Abstract
BACKGROUND:
Central sleep apnea (CSA) is common among patients with heart failure (HF) and is promoted by elevated CO2 chemosensitivity. Left atrial size is a marker of the hemodynamic severity of HF. The aim of this study was to determine if left atrial size predicts chemosensitivity to CO2 and CSA in patients with HF.
METHODS:
Patients with HF with left ventricular ejection fraction ≤ 35% underwent polysomnography for detection of CSA, echocardiography, and measurement of CO2 chemosensitivity. CSA was defined as an apnea-hypopnea index (AHI) ≥ 15/h with ≥ 50% central apneic events. The relation of clinical and echocardiographic parameters to chemosensitivity and CSA were evaluated by linear regression, estimation of ORs, and receiver operator characteristics.
RESULTS:
Of 46 subjects without OSA who had complete data for analysis, 25 had CSA. The only parameter that significantly correlated with chemosensitivity was left atrial volume index (LAVI) (r = 0.40, P < .01). LAVI was greater in those with CSA than those without CSA (59.2 mL/m2 vs 36.4 mL/m2, P < .001) and significantly correlated with log-transformed AHI (r = 0.46, P = .001). LAVI was the best predictor of CSA (area under the curve = 0.83). A LAVI ≤ 33 mL/m2 was associated with 22% risk for CSA, while LAVI ≥ 53 mL/m2 was associated with 92% risk for CSA.
CONCLUSIONS:
Increased LAVI is associated with heightened CO2 chemosensitivity and greater frequency of CSA. LAVI may be useful to guide referral for polysomnography for detection of CSA in patients with HF.
Heart failure (HF) is common, affecting 1% to 2% of the adult population.1 As many as 50% of patients with HF may have sleep-disordered breathing, most frequently central sleep apnea (CSA).2,3 CSA is associated with adverse prognosis,4,5 and treatment improves sleep architecture, cardiac function,6 exercise capacity, HF symptoms,7‐13 and may improve survival.6,13,14 However, diagnosis requires polysomnography (PSG), which is expensive and not always readily available. Some have advocated formal sleep testing for all patients with HF,15 although current guidelines do not recommend routine testing or screening for CSA.16‐18 History, physical examination, and symptoms are of limited usefulness for screening for CSA.19 Moreover, no method has been endorsed for routine application in patients with HF as a screening tool for CSA.20,21 Hence, the development of a simple screening strategy for CSA in patients with HF potentially would have wide utility.
CSA is associated with elevated pulmonary capillary wedge pressure.22‐24 It has been shown that pulmonary congestion promotes lung J-receptor stretch with increased reflex ventilatory response to CO225,26 and hyperventilation.22,27,28 Indeed, in patients with HF, CSA is manifested as cyclic hyperventilation with compensatory apnea and considered secondary to increased cardiac filling pressures.22
Central apnea frequency is related not only to pulmonary capillary wedge pressure but also to left atrial size,29 as patients with HF with CSA have greater left atrial dimension than patients with HF who do not have CSA.3,30,31 Assessment of left atrial size is a routine part of a comprehensive echocardiographic examination,32 a test that is widely recommended in the evaluation of patients with HF.16‐18 However, to our knowledge no previous study has reported whether left atrial size is predictive of CSA or chemosensitivity to CO2, which may promote CSA. The purpose of this study was to determine if left atrial size predicts chemosensitivity to CO2 and CSA in patients with HF. We hypothesized that left atrial size is sensitive and specific for the detection of CSA and associated with augmented CO2 chemosensitivity. Accordingly, our specific aims were to quantify left atrial volume by echocardiography in patients with HF who underwent measurement of CO2 chemosensitivity and PSG.
Materials and Methods
This study was conducted in accordance with the amended Declaration of Helsinki and approved by the Mayo Clinic Institutional Review Board (IRB#923-02). Written informed consent was obtained from all participants. Consecutive ambulatory outpatients were prospectively enrolled from the Mayo Clinic Heart Failure Clinic for participation in this study, which included laboratory-based, overnight, attended PSG; echocardiography; neurohormonal measurement; and assessment of chemosensitivity in all subjects. Patients were required to have stable HF with no changes of optimized medical therapy in the preceding 3 months and left ventricular ejection fraction (LVEF) ≤ 35% measured by echocardiography. New York Heart Association (NYHA) function class was assessed18; those with NYHA III-IV HF were defined as having “advanced heart failure.” BMI was computed as weight in kilograms divided by body surface area in square meters.
Echocardiography
All subjects underwent comprehensive transthoracic echocardiography. Measured parameters included LVEF, left ventricular end diastolic diameter (LVEDD), right ventricular systolic pressure (RVSP), mitral regurgitation (defined as moderate or more in severity by proximal isovelocity surface area), left atrial volume index (LAVI) (defined as left atrial volume to body surface area in mL/m2 by biplane two-dimensional echocardiography consistent with current guidelines32), mitral deceleration time, mitral E velocity, and the ratio of mitral E velocity to medial anulus e′ velocity (E/e′).
