Abstract
B cells are central pathogenic players in Systemic Lupus Erythematosus and multiple other autoinmune diseases through antibody production as well as antibody independent functiona. At the same time, B cells are known to play important regulatory functions that may protect against autoimmune manifestations. Yet, the functional role of different B cell populations and their contribution to disease remain to be understood. The advent of agents that specifically target B cells, in particular anti-CD20 and ant-BLyS antibodies, have demonstrated the efficacy of this approach for the treatment of human autoimmunity. The analysis of patients treated with these and other B cell agents provide a unique opportunity to understand the correlates of clinical response and the significance of different B cell subsets. Here we discuss this information and how it could be used to better understand SLE and improve the rational design of B cell directed therapies in this disease.
Keywords: SLE, B cell therapy, B cells, Plasma cells, Autoantibodies
Introduction
B cells are critical players in human immune responses including both protective responses during infections and vaccination and pathogenic responses in transplant rejection, allergic and autoimmune conditions [1]. The dual nature of B cells also applies to many other medical areas such as cardiovascular disease where B cells may adversely impact the outcome of acute myocardial infarction yet their natural products (antibodies), may play either a protective or a pathogenic cardiovascular role. The opposing roles of B cells in multiple biological systems and diseases have been reviewed in depth elsewhere [2].
Over the last 15 years, we have witnessed an explosion of interest in the use of B cell depletion in a growing number of diseases prominently including B cell malignancies, autoimmune diseases and transplantation. Spurred by the success of B cell depletion in Rheumatoid Arthritis [3] and ANCA-mediated vasculitis [4] and the relatively low toxicity of this intervention, multiple other agents that impact B cell survival and/or function have been introduced in the clinic or are in different stages of development. The most prominent example of agents that modulate B cell survival, the anti-BAFF monoclonal antibody Belimumab, has been recently approved by the FDA for the treatment of SLE thereby providing a second wind to the field of B cell targeting in this disease [5] after the failure of two randomized, placebo controlled clinical trials of Rituximab in non-renal lupus and lupus nephritis (EXPLORER and LUNAR, respectively) [6,7]. Given the very different mechanism of action of these two agents with dramatically different impact on B cells, the growing body of clinical and immunological information available provides an interesting opportunity to think through the rationale and application of different modalities of B cell targeting. Due to the plethora of excellent clinical reviews of anti-B cell therapies published over the last few years [1,8–10], here we shall focus on the immunological rationale for the different modalities. Moreover, we will discuss how to apply this knowledge to improve the use of current agents and to design new therapeutic strategies.
B cells in SLE. Rationale for B cell directed therapies
B cell diversity and division of labor
B cells are known to play multiple effector and regulatory functions through diverse mechanisms of action[2]. Such mechanisms include the defining B cell function, namely antibody production after differentiation into plasmablasts (PB; proliferative, blasting antibody secreting cells typically of short life-span) and plasma cells (PC; mature, resting antibody secreting cells some of which may have very long life spans after homing either to the bone marrow or the spleen) [11]. Spontaneous antibody production may also be a function of specific B cell subsets, in particular B1 cells. In addition, B cells may produce both, proinflammatory cytokines (including L-6, TNF and INFγ) [12], and regulatory cytokines, prominently including IL-10 [13]. Mouse models have demonstrated the ability of B cells to influence T cell activation and polarization into different effector T helper subsets including TH1, TH2 and TH17, a function that in autoimmune disease is likely of pathogenic consequence [12] [14–16]. On the other hand, B cells have also been reported to either induce or inhibit the generation of regulatory T cells [2,17,18,16]. Importantly, several B cell subsets are capable of inhibiting pro-inflammatory responses in macrophages and dendritic cells and the activation of effector T cells, to a large extent through the generation of IL-10. These regulatory B cell functions have been ascribed to different B cell subsets which have been variously labeled B regulatory cells (Bregs) and B10 cells, and will be further discussed below in the context of SLE and other human autoimmune diseases [19–22].
Finally, B cells are powerful antigen presenting cells with the ability to activate antigen-specific T cells and influence the development and/or the maintenance of T cell memory [23]. While some studies have provided experimental evidence for antigen-specific Bregs, the full extent of this phenomenon and the coordinated participation of the APC and IL-10 production functions remain to be fully elucidated.
Given the multiple functions played by B cells and their opposing effects in autoimmunity, it remains essential to understand whether there exists strict division of labor among different B cell subsets or whether instead, there is significant functional plasticity among multiple B cell subsets which could be induced by extrinsic cues in a disease specific fashion. Under the former model, it should be possible to assign a specific, function-linked, surface or transcriptional phenotype to distinct B cell subsets, analyze their frequency in a given disease and in individual patients and design therapeutic approaches to target the population of interest accordingly (whether to eliminate, inhibit or expand such population). Moreover, this could be done in an individualized, patient-directed fashion. Under the latter model, it would be more effective to target the extrinsic milieu responsible for pathogenic alteration of B cell functions. Of course, these two approaches need not be mutually exclusive and thus, one could envision global or selective elimination of specific pathogenic B cell subsets combined with strategies to modify the immunological environment in order to promote B cell protective functions.
Thus, a precise understanding of the phenotype, function and developmental programs of different human B cell subsets is of the essence for a rational design of B cell targeted therapies. The current state of knowledge of human B cell diversity will be discussed in the next section.
Human B cell heterogeneity and function
In a normal immune system, immature bone marrow B cells progressively mature through a transitional stage that can be broken down into several discreet subsets (transitional T1, T2 and T3) [24]. Of note, murine T3 cells have also been reported to represent hyporesponsive anergic B cells [25] that may be decreased in murine lupus [26]. Maturation of transitional cells into either naïve follicular B2 cells can happen either in the bone marrow or in the spleen. Transitional cells can also differentiate into marginal zone (MZ) B cells but this process is limited to the spleen and may involve a specific transitional cell precursor (T2-MZP) [27]. MZ B cells have been well defined in the human spleen with almost universal expression of high levels of the CD27 memory marker and intermediate levels of surface IgD and IgM isotypes [28,29]. In addition, a blood population of cells with a similar phenotype albeit with lower levels of CD27 has been proposed to represent a recirculating MZ counterpart [30]. While their existence and accordingly their phenotype has long been disputed in humans, recent work has identified a human B1 cell compartment although some controversy still exists as to the nature and frequency of these cells [31–33]. B1 cells can be functionally split as CD11b+ B1 cells spontaneously secrete IL-10 and act as powerful APC due in part to high expression of CD86. Somewhat counterintuitively, these cells have been reported to both promote T cell proliferation and inhibit T cell activation and on that basis have been termed B1 orchestrators (B1orc) [34,35]. In contrast, CD11b- B1 cells (representing 9o% of all B1 cells), spontaneously produce IgM antibodies (B1 secretors; B1sec). The role of B1 cells in human autoimmunity in general and SLE in particular remains to be defined.
