Abstract
BACKGROUND:
OSA is highly prevalent in children and usually initially treated by adenotonsillectomy. Nonsurgical alternatives for mild OSA primarily consisting of antiinflammatory approaches have emerged, but their efficacy has not been extensively assessed.
METHODS:
A retrospective review of clinically and polysomnographically diagnosed patients with OSA treated between 2007 and 2012 was performed to identify otherwise healthy children ages 2 to 14 years who fulfilled the criteria for mild OSA and who were treated with a combination of intranasal corticosteroid and oral montelukast (OM) for 12 weeks (ICS + OM). A subset of children continued OM treatment for 6 to 12 months.
RESULTS:
A total of 3,071 children were diagnosed with OSA, of whom 836 fulfilled mild OSA criteria and 752 received ICS + OM. Overall, beneficial effects occurred in > 80% of the children, with nonadherence being documented in 61 children and adenotonsillectomy being ultimately performed in 12.3%. Follow-up polysomnography in a subset of 445 patients showed normalization of sleep findings in 62%, while 17.1% showed either no improvement or worsening of their OSA. Among the latter, older children (aged > 7 years; OR, 2.3; 95% CI, 1.43-4.13; P < .001) and obese children (BMI z-score > 1.65; OR: 6.3; 95% CI, 4.23-11.18; P < .000001) were significantly more likely to be nonresponders.
CONCLUSIONS:
A combination of ICS + OM as initial treatment of mild OSA appears to provide an effective alternative to adenotonsillectomy, particularly in younger and nonobese children. These results support implementation of multicenter randomized trials to more definitively establish the role of ICS + OM treatment in pediatric OSA.
Since the initial description of OSA, this condition has emerged as being highly prevalent in children and as imposing potentially reversible neurocognitive, behavioral, cardiovascular, and metabolic morbidities.1 Adenotonsillar hypertrophy has been recognized as the major pathophysiologic contributor to OSA in children and has been customarily managed by surgical removal of enlarged adenoids and tonsils with overall favorable results reported for moderate to severe OSA.1‐3 More recently, however, surgical adenotonsillectomy (T&A) for mild OSA has come under scrutiny,1‐3 particularly regarding the possibility that a significant proportion of the polysomnographic abnormalities associated with milder forms of OSA may not normalize after surgery, thereby prompting the need for development of nonsurgical therapeutic alternatives.4 Based on such initial reports, preliminary evidence on the potential beneficial effects of oral montelukast (OM) and intranasal corticosteroids (ICSs) on improving breathing patterns during sleep in pediatric cases of mild OSA has emerged.5‐15 Furthermore, the biologic plausibility of the potential efficacy of these approaches has been substantiated,16,17 raising the possibility that randomized controlled trials (RCTs) using antiinflammatory approaches would be justified for pediatric OSA. However, the effects of combined topical steroid and montelukast in mild OSA have not yet been explored.
Here we report on the retrospective assessment of our clinical experience in a large cohort of patients diagnosed with mild OSA with polysomnography who were treated with a combination of ICS + OM for ≥ 12 weeks, followed by either no further treatment or by continued OM therapy for an additional 6 to 12 months.
Materials and Methods
Patients
This retrospective review study of our clinical experience was approved by the institutional human study review committees of the University of Louisville (protocol number 474.99) and the University of Chicago (protocol numbers 09-008-A and 10-615-A). The population for the study was identified by screening charts from the Sleep Center medical records at Kosair Children’s Hospital in Louisville, Kentucky, for the time period from January 2007 until December 2008; St. Mary Women and Children’s Hospital, Evansville, Indiana, from January 2007 until December 2012; and Comer Children’s Hospital at the University of Chicago, Chicago, Illinois, from January 2011 until December 2012. The charts of children aged 2 to 14 years who were referred by their primary care pediatricians or pediatric otolaryngologists and underwent overnight sleep studies for suspected OSA were identified. Exclusion criteria were as follows: past T&A, genetic disorders, neuromuscular diseases, craniofacial abnormalities, or current treatment with medications such as corticosteroids (either oral, inhaled, or intranasal) or OM.