Measurement of Neurohormones
Concentration of brain natriuretic peptide (BNP) was measured from serum drawn on the evening of PSG. Measurement of BNP was evaluated by either the Shionogi immunoradiometric assay (Shionogi & Co, Ltd) or DxI 800 immunoassay (Beckman Coulter Inc). The coefficient of variation of these two BNP assays was > 0.99.
Measurement of Chemosensitivity
CO2 chemosensitivity was measured by a modified rebreathing method as previously described.33 Subjects breathed from a mouthpiece connected to a 6-L rebreathing bag; the bag included 5% CO2 with balance oxygen. Ventilation was measured by a pneumotachograph. End-tidal oxygen and end-tidal CO2 (Petco2) were monitored by mass spectrometry for comparison with changes in minute ventilation (e). As the subject rebreathes, inspired CO2 in the rebreathing bag increases and the oxygen level falls. However, inspired oxygen levels do not fall below 500 mm Hg (approximately 70% oxygen). Rebreathing continues until Petco2 values reach 50 to 55 mm Hg (or about 8% CO2, requiring approximately 4 min). The slope of the plot of e vs Petco2 is used as an index of CO2 chemosensitivity (Δe/ΔPetco2). Three runs were performed for each subject, and values were reported as the mean.
Sleep Evaluation
Diagnostic PSG was performed in the Center for Translational Science Activity Sleep Core facility of the Clinical Research Unit and digitally recorded on Dimensions software (Network Concepts Inc) or PSG Online2 E-Series (Compumedics Ltd) and scored using Uniquant (Thermo Fisher Scientific Inc) or Profusion2 software (Compumedics USA Inc). Recorded parameters included three-channel EEGs, two-channel electrooculograms, oronasal airflow by pressure transducer and thermocouple sensors, submental and limb electromyograms, one-channel ECG, transcutaneous pulse oximetry (Ohmeda 3740; General Electric Co) and integrated pulse oximetry (Compumedics USA Inc), thoracic and abdominal respiratory effort by inductance plethysmography, snoring by tracheal microphone or piezo crystal sensor, and body position by closed-circuit video monitoring. Disordered breathing events were classified as apneas or hypopneas and as either obstructive or central. Apneas were defined as a cessation of airflow or > 90% reduction in airflow from baseline for > 10 s with an oxygen desaturation ≥ 4%. Hypopneas were defined as a ≥ 50% reduction in airflow with an oxygen desaturation ≥ 4%. Events were classified as central when the airflow criteria were met in the absence of respiratory effort as recorded by thoracic and abdominal inductance plethysmography and as obstructive when airflow criteria were met despite continued or increased respiratory effort. Per published guidelines, patients were considered to have CSA if the total apnea-hypopnea index (AHI) was ≥ 15/h with ≥ 50% disordered breathing events of central origin regardless of the presence or absence of respiratory periodicity.34 Subjects found to have OSA or mixed apneas in which ≥ 50% of disordered breathing events were obstructive were excluded from analysis.
Statistical Analysis
Differences among group means were tested for differences by two-sided t tests or Wilcoxon rank-sum tests, depending on distribution. Differences in proportions were tested by the χ2 or Fisher exact tests. The relationship of Δe/ΔPetco2 slope to continuous clinical variables was compared by linear regression and summarized using Pearson correlation coefficients. The primary analysis was logistic regression assessing clinical and echocardiographic variables for association with CSA, with results expressed as the ORs with 95% CIs. Variables associated with CSA were evaluated by receiver operator characteristic (ROC) analysis, with results presented as area under the curve (AUC) and 95% CIs derived by the Mann-Whitney statistic. Sensitivity, specificity, and positive and negative predictive values were used to estimate 2 × 2 decision statistics, and positive and negative likelihood ratios were calculated for several cutoff values of LAVI. Given the greater prevalence of CSA among men,19,35 we conducted a separate ROC analysis restricted to men to assess whether test performance characteristics differed by sex. Analyses were performed with JMP, version 8, and SAS, version 9.2 (SAS Institute Inc). For all comparisons, a two-tailed P value < .05 was considered significant.
Results
Of the 62 consecutive subjects with HF who were studied by PSG, 29 (47%) had CSA and seven (four men, three women) were found to have OSA or mixed apneas and were excluded. Of these 55 subjects without OSA, 46 had complete data available for analysis; 25 of these had CSA. Median time between PSG and echocardiography was 16 days (interquartile range, 0-41 days).