Of note, immature and transitional cells are subject to tolerance checkpoints that censor autoreactivity [36]. Yet, as described by our lab and others, mature naïve B cells still contain approximately 30% of autoreactive cells that are censored by anergy [37]. Such anergic B cells are unable to compete for BAFF-mediated signals responsible for follicular survival and excess BAFF results in autoimmunity [38–40]. Upon encountering antigen in the follicles, naïve B cells get activated through cognate BCR engagement (signal 1); T cell help (signal 2); and co-stimulatory factors (signal 3). The downstream consequences of naïve B cell activation are incompletely understood in humans. In mouse studies however, activated naïve B cells, first participate in an early extrafollicular reaction through interaction with macrophages, dendritic cells and extrafollicular T follicular helper (TFH) cells in an IL-21 independent fashion [41]. This phase generates early plasmablasts (PB), that are short-lived but may be enriched in high affinity cells and display significant mutation, whereas lower affinity clones migrate to the germinal centers (GC) for additional somatic hypermutation (SHM) and affinity maturation under the stimulation of IL-21-producing TFH cells [42,43]. In the GC, aN cells may differentiate into long-lived PC (LLPC) that migrate to the bone marrow (BM) [11,44], or undergo multiple rounds of division, SHM and antigen selection to generate memory cells with increased affinity for the offending antigen [43]. It should be noted however that the GC reaction also generates early IgM memory cells and plasmablasts (PB) and that memory B cells, including isotype switched memory cells (swM) can also be generated through GC-independent pathways [45–47]. In at least some mouse models, lasting IgM memory cells are generated by initial GC reactions formed in response to immunization which may persist for several months. These IgM memory cells may undergo additional GC reactions and experience isotype switch, progressive diversification and selection [45]. Antigen-specific responses can by enhanced by multiple polyclonal stimulators, including TLR signals, IL-21, IL-6, type I IFN, APRIL and IL-10, that may also expand non-specific memory cells through a bystander effect [48,49]. Moreover, multiple cytokines including IL-21, IL-17, IL-6 and IFN can promote dysregulated GC and autoimmunity [43]. Of interest, all these mediators have been implicated in the pathogenesis of human SLE and represent therapeutic targets for this disease [50–53].
As previously stated, these processes in general and the extra-follicular pathway in particular are less clearly understood in humans due to obvious experimental limitations and the lack of precise and consistent phenotypic definitions. Nevertheless, a substantial body of work, including our own [54,55], has contributed to the recognition of multiple markers that help identify human B cell subsets corresponding to the differentiation stages previously discussed including pro-B, pre-B, immature, transitional, naïve, memory and ASC including pre-PB, immature PB and mature PC (summarized in Table 1). Moreover, multiple subsets of human memory cells have been recognized which share the expression of CD27 which is considered a universal marker of human memory cells [54]. Thus, in addition to the MZ B cells previously discussed (also referred to as natural memory or unswitched memory), human CD27+ memory cells also include IgM-only cells lacking IgD (pre-switch memory), and isotype switched subsets including IgD+ (a small minority), IgG+ and IgA+ populations. Moreover, a population of isotype switched somatically mutated cells lacking expression of CD27 has been reported by different groups either in normal tonsils (FcRL4+, tissue-based memory) or chronic infections such as HIV and malaria (FcRL4+, prematurely exhausted cells) [56,57]. We originally reported large expansion of these cells (defined as IgD-CD27- double negative, DN, cells) in patients with active SLE [58]. The combination of our results and others strongly suggest that these cells derive either from extrafollicular reactions or the early phase of primary GC reactions [59,58].
Table 1.
Classification and phenotypic markers of human B cell populations.
| B cell population (CD19+ unless otherwise noted) | Markers | Function/Properties | SLE | ||
|---|---|---|---|---|---|
| Transitional | T1/T2 | CD24++CD38++CD10+CD27-IgM++ | Developmental precursor |
|
|
| T2-MZP | CD24++CD38++CD10+CD27-IgM++CD21+ | Regulatory (IL-10) MZ precursor |
 numbers |
||
| T3 | CD24+CD38+CD10+/-CD27-IgM++IgD+ | Developmental precursor |
|
||
| Naïve | Resting | CD24+/-CD38+/-CD27-IgM++/+IgD++CD21+ CD95- | Developmental precursor |
|
|
| Activated | CD24-CD38-CD27-IgM++ IgD++CD21-CD95+MTG+ | Precursor of short-lived PB and GC reactions |
|
||
| Anergic | CD24-CD38-CD27-IgMlow/-IgD+ | Hypo-responsive. Maintenance of tolerance |
|
||
| Memory | Unswitched | IgD+IgM+CD27+CD1c+ | Natural memory MZ equivalent |
|
|
| Pre-switched | IgM+IgD-CD27+ | Pre-switch memory Early IgM memory IgG memory precursor |
|
||
| Switched | Resting | IgG/IgA+CD27+CD21+ CD95- | Protective anti-microbial memory? |
|
|
| Activated | IgG/IgA+CD27+CD21-CD95+CD86+ | Pathogenic autoimmune memory? |
|
||
| Atypical memory | IgM/IgG/IgA+CD27-FcRL4+ | Tissue based-memory Exhausted memory? |
|
||
| B1 cells | CD11b+ (B1orc) | IgM+IgD++CD43+CD70- | CD86: T cell proliferation IL-10: T cell suppression |
|
|
| CD11b-(B1sec) | IgM+IgD++CD43+ CD70- | IgM production |
|
||
| Marginal zone | Spleen | IgD+IgM+CD27++ CD21++CD1c+ | Natural memory MZ equivalent |
?? | |
| Circulating | IgD+IgM+CD27+CD1c+ | Natural memory MZ equivalent |
|
||
| Antibody secreting cells | Circulating | Pre-PB | CD38++CD27+/-CD138-Ki67+ | Antibody secretion | ?? |
| PB | CD38++CD27++CD138-Ki67+ | Antibody secretion |
|
||
| PC | CD38++CD27++CD138+Ki-67+ | Antibody secretion |
|
||
| Bone marrow | Immature | CD38++CD27++CD138-Ki67- | Antibody secretion | ?? | |
| Mature | CD19+/-CD38++CD27++ CD138+Ki-67- | Antibody secretion | ?? | ||
| B regulatory cells | CD24hiCD38hi transitional cells | CD24++CD38++CD27- | IL-10: T cell suppression |
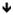 function |
|
| CD24++CD38++CD27-CD1d+ | iNKT cell induction |
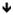 function |
|||
| B10 | CD24hiCD27+CD38+/-CD48++CD148++ IgM+/-IgD+/- | T cell and macrophage inhibition |
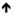 numbers |
||
Proposed classification of human B cell subsets with surface phenotypic markers derived from multiple studies and our own work [54,55,21,22,16,20,35,34,104,37,105,106,58]. Functions ascribed to these populations (or equivalent ones) in the literature are shown. Numeric or functional alterations demonstrated in at least some SLE studies are indicated.
It is also important to discuss the different phenotypes proposed by regulatory B cells, a population with protective effects in autoimmune conditions [60] and whose preservation or enhancement should presumably be an important consideration in the design of B cell targeting therapies. While Breg function has been proposed in the mouse for different cell types including B1 [61], MZ B cells [62], B10 cells with a CD1dhigh CD5+ intermediate phenotype [19] and transitional cells [63], in humans a Breg function has been proposed for naïve (IgD+CD27-) [64], transitional (CD24hiCD38hi) [21], B10 cells (CD24hiCD27+) [20] and B1orc cells (IgDhiCD43-CD27+CD70-CD11b+) [35].
Finally, consideration should be given to the phenotypic differentiation between short-lived PB and long-lived PC as the latter type is most likely responsible for the generation of important autoantibodies in SLE (including anti-RNA binding protein antibodies such as anti-Ro, Smith and U1-RNP that are powerful inducers of type I interferon production) and other autoimmune diseases. Given that long-lived PC survive current anti-B cell agents, with the possible exception of atacicept further discussed below, there is a need for the identification of markers unique to this population that could be specifically targeted by new agents.
