Abstract
Background
SAP can mediate the function of SLAM molecules, which have been proposed to be involved in the development of autoimmunity in mice.
Objective
We sought to determine if the SLAM/SAP pathway regulates the establishment of human B-cell tolerance and what mechanisms of B-cell tolerance could be affected by SAP deficiency.
Methods
We tested the reactivity of antibodies isolated from single B cells from SAP-deficient X-linked lymphoproliferative disease (XLP) patients. The expressions of SAP and SLAM family members were assessed in human bone marrow developing B cells. We also analyzed regulatory T cell (Treg) function in XLP patients and healthy controls.
Results
We found that new emigrant/transitional B cells from XLP patients were enriched in autoreactive clones, revealing a defective central B-cell tolerance checkpoint in the absence of functional SAP. In agreement with a B-cell intrinsic regulation of central tolerance, we identified SAP expression in a discrete subset of bone marrow immature B cells. SAP colocalized with SLAMF6 only in association with clustered B-cell receptors (BCRs) likely recognizing self-antigens, suggesting that SLAM/SAP regulate BCR-mediated central tolerance. In addition, XLP patients displayed defective peripheral B-cell tolerance, which is normally controlled by Tregs. Tregs in XLP patients seem functional but SAP-deficient T cells were resistant to Treg-mediated suppression. Indeed, SAP-deficient T cells were hyper-responsive to TCR stimulation, which resulted in increased secretion of interleukin-2, IFNγ and TNFα.
Conclusions
SAP expression is required for the counterselection of developing autoreactive B cells and prevents their T-cell dependent accumulation in the periphery.
Keywords: SAP, SLAM, B-cell tolerance, Regulatory T cells
INTRODUCTION
X-linked lymphoproliferative disease 1 (XLP1) is a primary immunodeficiency due to mutations/deletions in the SH2D1A gene, which encodes the SLAM-associated protein (SAP) (1–3). SAP is a single SH2 domain-containing molecule that plays a crucial role in the signaling of SLAM molecules. It may function as an adaptor for the Src family tyrosine kinase Fyn as well as a competitor for phosphatases such as SHP-1 and SHP-2, thereby modulating the function of SLAM family members (4). The SAP/SLAM pathway has been implicated in the development of autoimmunity. The mouse Sle1b locus, which has been linked to lupus susceptibility, contains genes encoding members of the SLAM family (5). In the lupus-prone mouse strain NZM2410, the expression of the Ly108.1 isoform leads to altered central B-cell tolerance mechanisms, including B-cell anergy, receptor editing and deletion (6). Although polymorphisms in SLAM family genes have been linked to lupus and rheumatoid arthritis in humans (7, 8), a direct role of the SAP/SLAM pathway in the control of B-cell tolerance in humans has not yet been demonstrated.
In healthy humans, most developing autoreactive B cells are removed at two discrete steps (9). First, a central tolerance checkpoint in the bone marrow between early immature and immature B cells removes most of developing B cells that express highly polyreactive antibodies. Then, a peripheral B cell tolerance checkpoint further counterselects autoreactive new emigrant B cells before they enter the mature naïve B-cell compartment (9). The central B-cell tolerance checkpoint seems to be mostly regulated by B-cell intrinsic pathways. Alterations of the B-cell receptor (BCR) signaling pathway in patients lacking functional BTK, or in healthy individuals carrying the C1858T PTPN22 risk allele result in the failure to counterselect developing autoreactive B cells in the bone marrow (10–12). In addition, mutations in genes encoding molecules such as IRAK-4, MyD88, UNC-93B and adenosine deaminase (ADA), which mediate and regulate the functions of Toll-like receptors (TLRs) potentially sensing self-antigens, also interfere with the establishment of central tolerance, especially towards nucleic acid containing antigens (11, 13, 14). While showing normal central B-cell tolerance, CD40L- and MHC class II-deficient patients display specific defects in the peripheral B-cell tolerance checkpoint, characterized by high frequencies of autoreactive mature naïve B cells correlating with low numbers of circulating CD4+CD25+CD127loFOXP3+ Tregs (15). Treg essential role in regulating the peripheral B-cell tolerance checkpoint was demonstrated in, FOXP3-deficient IPEX patients who display non-functional Tregs and harbor severe defects in the counterselection of autoreactive peripheral B cells (16). To determine the role of the SAP/SLAM pathway in the establishment of human B-cell tolerance checkpoints, we analyzed the repertoire and reactivity of antibodies expressed by single new emigrant/transitional and mature naive B cells from SAP-deficient XLP patients. We found that SAP is expressed by a discrete population of developing immature B cells and is required for central B-cell tolerance. We also found that SAP expression likely in T cells prevents the accumulation of autoreactive mature naive B cells, further suggesting the importance of B-T cell interactions for the establishment of peripheral B cell tolerance.
METHODS
Patients
XLP patients’ information is included in supplemental Table S1. Healthy donors were previously reported (9, 10, 12–17). None of the XLP patients experienced accelerated phases if they encountered EBV and none of the 4 XLP patients analyzed for antibody reactivity displayed the C1858T PTPN22 risk allele, which by itself interferes with the counterselection of developing autoreactive B cells (10–12). All samples were collected in accordance with institutional review board-reviewed protocols.
Cell staining and sorting, cDNA, RT-PCR, Antibody Production, ELISAs, and Indirect Fluorescence Assays
Single CD21loCD10++IgMhiCD27− new emigrant/transitional and CD21+CD10−IgM+CD27− peripheral mature naïve B cells from patients and control donors were sorted on a FACSVantage (Becton Dickinson) into 96-well PCR plates, and antibody reactivities were tested as previously described (9). The following antibodies were used for flow cytometry stainings: CD19-APC-Cy7, CD19-Pacific Blue, CD27-PerCP-Cy5.5, CD10-PE, CD10-PE-Cy7, IgM-FITC, CD21-APC, CD4-APC-Cy7, CD25-PE-Cy7, CD127-PerCP-Cy5.5, CD45RO-Pacific Blue, CD48-FITC, CD150-PE, CD352-PE, CD319-PE, CD244-APC (all from Biolegend), CD3-eFluor605NC (eBioscience), CD21-BDHorizonV450, CD84-PE (BD). The monoclonal anti-SAP antibody (clone1C9, Abnova) was biotinylated using the Fluoreporter Mini-biotin-XX protein labeling kit (Molecular Probes). Intracellular staining for FOXP3-AlexaFluor488 (clone PCH101, eBioscience) and Helios-AlexaFluor647 (Biolegend) were performed using the FOXP3/Transcription Factor Staining Buffer Set (eBioscience), according to the manufacturer’s instructions. For intracellular cytokine detection, CD4+ T responder cells activated for 4 days in vitro were then stimulated with 30 nM phorbol-12-myristate-13-acetate (PMA) and 200 nM ionomycin for 4 hours in the presence of GolgiStop (BD Biosciences) and intracellular staining of cytokines (IFNγ, TNFα), was performed with FOXP3 staining buffers (eBioscience) and the following antibodies: IFNγ (clone 4S.B3, eBioscience), TNFα (clone Mab11, eBioscience).