Men were significantly more likely to have CSA than women (65% vs 25%, P = .02), and subjects with CSA were older (68.5 years vs 59.3 years, P = .001). BNP concentration was significantly higher in those with CSA than in those without CSA (1,184 pg/mL vs 346 pg/mL, P < .001). No statistical differences in the proportion of patients with ischemic cardiomyopathy, atrial fibrillation, or in HF medications were observed on comparison of subjects with HF and CSA compared with those with HF and without CSA. By definition, subjects with CSA had significantly higher AHI (37.9/h vs 4.7/h, P < .01). As expected, mean oxygen saturation during sleep was lower in those with CSA, and those with CSA spent a greater proportion of sleep time with oxygen saturation < 90% (18.2% vs 1.3%, P < .001) (Table 1).
TABLE 1 .
] Subject Characteristics
| Characteristic | HF Without CSA | HF With CSA | P Value |
| Subjects, No. | 21 | 25 | … |
| Demographics | |||
| Male sex | 12 (57) | 22 (88) | .02 |
| Age, y | 59.3 ± 9.9 | 68.5 ± 8.1 | .001 |
| BMI, kg/m2 | 27.7 ± 3.5 | 29.0 ± 5.5 | .32 |
| Heart failure class | |||
| NYHA I-II | 7 (33) | 4 (16) | .17 |
| NYHA III-IV | 14 (66) | 21 (84) | … |
| Ischemic | 10 (48) | 13 (52) | .77 |
| Atrial fibrillation | 1 (5) | 5 (20) | .20 |
| BNP concentration, pg/mL | 345.7 ± 433.6 | 1,184.0 ± 1,588.4 | .02 |
| Medications | |||
| β-Blocker | 18 (86) | 22 (88) | .82 |
| ACE/ARB | 21 (100) | 24 (96) | 1.00 |
| Aldosterone antagonist | 5 (24) | 8 (32) | .54 |
| Diuretics | 14 (67) | 19 (76) | .48 |
| Digitalis | 14 (67) | 15 (60) | .64 |
| Nitrates | 1 (5) | 1 (4) | 1.0 |
| Sleep parameters | |||
| AHI, events/h | 4.7 ± 4.2 | 37.9 ± 15.5 | < .001 |
| Mean Sao2, % | 93.6 ± 3.6 | 92.2 ± 2.6 | .01 |
| T90% | 1.3 ± 2.2 | 18.2 ± 19.4 | < .001 |
Data given as mean ± SD or No. (%) unless otherwise specified. ACE/ARB = angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker; AHI = apnea-hyponea index; BNP = brain natriuretic peptide; CSA = central sleep apnea; HF = heart failure; NYHA = New York Heart Association; Sao2 = oxygen saturation of arterial blood hemoglobin; T90% = time with arterial oxygen saturation < 90%.
CO2 chemosensitivity (Δe/ΔPetco2 slope) was significantly greater in patients with HF with CSA than those without CSA (2.67 vs 1.83, respectively; P = .03) but not different in those with advanced HF (ie, NYHA III-IV) compared with those with nonadvanced HF (ie, NYHA I-II) (2.36 vs 2.00, respectively; P = .17). A nonsignificant difference in Δe/ΔPetco2 slope was seen between men and women (2.34 vs 2.09, respectively; P = .45). In addition, age (r = 0.29, P = .06) and BNP (r = 0.06, P = .69) concentration did not significantly correlate with Δe/ΔPetco2 slope. The only echocardiographic parameter that significantly correlated with Δe/ΔPetco2 slope was LAVI (r = 0.40, P = .006) (Table 2).
TABLE 2 .
] Correlations Between Clinical Variables and CO2 Chemosensitivity
| Characteristic | Correlation (r) | P Value |
| Age | 0.29 | .06 |
| BNP, pg/mL | −0.06 | .69 |
| LVEF, % | −0.13 | .39 |
| LVEDD, mm | −0.02 | .88 |
| RVSP, mm Hg | 0.26 | .08 |
| LAVI, mL/m2 | 0.40 | < .01 |
| Mitral deceleration time, ms | −0.28 | .10 |
| e′, medial annulus, m/s | −0.18 | .26 |
| E/e′, medial annulus | 0.22 | .16 |
e′ = medial anulus e′ velocity; E/e′ = ratio of mitral E velocity to medial anulus e′ velocity; LAVI = left atrial volume index; LVEDD = left ventricular end-diastolic dimension; LVEF = left ventricular ejection fraction; RVSP = right ventricular systolic pressure. See Table 1 legend for expansion of other abbreviation.
Subjects with CSA had more hemodynamically severe HF, as evidenced by lower LVEF (21.1% vs 25.3%, P = .03), higher RVSP (49.0 mm Hg vs 37.8 mm Hg, P = .02), higher LAVI (59.2 mL/m2 vs 36.4 mL/m2, P < .01), shorter mitral deceleration time (172.3 milliseconds vs 232.9 milliseconds, P = .03), lower medial anulus e′ velocity (e′) (0.04 vs 0.05, P = .02), and higher E/e′ (27.2 vs 16.7, P = .02). No significant difference in LVEDD or the proportion of subjects with mitral regurgitation was seen in the two study groups (Table 3).