B cells in SLE. Implications for therapy
The pathogenic significance of B cells in SLE is supported by the prominent presence of multiple autoantibodies that can recognize in excess of 100 different autoantigens [65]. SLE autoantibodies include disease-specific ones such as anti-dsDNA/chromatin/nucleosomes, anti-Sm and autoantibodies encoded by VH4-34 (recognized by the 9G4 anti-idiotype and accordingly referred to as 9G4 antibodies)[66,67]. Of great interest, with the exception of anti-Sm, SLE-specific autoantibodies tend to fluctuate with disease activity [68–70,66], thereby suggesting that they are produced by short-lived PB generated by ongoing immune responses, a feature of significant importance for the design and understanding of outcome of B cell therapies. The importance of B cell in SLE is also illustrated by multiple B cell abnormalities [71,67,58,72–75]; the concentration of SLE susceptibility genes on B cell pathways [76,77]; and the efficacy of B cell therapies [78,79,52,80]. From a cellular standpoint, SLE has arguably the most dramatic B cell changes of any human autoimmune disease thus far studied [55]. Naïve lymphopenia and increases in transitional, CD27+ switched memory (swM) cells in general and their activated CD21-CD95+ fraction, DN cells as well as PB have been reported in active SLE, in general with positive correlation with active disease. Moreover, multiple abnormalities of relevance to the putative Breg populations have also been reported including decreased Breg (CD24hiCD38hi) function despite increased cell numbers [21] and decreased circulating MZ cells (IgD+CD27+ unswitched memory cells). Of great interest, substantial abnormalities have also been reported for B10 and B1 cells in SLE. Their actual functional significance remains to be ascertained as both B10 and mostly their precursors (pro-B10 cells) are in SLE patients [20] as were the level of the IL-10 producing B1orc cells [34].
Overview of current B cell therapies in SLE
The clinical value of B cell targeted therapies in SLE and a growing number of other autoimmune diseases has been discussed in detail in a large number of publications including recent general reviews of the topic [81], and a more focused discussion of the rationale and the potential advantages of anti-CD19 antibodies [82]. Other reviews that provide a comprehensive discussion of different approaches to B cell targeting by multiple mechanisms of action are also available [10,52] and provide a summary of therapeutic modalities that directly or indirectly may result in strong anti-B cell and/or anti-plasma cell effects [10,53,52,83,82,8,1,23]. Overall, we find it useful to categorize B cell agents based on both, their breadth of B cell targeting (including their impact on antibody producing cells) and their mechanism of action [10]. As for the latter, it is most informative to separate B cell therapies into those that directly and quickly kill most B cells (best illustrated by anti-CD20 antibodies) and those that compromise the activation, differentiation and/or survival of B cells. The latter class of agents tends to target discreet B cell subsets and therefore, carries greater promise for application to disease subsets characterized by abnormalities in the corresponding B cell population.
Universal B cell depletion with anti-CD20 antibodies is the best studied modality and has achieved great success and FDA approval for both Rheumatoid Arthritis and ANCA-mediated Vasculitis [3,4]. This approach however, has failed to demonstrate added value over conventional therapy in both non-renal Lupus (EXPLORER) and Lupus Nephritis (LUNAR), randomized, placebo-controlled trials (RPCT) [7,6]. As discussed in many publications, these trials suffered from significant limitations including relatively small size which may have rendered them underpowered to detect significant differences in the context of highly effective background therapies and relatively short-duration. Moreover, it is important to note that pre-specified sub-group analysis demonstrated significant benefit in the Rituximab group in Hispanic and African-American patients in EXLORER and that the LUNAR study found, also in pre-specified analysis, a larger effect in African-American patients (75% vs 40%) although this substantail difference did not reach statistical significance due to the low number of such patients in the study (N=40). Of interest, the Rituximab group attained higher numbers of partial remission, an outcome that portends better long-term results in patients with lupus nephritis. Finally, it should also be noted that all patients that needed rescue therapy with cyclophosphamide belonged to the non-Rituximab group [84] Combined with multiple open observational studies (many with a high proportion of patients refractory to conventional therapy) and registries, there remains a strong possibility that anti-CD20 antibodies may be beneficial in at least some subsets of lupus patients.
In contrast to Rituximab, much larger studies of the anti-BLyS/BAFF antibody Belimumab (BLISS-52 and BLISS-76 RPCT) using patients with more limited background therapy and different outcome measurements, demonstrated a significant benefit of this modality in patients with moderate degrees of disease activity and exclusion of renal and CNS lupus. These studies also failed to assess the value of this therapy in African-American patients. These studies led to the FDA approval of Benlysta for the treatment of non-renal lupus. While the actual value of this therapy in the clinic continues to be established, subsequent studies have indicated that it may be of greater benefit in patients with significant clinical and serological activity [85]. Overall, Belimumab is also helpful to decrease SLE flares and corticosteroids needs.
Other direct anti-B cell agents that have been substantially tested in human SLE include antibodies against the inhibitory receptor CD22 (Epratuzumab) and combined BAFF/APRIL inhibitors (TACI-Ig or atacicept). Atacicept has been tested in a RPCT in combination with corticosteroids and mycophenolate mofetil. This treatment demonstrated a quick and powerful effect in serum antibody levels yet it had to be stopped due to severe infections in 3 out of 4 patients treated [86]. Epratuzumab illustrates the strategy of targeting inhibitory co-receptors that may dampen B cell activation and improve disease. Epratuzumab has been in two SLE studies, one of which was prematurely interrupted by lack of drug supply [87].
It should be noted that multiple other strategies can be envisioned to target B cells and plasma cells. Indeed, a large number of agents have been tested in animal models and in some human autoimmune diseases (such as Syk inhibitors in Rheumatoid Arthritis). These approaches have been discussed elsewhere [10,52].
Learning from B cell biology and the use of biological agents in SLE
The field of B cell therapies perfectly illustrate the rational application of immunological knowledge to the development of targeted therapies. However, as previously discussed in this review, this promise will only be fulfilled through a better understanding of the pathogenic and protective roles played by different B cell subsets and the clinical consequences of imbalances in different B cell populations and functions. Indeed, extant studies and analysis of the clinical experience gathered over the years with current B cell agents already provide important clues. In keeping with the diverse different roles previously discussed for B cells, the clinical impact of B cell therapies would depend on their effect on specific B cell populations and the contribution of these populations to antibody-dependent and antibody-independent pathogenic functions and to the promotion of B cell regulatory functions. Therefore, a significant difference exists between agents that induce general B cell depletion (such as anti-CD20 agents and anti-CD19 antibodies, the latter category targeting a larger swath of B cells including pro-B cells and a fraction of mature plasma cells) and those with more selective B cell targeting (such as anti-BAFF, anti-CD22 and anti-BCR signaling agents). Thus, the benefit of antibodies designed to directly kill as many B cells as possible will rest of the actual degree of depletion initially obtained and the type of repopulation achieved down the line. The depth of initial depletion is a matter of the greatest consequence and may determine the clinical outcome and the quality of repopulation. Thus, several studies have shown good correlation between peripheral depletion at the level of ≤0.01 CD19+ B cells/μl of blood and good responses [88,89]. Moreover, deep depletion minimizes the level of residual memory cells and plasma cells and may minimize also the preferential homeostatic proliferation and expansion of these residual cells in the absence of competing new bone marrow B cell output which can be suppressed by lingering amounts of anti-B cell antibodies and other poorly understood mechanisms. Finally, deep depletion of pre-existing activated cells may also promote a favorable reconstitution of the B cell compartment with a strong predominance with transitional cells with regulatory function and presumably restored enforcement of tolerance at the transitional-naïve developmental checkpoint. This ideal outcome could be promoted by regimens that maximize the initial deep of depletion including synergistic anti-B cell effects in combination with cyclophosphamide, a common clinical practice for the treatment of refractory lupus patient. The well-known anti-B cell activity of cyclophosphamide has been recently highlighted by the profound degree of B cell depletion induced by this agent in one of the arms of the RAVE study for ANCA-mediated vasculitis [90]. Moreover, favorable outcomes could be promoted by the sequential use of agents likely to enforce tolerance or decrease B cell activation during the reconstitution phase (such as anti-BAFF, anti-CD22 and anti-BCR and co-stimulatory signaling pathways). Finally, it is worth considering the possibility of Rituximan titration as it is possible that the depth of initial depletion might be improved with either higher or additional doses of this drug.
