prevalence of ILD in smokers with RA suggests that these individuals are now not only at an increased risk of cardiovascular disease and cancer but also the morbidity and mortality that accompanies RA-ILD. Approximately 50% of BRASS subjects are ever smokers, with a higher percentage of those individuals having ILD. In addition, smoking promotes citrullination in the lungs, which leads to generation of CCP antibodies, possibly contributing to the development of lung abnormalities early in the rheumatoid process,37 especially in individuals with the HLA-DRB1 shared epitope.38 Our data demonstrate a significantly higher percentage of anti-CCP antibodies in both ILAs and RA-ILD. This information underlines the importance of smoking cessation as a key component of the management of these individuals. This may be particularly true for those with ILAs, as smoking cessation may help to prevent or slow the progression to RA-ILD.
In addition to anti-CCP antibodies,39 high-titer RF40 and severity of joint disease5 have been associated with a higher incidence of RA-ILD and may be helpful in risk stratifying individuals who should be screened for ILD. Our study supports these findings by demonstrating that increasing levels of RF, MDHAQ scores, and total swollen joint counts are associated with increasing severity of ILD. This also highlights the fact that some patients with RA may not have sufficient joint functionality to develop respiratory symptoms, and, thus, the diagnosis of ILD may be missed. This finding may suggest that there is a subset of patients with RA and with severely limiting joint disease who would benefit from screening for ILD with PFTs or HRCT scans.
Taken together, our findings suggest that there should be a high suspicion of ILD in any patient with RA who has respiratory symptoms or functional decrements, especially if the patient is a smoker with more severe RA. In the future there may be a role for the risk stratification of asymptomatic patients with RA for ILAs,6 as it may represent the early stages of RA-ILD and could lead to closer monitoring and earlier treatment. Additional, larger, prospective studies are necessary to replicate and expand on these preliminary findings, with particular attention dedicated to the role of earlier identification of ILD in the RA population. Longitudinal studies could help explore the natural history of progressive subclinical disease and how early interventions, such as smoking cessation, will promote secondary prevention of RA-ILD, potentially leading to improved clinical outcomes.
Our study has several limitations. First, it was unclear if individuals in the ILA group who had a previous diagnosis of pulmonary fibrosis (n = 7) were truly representative of subclinical disease or if these individuals had more clinically significant disease. As such, we excluded these individuals from the primary analyses, although additional analyses including these individuals are included in the supplement. Second, there is significant bias in patient selection, as all subjects had clinical indications for CT scans, although many of those indications were unrelated to the diagnosis of ILD (ie, lung nodule or chest pain). Third, within an individual subject, the CT scan, PFT, and 6MWD data may have been separated by considerable variations in time, up to 6.6 years (e-Appendix 1 (415.4KB, pdf) ). Since RA-ILD is a progressive disease, it is hard to correlate functional data that are not temporally close to the CT scan. This important limitation should be addressed in subsequent, prospective studies. Fourth, not every subject included in this study who had a CT scan had PFT and 6MWD data available, adding another source of bias and limiting the generalizability of the results. Last, the limited number of subjects with data available for this study hindered our ability to perform multivariate analyses adjusting for such confounders as RA disease severity, comorbidities, and medication-induced lung changes.
Conclusions
In our study, subjects with ILAs and RA-ILD were older, reported an increased smoking history, had increased RA disease severity, and had a spectrum of functional and physiologic decrements. Additional prospective and longitudinal follow-up studies will be important to verify these abnormalities, to evaluate if the decrements present in subclinical disease are progressive, and to determine the best way to screen for RA-ILD in an outpatient clinical setting.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: T. J. D., M. L. F., C. K. I., and I. O. R. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. T. J. D. served as principal author. T. J. D., P. F. D., and K. B. contributed to data acquisition; T. J. D. contributed to data analysis and interpretation; H. H., M. N., M. E. W., D. P. A., G. R. W., G. M. H., A. M. K. C., N. A. S., and I. O. R. contributed to the statistical analysis and interpretation of the clinical data; M. L. F. and C. K. I. contributed to administrative, technical, or material support; T. J. D., M. E. W., D. P. A., G. M. H., A. M. K. C., N. A. S., and I. O. R. contributed to the drafting of the manuscript; and T. J. D., P. F. D., K. B., M. L. F., C. K. I., H. H., M. N., M. E. W., D. P. A., G. R. W., G. M. H., A. M. K. C., N. A. S., and I. O. R. contributed to the revision of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Weinblatt has received consulting fees and grant support from Bristol-Myers Squibb Co, MedImmune LLC, and Crescendo Bioscience Inc, and has received consulting fees from Stromedix/Biogen, Synovex Corp, and Sanofi SA. Dr Shadick receives research grant funding from Crescendo Biosciences Inc, MedImmune LLC, Bristol-Myers Squibb Co, Amgen Inc, Genentech Inc, and Abbott Laboratories, and has received consulting fees from Stromedix/Biogen, Synovex Corp, and Sanofi SA. Dr Rosas has received consulting fees from Stromedix/Biogen, Synovex Corp, and Sanofi SA. The remaining authors have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: This work was performed at Brigham and Women’s Hospital, Boston, MA.