TABLE 3 .
] Echocardiographic Findings
| Characteristic | HF Without CSA | HF With CSA | P Value |
| LVEF, % | 25.3 ± 6.5 | 21.1 ± 6.3 | .03 |
| LVEDD, mm | 70.0 ± 6.8 | 71.2 ± 9.0 | .60 |
| RVSP, mm Hg | 37.8 ± 14.9 | 49.0 ± 15.9 | .02 |
| Mitral regurgitation, % | 23.8 | 48.0 | .13 |
| LAVI, mL/m2 | 36.4 ± 9.7 | 59.2 ± 23.4 | < .001 |
| Mitral deceleration time, ms | 232.9 ± 93.9 | 172.3 ± 48.5 | .03 |
| e′, medial annulus, m/s | 0.05 ± 0.03 | 0.04 ± 0.01 | .02 |
| E/e′, medial annulus | 16.7 ± 8.4 | 27.2 ± 18.1 | .02 |
After adjusting for LAVI, no other echocardiographic parameter was significantly associated with the presence of CSA, and the association between LAVI and CSA persisted even after controlling for age, sex, and BNP concentration (OR, 1.08 per mL/m2; P = .03) (Table 4). LAVI also significantly correlated with log-AHI (r = 0.46, P = .001).
TABLE 4 .
] Multivariate Predictors of CSA
| Parameter | OR | 95% CI | P Value |
| Model 1: AUC = 0.88 (0.79, 0.98) | |||
| Age, per y | 1.12 | 1.01-1.25 | .03 |
| LAVI, per mL/m2 | 1.10 | 1.03-1.18 | .005 |
| Model 2: AUC = 0.89 (0.80, 0.98) | |||
| Age, per y | 1.11 | 0.99-1.25 | .07 |
| Male sex | 1.51 | 0.19-12.09 | .70 |
| LAVI, per mL/m2 | 1.09 | 1.02-1.17 | .01 |
| Model 3: AUC = 0.90 (0.81, 0.99) | |||
| Age, per y | 1.12 | 0.99-1.28 | .08 |
| Male sex | 2.07 | 0.21-20.81 | .54 |
| LAVI, per mL/m2 | 1.08 | 1.01-1.16 | .03 |
| BNP, per 200 pg/mL | 2.35 | 0.95-1.91 | .09 |
ROC analysis showed that the best predictor of CSA was LAVI (AUC = 0.83), although BNP (AUC = 0.79) and age (AUC = 0.77) performed similarly. A LAVI cutoff of 44 mL/m2 yielded a sensitivity of 80% and specificity of 81% (Table 5). Test performance was similar when the analysis was restricted to just men compared with the overall group in univariate analysis (AUC = 0.88 [95% CI, 0.74-1.00] vs AUC = 0.83 [95% CI, 0.70-0.95]) and in multivariate analysis adjusting for sex (AUC = 0.88 [95% CI, 0.76-1.00] vs AUC = 0.88 [95% CI 0.79-0.98]). Risk for CSA by LAVI cutoff values is shown in Figure 1.
TABLE 5 .