Real-time RT-PCR analysis
CD19-positive cells from fetal liver, fetal bone marrow or the peripheral blood of healthy donors were enriched using the CD19 magnetic beads (Miltenyi). Peripheral mature naïve B cells were further sorted to exclude the presence of CD3+ T cells, which represent the majority of circulating lymphocytes. Total RNA was then extracted using the Absolutely RNA Microprep Kit (Agilent Technologies) and 150ng RNA samples were reverse transcribed using random hexamers (Applied Biosystems) and SuperScript III Reverse Transcriptase kit (Invitrogen). For mRNA gene expression assays, probes were purchased from Applied Biosystems (SH2D1A (SAP): Hs00158978_m1, CD3E: Hs01062241_m1, HPRT1: Hs02800695_m1) and the reactions were run on a 7500 Real-Time PCR system (Applied Biosystems) in duplicate. Values are represented as the difference in Ct values normalized to HPRT1 for each sample before comparisons between fetal samples and peripheral B and T cells.
Immunofluorescence
PBMC depleted from CD20+ B cells, purified naive B cells (EasySep Naive B cell isolation kit) or pre-enriched CD34−CD19+ bone marrow B cells were washed in PBS 1X and deposited on poly-L-lysine-coated glass slides (Sigma-Aldrich) in a cytospin centrifuge (Thermo). Acetone-fixed cells were stained with mouse anti-IgM (Santa Cruz Biotechnology), -Igκ (BD Biosciences), -Igλ (BD Biosciences), VpreB (Biolegend) or -SLAMF6/CD352 (Biolegend); rat anti-SAP-PE (clone 1D12, Santa Cruz Biotechnology) or rat isotype control-PE (eBiosciences); DAPI or rabbit anti-Igκ and -Igλ (Dako Corporation). Primary antibodies were revealed with donkey anti-mouse AlexaFluor488 (Invitrogen Molecular Probes); goat anti-rat PE (Santa Cruz Biotechnology) followed by donkey anti-goat PE (Jackson ImmunoResearch); donkey anti-rabbit AlexaFluor350 (Invitrogen Molecular Probes). The percentage of cells presenting a clustered pattern of either IgM or Igκ/Igλ was estimated by counting the number of cells with clustered vs diffuse/non-clustered IgM or Igκ/Igλ cells in the SAP+ cells and in the SAP− cells. Slides were visualized under a Zeiss AxioObserver.Z1 microscope.
In vitro Treg suppression assay
CD4+T cells were enriched with the EasySep Human CD4+T cells kit (StemCell Technologies) and were either stained for FACS sort of CD4+CD25hiCD127lo/− Treg cells or depleted from CD25+ cells with anti-human CD25 microbeads (Miltenyi) to obtain the responder T (Tresp) cell fraction. CD4+CD25− Tresp cells were labeled with CellTrace CFSE (InVivogen). Co-cultures of Treg and Tresp at a 1:1 ratio with or without the addition of cytokines at 50 ng/mL concentration (Peprotech) were stimulated with the Treg Suppression Inspector Human kit (Miltenyi). Proliferation of the viable Tresp was analyzed by CFSE dilution at day 4.5.
Cytokine detection
Serum BAFF concentrations were determined by ELISA according to the manufacturer’s instruction (R&D Systems). Cytokines (IL-2, IL-4, IL-6, IL-7, IL-10, IL13, IFNγ, TNFα, GM-CSF) in plasma or culture supernatants were measured with the High Sensitivity Human Cytokine Magnetic Bead kit (Millipore), using a Luminex200. IL-21 was measured with the Human IL-21 ELISA Ready-Set-Go kit (eBiosciences).
KREC assay
The ratio of KREC joints (signal joint) to the Jκ-Cκ recombination genomic joints (coding joint) was determined as previously described (18, 19). The number of cell divisions was calculated by subtracting the cycle threshold of the PCR detecting the coding joint from that of the PCR detecting the signal joint.
Statistical analysis
Differences between patients and healthy donors were analyzed for statistical significance with unpaired Student’s t tests, using GraphPad Prism (GraphPad Software).
RESULTS
Defective central B-cell tolerance checkpoint in XLP patients
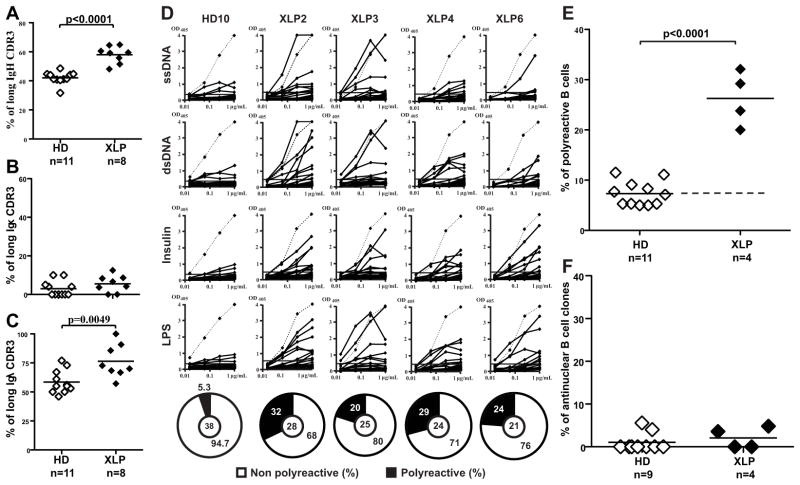
Most developing B cells that express polyreactive and antinuclear antibodies are removed at a central B-cell tolerance checkpoint in the bone marrow. To assess whether central tolerance is functional in SAP-deficient XLP patients, we cloned antibodies expressed by single CD19+CD10++CD21loIgMhiCD27− new emigrant/transitional B cells from 8 XLP patients (supplemental Tables S1–S9) and analyzed their immunoglobulin (Ig) gene repertoire. Although the immunoglobulin (Ig) heavy chain gene segment repertoires showed no differences between healthy donors and XLP patients (Fig. E1), we found that both Igκ and Igλ variable gene usages were biased in new emigrant/transitional B cells from XLP patients (Fig. E2). D reading frames favoring hydrophobic amino acids have been suggested to favor self-reactivity (10, 20). Remarkably, D6-6 gene is the only D gene that was virtually never found utilizing its hydrophobic reading frame in new emigrant B cells from 11 healthy donors (Fig. E3). In contrast, D6-6 was used as frequently in its hydrophobic and hydrophilic reading frame in XLP B cell counterparts, a feature that we previously observed in other patients with defective central B cell tolerance (10, 21). In addition, antibodies with long Ig heavy chain and Igλ but not Igκ CDR3s were frequently expressed in new emigrant/transitional B cells from XLP patients, another feature that favors self-reactivity normally counterselected in the bone marrow, suggesting an altered central B-cell tolerance checkpoint in these patients (Fig 1, A–C) (9). In agreement with these observations, we found that the frequency of polyreactive clones was significantly higher in all SAP-deficient XLP patients (average 26.3%) compared to healthy controls (average 7.3%) (p<0.0001);(Fig 1, D–E). However, the frequency of antinuclear antibody (ANA) clones was not increased, which suggests that ANA-expressing B cells are properly removed in the bone marrow of XLP patients (Fig 1, F). In conclusion, we found that the central B- cell tolerance checkpoint is impaired in the absence of functional SAP.
FIG 1. The central B-cell tolerance checkpoint is not functional in XLP patients.
The frequencies of long IgH (15 or more a.a.) (A), long Igκ (B) and Igλ (C) (11 or more a.a.) in new emigrant/transitional B cells are represented for 11 healthy donors and 8 XLP patients. Each diamond represents an individual. The average is shown with a bar. (D) Antibodies from new emigrant/transitional B cells from XLP patients were tested by ELISA for reactivity against ssDNA, dsDNA, insulin and lipopolysaccharide (LPS) and the frequencies of polyreactive (E) and anti-nuclear clones are displayed in (E) and (F), respectively. Dotted line: ED38-positive control. Horizontal line: cutoff OD405 for positive reactivity. For each individual, the frequency of polyreactive (filled area) and non polyreactive (open area) clones is summarized in pie charts, with the total number of clones tested indicated in the centers.