The period covered by this retrospective review corresponded to the implementation of a standard clinical management protocol whereby children with OSA and obstructive apnea-hypopnea index (AHI) > 5.0/h of total sleep time (TST) were referred for surgical T&A or occasionally for CPAP therapy, while those with obstructive AHI > 1.0/h TST but < 5.0/h TST were initially recommended treatment with ICS + OM, following which a second overnight sleep study was performed to assess clinical response to therapy. Children with an obstructive AHI < 1.0/h TST were considered to have primary snoring and did not receive treatment.
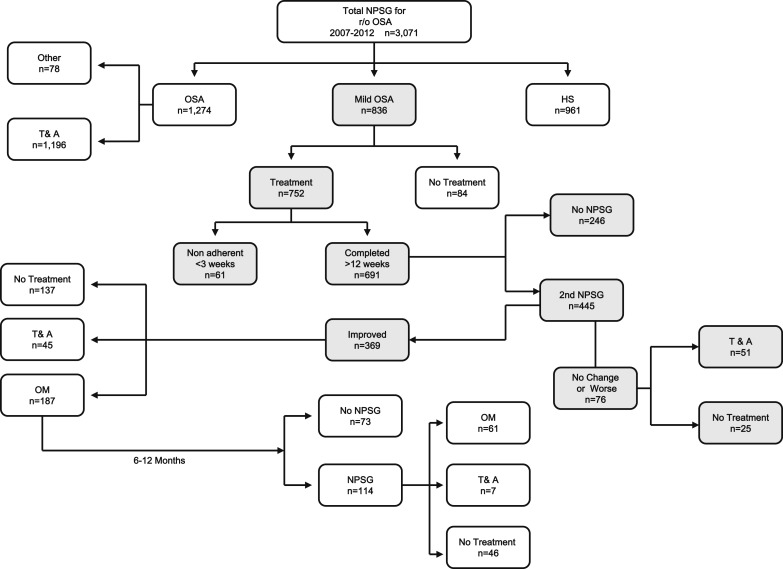
For children receiving ICS + OM, nonadherence was considered to be present if they received < 3 weeks of any of the two medications as indicated by the parents or based on the absence of prescription refills. Otherwise, if the second nocturnal polysomnography (NPSG) documented improvement, OM was usually continued for up to 12 months. If no changes or worsening of the NPSG results occurred, then T&A was recommended. A third NPSG was conducted after 6 to 12 months of OM, and based on the findings (ie, worsening OSA, persistent mild OSA, or normal NPSG results), T&A, OM, or no treatment were recommended, respectively (Fig 1).
Figure 1 .
– Schematic diagram of the overall clinical experience in treating 3,071 otherwise healthy children referred for evaluation of habitual snoring and suspected OSA. HS = habitual snoring; NPSG = nocturnal polysomnography; OM = oral montelukast; r/o = rule out; T&A = adenotonsillectomy.
In addition to demographic information including age, sex, and ethnicity, height and weight were extracted from all the charts. Tonsil size derived from a score of 0 (no tonsils present) to 4 (kissing tonsils),18 Mallampati score (Likert scale range, 1-4),19 and adenoid size as estimated from lateral neck radiographs based on the degree of choanal obstruction on a Likert scale range, 1 to 4 (4: 75% to 100%; 3: 50% to 75%; 2: 25% to 50%; and 1: 0% to 25%) were tabulated when available, as previously described.20,21
BMI z-Score Calculation
Height and weight were recorded when each child arrived for NPSG. BMI z-score was calculated using an online BMI z-score calculator provided by the US Centers for Disease Control and Prevention.22 Children with BMI z-score values > 1.67 were considered obese.23
Overnight Sleep Study
An NPSG was performed in the laboratory in the presence of a trained polysomnographic technologist at each sleep center using the computerized clinical-data-acquisition system in use at that site. Briefly, the bilateral electrooculogram, eight channels of EEG, chin and anterior tibial electromyograms, tracheal sounds, and analog output from a body position sensor were monitored, along with chest and abdominal wall movement, ECG, and airflow using nasal pressure catheter, end-tidal capnography, and an oronasal thermistor. Arterial oxygen saturation (Spo2) was assessed by pulse oximetry with simultaneous recording of the pulse waveform. In addition, a digital time-synchronized video recording was performed.
After removal of movement and technical artifacts, the studies were scored according to standard criteria as defined by the American Academy of Sleep Medicine in 2007, with all scoring technologists being supervised by one of the authors to ensure consistency across centers.24 The proportion of time spent in each sleep stage was expressed as percentage of TST (%TST). Central, obstructive, and mixed apneic events were counted, and hypopneas were assessed. Obstructive apnea was defined as the absence of airflow with continued chest wall and abdominal movement for duration of at least two breaths. Hypopneas were defined as a decrease in oronasal flow of > 50% on either the thermistor or nasal pressure transducer signal with a corresponding decrease in Spo2 of > 3% or arousal. The obstructive AHI was defined as the number of apneas and hypopneas per hour of TST, and an AHI < 1/h TST was considered within normal limits.25
Data Analysis
Data are presented as mean ± SD unless stated otherwise. Data were assessed for kurtosis and confirmed as being normally distributed. Statistical analyses were conducted using SPSS, version 18.0 (IBM). A P value < .05 was considered to achieve statistical significance.
Results
A total of 3,071 otherwise healthy children between the ages of 2 to 14 years were referred for evaluation of habitual snoring and suspected OSA and underwent a diagnostic NPSG. Table 1 shows the demographic, anthropometric, and polysomnographic characteristics of these children based on their final diagnosis—namely, moderate to severe OSA, mild OSA, and habitual primary snoring. There were no significant differences in any of the demographic characteristics of the three groups or in the overall proportion of obesity across the groups. There were, however, small, albeit significantly higher, Mallampati scores in the children with more severe OSA when compared with the other two groups (P < .001). Similarly, the obstructive AHI and arousal indexes were increased in moderate to severe OSA, and lower nadir Spo2 was also apparent compared with the other two groups. Mild OSA also differed from primary snoring in these polysomnographic measures (Table 1).
TABLE 1 .
] Demographic and Polysomnographic Characteristics of 3,071 Children Referred for Evaluation of Habitual Snoring
| Characteristic | Moderate to Severe OSA (n = 1,274) | Mild OSA (n = 836) | Primary Snorers (n = 961) |
| Age, y | 6.1 ± 1.3 | 6.4 ± 1.7 | 6.3 ± 1.9 |
| Male sex, % | 52.0 | 53.5 | 51.7 |
| White, % | 56.2 | 55.7 | 54.4 |
| Black, % | 27.7 | 26.3 | 25.8 |
| BMI z-score | 1.12 ± 0.76 | 1.17 ± 0.81 | 1.09 ± 0.87 |
| Obese (BMI z-score > 1.65), % | 37.3 | 38.2 | 34.8 |
| Tonsillar size | 2.37 ± 0.73 | 2.41 ± 0.82 | 2.29 ± 0.83 |
| Adenoid size | 2.3 ± 0.67 | 2.14 ± 0.71 | 2.13 ± 0.68 |
| Mallampati score (n) | 2.29 ± 0.54a,b (1,076) | 1.87 ± 0.52a (789) | 1.89 ± 0.58b (836) |
| TST, min | 469.5 ± 47.7 | 472.3 ± 47.8 | 464.1 ± 49.1 |
| Stage N1, % | 7.4 ± 2.8a,b | 4.4 ± 3.2a | 4.6 ± 3.4b |
| Stage N2, % | 38.1 ± 6.6 | 39.9 ± 7.5 | 39.2 ± 7.3 |
| Stage N3, % | 38.6 ± 14.1 | 41.4 ± 12.6 | 42.9 ± 12.3 |
| REM sleep, % | 19.3 ± 6.4 | 21.5 ± 7.8 | 26.7 ± 9.6c |
| Sleep latency, min | 22.6 ± 14.3b | 24.2 ± 15.2d | 28.7 ± 11.8 b,d |
| REM latency, min | 128.9 ± 51.0a,b | 137.9 ± 65.1a | 138.4 ± 55.2b |
| Total arousal index, events/h TSTe | 19.1 ± 7.8a | 14.7 ± 7.1a,d | 10.5 ± 5.7a,d |
| Respiratory arousal index, events/h TSTe | 5.9 ± 2.2a,d | 2.8 ± 1.3a,d | 0.6 ± 0.3a,d |
| Obstructive AHI, events/h TSTe | 13.5 ± 5.6a,d | 4.4 ± 0.1.9a,d | 0.7 ± 0.2a,d |
| Spo2 nadir, % | 82.6 ± 6.8a,d | 87.3 ± 2.5a,d | 94.8 ± 1.2a,d |
Data given as mean ± SD unless otherwise indicated. AHI = apnea-hypopnea index; REM = rapid eye movement; Spo2 = arterial oxygen saturation; TST = total sleep time.
OSA vs mild OSA or primary snorer: P < .01.
OSA vs primary snorer: P < .01.
Primary snorer vs OSA or mild OSA: P < .05.
Mild OSA vs OSA or primary snorer: P < .01.
Time spent in the sleep state during the nocturnal polysomnography.
Of the 836 children with mild OSA, 84 parents (10%) refused ICS + OM treatment and sought alternative treatments, primarily consisting of surgical T&A (n = 72, 8.4%). Thus, 752 children began ICS + OM treatment, with 61 of these children (8.1%) discontinuing the treatment within the first 3 weeks or not adhering to the treatment as reported by parents. In the majority of these children (n = 52), parents decided to pursue T&A despite initiating the therapy but not adhering to it. However, six patients (0.7%) reported side effects that prompted them to discontinue therapy (three with headaches, one with nausea and vomiting, and two with epistaxis). Of the 691 children who presumably completed the 12-week treatment course, only 445 children (64.4%) returned for their follow-up NPSG. The changes in the sleep study between diagnosis and following ICS + OM treatment are shown in Table 2. Overall, significant improvements occurred with ICS + OM treatment in the magnitude of respiratory disturbances during sleep. More importantly, 62% of these 445 children exhibited normalization of their sleep studies (ie, they had an obstructive AHI < 1/h TST after completion of ICS + OM treatment). However, 17.1% (n = 76) showed either no improvement or worsening of their OSA. Table 3 shows the potential differences between responders who normalized breathing patterns during sleep after ICS + OM treatment and nonresponders. In general, no differences were apparent in either the sex, ethnicity, or sleep study measures between responders and nonresponders before initiation of ICS + OM treatment. However, younger children (ie, < 7 years of age) were 2.3 times more likely to normalize their sleep studies after ICS + OM treatment than obese children (95% CI, 1.43-4.13; P < .001), and nonobese children were 6.3 times more likely to normalize their sleep studies after ICS + OM treatment than obese children (BMI z-score > 1.65; 95% CI, 4.23-11.18; P < .000001). It is also worth mentioning that among the 396 patients in whom either improvements or normalization of the sleep study occurred with ICS + OM treatment, a subset of 45 patients (11.4%) opted to undergo T&A, while in 137 children (34.6%), no further treatment was prescribed (Fig 1). In the remaining 187 children (47.2%), OM was continued for 6 to 12 months as consolidation therapy or with the intent to prevent recurrence of OSA, with such recommendation being consistently provided to parents who opted to either continue therapy or not. A third NPSG was obtained in 114 of these children (61%), with complete resolution of OSA being documented in 46 children (49.1%), persistently mild OSA being present in 61 children who elected to continue OM treatment (53.5%), and unchanged or worsening of OSA severity in seven children (6.2%) prompting surgical T&A. Thus, of the original cohort with mild OSA, a total of 175 children (20.9%) underwent T&A.
TABLE 2 .
] Changes in Polysomnographic Findings Following 12-Wk Treatment With an Intranasal Corticosteroid and Oral Montelukast in 445 Children
| Characteristic | Mild OSA Pretreatment (n = 445) | Mild OSA Posttreament (n = 445) | P Value |
| Age, y | 6.2 ± 1.9 | 6.6 ± 1.9 | … |
| Male sex, % | 55.1 | … | … |
| White, % | 56.5 | … | … |
| Black, % | 26.8 | … | … |
| BMI z-score | 1.17 ± 0.81 | … | … |
| Obese (BMI z-score > 1.65), % | 33.8 | … | … |
| Elapsed time between beginning treatmenta and second NPSG, mean, d | … | 114.8 ± 39.2 | … |
| Tonsillar size | 2.39 ± 0.77 | 1.87 ± 0.62 | < .01 |
| Adenoid size | 2.17 ± 0.77 | 1.34 ± 0.68 | < .001 |
| Mallampati score (n) | 1.89 ± 0.62 (412) | 1.83 ± 0.64 (412) | … |
| Total sleep duration, min | 472.1 ± 51.2 | 470.9 ± 49.1 | … |
| Stage 1, % | 4.7 ± 3.1 | 4.2 ± 3.4 | … |
| Stage 2, % | 37.8 ± 8.3 | 29.3 ± 9.7 | … |
| Stage 3, % | 40.6 ± 16.2 | 41.2 ± 15.8 | … |
| REM sleep, % | 19.3 ± 6.4 | 27.5 ± 7.8 | < .01 |
| Sleep latency, min | 24.7 ± 16.1 | 27.9 ± 17.2 | … |
| REM latency, min | 138.1 ± 54.7 | 135.3 ± 62.9 | … |
| Total arousal index, events/h TST | 15.1 ± 9.3 | 12.2 ± 8.7 | < .01 |
| Respiratory arousal index, events/h TST | 2.9 ± 1.7 | 0.8 ± 1.5 | < .001 |
| Obstructive AHI, events/h TST | 4.5 ± 2.0 | 1.4 ± 0.0.9 | < .01 |
| Spo2 nadir, % | 87.5 ± 3.1 | 92.3 ± 2.1 | < .001 |
| Patients with normal NPSG, No. (%) | … | 276 (62.0) | … |
Data given as mean ± SD unless otherwise indicated. NPSG = nocturnal polysomnography. See Table 1 legend for expansion of other abbreviations.
Intranasal corticosteroids plus oral montelukast for 12 wk.
TABLE 3 .
] Demographic, Anthropometric, and Polysomnographic Characteristics of Children Who Were “Cured” and “Nonresponders” After Treatment With Intranasal Corticosteroids and Oral Montelukast for 12 Wk
| Characteristic | “Cured” AHI < 1/h TST (n = 276) | Nonresponders AHI > 5/h TST (n = 76) | P Value |
| Age, y | 4.9 ± 2.1 | 8.1 ± 2.6 | < .0001 |
| Male sex, % | 54.3 | 53.9 | … |
| White, % | 54.3 | 56.5 | … |
| Black, % | 27.1 | 27.6 | … |
| BMI z-score | 1.01 ± 0.51 | 1.47 ± 0.63 | < .000001 |
| Obese (BMI z-score > 1.65), % | 13.0 | 48.7 | … |
| Elapsed time between beginning treatmenta and second NPSG, mean, d | 107.8 ± 13.7 | 113.8 ± 17.4 | … |
Discussion
This retrospective study on the clinical experience and long-term outcomes of combination therapy consisting of ICS + OM for management of mild OSA in children provides initial insights into the potential beneficial effects of this approach. Indeed, of the 836 children included in this clinical series with mild OSA, who would have normally undergone surgical removal of adenoids and tonsils in most centers in the United States as the first line of therapy, only 175 children (20.9%) ultimately required surgical intervention either based on a priori parental decision to refuse therapy or on response to therapy, with an additional 61 children (7.3%) being nonadherent to ICS + OM treatment and disappearing from follow-up. Thus, the overall success rate of the nonsurgical approach afforded by ICS and OM was 80.5%. Furthermore, we have now identified two readily identifiable patient characteristics that appear to adversely affect the favorable response to ICS + OM treatment: age > 7 years and the presence of obesity.
The rationale for implementing in our pediatric sleep center a clinical management paradigm consisting of nonsurgical treatment was twofold. First and foremost, the overall success rate of T&A resulting in normalization of NPSG abnormalities was found to be low in both our initial, prospective, single-center study and in a subsequent, multicenter, retrospective study.2,26 Similar, albeit slightly more favorable, results have been reported by others, further providing compelling evidence that improved selection of those patients with OSA who are most likely to demonstrate complete resolution is highly desirable, but currently unavailable.3,27 When these suboptimal outcomes are paired with the potential risks of T&A surgery,28 it becomes readily apparent that nonsurgical options could be highly desirable, at least for patients with milder OSA.
Upon implementation of the clinical protocol in our center, the criteria for proposing ICS + OM treatment options to parents relied on the NPSG findings, the latter fulfilling the criteria of mild OSA. However, despite the uniformity of the clinical approaches implemented during the period of time covered in this study, we cannot infer whether differences in the duration of disease were present and affected the response to therapy. Of note, there is also evidence indicating that watchful waiting may result in improvements in the severity of OSA, and such naturally occurring improvements could have occurred in our cohort as well.3 Second, the combined evidence from in vitro experiments showing marked reductions in tonsillar and adenoid tissue proliferation with application of corticosteroids or montelukast and the experience garnered from clinical trials using either ICS alone or OM alone further supported the rationale for implementation of nonsurgical options, even if appropriately RCTs are sorely lacking.5‐17 Notwithstanding the retrospective nature of the study and the potential for selection biases inherent to any retrospective study, current findings provide initial confirmation in the clinical setting that the combination of ICS and OM is a potentially effective intervention for treatment of mild OSA in children, and such findings need to be confirmed by prospective, multicenter, RCT approaches.
As mentioned, the subanalyses of the children presenting with worsening or unchanged polysomnographic findings after ICS + OM treatment raised the possibility that obese children and older children may not be as likely to respond to ICS + OM treatment. Although the specific reasons for such differences remain to be elucidated, there is some degree of plausibility to such findings. First, obesity is now a clearly established risk factor for OSA in children that not only imposes increased mass loading to the upper airway and respiratory system, but may also promote increased inflammation ultimately favoring proliferation of adenotonsillar tissues.1,29‐33 Therefore, similar to the poorer outcomes associated with T&A in obese children, administration of ICS + OM may have been less efficacious in alleviating the underlying processes that promoted the occurrence of OSA in these children. The putative explanations for the reduced likelihood of favorable results among older children are less apparent. It is possible that the presence of increased fibrotic and connective tissues in upper airway lymphadenoid tissues of older children may lead to better preservation of the overall structure of these tissues and reduced probability that such tissues will decrease in volume even if ICS + OM treatment effectively reduces the inflammatory cellularity component. Of course, we cannot exclude the possibility that these findings simply reflect a spurious association or, alternatively, reflect absence of adherence to ICS + OM treatment, since no oversight of adherence was implemented in this clinical population.
There are multiple methodologic limitations that preclude assertive affirmations on the efficacy of ICS + OM treatment in mild pediatric OSA. The retrospective nature of the study and the uncontrolled and open-label approach that are inherent to the clinical practice setting in which ICS + OM was administered markedly reduce the level of evidence and of the strength of potential recommendations that can be derived from this study.34,35 Nevertheless, the absence of significant side effects and the overall favorable safety profile associated with the use of either ICS36‐38 or OM39 and the possibility that based on the current encouraging results reported herein ICS + OM may ultimately replace T&A as the first line of treatment in mild OSA, provides major impetus for future, large-scale, multicenter RCTs. In summary, the retrospective analysis of our clinical experience associated with the implementation of ICS and OM in the management of mild OSA in children as an alternative to T&A is highly encouraging and supports prospective evaluation of this treatment modality as a potential alternative to T&A.
Acknowledgments
Author contributions: D. G. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. L. K.-G. was principal author of the manuscript. L. K.-G. and D. G. contributed to the conceptual framework for the study; L. K.-G. and D. G. contributed to data analysis; L. K.-G., R. B., and H. P. R. B. contributed to data acquisition; L. K.-G., R. B., H. P. R. B., and D. G. contributed to data interpretation; L. K.-G. drafted the initial manuscript; R. B. and H. P. R. B. contributed to the revision of the manuscript; D. G. provided critical editing of the initial manuscript; D. G. is responsible for the financial support of the project; and L. K.-G., R. B., H. P. R. B., and D. G. approved the final manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Kheirandish-Gozal is the recipient of an investigator-initiated grant from Merck & Co Inc on the effect of montelukast in the treatment of pediatric sleep apnea. Dr Gozal is the recipient of an investigator-initiated grant from ResMed Corp on urine biomarkers in adult sleep apnea. Drs Bhattacharjee and Bandla have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- ICS
intranasal corticosteroid
- NPSG
nocturnal polysomnography
- OM
oral montelukast
- RCT
randomized controlled trial
- Spo2
arterial oxygen saturation
- T&A
adenotonsillectomy
- TST
total sleep time
Footnotes
FUNDING/SUPPORT: Drs Kheirandish-Gozal and Gozal are supported by the US National Institutes of Health [Grants HL-65270, HL-086662, and HL-107160].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Marcus CL, Brooks LJ, Draper KA, et al. ; American Academy of Pediatrics. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714-e755 [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med. 2010;182(5):676-683 [DOI] [PubMed] [Google Scholar]
- 3.Marcus CL, Moore RH, Rosen CL, et al. ; Childhood Adenotonsillectomy Trial (CHAT). A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368(25):2366-2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kheirandish-Gozal L, Kim J, Goldbart AD, Gozal D. Novel pharmacological approaches for treatment of obstructive sleep apnea in children. Expert Opin Investig Drugs. 2013;22(1):71-85 [DOI] [PubMed] [Google Scholar]
- 5.Goldbart AD, Goldman JL, Veling MC, Gozal D. Leukotriene modifier therapy for mild sleep-disordered breathing in children. Am J Respir Crit Care Med. 2005;172(3):364-370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuhle S, Urschitz MS. Anti-inflammatory medications for obstructive sleep apnea in children. Cochrane Database Syst Rev. 2011;(1):CD007074. [DOI] [PubMed] [Google Scholar]
- 7.Goldbart AD, Greenberg-Dotan S, Tal A. Montelukast for children with obstructive sleep apnea: a double-blind, placebo-controlled study. Pediatrics. 2012;130(3):e575-e580 [DOI] [PubMed] [Google Scholar]
- 8.Kheirandish L, Goldbart AD, Gozal D. Intranasal steroids and oral leukotriene modifier therapy in residual sleep-disordered breathing after tonsillectomy and adenoidectomy in children. Pediatrics. 2006;117(1):e61-e66 [DOI] [PubMed] [Google Scholar]
- 9.Esteitie R, Emani J, Sharma S, Suskind DL, Baroody FM. Effect of fluticasone furoate on interleukin 6 secretion from adenoid tissues in children with obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2011;137(6):576-582 [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Mendoza-Sassi RA, César JA, Chadha NK. Intranasal corticosteroids for nasal airway obstruction in children with moderate to severe adenoidal hypertrophy. Cochrane Database Syst Rev. 2008;(3):CD006286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kheirandish-Gozal L, Gozal D. Intranasal budesonide treatment for children with mild obstructive sleep apnea syndrome. Pediatrics. 2008;122(1):e149-e155 [DOI] [PubMed] [Google Scholar]
- 12.Alexopoulos EI, Kaditis AG, Kalampouka E, et al. Nasal corticosteroids for children with snoring. Pediatr Pulmonol. 2004;38(2):161-167 [DOI] [PubMed] [Google Scholar]
- 13.Mintz M, Garcia J, Diener P, Liao Y, Dupclay L, Georges G. Triamcinolone acetonide aqueous nasal spray improves nocturnal rhinitis-related quality of life in patients treated in a primary care setting: the Quality of Sleep in Allergic Rhinitis study. Ann Allergy Asthma Immunol. 2004;92(2):255-261 [DOI] [PubMed] [Google Scholar]
- 14.Mansfield LE, Diaz G, Posey CR, Flores-Neder J. Sleep disordered breathing and daytime quality of life in children with allergic rhinitis during treatment with intranasal budesonide. Ann Allergy Asthma Immunol. 2004;92(2):240-244 [DOI] [PubMed] [Google Scholar]
- 15.Brouillette RT, Manoukian JJ, Ducharme FM, et al. Efficacy of fluticasone nasal spray for pediatric obstructive sleep apnea. J Pediatr. 2001;138(6):838-844 [DOI] [PubMed] [Google Scholar]
- 16.Dayyat E, Serpero LD, Kheirandish-Gozal L, et al. Leukotriene pathways and in vitro adenotonsillar cell proliferation in children with obstructive sleep apnea. Chest. 2009;135(5):1142-1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kheirandish-Gozal L, Serpero LD, Dayyat E, et al. Corticosteroids suppress in vitro tonsillar proliferation in children with obstructive sleep apnoea. Eur Respir J. 2009;33(5):1077-1084 [DOI] [PubMed] [Google Scholar]
- 18.Brodsky L, Moore L, Stanievich JF. A comparison of tonsillar size and oropharyngeal dimensions in children with obstructive adenotonsillar hypertrophy. Int J Pediatr Otorhinolaryngol. 1987;13(2):149-156 [DOI] [PubMed] [Google Scholar]
- 19.Mallampati SR, Gatt SP, Gugino LD, et al. A clinical sign to predict difficult tracheal intubation: a prospective study. Can Anaesth Soc J. 1985;32(4):429-434 [DOI] [PubMed] [Google Scholar]
- 20.Bhattacharjee R, Dayyat E, Kheirandish-Gozal L, Sans Capdevila O, Gozal D. Nocturnal polysomnographic characteristics of habitually snoring children initially referred to pediatric ENT or sleep clinics. Sleep Med. 2009;10(9):1031-1034 [DOI] [PubMed] [Google Scholar]
- 21.Dayyat E, Kheirandish-Gozal L, Sans Capdevila O, Maarafeya MM, Gozal D. Obstructive sleep apnea in children: relative contributions of body mass index and adenotonsillar hypertrophy. Chest. 2009;136(1):137-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.What is epi info? Centers for Disease Control and Prevention website. http://www.cdc.gov/epiinfo/. Accessed August 16, 2013
- 23.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;314(314):1-27 [PubMed] [Google Scholar]
- 24.Iber C, Chesson A, Quan SF; American Academy of Sleep Medicine. The AASM Manual for Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed.Westchester, IL: American Academy of Sleep Medicine; 2007 [Google Scholar]
- 25.Montgomery-Downs HE, O’Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117(3):741-753 [DOI] [PubMed] [Google Scholar]
- 26.Tauman R, Gulliver TE, Krishna J, et al. Persistence of obstructive sleep apnea syndrome in children after adenotonsillectomy. J Pediatr. 2006;149(6):803-808 [DOI] [PubMed] [Google Scholar]
- 27.Mitchell RB. Adenotonsillectomy for obstructive sleep apnea in children: outcome evaluated by pre- and postoperative polysomnography. Laryngoscope. 2007;117(10):1844-1854 [DOI] [PubMed] [Google Scholar]
- 28.Subramanyam R, Varughese A, Willging JP, Sadhasivam S. Future of pediatric tonsillectomy and perioperative outcomes. Int J Pediatr Otorhinolaryngol. 2013;77(2):194-199 [DOI] [PubMed] [Google Scholar]
- 29.Bixler EO, Vgontzas AN, Lin HM, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009;32(6):731-736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gozal D, Kheirandish-Gozal L. Childhood obesity and sleep: relatives, partners, or both?—a critical perspective on the evidence. Ann N Y Acad Sci. 2012;1264:135-141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verhulst SL, Schrauwen N, Haentjens D, et al. Sleep-disordered breathing in overweight and obese children and adolescents: prevalence, characteristics and the role of fat distribution. Arch Dis Child. 2007;92(3):205-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arens R, Sin S, Nandalike K, et al. Upper airway structure and body fat composition in obese children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2011;183(6):782-787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kheirandish-Gozal L. Fat and lymphadenoid tissues: a mutually obstructive combination. Am J Respir Crit Care Med. 2011;183(6):694-695 [DOI] [PubMed] [Google Scholar]
- 34.Grade Working Group. The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) Working Group website. http://www.gradeworkinggroup.org. Accessed November 10, 2013
- 35.Ansari MT, Tsertsvadze A, Moher D. Grading quality of evidence and strength of recommendations: a perspective. PLoS Med. 2009;6(9):e1000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ratner PH, Miller SD, Hampfel FC, Jr, Melchior A, Dunbar SA, Tantry SK. Once-daily treatment with beclomethasone dipropionate nasal aerosol does not affect hypothalamic-pituitary-adrenal axis function. Ann Allergy Asthma Immunol. 2012;109(5):336-341 [DOI] [PubMed] [Google Scholar]
- 37.Ratner PH, Meltzer EO, Teper Mometasone furoate nasal spray is safe and effective for 1-year treatment of children with perennial allergic rhinitis. Int J Pediatr Otorhinolaryngol. 2009;73(5):651-657 [DOI] [PubMed] [Google Scholar]
- 38.Sastre J, Mosges R. Local and systemic safety of intranasal corticosteroids. J Investig Allergol Clin Immunol. 2012;22(1):1-12 [PubMed] [Google Scholar]
- 39.Bisgaard H, Skoner D, Boza ML, et al. Safety and tolerability of montelukast in placebo-controlled pediatric studies and their open-label extensions. Pediatr Pulmonol. 2009;44(6):568-579 [DOI] [PubMed] [Google Scholar]