] Test Characteristics for Predictors of CSA
| Characteristic | Sensitivity | Specificity | PPV | NPV | LR+ | LR− |
| LAVI, mL/m2 | ||||||
| 33 | 92 (75-98) | 33 (17-55) | 62 (46-76) | 78 (45-94) | 1.38 (1.00-1.91) | 0.24 (0.06-1.03) |
| 44 | 80 (61-91) | 81 (60-92) | 83 (64-93) | 77 (57-90) | 4.20 (1.70-10.36) | 0.25 (0.11-0.56) |
| 53 | 52 (34-70) | 95 (77-99) | 93 (69-99) | 63 (45-77) | 10.92 (1.55-76.71) | 0.50 (0.33-0.77) |
| BNP, pg/mL | ||||||
| 148 | 92 (75-98) | 43 (25-64) | 66 (49-79) | 82 (52-95) | 1.61 (1.09-2.37) | 0.19 (0.05-0.77) |
| 336 | 84 (65-94) | 71 (50-86) | 78 (59-89) | 79 (57-92) | 2.94 (1.46-5.91) | 0.22 (0.09-0.57) |
| 1,149 | 36 (20-56) | 91 (71-97) | 82 (52-95) | 54 (38-70) | 3.78 (0.92-15.61) | 0.71 (0.51-0.98) |
| Age, y | ||||||
| 59 | 92 (75-98) | 38 (21-59) | 64 (48-78) | 80 (49-94) | 1.49 (1.04-2.12) | 0.21 (0.05-0.88) |
| 62 | 88 (70-96) | 57 (37-76) | 71 (53-84) | 80 (55-93) | 2.05 (1.23-3.44) | 0.21 (0.07-0.65) |
| 71 | 44 (27-63) | 91 (71-97) | 85 (58-96) | 58 (41-73) | 4.62 (1.15-18.56) | 0.62 (0.43-0.90) |
| e/Petco2 slope | ||||||
| 1.36 | 92 (74-98) | 10 (3-29) | 54 (39-68) | 50 (15-85) | 1.01 (0.84-1.22) | 0.88 (0.14-5.68) |
| 1.76 | 88 (69-96) | 52 (32-72) | 68 (50-81) | 79 (52-92) | 1.84 (1.15-2.95) | 0.24 (0.07-0.74) |
| 2.38 | 42 (25-61) | 91 (71-97) | 83 (55-95) | 58 (41-73) | 4.38 (1.08-17.75) | 0.65 (0.45-0.93) |
| Sex | ||||||
| Male | 88 (70-96) | 43 (25-64) | 65 (48-79) | 75 (47-91) | 1.54 (1.04-2.29) | 0.28 (0.09-0.90) |
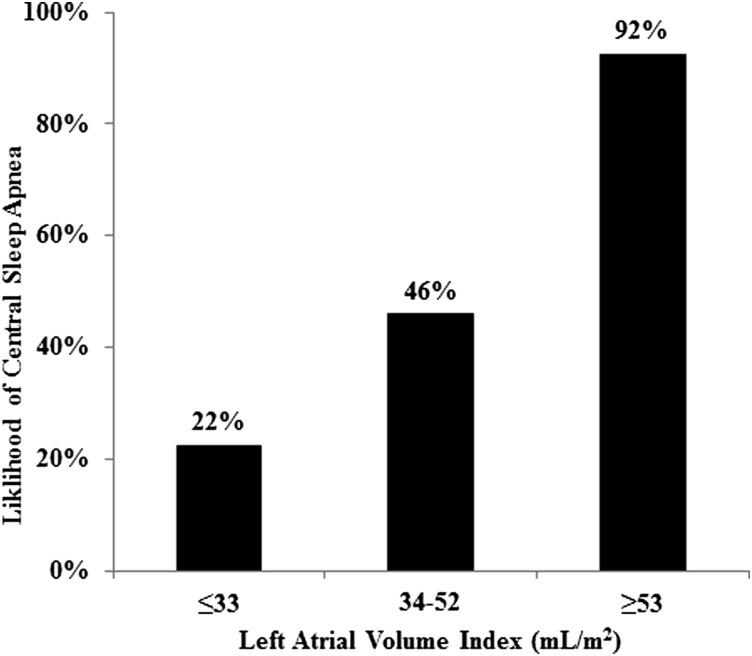
Figure 1 .
– Risk of central sleep apnea (CSA) was best predicted by the left atrial volume index (LAVI). Risk for CSA for those with LAVI ≤ 33 mL/m2 was 22% (95% CI, 3%-60%); with LAVI 34 to 52 mL/m2, 46% (95% CI, 26%-67%); and with LAVI ≥ 53 mL/m2, was 92% (95% CI, 64%-100%).
Discussion
The novel findings of this study were that LAVI is associated with enhanced CO2 chemosensitivity and the presence of CSA and may be useful as a screening tool for detection of CSA in patients with HF.
LAVI and CO2 Chemosensitivity
Enhanced CO2 chemosensitivity occurs in HF36 and correlates with sympathetic activation37 and natriuretic peptide elevation38,39 as well as HF mortality.38 Augmented chemosensitivity also promotes hyperventilation with reduction of CO2 concentration below the apneic threshold, thereby causing compensatory and cyclic hypopnea or apnea characteristic of CSA.25,26,40,41 Consistent with prior studies,25,26,40 we observed that CO2 chemosensitivity is augmented in patients with HF with CSA. To our knowledge, this is the first report to demonstrate a significant correlation between left atrial volume and CO2 chemosensitivity. Our findings may support a link between left atrial dimension; chronic pulmonary venous hypertension, which can promote pulmonary J-receptor stretch; and enhanced CO2 chemosensitivity and CSA in patients with HF.25,26 However, the modest correlation suggests that other factors are also likely important in affecting CO2 chemosensitivity and sleep-disordered breathing in patients with HF.
LAVI as a Screening Tool for CSA
Our study may have practical implications regarding the management of patients with HF. Multiple guidelines recommend consideration of sleep-disordered breathing as a comorbidity in HF.15‐18 However, diagnosis requires PSG, which is expensive, time consuming, and not always readily available. Herein we have shown that increased LAVI is predictive of CSA. Of all the clinical parameters considered, including age and sex, we found that LAVI most strongly correlated with the presence of CSA, with patients with LAVI ≥ 44 mL/m2 having a fourfold increased risk.
