Abstract
Autoimmune manifestations are paradoxical and frequent complications of primary immunodeficiencies, including T and/or B cell defects. Among pure B cell defects, the Activation-induced cytidine Deaminase (AID)-deficiency, characterized by a complete lack of immunoglobulin class switch recombination and somatic hypermutation, is especially complicated by autoimmune disorders. We summarized in this review the different autoimmune and inflammatory manifestations present in twelve patients out of a cohort of 45 patients. Moreover, we also review the impact of AID mutations on B-cell tolerance and discuss hypotheses that may explain why central and peripheral B-cell tolerance was abnormal in the absence of functional AID. Hence, AID plays an essential role in controlling autoreactive B cells in humans and prevents the development of autoimmune syndromes.
Keywords: Primary immunodeficiency, class switch recombination-deficiency, tolerance
Introduction
Immunoglobulin class switch recombination deficiencies (CSR-D) are rare primary immunodeficiencies with an estimated frequency of about 1 in 200,000 births. These deficiencies also referred to as hyper IgM (HIGM) syndromes are characterized by normal or elevated serum IgM levels and a decrease in (or an absence of) IgG, IgA and IgE (1). Defects in immunoglobulin class switch recombination (CSR) are often associated with faulty generation of somatic hypermutations (SHMs) in the Ig variable (V) region. The molecular identification and analysis of several CSR-D have provided new insights into the mechanisms underlying CSR and SHM, both of which are key elements in the maturation of antibody (Ab) responses (2).
Molecular basis of CSR-D
The genetic basis of CSR-D is diverse and is caused by defects in either the CD40L/CD40 pathway essential for B cell activation, germinal center (GC) formation and the induction of CSR, or the enzymes involved in CSR and SHM processes. Investigation of CD40L/CD40 deficiencies has provided evidence for an essential role for CD40 activation pathway in both CSR and SHM (3, 4). CD40L/CD40 interaction is also required for full T lymphocyte/ dendritic cell interaction, and CD40L and CD40-deficiencies are characterized by a defect in cellular immunity, leading to susceptibility to opportunistic and viral infections, which are not controlled by immunoglobulin substitution (5, 6). Other CSR-D are caused by an intrinsic B cell defect, affecting the CSR machinery itself. Patients suffer from recurrent bacterial infections but are generally well under immunoglobulin substitution. The most common of the CSR-D due to an intrinsic B cell defect is the autosomal recessive form due to mutations in AICDA gene encoding for activation-induced cytidine deaminase (AID). It is characterized by impairment of both CSR and SHM (7), emphasizing AID's master role in Ab maturation. The AID enzyme selectively modifies cytosine (C) residues into uracils (U) leading to the introduction of U:G mismatches on the single-strand DNA of transcribed switch (S) and V regions of the Ig, introducing a DNA lesion (8). AID can also deaminate the non-template strands in transcription bubbles (9) or through its interaction with the RNA exosome (10). However, it is very likely that AID plays an additional role in CSR, as indicated by the phenotype of patients harboring mutations in the C terminal part of AID that affect neither its catalytic activity nor the SHM process but abrogate the CSR (11). Moreover, C terminal mutations located in the nuclear export signal (NES) exert a dominant negative effect, likely because of the increased nuclear localization of truncated AID (12). Other CSR-D due to an intrinsic B cell defect are caused by DNA repair impairment, including the very rare Uracil N-glycosylase (UNG)-deficiency (13). This observation emphasizes the editing activity of AID since UNG removes the uracil residues from DNA leading to an abasic site. In V region, U:G mismatches’ repair involves also the MSH2/MSH6 complex (a component of the mismatch repair (MMR) machinery) and error-prone DNA polymerases for accomplishment of SHM (14). In contrast, the CSR process requires DNA double strand breaks for inter switch recombination: the UNG-induced abasic sites are eventually cleaved by apurinic-apyrimidic endonucleases (APEX), already characterized in mice but not in humans so far, that leads ultimately to the formation of single-strand DNA breaks (SSBs) which have to be processed into double-strand breaks (DSBs) (15). The MMR plays a role in the processing of the SSB into DSB in mice (14, 16-18) as in humans (19, 20). Thereafter the DSBs are sensed by several molecules, including the Ataxia Telangiectasia Mutated (ATM) protein, and repaired mostly through the classical, non-homologous end-joining (c-NHEJ) pathway; however, a recently described alternative end-joining pathway can also perform repair based on microhomology (21). Mutations in genes encoding MMR, ATM or NHEJ lead to different but severe phenotypes in which the CSR-D, although sometimes drastic, is only a side effect.
Immunologic features of AID-deficiency
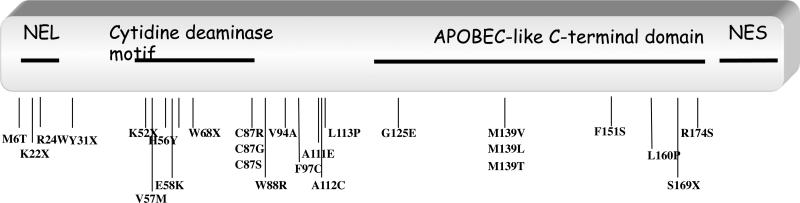
Although AID-deficiency is a very rare primary immunodeficiency, we could collect clinical data from 45 patients we diagnosed as affected by an autosomal recessive (AR) AID-deficiency and compared them to that of CD40L-deficient patients. Because of the rarity of the disease, patients are scattered all along the world and clinical information are sometimes sparse, especially when patients live in a developing country, whose tradition may include consanguineous marriages. All AR AID-deficient patients are characterized by a drastic defect in CSR (normal or increased IgM, lack of detectable IgG and IgA levels in serum). Mutations are scattered all along the gene, with no obvious hot spots of mutations (figure 1). SHM when evaluated were found negative except in two patients, both presenting mutations in splice sites in intron 4, leading in one patient to an homozygous in-frame insertion of 31 amino-acids and in the other one to heterozygous deletion of exon 4; this heterozygous change was associated with a missense mutation (F151S) on the second allele. NES is expected to be unaffected and AID catalytic activity was found normal (22), but CSR was defective, further revealing the role of the C terminal portion of AID in CSR in addition to the NES (23).
Figure 1.
Schematic representation of AICDA gene and localization of mutations observed in 45 AR AID-deficient patients. Deletions are not shown.
All AR AID-deficient patients suffer from bacterial infections, affecting mostly the upper respiratory and the gastro-intestinal tracts. No susceptibility to opportunistic infections is reported, which is in sharp contrast with CD40L or CD40-deficiencies as previously reported (4-6).
Lymphocyte numbers are normal in peripheral blood, with normal percentage of T and B cells, including CD27+ B cells. However, no switched IgM−IgD− B cells are detected, pinpointing to the complete absence of CSR (7). Strikingly, in this intrinsic B cell defect, CD4+/CD8+ ratio is <1. This unexpected weak decrease in CD4+ T cells could result from an exhaustion of T cells due to repeated infections before Ig replacement therapy. In addition, in CD40L-deficient patients as well as in AR AID-deficient patients, the number of CD3+CD4+CD25hiCD127loFOXP3+ Tregs was found significantly decreased (24, 25).
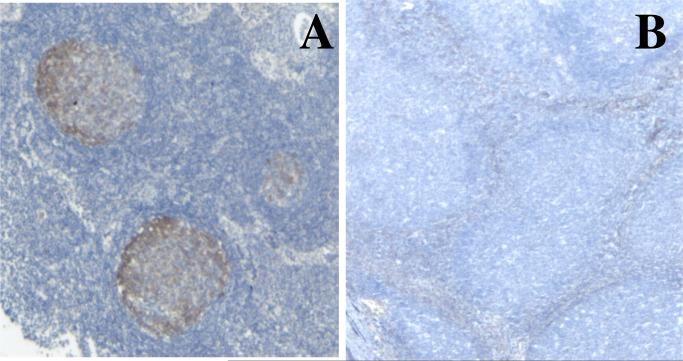
A hallmark of the disease is lymphadenopathy affecting 75% of patients (essentially cervical and mesenteric lymph nodes). The enlargement of lymph nodes is so impressive that patients undergo recurrent biopsies. All histological sections reveal the same aspect with a marked follicular hyperplasia, with giant germinal centres (GC) (5 to more than 10 times larger than GC from control reactive lymph nodes), filled with numerous proliferating (Ki67+) GC founder cells (CD38+sIgM+sIgD+ B cells). A dark zone and a lighter zone can be distinguished in some patients’ follicles on Ki67 staining. However this “light zone” also contains numerous cycling cells and sIgD+ B cells. The mantle zone (with normal B cell phenotype) and inter-follicular areas are present, although reduced in size (figure 2). IgM and IgD plasma cells are found in GC and T cell areas, but neither IgG nor IgA plasma cells. The high proliferation frequency of B cells in GC is associated with a dense network of macrophages filled with apoptotic bodies that gave the GC a starry sky appearance. The reason of such lymph node enlargement remains unknown, although hypertrophy of Peyers’ patches and of isolated lymphoid follicles in the lamina propria has been related to intestinal bacterial expansion in mice (26). In humans, such a correlation is unclear since lymphadenopathies can develop even in patients receiving efficient Ig substitution with no detectable infectious episode. Interestingly enough, no giant GC was observed in the lymph node biopsy of the patient with the homozygous splice site mutation in intron 4. Although often impressive, lymphadenopathies are not the most severe complication of AR AID-deficiency.
Figure 2.
Germinal centres in a control's reactive lymph node and in an AR AID-deficient patient's lymph node (magnification x25). Hist-immunochemical labeling with an anti-AID monoclonal antibody.
Autoimmune syndromes often develop in AR AID-deficiency
Besides increased susceptibility to infections, which is the hallmark of the disease, AR AID-deficient patients are prone to develop autoimmune manifestations (27). Autoimmune manifestations can occur before but also under appropriate Ig substitution and can be life-threatening, requiring steroid treatment, anti-CD20 monoclonal antibody and in some cases immunosuppressive agents administration. In our cohort of 45 patients with AR AID-deficiency for whom clinical data are available, autoimmune or inflammatory manifestations were found in 13 patients (29%) (Table 1).
Table 1.
AIHA: auto-immune hemolytic anemia, ITP: immune thrombocytopenia, Ab: antibodies, LKM: liver-kidney-microsome, AI: auto-immune, SLE: systemic lupus erythematous
| Patient | AID mutation | Clinical complications | Associated biological features |
|---|---|---|---|
| P1 | H56Y/H56Y | AIHA, ITP, AI hepatitis | IgM Ab: anti LKM, anti smooth muscle, anti liver membrane, anti cardiolipine, anti platelets and erythrocytes |
| P2 | Y31X/Y31X | ITP | cryoglobulinemia |
| P3 | M139V/M139V | AI hepatitis, arthritis | - |
| P4 | M139V/M139V | arthritis | - |
| P5 | F151S/F151S | arthritis | - |
| P6 | L160P/L160P | arthritis | - |
| P7 | V94A/V94A | cutaneous SLE | Rheumatoid factor, anti smooth muscle |
| P8 | F97C/F97C | multi-organ SLE | IgM Ab: anti nuclear factor, anti DNA Ab |
| P9 | W68X/W68X | Crohn's disease | - |
| P10 | deletion/deletion | destructive polyarthritis | - |
| P11 | W68X/W68X | chronic uveitis | - |
| P12 | W68X/deletion | - | cold agglutinins |
| P13 | Exon4 deletion/Exon4 deletion | ITP | anti cardiolipine |
P1 developed hemolytic anemia, thrombocytopenia and an auto-immune hepatitis with several autoantibodies of IgM isotype, including anti-hepatocyte, liver-kidney-microsome, smooth muscle, cardiolipin, erythrocyte (Coomb's test) and platelet antibodies. P2 presented with a thrombocytopenia and a cryoglobulinemia. P3 presents with chronic hepatitis and, though no auto-antibodies were found, its auto-immune origin was supported by a negative infection screen, histological findings and the efficacy of corticosteroids and immunosuppressive therapy. He suffered also from a non infectious arthritis. Three other patients were reported as affected by inflammatory arthritis (P4, P5 and P6). Two patients were diagnosed with systemic lupus erythematosus (SLE): P7 suffers only from a cutaneous SLE with photosensitivity, with no detectable anti-nuclear factor, but presence of rheumatoid factor and anti smooth muscle antibodies and controlled by hydroxychloroquine. P8 presents two severe episodes of SLE with multi organ failure, including the kidney and the central nervous system. Anti-nuclear factors, antibodies to SSA and RNP, anti-cardiolipine of the IgM isotype were found. The severity of the disease leads to treatment with long term azathioprin. P9 developed an inflammatory bowel disease mimicking Crohn's disease and treated by pentasalazin and low dose corticosteroids. P10 developed a chronic destructive, bilateral and symmetrical polyarthritis with the typical and radiological features of rheumatoid arthritis, which was controlled with low dose prednisone. No rheumatoid factor was detected. The biopsy of synovitis revealed a dense inflammatory infiltration (28). P11 had bilateral chronic uveitis, which required corticosteroids and cyclosoprin treatment, suggesting an autoimmune disorder although no autoantibody was found. P12 presented with cold agglutinins, however with no clinical consequences (27). P13 presented with a thrombocytopenia, and anti-cardiolipine autoantibodies.
Among this cohort of 45 patients affected by AR-AID deficiency, 2 patients were harbouring mutations located in the C terminal part of AID resulting in a defect in CSR but without affecting the SHM process and none of them have developed so far autoimmune complications.
Defective central B-cell tolerance checkpoint in AR AID-deficiency
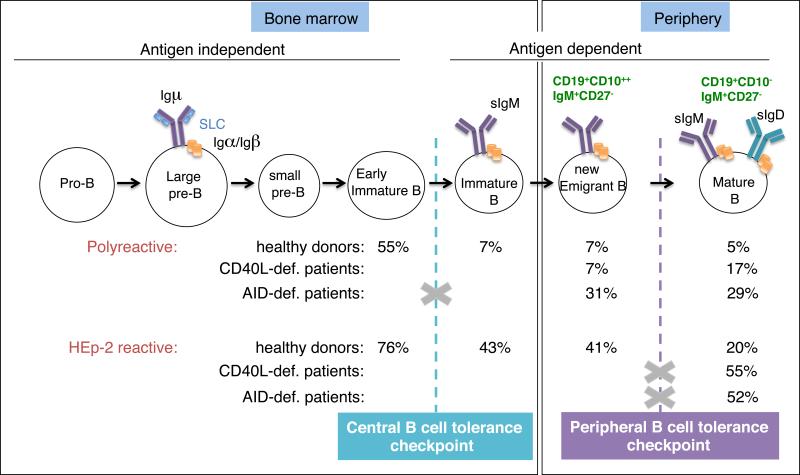
The observation that patients with CSR-D are prone to the development of autoimmune disease suggests that B-cell tolerance is not properly established and/or maintained in these patients (5, 6, 27). In humans, most developing autoreactive B cells generated during random V(D)J joining are removed at 2 discrete checkpoints during early B-cell development (29). First, a central checkpoint in the bone marrow between early immature and immature B cells removed most clones expressing polyreactive and anti-nuclear antibodies. Next, a peripheral checkpoint at the transition between new emigrant and mature naïve B cells further counterselected some autoreactive B cells that may have encountered peripheral autoantigens probably not expressed in the bone marrow environment. Defects in these early B-cell tolerance checkpoints were identified in CSR-D patients (24, 25) (Figure 3).
Figure 3.
Central and peripheral B-cell tolerance checkpoints in CD40L- and AR AID-deficient patients. The frequency of B cells expressing polyreactive and HEp-2-reactive antibodies in healthy controls, CD40L- and AID-deficient patients is indicated. Only peripheral B cell fractions were analyzed in the CD40L- and AID-deficient patients. The higher percentage of polyreactive B-cell clones in AID-def. patients versus healthy control new emigrant B cells reveals a defective central B-cell tolerance checkpoint in these patients. The increased frequency of HEp-2 reactive B-cell clones in both CD40L-and AID-deficient patients demonstrates a defective peripheral B-cell tolerance checkpoint in those patients.
The regulation of central B cell tolerance involves B cell receptor (BCR) signalling pathways that regulate recombination activating gene expression and central tolerance mechanisms such as receptor editing, anergy, and deletion in immature B cells (30), and is mostly controlled by B cell-intrinsic factors. Indeed, we have previously shown that alterations of the BCR signaling pathway in patients lacking functional BTK, or in healthy subjects carrying the R620W PTPN22 risk allele result in a defective central checkpoint and a failure to counter select developing autoreactive and polyreactive B cells (31). Mutations in the Toll-like receptor (TLR) pathway genes encoding molecules such as IRAK-4, MyD88, UNC-93B or Adenosine deaminase (ADA) also lead to defects in proper counter selection, especially towards nucleic acid containing antigens (32, 33).
Central B-cell tolerance was normally established in CD40L-deficient patients, with percentages of new emigrant B cell clones expressing polyreactive B cell receptors similar to what we find in HD ((25) and figure 3), suggesting that CD40L, which is not expressed in developing B cells, does not play an important role in the establishment of central B cell tolerance. In contrast, new emigrant/transitional B cells from AR AID-deficient patients express an abnormal immunoglobulin repertoire and a high frequency of polyreactive antibodies, demonstrating that AID is required for the establishment of central B cell tolerance ((24) and figure 3). The mechanisms by which AID affects central B cell tolerance are currently unknown but they seem to require AID expression but not induction of SHM or CSR, since all antibodies cloned from AID-deficient and healthy donor transitional B cells were of the IgM isotype and devoid of somatic mutation. Although AID expression was previously believed to be restricted to activated B cells and GCs, we and others have now detected AID transcripts in human and mouse immature B cells, further supporting an intrinsic role for AID during bone marrow B cell development (24, 34-37). In addition, AID-deficient mice also display abnormal central B-cell tolerance, further identifying a major and previously unexpected role for AID in the removal of developing autoreactive B cell clones in both mice and humans (24, 38).
Defective peripheral B-cell tolerance is common to AID- and CD40L-deficiencies
The factors that control the second B-cell tolerance checkpoint, eliminating autoreactive B cells in the periphery before they enter the CD19+CD10−IgM+CD21+CD27− mature naive B-cell compartment, are less well established. In both CD40L- and AID-deficiency, this checkpoint is impaired as evidenced by the significantly higher proportion of mature naïve B cells expressing polyreactive and autoreactive antibodies including anti-nuclear antibodies (ANAs) ((24, 25) and figure 3). Transgenic mouse models have suggested that CD4+ T cells may play an important role in the elimination of peripheral autoreactive B cells through MHC class II/T cell receptor; CD40/CD40L and Fas/FasL interactions (39, 40). Interestingly, CD4+CD25hiCD127loFOXP3+ regulatory T cell (Treg) numbers and frequencies are decreased in both CD40L- as in AID deficient patients (24, 25). This correlation may suggest the involvement of switched memory B cells in either the generation or the maintenance of some Treg cells in humans.
In addition, both CD40L- and AID-deficient patients display a 2 to 3 fold increase in B-cell activation factor (BAFF) in their serum (24, 25). BAFF is a critical B cell survival factor that controls the number of peripheral B cells (41). Mice overexpressing BAFF develop autoimmune disorders similar to SLE and Sjögren's syndrome characterized by the production of autoreactive antibodies including rheumatoid factor, anti-DNA and other ANAs (42). However, AID−/− mice do not display increased serum BAFF concentration (38). It remains to be determined whether these differences result from the patients exposure to pathogens or if the higher proportion of switched memory B cells in humans, which express several receptors for BAFF (BAFF-R and TACI) and which are absent in both HIGM syndromes, may account for increased BAFF concentration in both AID- and CD40L-deficient patients.
Increased secretion of autoreactive antibodies in AID-deficient patients
Since unexpected autoimmune manifestations occur in one fourth of the AR-AID-deficient patients, we examined if peripheral B-cell tolerance was further broken by analyzing their sera for autoreactive antibodies. Indeed, in all 13 out of 13 patients tested thus far, although not affected by any clinical autoimmune manifestation, ((24) and unpublished data) serum autoreactive antibodies could be observed. The sera reacted against cell structures of HEp-2 cells, similar to IgM autoreactive antibodies from SLE patients (23). In sharp contrast, none of the CD40L-deficient patients or healthy donors showed autoreactive IgM. We conclude that B-cell tolerance is further breached in AID-deficient patients compared with CD40L-deficient patients, and this breach correlates with the higher frequency and severity of autoimmune manifestations in AR AID-deficient patients (our personal observation).
Concluding remarks
The production of pathogenic IgG autoreactive antibodies have been long linked to the development of autoimmune diseases. It has been clearly demonstrated that these IgG autoreactive antibodies in both mice and humans display somatic hypermutations with patterns indicating that autoreactive B cell clones were actively selected by self-antigens (43-49). Because AID is responsible for both CSR and SHM, AID may therefore promote the development of autoimmune diseases by allowing the production of mutated autoreactive IgG B cells and autoreactive high affinity antibodies. Indeed, AID-deficiency in MRL/lpr mice abrogates lupus nephritis and a decrease in AID expression delays the development of such pathology (50, 51). Moreover, lupus-prone MRL/faslpr/lpr mice display increased AID expression, further supporting a role for AID in the development of autoimmune conditions in mice (52). However, our study of a large cohort of AID-deficient patients revealed that these patients do suffer from auto-immune or inflammatory disorders, suggesting that AID may also prevent the development of autoimmune syndromes. Indeed, 29 % of the patients from whom we can get a sufficient follow-up present such a complication, most of them early in life (during the first decade). Autoimmunity cannot be only related to increased serum IgM levels since these manifestations are more rare in CD40L-deficiency, in which serum IgM concentrations are also often elevated (5, 6). A correlation between the appearence of autoimmunity and infectious events is unclear in that AR AID-deficient patients on efficient Ig substitution can still develop autoimmune complications. In addition, organ specific auto-immune disease, linked to IgM antibodies to gastric antigens, has been reported in elderly AID−/− mice, independently of infections (53).
Hence, the pathogenesis of auto-immunity in AID-deficiency remains unclear and could be explained by:
It could be related to an intrinsic B cell defect. Low levels of AID expression in bone-marrow immature and transitional B cells may play a role in the control of central B-cell tolerance in both mice and humans (24, 38). In the absence of AID, immature and transitional B cells may be more resistant to apoptosis and thus more susceptible to expansion of autoreactive B lymphocytes (24, 38). Besides this role in central tolerance, AID could play also a role in the periphery by controlling mature B cell proliferation: AID has been shown to be involved in B cell apoptosis of mature B cells (54) and AID-deficient mice and humans display giant germinal centres filled of proliferating B cells, suggesting a lack of B cell proliferation control which can lead to the emergence of autoreactive B cells. Generation of tertiary lymphoid organs inside the gastric mucosa in the autoimmune gastric disease of AID−/− mice could also suggest a role of AID in B cell homeostasis. Expansion of autoimmune B cells could also be facilitated by the high levels of BAFF observed in AID-deficient patients’ serum. Finally, AID has been shown to deaminate methylated cytidines, and thus might play a role in the epigenetic regulation of gene expression (55-58).
A role for T cells is also suspected in AR AID- as well as CD40L-deficient patients. Tregs play a major role in the control of autoimmunity, as shown by Foxp3-deficient mice and humans (59, 60). Decreased numbers of Tregs in both AR AID- and CD40L-deficient patients may therefore directly contribute to the development of autoimmune conditions (24, 25). The autoimmune gastritis described in AID-deficient mice is associated with an infiltration of activated CD4+ T cells, which are able to transfer the gastritis when injected to a T-depleted recipient (53), emphasizing the role ot autoreactive T cells in organ-specific autoimmunity. One could also hypothesize that T cells are not fully normal in AID-deficiency. Indeed, novel data suggest that AID could be transiently expressed in T cells (61).
Both hypothesis are not exclusive since there is a cross-talk between T and B cells for autoimmune disease development. B cells have been shown to contribute to autoimmunity not only by secreting autoantibodies but also through activation of autoreactive T cells by presenting self-antigens (62), Using an antibody-independent autoimmune mouse model, Chan et al. could demonstrate that B cells could be involved in SLE pathogenesis as potential activators of autoreactive T cells and seem to be important for the maintenance of memory T cells (62). Anti-nuclear antibodies from an SLE mouse model require SHM to be generated (43-45, 48, 63). This observation is in apparent contradiction with our AID-deficient patients’ cohort in which autoreactive and anti-nuclear antibodies can be identified especially in AR AID-deficient patient 8 who suffer from SLE (Table 1). The 12 AR AID-deficient patients affected by autoimmune and/or inflammatory diseases were all harboring bi-allelic AICDA mutations located outside the C terminal part of AID, likely disturbing SHM. Because other patients were receiving immunosuppressive therapy at time of examination, only three out of the 12 (P1, P10 and P12) could be tested for SHM, that was found completely abrogated. These results suggest that IgM autoantibodies even devoid of somatic mutation can lead to tissue damage. Of note, none of the two patients with the splice site mutations in intron 4 (mutations preserving the cytidine deaminase activity and SHM as well as the NES, but modifying the C terminal part of AID leading to complete lack of CSR) present with autoimmunity. In addition, out of the 3 UNG-deficient patients described, one is suffering from severe auto-immune manifestations (AIHA, Sjogren's syndrome), and UNG-deficiency is not associated to defective SHM (although the nucleotide substitution pattern is biased)(13). Thus, our data suggest that the absence of CSR combined or not with a lack of SHM does not prevent the development of autoimmune manifestations in CSR-D patients.
In conclusion, AID appears to be a double-edge sword for the development of autoimmunity: on one hand AID can promote the development of autoimmunity and end-organ damage by promoting the generation of autoreactive mutated IgG B cells specific for self-antigens but on the other hand AID seems also to play an essential role in preventing the development of autoimmune syndromes in both mice and humans by favoring the elimination of developing autoreactive B cells during early B cell differentiation. Further analysis of B-cell tolerance in various primary immunodeficiencies may refine mechanisms leading to autoimmunity and potentially reveal how AID mediated its anti-autoimmune functions. Such approach may not only be important for a better understanding of autoimmunity in humans, but also for the prognosis and accurate follow-up of the patients.
Acknowledgments
This work was funded by grants from Institut National de la Santé et de la Recherche Médicale, the European Union's 7th RTD Framework Programme (EUROPAD contract 201549 and PID-IMMUNE contract 232809), Association Contre Le Cancer, ANR Blanc 2010-CSRD. This work was also supported by Grant Number AI061093, AI071087, AI082713 and AI095848 from NIH-NIAID (to E. M.). Tineke Cantaert was funded by a Rubicon grant from the Netherlands Organization for Scientific Research (NWO). S. K. is a scientist from the french Centre National de la Recherche Scientifique (CNRS).
References
- 1.Notarangelo LD, Duse M, Ugazio AG. Immunodeficiency with hyper-IgM (HIM]. Immunodefic Rev. 1992;3:101–121. [PubMed] [Google Scholar]
- 2.Durandy A, Taubenheim N, Peron S, Fischer A. Pathophysiology of B-cell intrinsic immunoglobulin class switch recombination deficiencies. Adv Immunol. 2007;94:275–306. doi: 10.1016/S0065-2776(06)94009-7. [DOI] [PubMed] [Google Scholar]
- 3.Korthauer U, Graf D, Mages HW, Briere F, Padayachee M, Malcolm S, Ugazio AG, Notarangelo LD, Levinsky RJ, Kroczek RA. Defective expression of T-cell CD40 ligand causes X-linked immunodeficiency with hyper-IgM. Nature. 1993;361:539–541. doi: 10.1038/361539a0. [DOI] [PubMed] [Google Scholar]
- 4.Ferrari S, Giliani S, Insalaco A, Al-Ghonaium A, Soresina AR, Loubser M, Avanzini MA, Marconi M, Badolato R, Ugazio AG, Levy Y, Catalan N, Durandy A, Tbakhi A, Notarangelo LD, Plebani A. Mutations of CD40 gene cause an autosomal recessive form of immunodeficiency with hyper IgM. Proc Natl Acad Sci USA. 2001;98:12614–12619. doi: 10.1073/pnas.221456898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy J, Espanol-Boren T, Thomas C, Fischer A, Tovo P, Bordigoni P, Resnick I, Fasth A, Baer M, Gomez L, Sanders EA, Tabone MD, Plantaz D, Etzioni A, Monafo V, Abinun M, Hammarstrom L, Abrabamsen T, Jones A, Finn A, Klemola T, DeVries E, Sanal O, Peitsch MC, Notarangelo LD. Clinical spectrum of X-linked hyper-IgM syndrome. J Pediatr. 1997;131:47–54. doi: 10.1016/s0022-3476(97)70123-9. [DOI] [PubMed] [Google Scholar]
- 6.Winkelstein JA, Marino MC, Ochs H, Fuleihan R, Scholl PR, Geha R, Stiehm ER, Conley ME. The X-linked hyper-IgM syndrome: clinical and immunologic features of 79 patients. Medicine (Baltimore) 2003;82:373–384. doi: 10.1097/01.md.0000100046.06009.b0. [DOI] [PubMed] [Google Scholar]
- 7.Revy P, Muto T, Levy Y, Geissmann F, Plebani A, Sanal O, Catalan N, Forveille M, Dufourcq-Labelouse R, Gennery A, Tezcan I, Ersoy F, Kayserili H, Ugazio AG, Brousse N, Muramatsu M, Notarangelo LD, Kinoshita K, Honjo T, Fischer A, Durandy A. Activation-induced cytidine deaminase (AID] deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2). Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 8.Petersen-Mahrt SK, Harris RS, Neuberger MS. AID mutates E. coli suggesting a DNA deamination mechanism for antibody diversification. Nature. 2002;418:99–104. doi: 10.1038/nature00862. [DOI] [PubMed] [Google Scholar]
- 9.Bransteitter R, Pham P, Scharff MD, Goodman MF. Activation-induced cytidine deaminase deaminates deoxycytidine on single-stranded DNA but requires the action of RNase. Proc Natl Acad Sci U S A. 2003;100:4102–4107. doi: 10.1073/pnas.0730835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basu U, Meng FL, Keim C, Grinstein V, Pefanis E, Eccleston J, Zhang T, Myers D, Wasserman CR, Wesemann DR, Januszyk K, Gregory RI, Deng H, Lima CD, Alt FW. The RNA exosome targets the AID cytidine deaminase to both strands of transcribed duplex DNA substrates. Cell. 2011;144:353–363. doi: 10.1016/j.cell.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imai K, Zhu Y, Revy P, Morio T, Mizutani S, Fischer A, Nonoyama S, Durandy A. Analysis of class switch recombination and somatic hypermutation in patients affected with autosomal dominant hyper-IgM syndrome type 2. Clin Immunol. 2005;115:277–285. doi: 10.1016/j.clim.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Geisberger R, Rada C, Neuberger MS. The stability of AID and its function in class-switching are critically sensitive to the identity of its nuclear-export sequence. Proc Natl Acad Sci U S A. 2009;106:6736–6741. doi: 10.1073/pnas.0810808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imai K, Slupphaug G, Lee WI, Revy P, Nonoyama S, Catalan N, Yel L, Forveille M, Kavli B, Krokan HE, Ochs HD, Fischer A, Durandy A. Human uracil-DNA glycosylase deficiency associated with profoundly impaired immunoglobulin class-switch recombination. Nat Immunol. 2003;4:1023–1028. doi: 10.1038/ni974. [DOI] [PubMed] [Google Scholar]
- 14.Rada C, Di Noia JM, Neuberger MS. Mismatch recognition and uracil excision provide complementary paths to both Ig switching and the A/T-focused phase of somatic mutation. Mol Cell. 2004;16:163–171. doi: 10.1016/j.molcel.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Guikema JE, Linehan EK, Tsuchimoto D, Nakabeppu Y, Strauss PR, Stavnezer J, Schrader CE. APE1- and APE2-dependent DNA breaks in immunoglobulin class switch recombination. J Exp Med. 2007;204:3017–3026. doi: 10.1084/jem.20071289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen HM, Tanaka A, Bozek G, Nicolae D, Storb U. Somatic hypermutation and class switch recombination in Msh6(−/−]Ung(−/−] doubleknockout mice. J Immunol. 2006;177:5386–5392. doi: 10.4049/jimmunol.177.8.5386. [DOI] [PubMed] [Google Scholar]
- 17.Ehrenstein MR, Rada C, Jones AM, Milstein C, Neuberger MS. Switch junction sequences in PMS2-deficient mice reveal a microhomology-mediated mechanism of Ig class switch recombination. Proc Natl Acad Sci U S A. 2001;98:14553–14558. doi: 10.1073/pnas.241525998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schrader CE, Vardo J, Stavnezer J. Role for Mismatch Repair Proteins Msh2, Mlh1, and Pms2 in Immunoglobulin Class Switching Shown by Sequence Analysis of Recombination Junctions. J Exp Med. 2002;195:367–373. doi: 10.1084/jem.20011877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peron S, Metin A, Gardes P, Alyanakian MA, Sheridan E, Kratz CP, Fischer A, Durandy A. Human PMS2 deficiency is associated with impaired immunoglobulin class switch recombination. J Exp Med. 2008;205:2465–2472. doi: 10.1084/jem.20080789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gardes P, Forveille M, Alyanakian MA, Aucouturier P, Ilencikova D, Leroux D, Rahner N, Mazerolles F, Fischer A, Kracker S, Durandy A. Human MSH6 deficiency is associated with impaired antibody maturation. J Immunol. 2012;188:2023–2029. doi: 10.4049/jimmunol.1102984. [DOI] [PubMed] [Google Scholar]
- 21.Yan CT, Boboila C, Souza EK, Franco S, Hickernell TR, Murphy M, Gumaste S, Geyer M, Zarrin AA, Manis JP, Rajewsky K, Alt FW. IgH class switching and translocations use a robust non-classical end-joining pathway. Nature. 2007;449:478–482. doi: 10.1038/nature06020. [DOI] [PubMed] [Google Scholar]
- 22.Ta VT, Nagaoka H, Catalan N, Durandy A, Fischer A, Imai K, Nonoyama S, Tashiro J, Ikegawa M, Ito S, Kinoshita K, Muramatsu M, Honjo T. AID mutant analyses indicate requirement for class-switch-specific cofactors. Nat Immunol. 2003;4:843–848. doi: 10.1038/ni964. [DOI] [PubMed] [Google Scholar]
- 23.Barreto V, Reina-San-Martin B, Ramiro AR, McBride KM, Nussenzweig MC. C-terminal deletion of AID uncouples class switch recombination from somatic hypermutation and gene conversion. Mol Cell. 2003;12:501–508. doi: 10.1016/s1097-2765(03)00309-5. [DOI] [PubMed] [Google Scholar]
- 24.Meyers G, Ng YS, Bannock JM, Lavoie A, Walter JE, Notarangelo LD, Kilic SS, Aksu G, Debre M, Rieux-Laucat F, Conley ME, Cunningham-Rundles C, Durandy A, Meffre E. Activation-induced cytidine deaminase (AID] is required for B-cell tolerance in humans. Proc Natl Acad Sci U S A. 2011;108:11554–11559. doi: 10.1073/pnas.1102600108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herve M, Isnardi I, Ng YS, Bussel JB, Ochs HD, Cunningham-Rundles C, Meffre E. CD40 ligand and MHC class II expression are essential for human peripheral B cell tolerance. J Exp Med. 2007;204:1583–1593. doi: 10.1084/jem.20062287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki K, Meek B, Doi Y, Muramatsu M, Chiba T, Honjo T, Fagarasan S. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc Natl Acad Sci U S A. 2004;101:1981–1986. doi: 10.1073/pnas.0307317101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quartier P, Bustamante J, Sanal O, Plebani A, Debre M, Deville A, Litzman J, Levy J, Fermand JP, Lane P, Horneff G, Aksu G, Yalcin I, Davies G, Tezcan I, Ersoy F, Catalan N, Imai K, Fischer A, Durandy A. Clinical, immunologic and genetic analysis of 29 patients with autosomal recessive hyper-IgM syndrome due to Activation-Induced Cytidine Deaminase deficiency. Clin Immunol. 2004;110:22–29. doi: 10.1016/j.clim.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Sibilia J, Durandy A, Schaeverbeke T, Fermand JP. Hyper-IgM syndrome associated with rheumatoid arthritis: report of RA in a patient with primary impaired CD40 pathway. Br J Rheumatol. 1996;35:282–284. doi: 10.1093/rheumatology/35.3.282. [DOI] [PubMed] [Google Scholar]
- 29.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 30.Nemazee D, Hogquist KA. Antigen receptor selection by editing or downregulation of V(D]J recombination. Curr Opin Immunol. 2003;15:182–189. doi: 10.1016/s0952-7915(03)00008-6. [DOI] [PubMed] [Google Scholar]
- 31.Menard L, Saadoun D, Isnardi I, Ng YS, Meyers G, Massad C, Price C, Abraham C, Motaghedi R, Buckner JH, Gregersen PK, Meffre E. The PTPN22 allele encoding an R620W variant interferes with the removal of developing autoreactive B cells in humans. J Clin Invest. 2011;121:3635–3644. doi: 10.1172/JCI45790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isnardi I, Ng YS, Srdanovic I, Motaghedi R, Rudchenko S, von Bernuth H, Zhang SY, Puel A, Jouanguy E, Picard C, Garty BZ, Camcioglu Y, Doffinger R, Kumararatne D, Davies G, Gallin JI, Haraguchi S, Day NK, Casanova JL, Meffre E. IRAK-4- and MyD88-dependent pathways are essential for the removal of developing autoreactive B cells in humans. Immunity. 2008;29:746–757. doi: 10.1016/j.immuni.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sauer AV, Morbach H, Brigida I, Ng YS, Aiuti A, Meffre E. Defective B cell tolerance in adenosine deaminase deficiency is corrected by gene therapy. J Clin Invest. 2012;122:2141–2152. doi: 10.1172/JCI61788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao C, Jiang L, Melo-Jorge M, Puthenveetil M, Zhang X, Carroll MC, Imanishi-Kari T. T cell-independent somatic hypermutation in murine B cells with an immature phenotype. Immunity. 2004;20:133–144. doi: 10.1016/s1074-7613(04)00019-6. [DOI] [PubMed] [Google Scholar]
- 35.Han JH, Akira S, Calame K, Beutler B, Selsing E, Imanishi-Kari T. Class switch recombination and somatic hypermutation in early mouse B cells are mediated by B cell and Toll-like receptors. Immunity. 2007;27:64–75. doi: 10.1016/j.immuni.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueda Y, Liao D, Yang K, Patel A, Kelsoe G. T-independent activation-induced cytidine deaminase expression, class-switch recombination, and antibody production by immature/transitional 1 B cells. J Immunol. 2007;178:3593–3601. doi: 10.4049/jimmunol.178.6.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuraoka M, Liao D, Yang K, Allgood SD, Levesque MC, Kelsoe G, Ueda Y. Activation-induced cytidine deaminase expression and activity in the absence of germinal centers: insights into hyper-IgM syndrome. J Immunol. 2009;183:3237–3248. doi: 10.4049/jimmunol.0901548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuraoka M, Holl TM, Liao D, Womble M, Cain DW, Reynolds AE, Kelsoe G. Activation-induced cytidine deaminase mediates central tolerance in B cells. Proc Natl Acad Sci U S A. 2011;108:11560–11565. doi: 10.1073/pnas.1102571108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rathmell JC, Cooke MP, Ho WY, Grein J, Townsend SE, Davis MM, Goodnow CC. CD95 (Fas]-dependent elimination of self-reactive B cells upon interaction with CD4+ T cells. Nature. 1995;376:181–184. doi: 10.1038/376181a0. [DOI] [PubMed] [Google Scholar]
- 40.Rathmell JC, Townsend SE, Xu JC, Flavell RA, Goodnow CC. Expansion or elimination of B cells in vivo: dual roles for CD40- and Fas (CD95]-ligands modulated by the B cell antigen receptor. Cell. 1996;87:319–329. doi: 10.1016/s0092-8674(00)81349-5. [DOI] [PubMed] [Google Scholar]
- 41.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 42.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shlomchik MJ, Marshak-Rothstein A, Wolfowicz CB, Rothstein TL, Weigert MG. The role of clonal selection and somatic mutation in autoimmunity. Nature. 1987;328:805–811. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- 44.Paul E, Manheimer-Lory A, Livneh A, Solomon A, Aranow C, Ghossein C, Shefner R, Offen D, Pillinger M, Diamond B. Pathogenic anti-DNA antibodies in SLE: idiotypic families and genetic origins. Int Rev Immunol. 1990;5:295–313. doi: 10.3109/08830189009056736. [DOI] [PubMed] [Google Scholar]
- 45.van Es JH, Gmelig Meyling FH, van de Akker WR, Aanstoot H, Derksen RH, Logtenberg T. Somatic mutations in the variable regions of a human IgG anti-double-stranded DNA autoantibody suggest a role for antigen in the induction of systemic lupus erythematosus. J Exp Med. 1991;173:461–470. doi: 10.1084/jem.173.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stott DI, Hiepe F, Hummel M, Steinhauser G, Berek C. Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. The salivary glands of patients with Sjogren's syndrome. J Clin Invest. 1998;102:938–946. doi: 10.1172/JCI3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sims GP, Shiono H, Willcox N, Stott DI. Somatic hypermutation and selection of B cells in thymic germinal centers responding to acetylcholine receptor in myasthenia gravis. J Immunol. 2001;167:1935–1944. doi: 10.4049/jimmunol.167.4.1935. [DOI] [PubMed] [Google Scholar]
- 48.Wellmann U, Letz M, Herrmann M, Angermuller S, Kalden JR, Winkler TH. The evolution of human anti-double-stranded DNA autoantibodies. Proc Natl Acad Sci U S A. 2005;102:9258–9263. doi: 10.1073/pnas.0500132102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang C, Zhao ML, Waters KM, Diaz M. Activation-induced deaminase contributes to the antibody-independent role of B cells in the development of autoimmunity. Autoimmunity. 2012;45:440–448. doi: 10.3109/08916934.2012.682668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang C, Foley J, Clayton N, Kissling G, Jokinen M, Herbert R, Diaz M. Abrogation of lupus nephritis in activation-induced deaminase-deficient MRL/lpr mice. J Immunol. 2007;178:7422–7431. doi: 10.4049/jimmunol.178.11.7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang C, Zhao ML, Diaz M. Activation-induced deaminase heterozygous MRL/lpr mice are delayed in the production of high-affinity pathogenic antibodies and in the development of lupus nephritis. Immunology. 2009;126:102–113. doi: 10.1111/j.1365-2567.2008.02882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zan H, Zhang J, Ardeshna S, Xu Z, Park SR, Casali P. Lupus-prone MRL/faslpr/lpr mice display increased AID expression and extensive DNA lesions, comprising deletions and insertions, in the immunoglobulin locus: concurrent upregulation of somatic hypermutation and class switch DNA recombination. Autoimmunity. 2009;42:89–103. doi: 10.1080/08916930802629554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hase K, Takahashi D, Ebisawa M, Kawano S, Itoh K, Ohno H. Activation-induced cytidine deaminase deficiency causes organ-specific autoimmune disease. PLoS One. 2008;3:e3033. doi: 10.1371/journal.pone.0003033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zaheen A, Boulianne B, Parsa JY, Ramachandran S, Gommerman JL, Martin A. AID constrains germinal center size by rendering B cells susceptible to apoptosis. Blood. 2009;114:547–554. doi: 10.1182/blood-2009-03-211763. [DOI] [PubMed] [Google Scholar]
- 55.Morgan HD, Dean W, Coker HA, Reik W, Petersen-Mahrt SK. Activation-induced cytidine deaminase deaminates 5-methylcytosine in DNA and is expressed in pluripotent tissues: implications for epigenetic reprogramming. J Biol Chem. 2004;279:52353–52360. doi: 10.1074/jbc.M407695200. [DOI] [PubMed] [Google Scholar]
- 56.Rai K, Huggins IJ, James SR, Karpf AR, Jones DA, Cairns BR. DNA demethylation in zebrafish involves the coupling of a deaminase, a glycosylase, and gadd45. Cell. 2008;135:1201–1212. doi: 10.1016/j.cell.2008.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhutani N, Brady JJ, Damian M, Sacco A, Corbel SY, Blau HM. Reprogramming towards pluripotency requires AID-dependent DNA demethylation. Nature. 2010;463:1042–1047. doi: 10.1038/nature08752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Popp C, Dean W, Feng S, Cokus SJ, Andrews S, Pellegrini M, Jacobsen SE, Reik W. Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency. Nature. 2010;463:1101–1105. doi: 10.1038/nature08829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX] is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 60.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 61.Qin H, Suzuki K, Nakata M, Chikuma S, Izumi N, Huong le T, Maruya M, Fagarasan S, Busslinger M, Honjo T, Nagaoka H. Activation-induced cytidine deaminase expression in CD4+ T cells is associated with a unique IL-10-producing subset that increases with age. PLoS One. 2011;6:e29141. doi: 10.1371/journal.pone.0029141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo W, Smith D, Aviszus K, Detanico T, Heiser RA, Wysocki LJ. Somatic hypermutation as a generator of antinuclear antibodies in a murine model of systemic autoimmunity. J Exp Med. 2010;207:2225–2237. doi: 10.1084/jem.20092712. [DOI] [PMC free article] [PubMed] [Google Scholar]