SAP is expressed in a discrete population of immature B cells
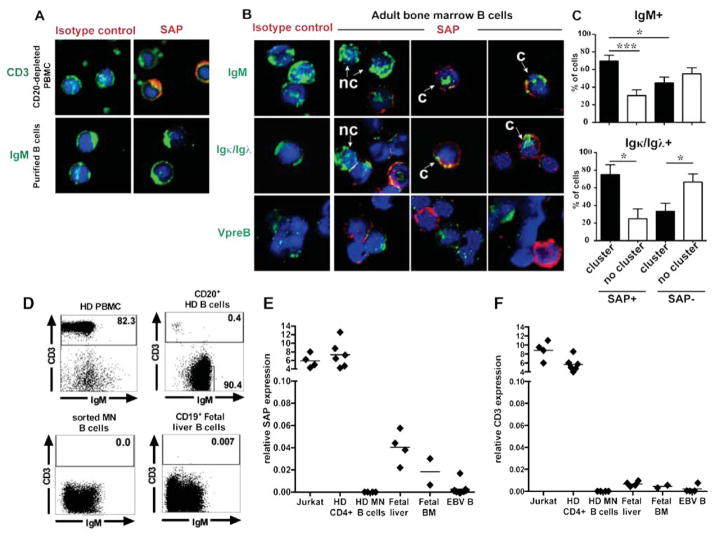
Central B-cell tolerance is believed to be regulated by intrinsic B-cell factors including BCRs and TLRs, which sense and bind to self-antigens at the immature B cell stage in the bone marrow (11). SAP expression in B cells is controversial and may be restricted to a subset of activated germinal center B cells (22–24). However, SAP expression in bone marrow B cells has not been characterized. Purified CD19+ precursor B cells from fetal or adult bone marrow showed SAP expression in about 10% of IgM+ cells, whereas SAP expression was not detected in peripheral blood B cells and only found in CD3+ T cells (Fig 2, A and B; Fig. E4A) (23, 24). SAP expression was restricted to Igκ+/Igλ+ immature B cells and not found in VpreB+ pre-B cells (Fig 2, B and Fig. E4, B and C). In addition, SAP+ immature B cells from fetal and adult bone marrow preferentially showed a pattern of clustered IgM or Igκ/Igλ, suggesting that these BCRs were crosslinked by self-antigens (Fig 2, C and Fig. E4, B and C). In contrast, SAP− immature B cells displayed non-clustered BCRs, suggesting that these B cells do not bind self-antigens (Fig 2, C and Fig. E4, B and C). To further identify SAP expression in human B cell precursors, we assessed the presence of SAP transcripts in fetal liver, fetal bone marrow CD19+ B cell precursors and mature naïve B cells that were further sorted to exclude any T cell contamination (Fig 2, D). We found that SAP transcripts could be detected in CD19+ B cell precursors from fetal liver and bone marrow but not in peripheral mature naïve B cells (Fig. 2, E). The absence of CD3E transcripts in all these CD19+ fractions further attested the absence of T cell contamination (Fig. 2, F). SAP expression was 100 fold lower in CD19+ fetal liver and bone marrow than in T cells but is consistent with SAP being only expressed in a discrete population (10%) of CD19+IgM+Igκ+/Igλ+ immature B cells, which itself, only represents about 20–30% of CD19+ cells (Fig. 2, E and Fig. E4D). Hence, SAP is expressed by a discrete subset of developing immature B cells, which are likely autoreactive and undergoing central selection.
FIG 2. SAP is expressed by some immature B cells.
(A) PBMC depleted from CD20+ B cells or purified naive B cells were stained for CD3 or IgM (green), SAP or isotype control (red). (B) CD19+ adult bone marrow cells were stained for IgM, Igκ and Igλ, VpreB (green) and SAP or isotype control (red). The proportion of cells in SAP+ vs SAP− cells that displayed a clustered (c) vs not clustered (n.c) IgM and Igκ/Igλ staining pattern is shown in (C). *:p-value<0.05; **: p-value<0.01. ***: p-value<0.001. (D) Dot plots show the proportion of CD3+ T cells in peripheral blood mononuclear cells from healthy donors (HD PBMC), CD20-enriched B cells using magnetic beads, sorted mature naive (MN) B cells and CD19+ fetal liver cells. SAP (E) and CD3E (F) gene expression was assessed by quantitative RT-PCR in MN B cells, CD19+ fetal liver and bone marrow cells and EBV cell lines. Jurkat and healthy donor (HD) CD4+ T cells were used as positive control for both SAP and CD3E expression.
SLAMF6 colocalizes with SAP when segregating with autoreactive BCRs
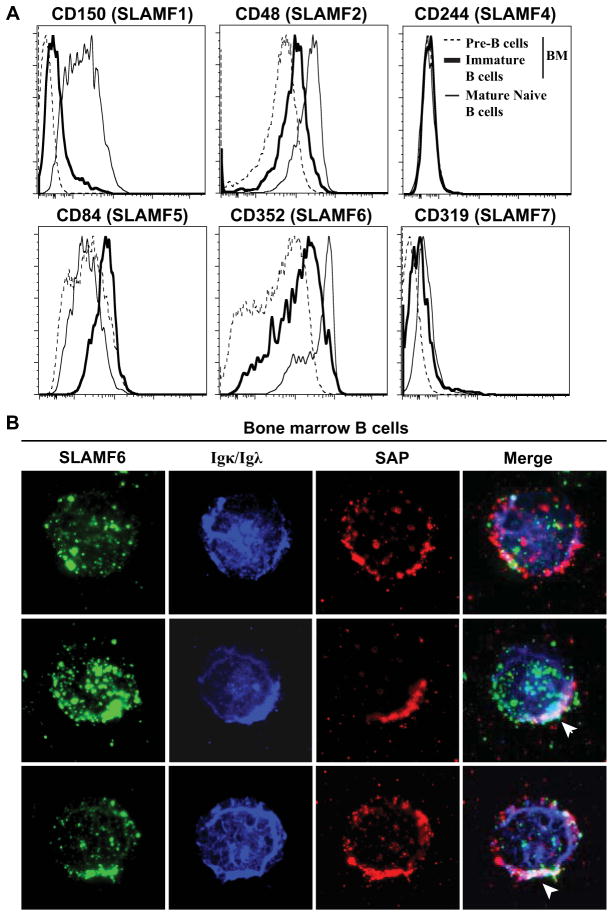
We assessed the expression during B-cell differentiation of SLAM family members that may require SAP to mediate their function (4). SLAMF1/CD150, SLAMF2/CD48 and SLAMF6/CD352 (human NTB-A/mouse Ly108) expression increased with B-cell maturation (low in pre-B cells, intermediate in immature B cells in the bone marrow and high in mature naive B cells in peripheral blood), whereas SLAMF5/CD84 expression seems to be increased at the immature B-cell stage (Fig 3, A). SLAMF4/CD244 and SLAMF7/CD319 (CRACC) are not expressed, or expressed at very low levels, respectively, suggesting that these SLAM members are unlikely to play a role in central B-cell selection (Fig 3, A). Since Slamf6 (mouse Ly108) is required for proper central B-cell tolerance mechanisms in mice (6) and because SLAMF6 (human NTB-A) seems to be one of the SLAM family members expressed at the highest level on immature B cells, we analyzed its expression by immunofluorescence in SAP+Igκ/Igλ+ immature B cells. We found in all analyzed cells that SLAMF6/CD352 and SAP only associated with each other when co-segregating with clustered BCRs (Fig 3 middle and bottom). In contrast, SLAMF6/CD352 and SAP never co-localized in immature B cells with diffuse Igκ/Igλ expression (Fig 3, B top). We conclude that SAP may be recruited by SLAMF6/CD352 to BCR clusters in autoreactive immature B cells, and may regulate BCR signaling and the induction of central B-cell tolerance mechanisms in human bone marrow.