As this study included consecutive, nonselected, ambulatory patients from a HF clinic, the assessment of positive and negative predictive values likely approximates the post-test probability of CSA in similar populations. A LAVI ≤ 33 mL/m2, a value at approximately the 25th percentile of our study population, was associated with 22% risk for CSA. Whether this is considered an acceptably low risk for CSA to defer PSG is unclear and would likely depend on the clinical situation and the perceived benefits of treatment. By comparison, LAVI ≥ 53 mL/m2, a value at approximately the 75th percentile of our study cohort, was associated with 92% risk for CSA. Thus, the main utility of assessment of left atrial size would seem to be in selecting those most at risk for CSA.
Limitations
The sample size was modest and included only patients with LVEF ≤ 35%, the majority of whom had advanced HF. These findings need to be confirmed in a larger study with a broader spectrum of disease severity. The subject population had a high prevalence of CSA, likely because the majority of subjects had advanced HF. Whether our findings apply to those with less advanced HF is unclear, although the prevalence of CSA also appears high in other studies.3,35 We excluded subjects with HF who had OSA or mixed apnea with a significant obstructive component, which may limit the generalizability of our findings to the overall HF population in which obstructive events are not rare.3,35 In nine subjects enrolled in the study, datasets were incomplete due to either inability to quantify left atrial volume (n = 4) or no BNP measurement performed on the day of PSG (n = 5). Of these nine subjects, four had CSA, and five did not have CSA or OSA. Including these nine subjects in the overall analyses did not cause changes in the significance of the outcomes as summarized in Tables 1-5.
Variation in LAVI or PSG findings may have influenced our results, though this seems unlikely as the majority of subjects underwent echocardiography within 30 days of PSG and all were clinically stable and on optimized medical therapy for > 3 months. While we found LAVI to be related to risk for CSA independent of sex, the degree to which our results apply to women is unclear, as our study included few women. However, prior research has suggested that risk factors for CSA and their relative importance are similar between men and women,19 and our estimates of the utility of LAVI for detection of CSA were similar when the analysis was restricted to men. It is also unclear if these findings apply to other groups of patients without HF where CSA may be present. Finally, the study design was observational and not designed to evaluate the specific physiologic and molecular mechanisms that may link left atrial volume increase to augmented CO2 chemosensitivity and CSA. In conclusion, these data show that left atrial size is significantly associated with elevated CO2 chemosensitivity and CSA in patients with HF and that left atrial dimension may be useful to guide referral for definitive diagnosis of CSA by PSG.
Acknowledgments
Author contributions: L. J. O. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. A. D. C. served as principal author. A. D. C. contributed to the analysis of the data; A. D. C., V. K. S., B. D. J., and L. J. O. contributed to the interpretation of the data; C. G. S. contributed to the statistical analysis and reporting of the data; L. J. O. contributed to the planning of the study; A. D. C., C. G. S., and L. J. O. contributed to the writing of the manuscript; and V. K. S. and B. D. J. contributed to the revision of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Somers has served as a consultant for Neu Pro; Respircardia, Inc; Sorin Inc; Price Waterhouse; and ResMed and has received grant support from Philips-Respironics Foundation. The other authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the National Center for Research Resources or the National Institutes of Health. The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AUC
area under the curve
- BNP
brain natriuretic peptide
- CSA
central sleep apnea
- e′
medial anulus e′ velocity
- E/e′
the ratio of mitral E velocity to medial anulus e′ velocity
- HF
heart failure
- LAVI
left atrial volume index
- LVEDD
left ventricular end-diastolic dimension
- LVEF
left ventricular ejection fraction
- NYHA
New York Heart Association
- Petco2
end tidal CO2
- PSG
polysomnography
- ROC
receiver operator characteristic
- RVSP
right ventricular systolic pressure
- e
minute ventilation
Footnotes
FUNDING/SUPPORT: This work was supported by the Mayo Clinic Clinician-Investigator Training Program (Dr Calvin); Mayo Foundation; American Heart Association [Grant 04-50103Z]; National Heart, Lung, and Blood Institute [Grants HL65176, HL70302, and HL73211]; and the National Center for Research Resources [Grant 1ULI RR024150], a component of the National Institutes of Health (NIH) and the NIH Roadmap for Medical Research.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.McMurray JJV, Pfeffer MA. Heart failure. Lancet. 2005;365(9474):1877-1889 [DOI] [PubMed] [Google Scholar]
- 2.Javaheri S, Parker TJ, Wexler L, et al. Occult sleep-disordered breathing in stable congestive heart failure. Ann Intern Med. 1995;122(7):487-492 [DOI] [PubMed] [Google Scholar]
- 3.Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Töpfer V. Sleep-disordered breathing in patients with symptomatic heart failure: a contemporary study of prevalence in and characteristics of 700 patients. Eur J Heart Fail. 2007;9(3):251-257 [DOI] [PubMed] [Google Scholar]
- 4.Lanfranchi PA, Braghiroli A, Bosimini E, et al. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation. 1999;99(11):1435-1440 [DOI] [PubMed] [Google Scholar]
- 5.Hanly PJ, Zuberi-Khokhar NS. Increased mortality associated with Cheyne-Stokes respiration in patients with congestive heart failure. Am J Respir Crit Care Med. 1996;153(1):272-276 [DOI] [PubMed] [Google Scholar]
- 6.Aurora RN, Chowdhuri S, Ramar K, et al. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses. Sleep. 2012;35(1):17-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Philippe C, Stoïca-Herman M, Drouot X, et al. Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne-Stokes respiration in heart failure over a six month period. Heart. 2006;92(3):337-342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szollosi I, O’Driscoll DM, Dayer MJ, Coats AJ, Morrell MJ, Simonds AK. Adaptive servo-ventilation and deadspace: effects on central sleep apnoea. J Sleep Res. 2006;15(2):199-205 [DOI] [PubMed] [Google Scholar]
- 9.Kasai T, Narui K, Dohi T, et al. First experience of using new adaptive servo-ventilation device for Cheyne-Stokes respiration with central sleep apnea among Japanese patients with congestive heart failure: report of 4 clinical cases. Circ J. 2006;70(9):1148-1154 [DOI] [PubMed] [Google Scholar]
- 10.Teschler H, Döhring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164(4):614-619 [DOI] [PubMed] [Google Scholar]
- 11.Noda A, Izawa H, Asano H, et al. Beneficial effect of bilevel positive airway pressure on left ventricular function in ambulatory patients with idiopathic dilated cardiomyopathy and central sleep apnea-hypopnea: a preliminary study. Chest. 2007;131(6):1694-1701 [DOI] [PubMed] [Google Scholar]
- 12.Naughton MT, Benard DC, Liu PP, Rutherford R, Rankin F, Bradley TD. Effects of nasal CPAP on sympathetic activity in patients with heart failure and central sleep apnea. Am J Respir Crit Care Med. 1995;152(2):473-479 [DOI] [PubMed] [Google Scholar]
- 13.Arzt M, Floras JS, Logan AG, et al. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure: a post hoc analysis of the Canadian Continuous Positive Airway Pressure for Patients with Central Sleep Apnea and Heart Failure Trial (CANPAP). Circulation. 2007;115(25):3173-3180 [DOI] [PubMed] [Google Scholar]
- 14.Sin DD, Logan AG, Fitzgerald FS, Liu PP, Bradley TD. Effects of continuous positive airway pressure on cardiovascular outcomes in heart failure patients with and without Cheyne-Stokes respiration. Circulation. 2000;102(1):61-66 [DOI] [PubMed] [Google Scholar]
- 15.Oldenburg O, Lamp B, Freudenberg G, Horstkotte D. Screening for sleep-disordered breathing is recommended in patients with chronic heart failure. Eur Respir J. 2007;30(5):1023; author reply 1023-1024 [DOI] [PubMed] [Google Scholar]
- 16.Heart Failure Society of America. Executive Summary: HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16(6):475-539 [Google Scholar]
- 17.Dickstein K, Cohen-Solal A, Filippatos G, et al. ; ESC Committee for Practice Guidelines (CPG). ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail. 2008;10(10):933-989 [DOI] [PubMed] [Google Scholar]
- 18.Hunt SA, Abraham WT, Chin MH, et al. ; American College of Cardiology; American Heart Association Task Force on Practice Guidelines; American College of Chest Physicians; International Society for Heart and Lung Transplantation; Heart Rhythm Society. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Writing Committee to update the 2001 guidelines for the evaluation and management of heart failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112(12):e154-e235 [DOI] [PubMed] [Google Scholar]
- 19.Sin DD, Fitzgerald FS, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160(4):1101-1106 [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Touchard A, Somers VK, Olson LJ, Caples SM. Central sleep apnea: implications for congestive heart failure. Chest. 2008;133(6):1495-1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradley TD, Floras JS. Sleep apnea and heart failure: part II: central sleep apnea. Circulation. 2003;107(13):1822-1826 [DOI] [PubMed] [Google Scholar]
- 22.Solin P, Bergin P, Richardson M, Kaye DM, Walters EH, Naughton MT. Influence of pulmonary capillary wedge pressure on central apnea in heart failure. Circulation. 1999;99(12):1574-1579 [DOI] [PubMed] [Google Scholar]
- 23.Mansfield D, Kaye DM, Brunner La Rocca H, Solin P, Esler MD, Naughton MT. Raised sympathetic nerve activity in heart failure and central sleep apnea is due to heart failure severity. Circulation. 2003;107(10):1396-1400 [DOI] [PubMed] [Google Scholar]
- 24.Olson TP, Frantz RP, Snyder EM, et al. Effects of acute changes in pulmonary wedge pressure on periodic breathing at rest in heart failure patients. Am Heart J. 2007;153(1):104.e1-104.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Javaheri S. A mechanism of central sleep apnea in patients with heart failure. N Engl J Med. 1999;341(13):949-954 [DOI] [PubMed] [Google Scholar]
- 26.Solin P, Roebuck T, Johns DP, Walters EH, Naughton MT. Peripheral and central ventilatory responses in central sleep apnea with and without congestive heart failure. Am J Respir Crit Care Med. 2000;162(6):2194-2200 [DOI] [PubMed] [Google Scholar]
- 27.Lorenzi-Filho G, Azevedo ER, Parker JD, et al. Relationship of carbon dioxide tension in arterial blood to pulmonary wedge pressure in heart failure. Eur Respir J. 2002;19(1):37-40 [DOI] [PubMed] [Google Scholar]
- 28.Oldenburg O, Bitter T, Wiemer M, Langer C, Horstkotte D, Piper C. Pulmonary capillary wedge pressure and pulmonary arterial pressure in heart failure patients with sleep-disordered breathing. Sleep Med. 2009;10(7):726-730 [DOI] [PubMed] [Google Scholar]
- 29.Dini FL, Ballo P, Badano L, et al. Validation of an echo-Doppler decision model to predict left ventricular filling pressure in patients with heart failure independently of ejection fraction. Eur J Echocardiogr. 2010;11(8):703-710 [DOI] [PubMed] [Google Scholar]
- 30.Vazir A, Hastings PC, Dayer M, et al. A high prevalence of sleep disordered breathing in men with mild symptomatic chronic heart failure due to left ventricular systolic dysfunction. Eur J Heart Fail. 2007;9(3):243-250 [DOI] [PubMed] [Google Scholar]
- 31.Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail. 2009;11(6):602-608 [DOI] [PubMed] [Google Scholar]
- 32.Lang RM, Bierig M, Devereux RB, et al. ; Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440-1463 [DOI] [PubMed] [Google Scholar]
- 33.Olson LJ, Snyder EM, Beck KC, Johnson BD. Reduced rate of alveolar-capillary recruitment and fall of pulmonary diffusing capacity during exercise in patients with heart failure. J Card Fail. 2006;12(4):299-306 [DOI] [PubMed] [Google Scholar]
- 34.Iber C, Ancoli-Israel S, Chesson A, Quan SF, for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. 1st ed.Westchester, IL: American Academy of Sleep Medicine;2007 [Google Scholar]
- 35.Yumino D, Wang H, Floras JS, et al. Prevalence and physiological predictors of sleep apnea in patients with heart failure and systolic dysfunction. J Card Fail. 2009;15(4):279-285 [DOI] [PubMed] [Google Scholar]
- 36.Chua TP, Clark AL, Amadi AA, Coats AJ. Relation between chemosensitivity and the ventilatory response to exercise in chronic heart failure. J Am Coll Cardiol. 1996;27(3):650-657 [DOI] [PubMed] [Google Scholar]
- 37.Narkiewicz K, Pesek CA, van de Borne PJH, Kato M, Somers VK. Enhanced sympathetic and ventilatory responses to central chemoreflex activation in heart failure. Circulation. 1999;100(3):262-267 [DOI] [PubMed] [Google Scholar]
- 38.Giannoni A, Emdin M, Bramanti F, et al. Combined increased chemosensitivity to hypoxia and hypercapnia as a prognosticator in heart failure. J Am Coll Cardiol. 2009;53(21):1975-1980 [DOI] [PubMed] [Google Scholar]
- 39.Giannoni A, Emdin M, Poletti R, et al. Clinical significance of chemosensitivity in chronic heart failure: influence on neurohormonal derangement, Cheyne-Stokes respiration and arrhythmias. Clin Sci (Lond). 2008;114(7):489-497 [DOI] [PubMed] [Google Scholar]
- 40.Arzt M, Harth M, Luchner A, et al. Enhanced ventilatory response to exercise in patients with chronic heart failure and central sleep apnea. Circulation. 2003;107(15):1998-2003 [DOI] [PubMed] [Google Scholar]
- 41.Javaheri S, Corbett WS. Association of low PaCO2 with central sleep apnea and ventricular arrhythmias in ambulatory patients with stable heart failure. Ann Intern Med. 1998;128(3):204-207 [DOI] [PubMed] [Google Scholar]