Additional information: The e-Appendix, e-Figures, and e-Tables can be found in the Supplemental Materials section of the online article.
ABBREVIATIONS
- 6MWD
6-min walk distance
- BRASS
Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Study
- CCP
cyclic citrullinated peptide
- Dlco
diffusion capacity of the lung for carbon monoxide
- HRCT
high-resolution CT
- ILA
interstitial lung abnormality
- ILD
interstitial lung disease
- IPF
idiopathic pulmonary fibrosis
- MDHAQ
Multidimensional Health Assessment Questionnaire
- PFT
pulmonary function test
- RA
rheumatoid arthritis
- RA-ILD
rheumatoid arthritis-associated interstitial lung disease
- RF
rheumatoid factor
- TNF-α
tumor necrosis factor-α
Footnotes
FOR EDITORIAL COMMENT SEE PAGE 8
FUNDING/SUPPORT: Dr Doyle is supported by the KL2/Catalyst MeRIT Program [Grant 8KL2TR000168-05]. Drs Nishino, Hunninghake, and Rosas are supported by the US National Institutes of Health (NIH) [Grant K23 CA157631 (National Cancer Institute) to Dr Nishino, Grants K08 HL092222 and R01 HL111024 to Dr Hunninghake, and Grant K23 HL087030 to Dr Rosas]. The Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Study is currently sponsored by Crescendo Bioscience Inc, MedImmune LLC, and Bristol-Myers Squibb Co.
Parts of this article have been presented or published in abstract form (17th International Colloquium on Lung & Airway Fibrosis, October 1, 2012, Modena, Italy, and Doyle TJ, Batra K, Frits ML, et al. Am J Resp Crit Care Med. 2013;187[1_MeetingAbstracts]:A22).
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315-324 [DOI] [PubMed] [Google Scholar]
- 2.Brown KK. Rheumatoid lung disease. Proc Am Thorac Soc. 2007;4(5):443-448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olson AL, Swigris JJ, Sprunger DB, et al. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med. 2011;183(3):372-378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turesson C, O’Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Extra-articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Ann Rheum Dis. 2003;62(8):722-727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010;62(6):1583-1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doyle TJ, Hunninghake GM, Rosas IO. Subclinical interstitial lung disease: why you should care. Am J Respir Crit Care Med. 2012;185(11):1147-1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gochuico BR, Avila NA, Chow CK, et al. Progressive preclinical interstitial lung disease in rheumatoid arthritis. Arch Intern Med. 2008;168(2):159-166 [DOI] [PubMed] [Google Scholar]
- 8.Dawson JK, Fewins HE, Desmond J, Lynch MP, Graham DR. Predictors of progression of HRCT diagnosed fibrosing alveolitis in patients with rheumatoid arthritis. Ann Rheum Dis. 2002;61(6):517-521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shadick NA, Heller JE, Weinblatt ME, et al. Opposing effects of the D70 mutation and the shared epitope in HLA-DR4 on disease activity and certain disease phenotypes in rheumatoid arthritis. Ann Rheum Dis. 2007;66(11):1497-1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iannaccone CK, Lee YC, Cui J, et al. Using genetic and clinical data to understand response to disease-modifying anti-rheumatic drug therapy: data from the Brigham and Women’s Hospital Rheumatoid Arthritis Sequential Study. Rheumatology (Oxford). 2011;50(1):40-46 [DOI] [PubMed] [Google Scholar]
- 11.Washko GR, Lynch DA, Matsuoka S, et al. Identification of early interstitial lung disease in smokers from the COPDGene Study. Acad Radiol. 2010;17(1):48-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Washko GR, Hunninghake GM, Fernandez IE, et al. ; COPDGene Investigators. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364(10):897-906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159(1):179-187 [DOI] [PubMed] [Google Scholar]
- 14.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111-117 [DOI] [PubMed] [Google Scholar]
- 15.Doyle TJ, Washko GR, Fernandez IE, et al. ; COPDGene Investigators. Interstitial lung abnormalities and reduced exercise capacity. Am J Respir Crit Care Med. 2012;185(7):756-762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mannino DM, Holguin F, Pavlin BI, Ferdinands JM. Risk factors for prevalence of and mortality related to restriction on spirometry: findings from the First National Health and Nutrition Examination Survey and follow-up. Int J Tuberc Lung Dis. 2005;9(6):613-621 [PubMed] [Google Scholar]