From an antibody standpoint, despite the lack of direct effect Rituximab on plasma cells, this therapy diminishes the levels of some autoantibodies but not others presumably due to survival of CD20- long-lived plasma cells [23,91]. While autoantibody decrease should most certainly contribute substantially to disease amelioration, both the clinical improvement that ensues before substantial antibody decline and the strong clinical response observed in patients that maintain their autoantibodies suggest the contribution of other important mechanisms as well including the restoration of a favorable B cell compartment dominated by transitional and naïve B cells [79,92,4]. While it remains to be determined whether such balance result from the lack of pathogenic cells or by the expansion of regulatory B cells [50], recent studies support a role for the latter possibility. Thus, in SLE Rituximab-induced B cell depletion induces a population of CD1d+ CD38hiCD24hi transitional B cells that in turn induce suppressive invariant NKT cells [22]. Of note, SLE CD24hiCD38hi transitional cells are deficient in IL-10 mediated regulatory function [21] and phenotypically, represent the main B cell reconstituting population in SLE patients with good response to Rituximab [93]. The same cells can induce T regulatory cells and suppress Th1 and TH17 development in RA [16]. However, whether either IL-10 mediated suppression, Treg induction or suppression of TH1/TH17 cells by CD24hiCD38hi transitional B cells during the reconstitution phase is a major mechanism of action of Rituximab remains to be formally addressed. It should be noted however while the actual mechanisms may remain unclear, rituximab-induced B cell depletion has been shown to result, at least in some studies, in attenuation of T cell activation [94,95] and decreased TH1 and TH17 development [96]. Similarly, B cell depletion may be followed by expansion of T regulatory cells following B cell depletion has also been described [97].
Given the above considerations, what to make then of the clinical benefit observed with agents that fail to deplete large fractions of B cells and may in fact, target the very cells proposed to exert regulatory functions, in particular transitional B cells? Indeed, Belimumab decreases total B cells by 50% through 76 weeks of treatment with significant reductions demonstrated as early as 8 weeks of treatment [98]. B cell reduction is largely accounted for by a decline in naïve cells by a median of 40% at 8 weeks and by 75% at 76 weeks, without significant memory cell reductions. In a different study, sustained decreases in transitional cells were reached after 3 months of treatment [99]. Importantly, only the decrease of CD20+CD27- cells (which would include both transitional and naïve cells as well as a population of isotype switched CD27- cells known to correlate with active SLE) correlated with clinical improvement and lower risk of severe flare.
Similar to belimumab, the anti-CD22 antibody epratuzumab has shown clinical efficacy in SLE studies [87,100]. In these studies, epratuzumab induced a 40% reduction in total B cells with preferential elimination of transitional and naïve B cells and attenuated expression of surface CD22 on these cells [101,100]. However, epratuzumab did not significantly decrease memory cells levels nor it induced significant changes in antibody levels [100].
Combined, the experience with both Belimumab and epratuzumab would appear counterintuitive with current models of SLE pathogenesis in which disease activity would be mediated by activated memory cells and autoantibody-secreting cells. They should also raise important questions regarding the importance of Breg mechanisms, the type of B cells that play regulatory roles and the impact of different B cell therapies on these functions. Indeed, given the regulatory potential of transitional and naïve cells and the substantial numerical contraction experienced by these compartments with both Belimumab and Epratuzumab despite the clinical improvement observed with these agents, it will be critical to examine if their regulatory function is enhanced by these treatments. Alternatively, it is important to consider that BAFF levels set the threshold for negative selection of early autoreactive B cells and that excess BAFF facilitates their selection into the mature compartment [38]. Similarly, Epratuzumab might enhance the CD22 inhibitory function on mature B cells thereby decreasing B cell activation. Therefore, both agents might play protective roles by decreasing B cell activation and enforcing tolerance. Moreover, given that CD22 enhances expression of L-selectin and β7 and β1 integrins and facilitates CXCL12-induced migration of naive B cells [102], it is likely that epratuzumab could also inhibit B cell migration to different tissues possibly including germinal centers [103].
It is worth noting however that belimumab has been reported to induce significant and rather quick reductions of total serum IgG levels and anti-ds DNA antibody titers. Moreover, anti-Sm antibodies were lost in approximately 30% of patients treated with high-dose Belimumab [98]. In view of the predominant impact of this agent of pre-germinal center B cells, these observations would be consistent with the notion that activation of naïve B cells and their recruitment into the antibody-secreting compartment play an important pathogenic role in SLE that can be interrupted by available agents. This model is supported by recent experimental data from our laboratory [37](Tam and Chris ACR abstract) and suggests the benefit of combination therapies with sequential used of induction therapy with generalized B cell depletion followed by maintenance therapy with more specific agents such as belimumab or epratuzumab in order to promote tolerance and decrease the activation of newly generated naïve B cells during the reconstitution phase. It is likely that a similar favorable B cell profile could be achieved during reconstitution with other agents capable of decreasing B cell activation including Syk and Btk inhibitors and other agents that target the BCR signaling and co-stimulatory pathways [10].
Final considerations. Challenges and opportunities
The next few years will witness a much better definition of the heterogeneity of human B cell subsets and their participation in SLE pathogenesis with a precise understanding of their mechanisms of action. This knowledge will be brought about by the confluence of better subset discrimination by multi-chromatic flow cytometry and an understanding of the autoreactivity mediated by such populations, facilitated by new technologies that enable the high-throughput interrogation of antigenic reactivity at the single cell level. When applied to large, well characterized patient cohorts, this information will greatly enhance the power of genetics, epigenetic and molecular studies to segment SLE into different subsets. Combined with the ability of the pharmaceutical industry to develop a large variety of agents capable of targeting specific cell subsets and genetic and molecular pathways through biologics, small molecules, RNA interference and other approaches, it should be finally possible to realize the thus far elusive dream of precision medicine in SLE. In addition to better and safer treatments of ongoing disease, patient segmentation based on cellular, genetic and molecular abnormalities should also provide useful biomarkers to estimate risk of disease progression and flares and facilitate early treatment to improve long-term disease outcome. Ultimately, these approaches should also enable to predict disease development in high-risk subjects and provide safe and effective treatments capable of preventing the progression of pre-clinical autoimmunity into autoimmune disease.
References
- 1.Chan AC. B cell immunotherapy in autoimmunity - 2010 update. Molecular Immunology. 2011;48 (11):1344–1347. doi: 10.1016/j.molimm.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 2.Manjarrez-Orduno N, Quach TD, Sanz I. B Cells and Immunological Tolerance. J Invest Dermatol. 2009;129 (2):278–288. doi: 10.1038/jid.2008.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, Stevens RM, Shaw T. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 4.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, Kallenberg CG, St Clair EW, Turkiewicz A, Tchao NK, Webber L, Ding L, Sejismundo LP, Mieras K, Weitzenkamp D, Ikle D, Seyfert-Margolis V, Mueller M, Brunetta P, Allen NB, Fervenza FC, Geetha D, Keogh KA, Kissin EY, Monach PA, Peikert T, Stegeman C, Ytterberg SR, Specks U. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363(3):221–232. doi: 10.1056/NEJMoa0909905[doi]. 363/3/221 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hahn BH. Belimumab for Systemic Lupus Erythematosus. New England Journal of Medicine. 2013;368 (16):1528–1535. doi: 10.1056/NEJMct1207259. [DOI] [PubMed] [Google Scholar]
- 6.Merrill JT, Buyon JP, Furie RA, Latinis KM, Gordon C, Hsieh H-J, Brunetta PG. Assessment of flares in lupus patients enrolled in a phase II/III study of rituximab (EXPLORER) Lupus. 2011 doi: 10.1177/0961203310395802. [DOI] [PubMed] [Google Scholar]
- 7.Rovin BH, Furie R, Latinis K, Looney RJ, Fervenza FC, Sanchez-Guerrero J, Maciuca R, Zhang D, Garg JP, Brunetta P, Appel G, Group LI. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: The Lupus Nephritis Assessment with Rituximab study. Arthritis & Rheumatism. 2012;64 (4):1215–1226. doi: 10.1002/art.34359. [DOI] [PubMed] [Google Scholar]
- 8.Dörner T, Isenberg D, Jayne D, Wiendl H, Zillikens D, Burmester G. Current status on B-cell depletion therapy in autoimmune diseases other than rheumatoid arthritis. Autoimmunity Reviews. 2009;9 (2):82–89. doi: 10.1016/j.autrev.2009.08.007. http://dx.doi.org/10.1016/j.autrev.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Faurschou M, Jayne DRW. Anti–B Cell Antibody Therapies for Inflammatory Rheumatic Diseases. Annual Review of Medicine. 2013 doi: 10.1146/annurev-med-070912-133235. [DOI] [PubMed] [Google Scholar]
- 10.Sanz I. Pharmacological Effects and Mechanisms of Action of Agents Blocking B Cells. In: Bosch X, Ramos-Casals M, Khamashta MA, editors. Drugs Targeting B-Cells in Autoimmune Diseases. Milestones in Drug Therapy. Springer; Basel: 2014. pp. 37–64. [DOI] [Google Scholar]
- 11.Hiepe F, Dorner T, Hauser AE, Hoyer BF, Mei H, Radbruch A. Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat Rev Rheumatol. 2011 doi: 10.1038/nrrheum.2011.1. advance online publication. [DOI] [PubMed] [Google Scholar]
- 12.Lund FE. Cytokine-producing B lymphocytes -- key regulators of immunity. Current Opinion in Immunology. 2008;20 (3):332–338. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fillatreau S, Gray D, Anderton SM. Not always the bad guys: B cells as regulators of autoimmune pathology. Nature Reviews Immunology. 2008;8:391–397. doi: 10.1038/nri2315. [DOI] [PubMed] [Google Scholar]
- 14.Zhong X, Gao W, Degauque N, Bai C, Lu Y, Kenny J, Oukka M, Strom TB, Rothstein TL. Reciprocal generation of Th1/Th17 and Treg by B1 and B2 B cells. European Journal of Immunology. 2007;9999(9999):NA. doi: 10.1002/eji.200737296. [DOI] [PubMed] [Google Scholar]
- 15.Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nature Immunology. 2000;1 (6):475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 16.Flores-Borja F, Bosma A, Ng D, Reddy V, Ehrenstein MR, Isenberg DA, Mauri C. CD19+CD24hiCD38hi B Cells Maintain Regulatory T Cells While Limiting TH1 and TH17 Differentiation. Science Translational Medicine. 2013;5 (173):173ra123. doi: 10.1126/scitranslmed.3005407. [DOI] [PubMed] [Google Scholar]
- 17.Chen X, Jensen PE. Cutting Edge: Primary B Lymphocytes Preferentially Expand Allogeneic FoxP3+ CD4 T Cells. J Immunol. 2007;179 (4):2046–2050. doi: 10.4049/jimmunol.179.4.2046. [DOI] [PubMed] [Google Scholar]
- 18.Olson TS, Bamias G, Naganuma M, Rivera-Nieves J, Burcin TL, Ross W, Morris MA, Pizarro TT, Ernst PB, Cominelli F, Ley K. Expanded B cell population blocks regulatory T cells and exacerbates ileitis in a murine model of Crohn disease. J Clin Invest. 2004;114 (3):389–398. doi: 10.1172/jci200420855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanaba K, Bouaziz J-D, Haas KM, Poe JC, Fujimoto M, Tedder TF. A Regulatory B Cell Subset with a Unique CD1dhiCD5+ Phenotype Controls T Cell-Dependent Inflammatory Responses. Immunity. 2008;28 (5):639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Iwata Y, Matsushita T, Horikawa M, DiLillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD, Hall RP, St Clair EW, Tedder TF. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117 (2):530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32(1):129–140. doi: 10.1016/j.immuni.2009.11.009. S1074-7613(09)00547-0 [pii] [DOI] [PubMed] [Google Scholar]
- 22.Bosma A, Abdel-Gadir A, Isenberg David A, Jury Elizabeth C, Mauri C. Lipid-Antigen Presentation by CD1d+ B Cells Is Essential for the Maintenance of Invariant Natural Killer T Cells. Immunity. 2012;36 (3):477–490. doi: 10.1016/j.immuni.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin F, Chan AC. B Cell Immunobiology in Disease: Evolving Concepts from the Clinic. Annual Review of Immunology. 2006;24(1) doi: 10.1146/annurev.immunol.24.021605.090517. [DOI] [PubMed] [Google Scholar]
- 24.Lindsley RC, Thomas M, Srivastava B, Allman D. Generation of peripheral B cells occurs via two spatially and temporally distinct pathways. Blood. 2007;109 (6):2521–2528. doi: 10.1182/blood-2006-04-018085. [DOI] [PubMed] [Google Scholar]
- 25.Merrell KT, Benschop RJ, Gauld SB, Aviszus K, Decote-Ricardo D, Wysocki LJ, Cambier JC. Identification of Anergic B Cells within a Wild-Type Repertoire. Immunity. 2006;25 (6):953–962. doi: 10.1016/j.immuni.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 26.Teague BN, Pan Y, Mudd PA, Nakken B, Zhang Q, Szodoray P, Kim-Howard X, Wilson PC, Farris AD. Cutting Edge: Transitional T3 B Cells Do Not Give Rise to Mature B Cells, Have Undergone Selection, and Are Reduced in Murine Lupus. J Immunol. 2007;178 (12):7511–7515. doi: 10.4049/jimmunol.178.12.7511. [DOI] [PubMed] [Google Scholar]
- 27.Srivastava B, Quinn WJ, III, Hazard K, Erikson J, Allman D. Characterization of marginal zone B cell precursors. J Exp Med. 2005;202 (9):1225–1234. doi: 10.1084/jem.20051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ettinger R, Sims GP, Robbins R, Withers D, Fischer RT, Grammer AC, Kuchen S, Lipsky PE. IL-21 and BAFF/BLyS Synergize in Stimulating Plasma Cell Differentiation from a Unique Population of Human Splenic Memory B Cells. J Immunol. 2007;178 (5):2872–2882. doi: 10.4049/jimmunol.178.5.2872. [DOI] [PubMed] [Google Scholar]
- 29.Weller C-ARJ-CW. Splenic marginal zone B cells in humans: Where do they mutate their Ig receptor? European Journal of Immunology. 2005;35 (10):2789–2792. doi: 10.1002/eji.200535446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, Plebani A, Kumararatne DS, Bonnet D, Tournilhac O, Tchernia G, Steiniger B, Staudt LM, Casanova J-L, Reynaud C-A, Weill J-C. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a pre-diversified immunoglobulin repertoire. Blood. 2004 doi: 10.1182/blood-2004-01-0346. 2004-2001-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griffin D, Rothstein TL. Human B1 cell Frequency: Isolation and Analysis of Human B1 Cells. Frontiers in immunology. 2012:3. doi: 10.3389/fimmu.2012.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Descatoire M, Weill J-C, Reynaud C-A, Weller S. A human equivalent of mouse B-1 cells? The Journal of Experimental Medicine. 2011;208 (13):2563–2564. doi: 10.1084/jem.20112232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tangye SG. To B1 or not to B1: that really is still the question! Blood. 2013;121(26):5109–5110. doi: 10.1182/blood-2013-05-500074. [DOI] [PubMed] [Google Scholar]
- 34.Griffin DO, Rothstein TL. A small CD11b+ human B1 cell subpopulation stimulates T cells and is expanded in lupus. The Journal of Experimental Medicine. 2011;208 (13):2591–2598. doi: 10.1084/jem.20110978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffin DO, Rothstein TL. Human “orchestrator” CD11b(+) B1 cells spontaneously secrete interleukin-10 and regulate T-cell activity. Mol Med. 2012;18:1003–1008. doi: 10.2119/molmed.2012.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201 (5):703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quách TD, Manjarrez-Orduño N, Adlowitz DG, Silver L, Yang H, Wei C, Milner ECB, Sanz I. Anergic Responses Characterize a Large Fraction of Human Autoreactive Naive B Cells Expressing Low Levels of Surface IgM. The Journal of Immunology. 2011;186 (8):4640–4648. doi: 10.4049/jimmunol.1001946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lesley R, Xu Y, Kalled SL, Hess DM, Schwab SR, Shu HB, Cyster JG. Reduced Competitiveness of Autoantigen-Engaged B Cells due to Increased Dependence on BAFF. Immunity. 2004;20 (4):441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 39.Cancro MP. Tipping the scales of selection with BAFF. Immunity. 2004;20 (6):655–656. doi: 10.1016/j.immuni.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 40.Scholz J, Oropallo M, Sindhava V, Goenka R, Cancro M. The role of B lymphocyte stimulator in B cell biology: implications for the treatment of lupus. Lupus. 2013;22 (4):350–360. doi: 10.1177/0961203312469453. [DOI] [PubMed] [Google Scholar]
- 41.Chan TD, Gatto D, Wood K, Camidge T, Basten A, Brink R. Antigen Affinity Controls Rapid T-Dependent Antibody Production by Driving the Expansion Rather than the Differentiation or Extrafollicular Migration of Early Plasmablasts. The Journal of Immunology. 2009;183 (5):3139–3149. doi: 10.4049/jimmunol.0901690. [DOI] [PubMed] [Google Scholar]
- 42.Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. The Journal of Experimental Medicine. 2006;203 (4):1081–1091. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol. 2009;9(12):845–857. doi: 10.1038/nri2637. nri2637 [pii] [DOI] [PubMed] [Google Scholar]
- 44.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KGC, Dorner T, Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6 (10):741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 45.Dogan I, Bertocci B, Vilmont V, Delbos F, Megret J, Storck S, Reynaud C-A, Weill J-C. Multiple layers of B cell memory with different effector functions. Nat Immunol. 2009;10 (12):1292–1299. doi: 10.1038/ni.1814. http://www.nature.com/ni/journal/v10/n12/suppinfo/ni.1814_S1.html. [DOI] [PubMed] [Google Scholar]
- 46.Kaji T, Ishige A, Hikida M, Taka J, Hijikata A, Kubo M, Nagashima T, Takahashi Y, Kurosaki T, Okada M, Ohara O, Rajewsky K, Takemori T. Distinct cellular pathways select germline-encoded and somatically mutated antibodies into immunological memory. J Exp Med. 2012;209 (11):2079–2097. doi: 10.1084/jem.20120127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor JJ, Pape KA, Jenkins MK. A germinal center–independent pathway generates unswitched memory B cells early in the primary response. The Journal of Experimental Medicine. 2012;209 (3):597–606. doi: 10.1084/jem.20111696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: upregulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 2003 doi: 10.1182/blood-2002-11-3569. 2002–2011–3569. [DOI] [PubMed] [Google Scholar]
- 49.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298 (5601):2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 50.Calero I, Nieto JA, Sanz I. B Cell Therapies for Rheumatoid Arthritis: Beyond B cell Depletion. Rheumatic Disease Clinics of North America. 2010;36 (2):325–343. doi: 10.1016/j.rdc.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 51.Calero I, Sanz I. Targeting B cells for the treatment of SLE: the beginning of the end or the end of the beginning? Discov Med. 2010;10 (54):416–424. [PMC free article] [PubMed] [Google Scholar]
- 52.Sanz I, Lee FE. B cells as therapeutic targets in SLE. Nat Rev Rheumatol. 2010;6(6):326–337. doi: 10.1038/nrrheum.2010.68. nrrheum.2010.68 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramos-Casals M, Sanz I, Bosch X, Stone JH, Khamashta MA. B-cell-depleting Therapy in Systemic Lupus Erythematosus. The American Journal of Medicine. 2012;125 (4):327–336. doi: 10.1016/j.amjmed.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaminski DA, Wei C, Qian Y, Rosenberg AF, Sanz I. Advances in human B cell phenotypic profiling. Frontiers in immunology. 2012;3:302. doi: 10.3389/fimmu.2012.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaminski DA, Wei C, Rosenberg AF, Lee FE, Sanz I. Multiparameter flow cytometry and bioanalytics for B cell profiling in systemic lupus erythematosus. Methods Mol Biol. 2012;900:109–134. doi: 10.1007/978-1-60761-720-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ehrhardt GRA, Hsu JT, Gartland L, Leu C-M, Zhang S, Davis RS, Cooper MD. Expression of the immunoregulatory molecule FcRH4 defines a distinctive tissue-based population of memory B cells. The Journal of Experimental Medicine. 2005;202 (6):783–791. doi: 10.1084/jem.20050879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, Chun T-W, Fauci AS. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. The Journal of Experimental Medicine. 2008;205 (8):1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei C, Anolik J, Cappione A, Zheng B, Pugh-Bernard A, Brooks J, Lee EH, Milner EC, Sanz I. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J Immunol. 2007;178(10):6624–6633. doi: 10.4049/jimmunol.178.10.6624. 178/10/6624 [pii] [DOI] [PubMed] [Google Scholar]
- 59.Berkowska MA, Driessen GJA, Bikos V, Grosserichter-Wagener C, Stamatopoulos K, Cerutti A, He B, Biermann K, Lange JF, van der Burg M, van Dongen JJM, van Zelm MC. Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood. 2011;118 (8):2150–2158. doi: 10.1182/blood-2011-04-345579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3 (10):944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 61.O’Garra A, Chang R, Go N, Hastings R, Haughton G, Howard M. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. European Journal of Immunology. 1992;22 (3):711–717. doi: 10.1002/eji.1830220314. [DOI] [PubMed] [Google Scholar]
- 62.Lenert P, Brummel R, Field E, Ashman R. TLR-9 Activation of Marginal Zone B Cells in Lupus Mice Regulates Immunity Through Increased IL-10 Production. J Clin Immunol. 2005;25 (1):29–40. doi: 10.1007/s10875-005-0355-6. [DOI] [PubMed] [Google Scholar]
- 63.Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, Mauri C. Novel Suppressive Function of Transitional 2 B Cells in Experimental Arthritis. J Immunol. 2007;178 (12):7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- 64.Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H, Kim HJ, Bar-Or A. Distinct Effector Cytokine Profiles of Memory and Naive Human B Cell Subsets and Implication in Multiple Sclerosis. J Immunol. 2007;178 (10):6092–6099. doi: 10.4049/jimmunol.178.10.6092. [DOI] [PubMed] [Google Scholar]
- 65.Tan EM. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Advances in Immunology. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- 66.Jenks SA, Palmer EM, Marin EY, Hartson L, Chida AS, Richardson C, Sanz I. 9G4+ Autoantibodies Are an Important Source of Apoptotic Cell Reactivity Associated With High Levels of Disease Activity in Systemic Lupus Erythematosus. Arthritis & Rheumatism. 2013;65 (12):3165–3175. doi: 10.1002/art.38138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cappione A, 3rd, Anolik JH, Pugh-Bernard A, Barnard J, Dutcher P, Silverman G, Sanz I. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J Clin Invest. 2005;115(11):3205–3216. doi: 10.1172/JCI24179[doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Isenberg DA, Garton M, Reichlin MW, Reichlin M. Long-term follow-up of autoantibody profiles in black female lupus patients and clinical comparison with Caucasian and Asian patients. British Journal of Rheumatology. 1997;36 (2):229–233. doi: 10.1093/rheumatology/36.2.229. [DOI] [PubMed] [Google Scholar]
- 69.van Vollenhoven RF, Bieber MM, Powell MJ, Gupta PK, Bhat NM, Richards KL, Albano SA, Teng NN. VH4–34 encoded antibodies in systemic lupus erythematosus: a specific diagnostic marker that correlates with clinical disease characteristics. Journal of Rheumatology. 1999;26 (8):1727–1733. [PubMed] [Google Scholar]
- 70.Cappione A, Pugh-Bernard A, Sanz A. Lupus VH4.34-Encoded Antibodies Bind to a B220-Specific Glycoform of CD45 on the Surface of Human B Lymphocytes. Arthritis & Rheumatism. 2002;46:S222. [Google Scholar]
- 71.Pugh-Bernard AE, Silverman GJ, Cappione AJ, Villano ME, Ryan DH, Insel RA, Sanz I. Regulation of inherently autoreactive VH4–34 B cells in the maintenance of human B cell tolerance. J Clin Invest. 2001;108(7):1061–1070. doi: 10.1172/JCI12462[doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anolik JH, Looney RJ, Lund FE, Randall TD, Sanz I. Insights into the heterogeneity of human B cells: diverse functions, roles in autoimmunity, and use as therapeutic targets. Immunol Res. 2009 doi: 10.1007/s12026-009-8096-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jenks SA, Sanz I. Altered B cell receptor signaling in human systemic lupus erythematosus. Autoimmunity Reviews. 2009;8 (3):209–213. doi: 10.1016/j.autrev.2008.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Palanichamy A, Barnard J, Zheng B, Owen T, Quach T, Wei C, Looney RJ, Sanz I, Anolik JH. Novel human transitional B cell populations revealed by B cell depletion therapy. J Immunol. 2009;182(10):5982–5993. doi: 10.4049/jimmunol.0801859. 182/10/5982 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jacobi AM, Reiter K, Mackay M, Aranow C, Hiepe F, Radbruch A, Hansen A, Burmester G, Diamond B, ELP, Dörner T. Activated memory B cell subsets correlate with disease activity in systemic lupus erythematosus: Delineation by expression of CD27, IgD, and CD95. Arthritis & Rheumatism. 2008;58 (6):1762–1773. doi: 10.1002/art.23498. [DOI] [PubMed] [Google Scholar]
- 76.Vaughn SE, Kottyan LC, Munroe ME, Harley JB. Genetic susceptibility to lupus: the biological basis of genetic risk found in B cell signaling pathways. Journal of Leukocyte Biology. 2012;92 (3):577–591. doi: 10.1189/jlb.0212095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cambier JC. Autoimmunity risk alleles: hotspots in B cell regulatory signaling pathways. J Clin Invest. 2013:1–4. doi: 10.1172/JCI69289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Anolik JH, Barnard J, Cappione A, Pugh-Bernard AE, Felgar RE, Looney RJ, Sanz I. Rituximab improves peripheral B cell abnormalities in human systemic lupus erythematosus. Arthritis & Rheumatism. 2004;50 (11):3580–3590. doi: 10.1002/art.20592. [DOI] [PubMed] [Google Scholar]
- 79.Looney RJ, Anolik JH, Campbell D, Felgar RE, Young F, Arend LJ, Sloand JA, Rosenblatt J, Sanz I. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis & Rheumatism. 2004;50 (8):2580–2589. doi: 10.1002/art.20430. [DOI] [PubMed] [Google Scholar]
- 80.Ching KH, Burbelo PD, Gonzalez-Begne M, Roberts ME, Coca A, Sanz I, Iadarola MJ. Salivary anti-Ro60 and anti-Ro52 Antibody Profiles to Diagnose Sjogren’s Syndrome. J Dent Res. 2011 doi: 10.1177/0022034510390811. 0022034510390811 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Faurschou M, Jayne DRW. Anti–B Cell Antibody Therapies for Inflammatory Rheumatic Diseases. Annual Review of Medicine. 2014;65(1) doi: 10.1146/annurev-med-070912-133235. null. [DOI] [PubMed] [Google Scholar]
- 82.Mei H, Schmidt S, Dorner T. Rationale of anti-CD19 immunotherapy: an option to target autoreactive plasma cells in autoimmunity. Arthritis Research & Therapy. 2012;14 (Suppl 5):S1. doi: 10.1186/ar3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sanz I. Indications for Rituximab in Autoimmune Diseases. Drug Discovery Today: Therapeutic Strategies. 2009;6 (1):13–19. doi: 10.1016/j.ddstr.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lightstone L. The landscape after LUNAR: Rituximab’s crater-filled path. Arthritis & Rheumatism. 2012;64 (4):962–965. doi: 10.1002/art.34362. [DOI] [PubMed] [Google Scholar]
- 85.van Vollenhoven RF, Petri MA, Cervera R, Roth DA, Ji BN, Kleoudis CS, Zhong ZJ, Freimuth W. Belimumab in the treatment of systemic lupus erythematosus: high disease activity predictors of response. Annals of the Rheumatic Diseases. 2012;71 (8):1343–1349. doi: 10.1136/annrheumdis-2011-200937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ginzler E, Wax S, Rajeswaran A, Copt S, Hillson J, Ramos E, Singer N. Atacicept in combination with MMF and corticosteroids in lupus nephritis: results of a prematurely terminated trial. Arthritis Research & Therapy. 2012;14 (1):R33. doi: 10.1186/ar3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wallace DJ, Kalunian K, Petri MA, Strand V, Houssiau FA, Pike M, Kilgallen B, Bongardt S, Barry A, Kelley L, Gordon C. Efficacy and safety of epratuzumab in patients with moderate/severe active systemic lupus erythematosus: results from EMBLEM, a phase IIb, randomised, double-blind, placebo-controlled, multicentre study. Annals of the Rheumatic Diseases. 2013 doi: 10.1136/annrheumdis-2012-202760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Looney RJ, Anolik JH, Campbell D, Felgar RE, Young F, Arend LJ, Sloand J, Rosenblatt J, Sanz I. B cell depletion as a novel treatment for systemic lupus erythematosus: A phase I/II dose-escalation trial of rituximab. Arthritis & Rheumatism. 2004;50 (8):2580–2589. doi: 10.1002/art.20430. [DOI] [PubMed] [Google Scholar]
- 89.Vital EM, Dass S, Buch MH, Henshaw K, Pease CT, Martin MF, Ponchel F, Rawstron AC, Emery P. B cell biomarkers of rituximab responses in systemic lupus erythematosus. Arthritis & Rheumatism. 2011;63 (10):3038–3047. doi: 10.1002/art.30466. [DOI] [PubMed] [Google Scholar]
- 90.Miloslavsky EM, Specks U, Merkel PA, Seo P, Spiera R, Langford CA, Hoffman GS, Kallenberg CGM, St Clair EW, Tchao NK, Viviano L, Ding L, Sejismundo LP, Mieras K, Ikle D, Jepson B, Mueller M, Brunetta P, Allen NB, Fervenza FC, Geetha D, Keogh K, Kissin EY, Monach PA, Peikert T, Stegeman C, Ytterberg SR, Stone H for the R-ITNRG. Clinical outcomes of remission induction therapy for severe ANCA-Associated vasculitis. Arthritis & Rheumatism. 2013:n/a–n/a. doi: 10.1002/art.38044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cambridge G, Leandro MJ, Teodorescu M, Manson J, Rahman A, Isenberg DA, Edwards JC. B cell depletion therapy in systemic lupus erythematosus: Effect on autoantibody and antimicrobial antibody profiles. Arthritis Rheum. 2006;54 (11):3612–3622. doi: 10.1002/art.22211. [DOI] [PubMed] [Google Scholar]
- 92.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, Langer-Gould A, Smith CH, Group HT. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis.[see comment] New England Journal of Medicine. 2008;358 (7):676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 93.Anolik J, Barnard J, Owen T, Zheng B, Kemshett S, Looney J, Sanz I. Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis & Rheumatism. 2007;56 (9):3044–3056. doi: 10.1002/art.22810. [DOI] [PubMed] [Google Scholar]
- 94.Iwata S, Saito K, Tokunaga M, Yamaoka K, Nawata M, Yukawa S, Hanami K, Fukuyo S, Miyagawa I, Kubo S, Tanaka Y. Phenotypic Changes of Lymphocytes in Patients with Systemic Lupus Erythematosus Who Are in Longterm Remission After B Cell Depletion Therapy with Rituximab. The Journal of Rheumatology. 2010 doi: 10.3899/jrheum.100729. [DOI] [PubMed] [Google Scholar]
- 95.Sfikakis PP, Boletis JN, Lionaki S, Vigklis V, Fragiadaki V, Iniotaki A, Moutsopoulos HM. Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down-regulation of the T cell costimulatory molecule CD40 ligand: An open-label trial. Arthritis & Rheumatism. 2005;52 (2):501–513. doi: 10.1002/art.20858. [DOI] [PubMed] [Google Scholar]
- 96.van de Veerdonk FL, Lauwerys B, Marijnissen RJ, Timmermans K, Di Padova F, Koenders MI, Gutierrez-Roelens I, Durez P, Netea MG, van der Meer JWM, van den Berg WB, Joosten LAB. The anti-CD20 antibody rituximab reduces the Th17 cell response. Arthritis & Rheumatism. 2011;63 (6):1507–1516. doi: 10.1002/art.30314. [DOI] [PubMed] [Google Scholar]
- 97.Stasi R, Cooper N, Del Poeta G, Stipa E, Evangelista ML, Abruzzese E, Amadori S. Analysis of regulatory T cell changes in patients with idiopathic thrombocytopenic purpura receiving B-cell depleting therapy with rituximab. Blood. 2008 doi: 10.1182/blood-2007-12-129262. blood-2007-2012-129262. [DOI] [PubMed] [Google Scholar]
- 98.Stohl W, Hiepe F, Latinis KM, Thomas M, Scheinberg MA, Clarke A, Aranow C, Wellborne FR, Abud-Mendoza C, Hough DR, Pineda L, Migone T-S, Zhong ZJ, Freimuth WW, Chatham WW on behalf of the B, Groups B-S. Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus. Arthritis & Rheumatism. 2012;64 (7):2328–2337. doi: 10.1002/art.34400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jacobi AM, Huang W, Wang T, Freimuth W, Sanz I, Furie R, Mackay M, Aranow C, Diamond B, Davidson A. Effect of long-term belimumab treatment on b cells in systemic lupus erythematosus: Extension of a phase II, double-blind, placebo-controlled, dose-ranging study. Arthritis & Rheumatism. 2010;62 (1):201–210. doi: 10.1002/art.27189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wallace DJ, Gordon C, Strand V, Hobbs K, Petri M, Kalunian K, Houssiau F, Tak PP, Isenberg DA, Kelley L, Kilgallen B, Barry AN, Wegener WA, Goldenberg DM. Efficacy and safety of epratuzumab in patients with moderate/severe flaring systemic lupus erythematosus: results from two randomized, double-blind, placebo-controlled, multicentre studies (ALLEVIATE) and follow-up. Rheumatology (Oxford) 2013 doi: 10.1093/rheumatology/ket129. [DOI] [PubMed] [Google Scholar]
- 101.Jacobi AM, Goldenberg DM, Hiepe F, Radbruch A, Burmester GR, Dörner T. Differential effects of epratuzumab on peripheral blood B cells of patients with systemic lupus erythematosus versus normal controls. Annals of the Rheumatic Diseases. 2008;67 (4):450–457. doi: 10.1136/ard.2007.075762. [DOI] [PubMed] [Google Scholar]
- 102.Daridon C, Blassfeld D, Reiter K, Mei H, Giesecke C, Goldenberg D, Hansen A, Hostmann A, Frolich D, Dorner T. Epratuzumab targeting of CD22 affects adhesion molecule expression and migration of B-cells in systemic lupus erythematosus. Arthritis Research & Therapy. 2010;12 (6):R204. doi: 10.1186/ar3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Allen CDC, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N, Cyster JG. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol. 2004;5 (9):943–952. doi: 10.1038/ni1100. http://www.nature.com/ni/journal/v5/n9/suppinfo/ni1100_S1.html. [DOI] [PubMed] [Google Scholar]
- 104.Wei C, Jung J, Sanz I. OMIP-003: Phenotypic analysis of human memory B cells. Cytometry Part A. 2011:n/a–n/a. doi: 10.1002/cyto.a.21112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qian Y, Wei C, Eun-Hyung Lee F, Campbell J, Halliley J, Lee JA, Cai J, Kong YM, Sadat E, Thomson E, Dunn P, Seegmiller AC, Karandikar NJ, Tipton CM, Mosmann T, Sanz I, Scheuermann RH. Elucidation of seventeen human peripheral blood B-cell subsets and quantification of the tetanus response using a density-based method for the automated identification of cell populations in multidimensional flow cytometry data. Cytometry Part B: Clinical Cytometry. 2010;78B (S1):S69–S82. doi: 10.1002/cyto.b.20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Palanichamy A, Bernard J, Owen T, Zheng B, Conley T, Quach T, Wei C, Looney J, Sanz I, Anolik JH. Characterization of human late transitional B cells: Implications for systemic lupus. Arthritis & Rheumatism. 2008;58 (9):S446. [Google Scholar]