FIG 3. SAP and SLAMF6 co-localize in B cells with aggregated BCRs.
(A) Overlays display the expression of SLAM family members by CD19+CD27−CD10+IgM− pre-B cells (dotted line) and CD19+CD27−CD10+IgM+ immature B cells (bold line) from bone marrow and peripheral CD19+CD27−CD10−IgM+ mature naive B cells (thin line). (B) CD19+ enriched bone marrow B cells were stained for SLAMF6 (green), Igκ/Igλ (blue) and SAP (red). Representative cells without (top row) or with (middle and bottom row) co-staining of SLAMF6, Igκ/Igλ and SAP are shown. Clustered Igκ/Igλ, SLAMF6 and SAP appear white as the superposition of green, red and blue and is indicated by an arrow.
Defective peripheral B-cell tolerance checkpoint in XLP patients
To analyze the impact of SAP-deficiency on the peripheral B-cell tolerance checkpoint, we tested by ELISA the reactivity of recombinant antibodies from CD19+CD10− CD21+IgM+CD27− mature naive B cells from XLP patients (supplemental Tables S10–S13) against HEp-2 cell lysates (Fig 4, A). We found that the frequency of mature naive B cells that expressed HEp-2 reactive antibodies was significantly increased in all four XLP patients tested (38–64%) compared to healthy donors (17–26%, p<0.0001; Fig 4, B). Consistent with these data, the frequency of mature naive polyreactive B cells was higher in XLP patients compared with healthy donors (p<0.0001) (Fig 4, C). However, there was no increase in ANA clones in the mature naive B-cell compartment of XLP patients (Fig 4, D). Thus, the high frequencies of HEp-2 reactive and polyreactive mature naive B cells in XLP patients reveal a defective peripheral B-cell tolerance checkpoint in the absence of functional SAP expression.
FIG 4. Defective peripheral B-cell tolerance checkpoint in XLP patients.
(A) Antibodies from mature naive B cells from XLP patients were tested by ELISA for anti-HEp-2 cell reactivity and the frequencies of HEp-2 reactive, polyreactive, and antinuclear mature naive B cells are shown in (B), (C) and (D). Dotted line: ED38-positive control. Horizontal lines: cutoff OD405 for positive reactivity. For each individual, the frequency of HEp-2 reactive (filled area) and non HEp-2 reactive (open area) clones is summarized in pie charts, with the total number of clones tested indicated in the centers.
XLP patients display normal serum BAFF concentration and Treg frequency
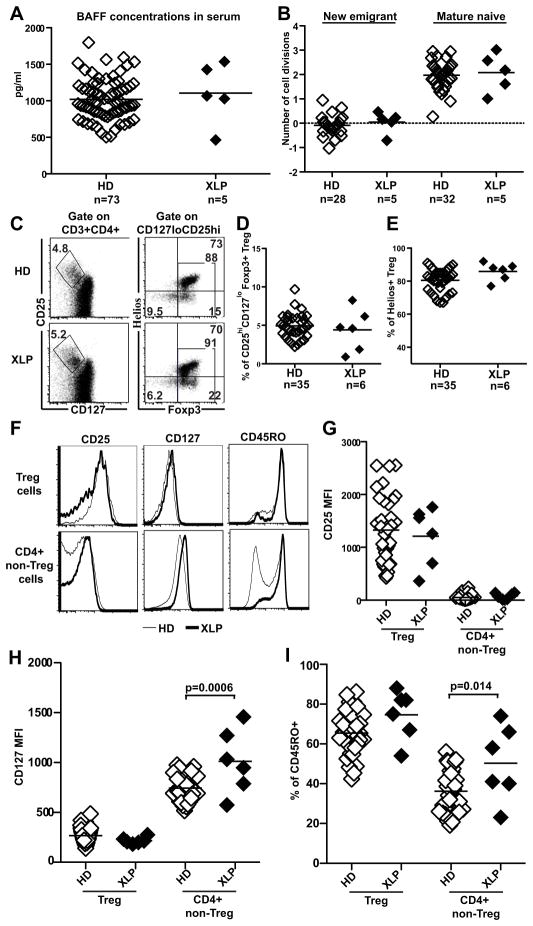
Defects in human peripheral B-cell tolerance checkpoint seem to correlate with increased serum BAFF concentrations and decreased Treg frequency (11). However, more recent analyses revealed that these defects may occur in the absence of BAFF dysregulation (15, 16). Indeed, we found that XLP patients displayed normal serum BAFF concentrations comparable to healthy donors, revealing that BAFF does not account for the defective peripheral B-cell tolerance checkpoint in XLP patients (Fig 5, A). Tregs play an essential role in preventing the accumulation of autoreactive mature naive B cells in the periphery and may control their homeostatic expansion, as is demonstrated by FOXP3-deficient IPEX patients who display a defective peripheral B cell tolerance checkpoint (16). SAP-deficient naïve B cell subsets showed a normal proliferative history, suggesting normal Treg functions in XLP patients (Fig 5, B). In agreement with this observation, normal Treg frequencies and phenotypes were found in XLP patients, as evidenced by the normal expression of Helios, CD25/IL-2Rα, CD127/IL-7Rα and CD45RO (Fig 5, C–I). In contrast, non-Treg FOXP3−Helios−CD4+ T-cell populations from XLP patients displayed a dysregulated phenotype characterized by an increased expression of CD127/IL-7Rα (XLP, MFI=1012 vs. healthy donors, MFI=744; p-value=0.0006) and elevated frequencies of CD45RO+ memory cells (Fig 5, F–I). Hence, SAP-deficient Tregs display a normal phenotype, suggesting that these T cells may be functional in XLP patients, whereas non-Treg CD4+ T cells show an altered phenotype in these patients.
FIG 5. XLP patients display normal serum BAFF concentrations and Treg frequencies.
(A). Serum BAFF concentrations (pg/ml) in healthy donors (n=73) and XLP patients (n=5). (B) Evaluation of the number of cell divisions undergone in vivo by KREC analysis on new emigrant and mature naive B cells of healthy donors (n=28 and n=32 respectively) and XLP patients (n=5). (C) Representative CD25 and CD127 (left) and Helios and FOXP3 staining (right) on CD3+CD4+ T cells from a healthy donor and an XLP patient. (D) CD3+CD4+CD25hiCD127loFOXP3+ Treg cell frequencies in 35 healthy donors and 6 XLP patients. (E) Frequencies of FOXP3+Helios+ cells in the CD3+CD4+CD25hiCD127loFOXP3+ population in 35 healthy donors and 6 XLP patients. (F) Representative staining of CD25, CD127 and CD45RO expression in an XLP patient (bold line) and HD (thin line) for FOXP3+Helios+ Treg cells and FOXP3−Helios− CD4+ T cells. MFI of CD25 (G), CD127 (H) and frequency of CD45RO+ cells (I) for Helios+FOXP3+ Treg and Helios−FOXP3− CD4+ non-Treg T cells from healthy donors and XLP patients.
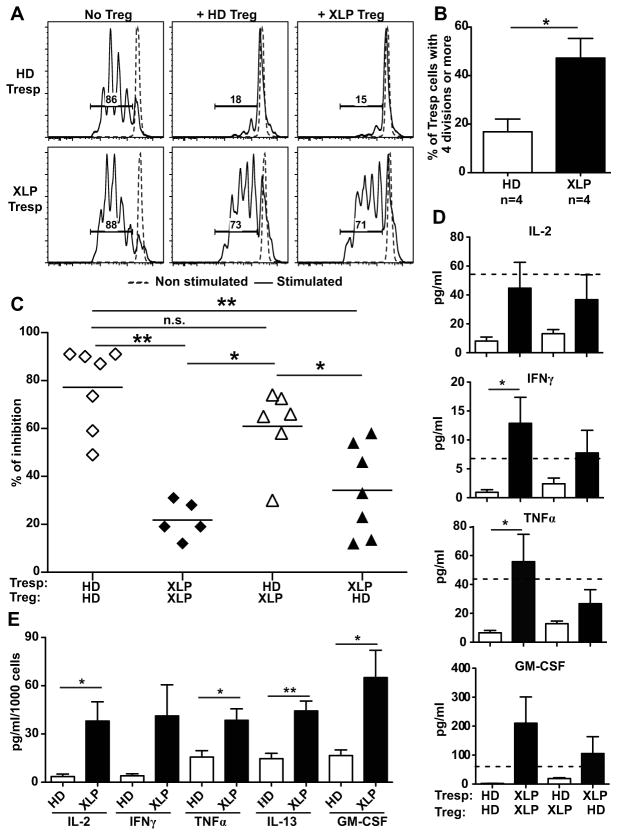
SAP-deficient T cells are resistant to in vitro suppression by Treg
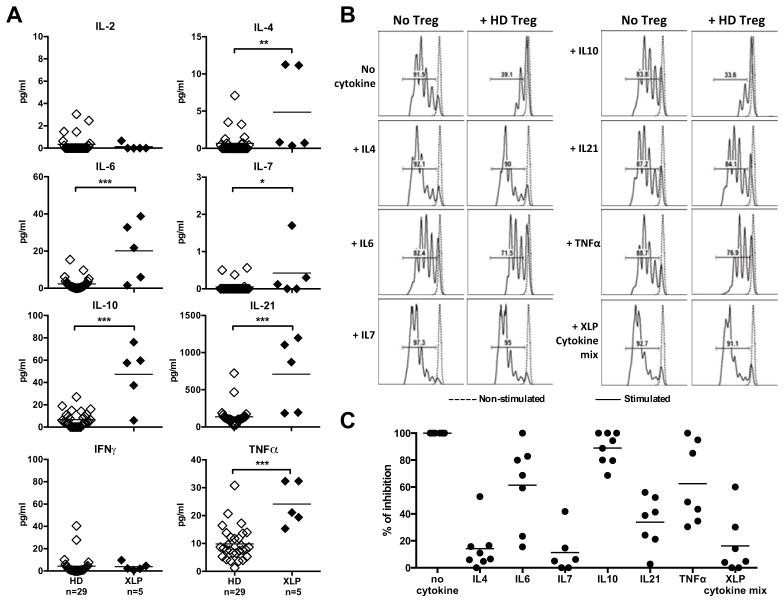
Control and SAP-deficient Tregs were then tested in vitro for their ability to suppress responder T (Tresp) cell proliferation. CD3+CD4+CD25hiCD127−/lo Tregs were sorted by flow cytometry and cultured with CFSE-labeled CD3+CD4+CD25− Tresp cells in the presence of anti-CD2, anti-CD3 and anti-CD28 coated beads. The proliferation of healthy donor Tresp cells was inhibited by addition of Treg, either from healthy controls or XLP individuals (Fig 6, A, C). Thus, SAP-deficient Tregs have normal suppressive functions. However, the proliferation of XLP Tresp cells was not inhibited by either HD or XLP Tregs, thereby showing that XLP Tresp are resistant to in vitro suppression by Treg (Fig 6, A, C). XLP Tresp were hyper-responsive to stimulation because 47 % of XLP Tresp cells had undergone 4 divisions after 4.5 days in culture vs only 17% of Tresp from healthy controls (Fig 6, B). This increased proliferation of SAP-deficient Tresp cells was linked to increased concentrations of cytokines such as IL-2, IFNγ, TNFα and GM-CSF, which were detected in the supernatants of these in vitro cultures in the presence of Tregs from either HD or XLP patients (Fig 6, D). These cytokines originate from Tresp cells themselves, as illustrated by the increased production of Th1 cytokines, IL-2, IFNγ, TNFα and GM-CSF, as well as the Th2 cytokine IL-13 by in vitro stimulated XLP Tresp cells compared to healthy donor controls (Fig 6, E). Increased production of IFNγ and TNFα by Tresp cells from XLP patients activated for 4.5 days in vitro was further evidenced by intracytoplasmic detection of these cytokines by flow cytometry (Fig. E5). The serum of XLP patients displayed increased concentrations of several cytokines, further reflecting the hyperactive T-cell phenotype of SAP-deficiency. Although we did not detect increased serum IL-2 and IFNγ, TNFα and IL-7 concentrations were higher in XLP patients (Fig 7). In addition, the sera of XLP patients were especially enriched in IL-4, IL-6, IL-10 and IL-21, which favor B-cell survival and activation (Fig 7, A). Moreover, we found that IL-4, IL-7 and IL-21 and to a lesser extent IL-6 and TNFα blocked by themselves in vitro Treg suppression (Fig. 7, B and C). When all the cytokines found elevated in XLP patients (XLP cytokine mix) were added to the Treg assay, we observed an inability for Tregs to suppress refractory Tresp cell proliferation similarly to Treg-Tresp isolated from XLP patients (Fig 7C and 6C). Hence, SAP deficiency affects peripheral CD4+ T cells, which are hyper-responsive to TCR stimulation and prone to secrete many Th1 and Th2/Tfh cytokines, rendering these T cells resistant to Treg suppression.
FIG 6. Increased resistance of SAP-deficient responder CD4+ T cells to suppression by Tregs.
(A) Representative histograms of Treg mediated suppression of autologous and heterologous CFSE labeled Tresp cells on day 4.5 from a XLP patient compared to a healthy donor (HD). Dashed line displays non-stimulated Tresp. (B) Increased percentage of Tresp that divided 4 times or more in XLP patients compared to HD in the ‘Tresp only’ culture after 4.5 days. The autologous and heterologous suppressive activities of Tregs from HD and XLP patients are summarized in (C). (D) Increased concentrations of IL-2, IFNγ, TNFα and GM-CSF in supernatants of XLP ‘Tresp+Treg’ cocultures. The doted line represents the level of each cytokine in the supernatant of the stimulated ‘HD Tresp only’ well. (E) displays concentrations of the indicated cytokines in stimulated HD or XLP CD4+ T cells monocultures after 4 days. *:p-value<0.05; **: p-value<0.01.
FIG 7. Elevated Th2/Tfh cytokine concentrations in the serum of XLP patients.
(A) IL-2, IFNγ, TNFα, IL-7, IL-4, IL-6, IL-10, IL-21 cytokine concentrations in the serum of 5 XLP patients and 29 healthy donors were measured by Luminex or ELISA. *:p-value<0.05; **: p-value<0.01. ****: p-value<0.0001. (B) Representative histograms of Treg mediated suppression of autologous CFSE labeled Tresp cells on day 4.5 from a healthy donor in the presence of the indicated cytokines. Dashed line displays non-stimulated Tresp and the suppressive activities of Tregs in the presence of diverse cytokines are summarized in (C). The XLP cytokine mix includes IL-4, IL-6, IL-7, IL-10, IL-21 and TNFα found elevated in XLP patients.
DISCUSSION
We report here that SAP/SLAMs play a major role in the establishment of central and peripheral B-cell tolerance in humans. Many transitional B cells that recently emigrated from the bone marrow of XLP patients expressed BCRs characterized by an abnormal immunoglobulin repertoire associated with self-reactivity, thereby demonstrating a defective central B-cell tolerance checkpoint in the absence of functional SAP. Central B-cell tolerance is normally regulated by intrinsic B-cell pathways involved in sensing the binding of self-antigens and inducing tolerance mechanisms such as receptor editing, anergy or deletion (11). Defective BCR and TLR signaling and function result in a failure to remove developing autoreactive B cells in the bone marrow (10–12). We identified SAP expression in a discrete population of B cell precursors that expressed both heavy and light chains and therefore belonged to the immature B cell compartment in which the reactivity of newly synthesized BCRs is assessed for binding to self-antigens. In addition, the preferential expression of SAP in immature B cells displaying aggregated BCRs likely binding self-antigens suggests that SAP expression may be mostly restricted to autoreactive clones. SAP expression in immature B cells may therefore be important for the establishment of central B cell tolerance by potentially regulating BCR signaling threshold and modifying SLAM functions. In T cells, the SAP/Ly108 pathway enhances the signaling strength of the TCR to reach the threshold required for restimulation-induced apoptosis and cytolytic function (25, 26). Indeed, in the absence of SAP, many SLAMs bind SHP-1, which is a negative regulator of TCR signaling (27). When SAP is expressed, SAP competes with and displaces SHP-1 binding to SLAMs and switches off Ly108/SLAMF6 inhibitory function (27, 28). Interestingly, we found that SLAMF6 is expressed in immature B cells and co-localized with SAP in BCR clusters. Moreover, Ly108, the mouse homologue to SLAMF6, regulates mechanisms of central B-cell tolerance by modulating the strength of the BCR signaling (6). Hence, SAP expression at the immature B-cell stage may allow SLAM proteins including SLAMF6 to modulate BCR signaling strength and thereby participate in the counter-selection of autoreactive B cells. We conclude that SLAM/SAP play an essential role in mediating central B-cell tolerance in humans, potentially by favoring the induction of apoptosis in immature autoreactive B cells as reported in B-cell derived cell lines (29).
In contrast to central tolerance, the establishment of peripheral B cell tolerance seems to rely on B-cell extrinsic factors. Indeed, defects in the expression of functional CD40L and FOXP3 mostly restricted to the T cell lineage result in a specific abnormal peripheral B-cell tolerance checkpoint (15, 16). The failure in XLP patients to counterselect autoreactive B cells between the new emigrant/transitional and mature naïve B cell stages at which SAP is no longer expressed in B cells further supports the B-cell extrinsic regulation of peripheral B-cell tolerance. Defects in the peripheral B-cell tolerance checkpoint often correlate with decreased Treg cell frequencies and increased serum BAFF concentrations (15). Recently, we identified similar peripheral B-cell tolerance abnormalities in FOXP3-deficient IPEX patients, who do not have functional Tregs and display normal serum BAFF concentrations. This observation further suggests that defective Treg functions in these patients, but not serum BAFF concentrations, are responsible for the impairment in preventing the accumulation of autoreactive B cells in the mature naïve B cell compartment (16). The normal serum BAFF concentrations in most XLP patients who display defective peripheral B-cell tolerance also suggest that BAFF is not responsible for the impaired peripheral selection of autoreactive B cells. However, we did not observe any decrease in Treg frequencies in SAP-deficient patients or any unusual phenotype of these T cells that could infer compromised functions. In addition, we found that Tregs retained their in vitro suppressive function in the absence of functional SAP. It has been proposed that Tregs may directly interact with autoreactive B cells by recognizing self-antigens presented by these B cells through MHC class II, leading to their elimination (30). Interestingly, examination of SAP-deficient mice revealed that functional interactions between B and T cells do not form in the absence of SAP, whereas T-dendritic cell interactions are not affected (27, 31–33). Hence, the defective peripheral B-cell tolerance checkpoint in XLP patients may be due to the inability of T cells and potentially Tregs to interact directly with autoreactive B cells and mediate their removal in the absence of functional SAP, independently of dendritic cells and other antigen-presenting cells.
Alternatively, increased cytokine secretion by SAP-deficient T cells may be responsible for a break in peripheral B-cell tolerance. Enhanced cytokine secretion in the absence of functional SAP detected in vitro in activation experiments as well as in vivo directly in the patients’ sera contrasted with the hypo-responsive phenotype reported in SAP-deficient T cell lines obtained from two XLP patients or in engineered Jurkat cells (34, 35). However, these artificial models may not be representative of polyclonal responses induced in freshly isolated T cells from XLP patients. Indeed, enhanced T cell responses in XLP patients were recently reported in two asymptomatic XLP patients (36). In addition, increased IFNγ secretion has been reported during acute EBV infection in XLP patients and in SAP-deficient mice infected with lymphocytic choriomeningitis virus or T. gondii (37–39). The increased IL-2, IFNγ and TNFα secretion may account for the ineffective suppression of Tresp proliferation by Tregs as suggested by several reports (40–42). In agreement with this hypothesis, we found that cytokines such as IL-4, IL-7 and IL-21 and to a lesser extent IL-6 and TNFα were able to inhibit by themselves in vitro Treg suppression. SAP-deficiency recapitulates defective suppressive scenarios observed in type 1 diabetes (T1D) and multiple sclerosis (MS). Indeed, T1D patients also display Tresp cells that are refractory to Treg suppression in vitro associated with a defective peripheral B-cell tolerance checkpoint (12, 43). IFNγ secretion by Tregs from MS patients results in a loss of in vitro suppressive activity and correlates with an abnormal peripheral B-cell tolerance checkpoint in these patients (19, 40). A defective suppression of T cells by Treg is also consistent with the overwhelming and uncontrolled B-cell and T-cell responses to EBV infection in XLP patients (39). In addition to rendering Tresp cells refractory to Tregs and perhaps directly affecting Treg suppressive function, Th2/Tfh cytokines including IL-4, IL-6, IL-10 and IL-21 favor B-cell activation, isotype switch and plasma cell development (42, 44). Paradoxically, XLP patients display severely decreased isotype-switched B cell frequencies likely resulting from defective interactions with CD4+ T cells (23, 24, 45). Nonetheless, SAP-deficiency could also interfere with the establishment of peripheral B-cell tolerance by favoring the survival of autoreactive B cells through enhanced serum cytokine concentrations.
SAP is essential for NKT cell development (46, 47). Consequently, XLP patients lack NKT cells. NKT cells express an invariant TCR that recognize lipid self-antigens and are able to provide cognate help to B cells in a SAP-dependent manner potentially preventing the activation of autoreactive B cells (48). Lower NKT numbers and function have been linked to the development of type 1 diabetes and lupus in NOD mice with genetic defects of slamf1 and slamf6 combined with altered SLAM expression (49). Therefore, we cannot exclude the involvement of NKT cells in the control of human peripheral B-cell tolerance. The absence of NKT cells may contribute to the improper removal of autoreactive clones from the mature naive B cell compartment of SAP-deficient XLP patients.
In conclusion, we found that SAP is required for the establishment of B-cell tolerance in humans. Transient SAP expression in bone marrow developing B cells is required for a functional central B-cell tolerance whereas, in the periphery, SAP expression in T cells is likely necessary for preventing the accumulation of autoreactive mature naïve B cells by ensuring stable B/T interactions and repressing uncontrolled cytokine secretion by human T cells.
Supplementary Material
Key messages.
SAP expression in human autoreactive immature B cells is required for the establishment of central B-cell tolerance in the bone marrow.
SAP expression by T cells prevents the accumulation of autoreactive mature naive B cells in the periphery.
Acknowledgments
Supported by National Institute of Health (NIH)/National Institute of Allergy and Infectious Diseases (NAID) grants AI061093, AI071087, AI082713, AI095848 (to E. M.), T32 AI089704 (to L.M.), the Division of Intramural Research, NIH/NAID (to A.K.) and a Rubicon grant from Netherlands Organization for Scientific Research (to T.C.).
We thank Dr. S. Rudchenko, L. Devine and C. Wang for cell sorting. This work was supported by Grant Number AI071087, AI082713 and AI095848 from NIH-NIAID (to E. M.) and AI061093 from NIH-NIAID (to E.M and C.C.R.). L.M was supported by T32 AI089704 and T.C. received support from Rubicon, Netherlands Organization for Scientific Research.
Abbreviations
- SLAM
Signaling Lymphocyte Activated Molecule
- SAP
SLAM-Associated Protein
- XLP
X-linked lymphoproliferative disease
- BCR
B-cell receptor
- TLR
Toll-like receptor
- Treg
Regulatory T cell
- Tresp
Responder T cell
- ADA
Adenosine deaminase
- FACS
Fluorescence-activated cell sorting
- ANA
Anti-nuclear antibody
- Ig
Immunoglobulin
- MFI
Mean Fluorescence Intensity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nichols KE, Harkin DP, Levitz S, Krainer M, Kolquist KA, Genovese C, et al. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc Natl Acad Sci U S A. 1998 Nov 10;95(23):13765–70. doi: 10.1073/pnas.95.23.13765. [Research Support, U.S. Gov’t, P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coffey AJ, Brooksbank RA, Brandau O, Oohashi T, Howell GR, Bye JM, et al. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat Genet. 1998 Oct;20(2):129–35. doi: 10.1038/2424. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 3.Sayos J, Wu C, Morra M, Wang N, Zhang X, Allen D, et al. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 1998 Oct 1;395(6701):462–9. doi: 10.1038/26683. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 4.Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol. 2011;29:665–705. doi: 10.1146/annurev-immunol-030409-101302. [Review] [DOI] [PubMed] [Google Scholar]
- 5.Wandstrat AE, Nguyen C, Limaye N, Chan AY, Subramanian S, Tian XH, et al. Association of extensive polymorphisms in the SLAM/CD2 gene cluster with murine lupus. Immunity. 2004 Dec;21(6):769–80. doi: 10.1016/j.immuni.2004.10.009. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 6.Kumar KR, Li L, Yan M, Bhaskarabhatla M, Mobley AB, Nguyen C, et al. Regulation of B cell tolerance by the lupus susceptibility gene Ly108. Science. 2006 Jun 16;312(5780):1665–9. doi: 10.1126/science.1125893. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 7.Cunninghame Graham DS, Vyse TJ, Fortin PR, Montpetit A, Cai YC, Lim S, et al. Association of LY9 in UK and Canadian SLE families. Genes Immun. 2008 Mar;9(2):93–102. doi: 10.1038/sj.gene.6364453. [Comparative Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 8.Suzuki A, Yamada R, Kochi Y, Sawada T, Okada Y, Matsuda K, et al. Functional SNPs in CD244 increase the risk of rheumatoid arthritis in a Japanese population. Nat Genet. 2008 Oct;40(10):1224–9. doi: 10.1038/ng.205. [Comparative Study Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 9.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003 Sep 5;301(5638):1374–7. doi: 10.1126/science.1086907. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 10.Ng YS, Wardemann H, Chelnis J, Cunningham-Rundles C, Meffre E. Bruton’s tyrosine kinase is essential for human B cell tolerance. J Exp Med. 2004 Oct 4;200(7):927–34. doi: 10.1084/jem.20040920. [Comparative Study Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meffre E. The establishment of early B cell tolerance in humans: lessons from primary immunodeficiency diseases. Ann N Y Acad Sci. 2011 Dec;1246:1–10. doi: 10.1111/j.1749-6632.2011.06347.x. [Research Support, N.I.H., Extramural Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menard L, Saadoun D, Isnardi I, Ng YS, Meyers G, Massad C, et al. The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J Clin Invest. 2011 Sep;121(9):3635–44. doi: 10.1172/JCI45790. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isnardi I, Ng YS, Srdanovic I, Motaghedi R, Rudchenko S, von Bernuth H, et al. IRAK-4- and MyD88-dependent pathways are essential for the removal of developing autoreactive B cells in humans. Immunity. 2008 Nov 14;29(5):746–57. doi: 10.1016/j.immuni.2008.09.015. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sauer AV, Morbach H, Brigida I, Ng YS, Aiuti A, Meffre E. Defective B cell tolerance in adenosine deaminase deficiency is corrected by gene therapy. J Clin Invest. 2012 Jun 1;122(6):2141–52. doi: 10.1172/JCI61788. [Clinical Trial Multicenter Study Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herve M, Isnardi I, Ng YS, Bussel JB, Ochs HD, Cunningham-Rundles C, et al. CD40 ligand and MHC class II expression are essential for human peripheral B cell tolerance. J Exp Med. 2007 Jul 9;204(7):1583–93. doi: 10.1084/jem.20062287. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kinnunen T, Chamberlain N, Morbach H, Choi J, Kim S, Craft J, et al. Accumulation of peripheral autoreactive B cells in the absence of functional human regulatory T cells. Blood. 2013;121(9):1595–603. doi: 10.1182/blood-2012-09-457465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isnardi I, Ng YS, Menard L, Meyers G, Saadoun D, Srdanovic I, et al. Complement receptor 2/CD21- human naive B cells contain mostly autoreactive unresponsive clones. Blood. 2010 Jun 17;115(24):5026–36. doi: 10.1182/blood-2009-09-243071. [Research Support, N.I.H., Extramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Zelm MC, Szczepanski T, van der Burg M, van Dongen JJ. Replication history of B lymphocytes reveals homeostatic proliferation and extensive antigen-induced B cell expansion. J Exp Med. 2007 Mar 19;204(3):645–55. doi: 10.1084/jem.20060964. [Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinnunen T, Chamberlain N, Morbach H, Cantaert T, Lynch M, Preston-Hurlburt P, et al. Specific peripheral B cell tolerance defects in patients with multiple sclerosis. J Clin Invest. 2013 Jun 3;123(6):2737–41. doi: 10.1172/JCI68775. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meffre E, Schaefer A, Wardemann H, Wilson P, Davis E, Nussenzweig MC. Surrogate light chain expressing human peripheral B cells produce self-reactive antibodies. J Exp Med. 2004 Jan 5;199(1):145–50. doi: 10.1084/jem.20031550. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyers G, Ng YS, Bannock JM, Lavoie A, Walter JE, Notarangelo LD, et al. Activation-induced cytidine deaminase (AID) is required for B-cell tolerance in humans. Proc Natl Acad Sci U S A. 2011 Jul 12;108(28):11554–9. doi: 10.1073/pnas.1102600108. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shlapatska LM, Mikhalap SV, Berdova AG, Zelensky OM, Yun TJ, Nichols KE, et al. CD150 association with either the SH2-containing inositol phosphatase or the SH2-containing protein tyrosine phosphatase is regulated by the adaptor protein SH2D1A. J Immunol. 2001 May 1;166(9):5480–7. doi: 10.4049/jimmunol.166.9.5480. [Comparative Study Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 23.Ma CS, Pittaluga S, Avery DT, Hare NJ, Maric I, Klion AD, et al. Selective generation of functional somatically mutated IgM+CD27+, but not Ig isotype-switched, memory B cells in X-linked lymphoproliferative disease. J Clin Invest. 2006 Feb;116(2):322–33. doi: 10.1172/JCI25720. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veillette A, Zhang S, Shi X, Dong Z, Davidson D, Zhong MC. SAP expression in T cells, not in B cells, is required for humoral immunity. Proc Natl Acad Sci U S A. 2008 Jan 29;105(4):1273–8. doi: 10.1073/pnas.0710698105. [Comparative Study Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snow AL, Marsh RA, Krummey SM, Roehrs P, Young LR, Zhang K, et al. Restimulation-induced apoptosis of T cells is impaired in patients with X-linked lymphoproliferative disease caused by SAP deficiency. J Clin Invest. 2009 Oct;119(10):2976–89. doi: 10.1172/JCI39518. [Research Support, N.I.H., Extramural Research Support, N.I.H., Intramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao F, Cannons JL, Dutta M, Griffiths GM, Schwartzberg PL. Positive and negative signaling through SLAM receptors regulate synapse organization and thresholds of cytolysis. Immunity. 2012 Jun 29;36(6):1003–16. doi: 10.1016/j.immuni.2012.05.017. [Comparative Study Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kageyama R, Cannons JL, Zhao F, Yusuf I, Lao C, Locci M, et al. The receptor Ly108 functions as a SAP adaptor-dependent on-off switch for T cell help to B cells and NKT cell development. Immunity. 2012 Jun 29;36(6):986–1002. doi: 10.1016/j.immuni.2012.05.016. [Research Support, N.I.H., Intramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palendira U, Low C, Chan A, Hislop AD, Ho E, Phan TG, et al. Molecular pathogenesis of EBV susceptibility in XLP as revealed by analysis of female carriers with heterozygous expression of SAP. PLoS Biol. 2011 Nov;9(11):e1001187. doi: 10.1371/journal.pbio.1001187. [Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagy N, Matskova L, Kis LL, Hellman U, Klein G, Klein E. The proapoptotic function of SAP provides a clue to the clinical picture of X-linked lymphoproliferative disease. Proc Natl Acad Sci U S A. 2009 Jul 21;106(29):11966–71. doi: 10.1073/pnas.0905691106. [Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao DM, Thornton AM, DiPaolo RJ, Shevach EM. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood. 2006 May 15;107(10):3925–32. doi: 10.1182/blood-2005-11-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008 Oct 9;455(7214):764–9. doi: 10.1038/nature07345. [Research Support, N.I.H., Intramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, Cheng J, et al. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 2010 Feb 26;32(2):253–65. doi: 10.1016/j.immuni.2010.01.010. [Research Support, N.I.H., Intramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R, et al. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity. 2010 Aug 27;33(2):241–53. doi: 10.1016/j.immuni.2010.07.015. [Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baldanzi G, Pighini A, Bettio V, Rainero E, Traini S, Chianale F, et al. SAP-mediated inhibition of diacylglycerol kinase alpha regulates TCR-induced diacylglycerol signaling. J Immunol. 2011 Dec 1;187(11):5941–51. doi: 10.4049/jimmunol.1002476. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanzone S, Zeyda M, Saemann MD, Soncini M, Holter W, Fritsch G, et al. SLAM-associated protein deficiency causes imbalanced early signal transduction and blocks downstream activation in T cells from X-linked lymphoproliferative disease patients. J Biol Chem. 2003 Aug 8;278(32):29593–9. doi: 10.1074/jbc.M300565200. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 36.Coraglia A, Felippo M, Schierloh P, Malbran A, de Bracco MM. CD4+ T Lymphocytes with follicular helper phenotype (T(FH)) in patients with SH2D1A deficiency (XLP) Clin Immunol. 2011 Dec;141(3):357–64. doi: 10.1016/j.clim.2011.09.007. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 37.Czar MJ, Kersh EN, Mijares LA, Lanier G, Lewis J, Yap G, et al. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proc Natl Acad Sci U S A. 2001 Jun 19;98(13):7449–54. doi: 10.1073/pnas.131193098. [Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu C, Nguyen KB, Pien GC, Wang N, Gullo C, Howie D, et al. SAP controls T cell responses to virus and terminal differentiation of TH2 cells. Nat Immunol. 2001 May;2(5):410–4. doi: 10.1038/87713. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 39.Nichols KE, Ma CS, Cannons JL, Schwartzberg PL, Tangye SG. Molecular and cellular pathogenesis of X-linked lymphoproliferative disease. Immunol Rev. 2005 Feb;203:180–99. doi: 10.1111/j.0105-2896.2005.00230.x. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. Review] [DOI] [PubMed] [Google Scholar]
- 40.Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med. 2011 Jun;17(6):673–5. doi: 10.1038/nm.2389. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006 Jul 1;108(1):253–61. doi: 10.1182/blood-2005-11-4567. [Research Support, N.I.H., Intramural] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruprecht CR, Gattorno M, Ferlito F, Gregorio A, Martini A, Lanzavecchia A, et al. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med. 2005 Jun 6;201(11):1793–803. doi: 10.1084/jem.20050085. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schneider A, Rieck M, Sanda S, Pihoker C, Greenbaum C, Buckner JH. The effector T cells of diabetic subjects are resistant to regulation via CD4+ FOXP3+ regulatory T cells. J Immunol. 2008 Nov 15;181(10):7350–5. doi: 10.4049/jimmunol.181.10.7350. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peluso I, Fantini MC, Fina D, Caruso R, Boirivant M, MacDonald TT, et al. IL-21 counteracts the regulatory T cell-mediated suppression of human CD4+ T lymphocytes. J Immunol. 2007 Jan 15;178(2):732–9. doi: 10.4049/jimmunol.178.2.732. [Research Support, Non-U.S. Gov’t] [DOI] [PubMed] [Google Scholar]
- 45.Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. SAP is required for generating long-term humoral immunity. Nature. 2003 Jan 16;421(6920):282–7. doi: 10.1038/nature01318. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PubMed] [Google Scholar]
- 46.Nichols KE, Hom J, Gong SY, Ganguly A, Ma CS, Cannons JL, et al. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat Med. 2005 Mar;11(3):340–5. doi: 10.1038/nm1189. [Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pasquier B, Yin L, Fondaneche MC, Relouzat F, Bloch-Queyrat C, Lambert N, et al. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J Exp Med. 2005 Mar 7;201(5):695–701. doi: 10.1084/jem.20042432. [Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wermeling F, Lind SM, Jordo ED, Cardell SL, Karlsson MC. Invariant NKT cells limit activation of autoreactive CD1d-positive B cells. J Exp Med. 2010 May 10;207(5):943–52. doi: 10.1084/jem.20091314. [Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baev DV, Caielli S, Ronchi F, Coccia M, Facciotti F, Nichols KE, et al. Impaired SLAM-SLAM homotypic interaction between invariant NKT cells and dendritic cells affects differentiation of IL-4/IL-10-secreting NKT2 cells in nonobese diabetic mice. J Immunol. 2008 Jul 15;181(2):869–77. doi: 10.4049/jimmunol.181.2.869. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.