- 17.Lederer DJ, Enright PL, Kawut SM, et al. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study. Am J Respir Crit Care Med. 2009;180(5):407-414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosas IO, Ren P, Avila NA, et al. Early interstitial lung disease in familial pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176(7):698-705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz de Leon A, Cronkhite JT, Yilmaz C, et al. Subclinical lung disease, macrocytosis, and premature graying in kindreds with telomerase (TERT) mutations. Chest. 2011;140(3):753-763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kashiwabara K. Characteristics and disease activity of early interstitial lung disease in subjects with true parenchymal abnormalities in the posterior subpleural aspect of the lung. Chest. 2006;129(2):402-406 [DOI] [PubMed] [Google Scholar]
- 21.Dawson JK, Fewins HE, Desmond J, Lynch MP, Graham DR. Fibrosing alveolitis in patients with rheumatoid arthritis as assessed by high resolution computed tomography, chest radiography, and pulmonary function tests. Thorax. 2001;56(8):622-627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HK, Kim DS, Yoo B, et al. Histopathologic pattern and clinical features of rheumatoid arthritis-associated interstitial lung disease. Chest. 2005;127(6):2019-2027 [DOI] [PubMed] [Google Scholar]
- 23.Akira M, Sakatani M, Hara H. Thin-section CT findings in rheumatoid arthritis-associated lung disease: CT patterns and their courses. J Comput Assist Tomogr. 1999;23(6):941-948 [DOI] [PubMed] [Google Scholar]
- 24.Raghu G, Collard HR, Egan JJ, et al. ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788-824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American Thoracic Society; European Respiratory Society International. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277-304 [DOI] [PubMed] [Google Scholar]
- 26.Kim EJ, Collard HR, King TE., Jr Rheumatoid arthritis-associated interstitial lung disease: the relevance of histopathologic and radiographic pattern. Chest. 2009;136(5):1397-1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim EJ, Elicker BM, Maldonado F, et al. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J. 2010;35(6):1322-1328 [DOI] [PubMed] [Google Scholar]
- 28.Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183(4):431-440 [DOI] [PubMed] [Google Scholar]
- 29.Antoniou KM, Hansell DM, Rubens MB, et al. Idiopathic pulmonary fibrosis: outcome in relation to smoking status. Am J Respir Crit Care Med. 2008;177(2):190-194 [DOI] [PubMed] [Google Scholar]
- 30.Vassallo R, Ryu JH. Tobacco smoke-related diffuse lung diseases. Semin Respir Crit Care Med. 2008;29(6):643-650 [DOI] [PubMed] [Google Scholar]
- 31.Patel RR, Ryu JH, Vassallo R. Cigarette smoking and diffuse lung disease. Drugs. 2008;68(11):1511-1527 [DOI] [PubMed] [Google Scholar]
- 32.Baumgartner KB, Samet JM, Stidley CA, Colby TV, Waldron JA. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1997;155(1):242-248 [DOI] [PubMed] [Google Scholar]
- 33.Saag KG, Kolluri S, Koehnke RK, et al. Rheumatoid arthritis lung disease. Determinants of radiographic and physiologic abnormalities. Arthritis Rheum. 1996;39(10):1711-1719 [DOI] [PubMed] [Google Scholar]
- 34.King TE., Jr Smoking and subclinical interstitial lung disease. N Engl J Med. 2011;364(10):968-970 [DOI] [PubMed] [Google Scholar]
- 35.Sverzellati N, Guerci L, Randi G, et al. Interstitial lung diseases in a lung cancer screening trial. Eur Respir J. 2011;38(2):392-400 [DOI] [PubMed] [Google Scholar]
- 36.Flaherty KR, Hunninghake GG. Smoking: an injury with many lung manifestations. Am J Respir Crit Care Med. 2005;172(9):1070-1071 [DOI] [PubMed] [Google Scholar]
- 37.Malik S, Saravanan V, Kelly C. Interstitial lung disease in rheumatoid arthritis: an update on diagnosis and management. Int J Clin Rheumatol. 2012;7(3):297-308 [Google Scholar]
- 38.Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54(1):38-46 [DOI] [PubMed] [Google Scholar]
- 39.Aubart F, Crestani B, Nicaise-Roland P, et al. High levels of anti-cyclic citrullinated peptide autoantibodies are associated with co-occurrence of pulmonary diseases with rheumatoid arthritis. J Rheumatol. 2011;38(6):979-982 [DOI] [PubMed] [Google Scholar]
- 40.Luukkainen R, Saltyshev M, Pakkasela R, Nordqvist E, Huhtala H, Hakala M. Relationship of rheumatoid factor to lung diffusion capacity in smoking and non-smoking patients with rheumatoid arthritis. Scand J Rheumatol. 1995;24(2):119-120 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement