The primary objective was to evaluate outcome of consecutive cervical cancer patients treated over 4 years at the only radiotherapy center in Ethiopia. Two-year overall survival was 73.6%. Promoting early detection and increasing ... Increasing the capacity for external-beam radiation as well as options for brachytherapy would facilitate treatment with curative intention.
Keywords: Uterine cervical neoplasms, Africa, Ethiopia, Survival, Prognosis
Abstract
Background.
Almost 500,000 women are newly diagnosed with cervical cancer (CC) every year, the majority from developing countries. There is little information on the survival of these patients. Our primary objective was to evaluate consecutive CC patients presenting over 4 years at the only radiotherapy center in Ethiopia.
Methods.
All patients with CC from September 2008 to September 2012 who received radiotherapy and/or surgery were included (without brachytherapy). Vital status was obtained through telephone contact or patient cards.
Results.
Of 2,300 CC patients, 1,059 patients with standardized treatment were included. At the end of the study, 249 patients had died; surviving patients had a median follow-up of 16.5 months; the 10% and 90% percentiles were 3.0 and 32.7 months, respectively. Mean age was 49 years (21–91 years). The majority of patients presented with International Federation of Gynecology and Obstetrics stage IIb–IIIa (46.7%). Because of progression during the waiting time (median 3.8 months), this proportion declined to 19.3% at the beginning of radiotherapy. The 1- and 2-year overall survival probabilities were 90.4% and 73.6%. If assuming a worst-case scenario (i.e., if all patients not available for follow-up after 6 months had died), the 2-year survival probability would be 45.4%.
Conclusion.
This study gives a thorough 4-year overview of treated patients with CC in Ethiopia. Given the limited treatment availability, a relatively high proportion of patients survived 2 years. More prevention and early detection at all levels of the health care system are needed. Increasing the capacity for external-beam radiation as well as options for brachytherapy would facilitate treatment with curative intention.
Implications for Practice:
This study analyzes 1,059 patients from Ethiopia with newly diagnosed cervical cancer who were treated by radiotherapy in the country’s only oncologic referral center. Overall survival after 2 years was considerably high (74%) compared with data from African cancer registries, underlining the usefulness of radiotherapy. The survival was still lower than that of patients from higher-resource settings, probably because of the lack of brachytherapy. Therefore, brachytherapy for cervical cancer patients should be of high priority. Patients with earlier stages of disease had better outcome compared with those with later stages. Awareness and early detection programs are needed in the Ethiopian setting.
Introduction
Every year, almost 500,000 women worldwide are estimated to be newly diagnosed with cancer of the cervix uteri [1]. The majority of cases are found in developing countries; in Africa almost 60,000 women die of the disease each year [2]. In 2004, cervical cancer contributed to 3.4 million years of life lost (YLL) worldwide and was the greatest single cause of YLL from cancer in women from low-income countries [3]. This mainly reflects the absence of national cancer control programs, including vaccination, screening, and early detection, in most African countries. Cervical cancer accounts for 22% of cancer deaths in women aged 15–59 years, making it a symbol for global health disparity; it burdens young women from the poorest countries and the most disadvantaged populations [4, 5]. Cancer patients in sub-Saharan Africa tend to present with advanced disease [6]. Despite this, in 2010 radiotherapy was available in only 23 of 52 African countries—mostly in the northern and southern states of the continent. Brachytherapy was available in only 20 countries [7]. Only a small amount of epidemiological data on cervical cancer is currently available [8]; only five African cancer registries were included in the World Health Organization publication “Cancer Incidence in Five Continents, Volume IX” [9]. Variations in incidence are expected within countries between rural and urban settings [10], as well as between different regions [11]. Research on cancer in Africa is increasingly being facilitated through various initiatives (e.g., the Global Initiative for Cancer Registry Development in Low- and Middle-Income Countries and the African Organisation for Research and Training in Cancer) [12].
Ethiopia is the second most populated country in sub-Saharan Africa, with more than 42 million females [13]. Ethiopia is one of the least urbanized countries in the world, with only 16% of the population living in urban areas [14]. There are an estimated 7,000 new cases of cervical cancer in Ethiopia per year; nearly 5,000 people are estimated to die of the disease per year [15]. Public oncological treatment in Ethiopia, including radiotherapy, is limited to the Radiotherapy Center at Tikur Anbessa University Hospital, which is staffed by four radiation oncologists. Treatment options for patients with cervical cancer are radical hysterectomy (Wertheim operation) when in the early stages, at the Department of Gynaecology at Tikur Anbessa Hospital. External-beam radiation can be given combined with chemotherapy at the Radiotherapy Department. Brachytherapy is not available in Ethiopia. When attending the hospital, patients first have to register at the Radiotherapy Department for an appointment with the radiation oncologist. At this appointment, evaluation and planning of radiotherapy is performed by the radiation oncologist. Thereafter, patients receive an appointment to start radiotherapy. Because of huge patient loads, a considerable amount of time may pass between these appointments. Patients with acute bleeding receive priority for appointments for emergency radiation. Little is known about the outcome of cervical cancer patients who receive therapy in such settings with limited resources. Recent publications point toward the need for more epidemiological data on noncommunicable diseases, including cancer, for example from cohort studies [16, 17].
The primary aim of this study was to estimate the overall survival of women with cancer of the cervix uteri whose diagnosis and treatment by surgery and/or radiotherapy at the Radiotherapy Center Addis Ababa took place between September 10, 2008 and September 11, 2012.
Materials and Methods
Patients and Methods
Women with histologically verified cancer of the cervix uteri (International Classification of Disease-Oncology (ICD-O-3) codes C53.0–9) who were diagnosed and treated between September 10, 2008 and September 11, 2012 at the Radiotherapy Center at Tikur Anbessa Hospital in Addis Ababa, Ethiopia, were included in the study. Treatment consisted of radiotherapy and/or surgery. All patient characteristics, tumor characteristics, and information concerning therapy and outcome were extracted from the patients’ files. Waiting time was calculated between the date of the first presentation at the hospital and start of radiotherapy. All patients with telephone numbers available were contacted by telephone. Information on the date of last contact and survival status was obtained by telephone from the patients or—in case of death—from relatives. If patients or relatives were not reached by telephone, the last date of personal contact was taken from the patients’ files.
Staging
Tumors were classified according to the International Federation of Gynecology and Obstetrics (FIGO) staging system [18]. The FIGO stage was based on clinical examination by at least one of the four radiation oncologists from the Radiotherapy Center (by authors A.M., T.W., and A.A.) and was documented in the patients’ files. The FIGO stage at first presentation to the hospital was used for further analysis. In cases of discrepancy between examinations, another radiation oncologist was consulted. In a few cases of additional radiologic or sonographic detection of hydronephrosis around the time of diagnosis, the FIGO stage was classified as stage IIIb; in cases of distant metastasis, the FIGO stage was classified as stage IVb (by authors U.M. and M.B.). Patients with lack of findings on routinely performed chest x-ray and abdominal ultrasound were considered “free of distant metastasis.” The histological results were documented according to written notes from pathology reports.
Treatment Modalities
Patients with cervical cancer were referred from all over Ethiopia to Tikur Anbessa Hospital for radiotherapy. This hospital has one cobalt-60 teletherapy unit. Patients with early-stage disease residing in Addis Ababa were referred for surgery as well. The surgical treatment was radical hysterectomy with pelvic lymphadenectomy (Wertheim). This surgery was performed in cases of FIGO stages Ia, Ib, and IIa. Tikur Anbessa Hospital is the only hospital in Ethiopia regularly performing Wertheim surgery and the only facility administering combination radiochemotherapy. Patients with a FIGO stage lower than IIb and clear surgical margins and negative lymph nodes did not receive radiotherapy. Also, patients with renal failure did not receive radiotherapy.
Adjuvant, radical, and palliative radiotherapy were applied in two phases. In the first phase, opposing-field techniques (anterior-posterior/posterior-anterior) were used, and in the second phase, four-field box techniques were applied. Typically, opposing fields in the first phase were 20–22 by 20–22 cm in size and included the gross tumor volume and the pelvic lymph nodes (upper border: L5/S1, lower border: 2–3 cm below palpable tumor, lateral borders to include inguinal nodes). In the second-phase, boost series directed only at the gross tumor volume, the typical size of an anterior field in the four-field box technique was 12 by 14 cm.
Adjuvant radiotherapy was given to patients after surgery without clear surgical margins or with positive lymph nodes and/or parametrium involvement. The patients received 40 Gy in 20 fractions of 2.0 Gy within 4–5 weeks in the first phase. Depending on tumor response, adverse effects, and compliance of the patients, a boost dose of 20–26 Gy was applied in 10–13 fractions of 2.0 Gy within 2–3 weeks in the second phase. In cases of FIGO stage IIb or IIIa, as well as cases of FIGO stage less than IIb without surgery, primary radical radiotherapy was given. The patients received 46 Gy in 23 fractions of 2.0 Gy within 5–6 weeks in the first phase and 26 Gy in 13 fractions within 2–4 weeks in the second phase. Patients with FIGO stage IIIb or 4a without bilateral hydronephrosis or clinical fistula were given palliative radiotherapy with a larger dose per fraction: 32 Gy in 8 fractions of 4.0 Gy within 4 weeks in the first phase followed by a second phase of 18 Gy (6 fractions of 3.0 Gy) or 12 Gy (4 fractions of 3.0 Gy) within 2–3 weeks. In cases of FIGO stage IVa or IIIb with bilateral hydronephrosis, stage IVa with clinical fistula, or FIGO stage IVb, the patients received two single fractions of 10 Gy each, and depending on response, performance status, and site of metastasis, they received additional radiotherapy or palliative chemotherapy.
The waiting time for application of single-fraction radiotherapy was short (1–2 days), and therefore this concept was also applied for FIGO stage I–II patients who were unable to stay in Addis Ababa for longer periods of time because of their socioeconomic background. This applied to 12% of patients with FIGO stage Ia–IIa and 15% of patients with FIGO stage IIb–IIIa. Hemostatic radiotherapy (12 Gy in 4 fractions) was administered independently of FIGO stage because of massive vaginal bleeding and decline in hematocrit. In curative concepts, chemotherapy was recommended simultaneous with curative radiotherapy or rarely neoadjuvant to surgery (cisplatin, 60 mg/m2, 3–6 cycles). Palliative chemotherapy with cisplatin and 5-fluorouracil (50 mg/m2, 6 cycles) was recommended to patients with FIGO stage IIIb–IVb. Because of the limited availability of the substances and the financial limitations of the patients, chemotherapy was not administered on a regular basis.
Statistical Analysis
The primary endpoint of this study was overall survival. Person time equaled the time from the date of pathologic diagnosis to death or to closing date for follow-up (August 7, 2013), whichever came first. Women were right-censored at the date of last contact before the closing date. The survival probabilities in months were estimated using the Kaplan-Meier method. The 95% confidence intervals at year 2 are shown. Kaplan-Meier estimates were compared using the log-rank test. Analyses were conducted using SPSS Statistics, version 19 (SPSS software, IBM Corp., Armonk, NY, http://www-01.ibm.com/software/analytics/spss/) and SAS (SAS Institute, Inc., Cary, NC, http://www.sas.com), version 9.3. The median follow-up time for surviving patients was 16.5 months (range, 0.1–53.0 months). In the first analysis, we assumed right censoring to be unrelated to the risk of distant metastasis. Worst-case analysis assumed that all patients who were not available for follow-up after 6 months had died.
Ethics
For this study, ethical approval was obtained from the Institutional Review Boards of Addis Ababa University School of Medicine and Martin Luther University Halle. The study was conducted without individual informed consent because the study relied on retrospective data collected as part of routine patient care.
Results
Description of the Study Population
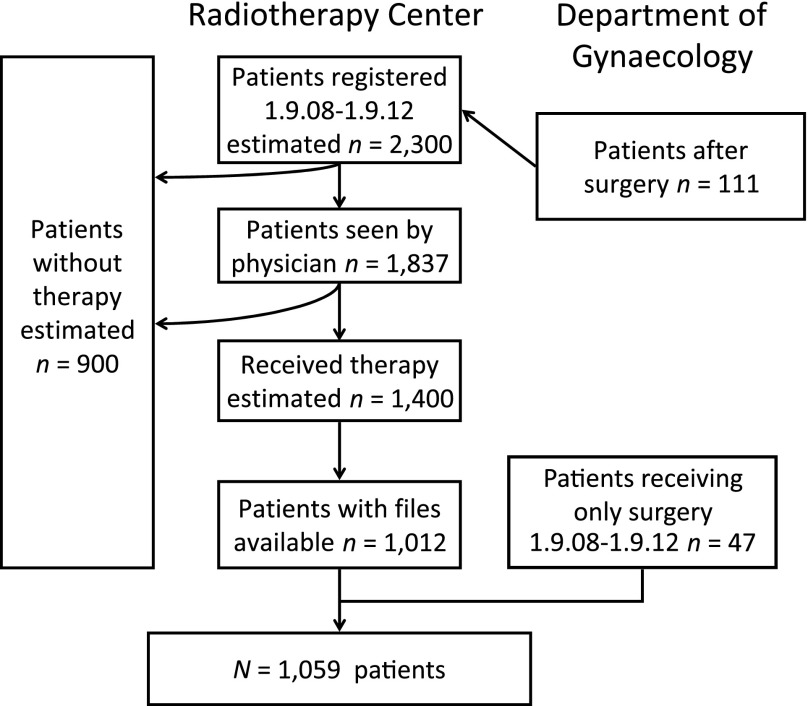
Between September 9, 2008 and September 11, 2012, an estimated 2,300 patients with cancer of the cervix uteri were registered at the Radiotherapy Center. This includes 111 patients who received surgery for the cancer. Of those, 1,837 were seen by the radiation oncologist, and radiotherapy was planned. The estimated number of patients who received radiotherapy was 1,400. Of those, 1,012 patient files could be retrieved. An additional 47 patients who received only surgery for early-stage cervical cancer were included in the study. Note that an estimated 900 patients who registered never received any therapy (Fig. 1).
Figure 1.
CONSORT diagram. Patients diagnosed with cancer of the uterine cervix included in this study are a subgroup of all who presented at the Radiotherapy Center or Department of Gynecology of Tikur Anbessa University Hospital, Addis Ababa.
Patient Characteristics
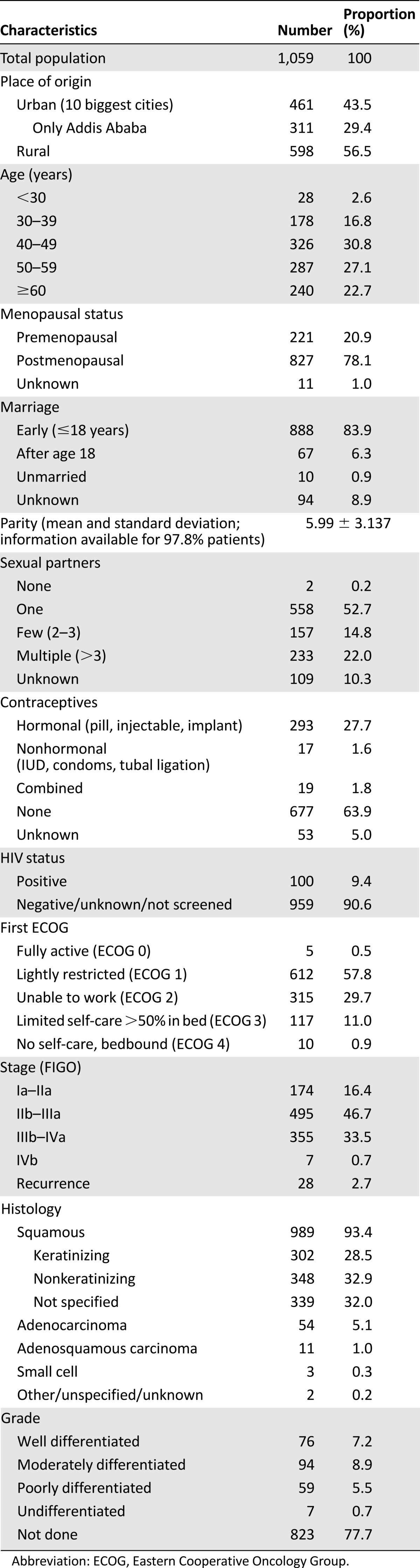
Most patients came from rural areas (56.5%). The largest group of patients were 40–49 years old (30.9%). The majority of patients (78.1%) were postmenopausal. The mean number of children was 6.0, with 10% and 90% percentiles of 2 and 10, respectively. Many women reported only one lifetime sexual partner (52.7%), as well as marriage before the age of 18 (83.9%). The proportion of women using contraceptives was 31.1%. A total of 9.4% were known to be HIV-positive. The Eastern Cooperative Oncology Group performance status 1 at first presentation was common (57.8%). The largest group of patients presented with FIGO stage IIb–IIIa (46.7%) and squamous cell carcinoma (93.4%) (Table 1).
Table 1.
Basic demographic data of the 1,059 patients when first seen by a physician
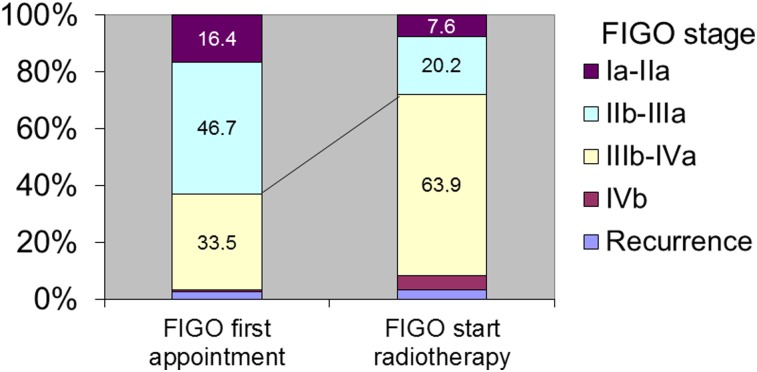
An important piece of baseline information is change in FIGO stage between pathological diagnosis and the start of radiotherapy, during which time a considerable number of patients died while waiting for treatment. However, information about these patients was not available. Because of the huge patient load, the median waiting time was 1.8 months between first registration at the Radiotherapy Center and the first appointment with the radiation oncologist (10th and 90th percentiles were 0.2 and 5.2 months, respectively). The median waiting time between the appointment with the radiation oncologist and the start of radiotherapy was 0.2 months for emergency radiotherapy, 1.7 months for curative radiotherapy, and 2.3 months for palliative radiotherapy (supplemental online Fig. 1). The proportion of patients with advanced stage IIIb and above increased between the appointment with the radiation oncologist (44.2%) and the start of radiotherapy (68.3%) (Fig. 2).
Figure 2.
Changes in FIGO stage during waiting time. FIGO stage was evaluated at the first visit to the radiation oncologist at the Radiotherapy Department and then at the start of radiotherapy. The proportion of higher FIGO stages in the cohort increased during this period.
Abbreviation: FIGO, International Federation of Gynecology and Obstetrics.
Treatment Modalities
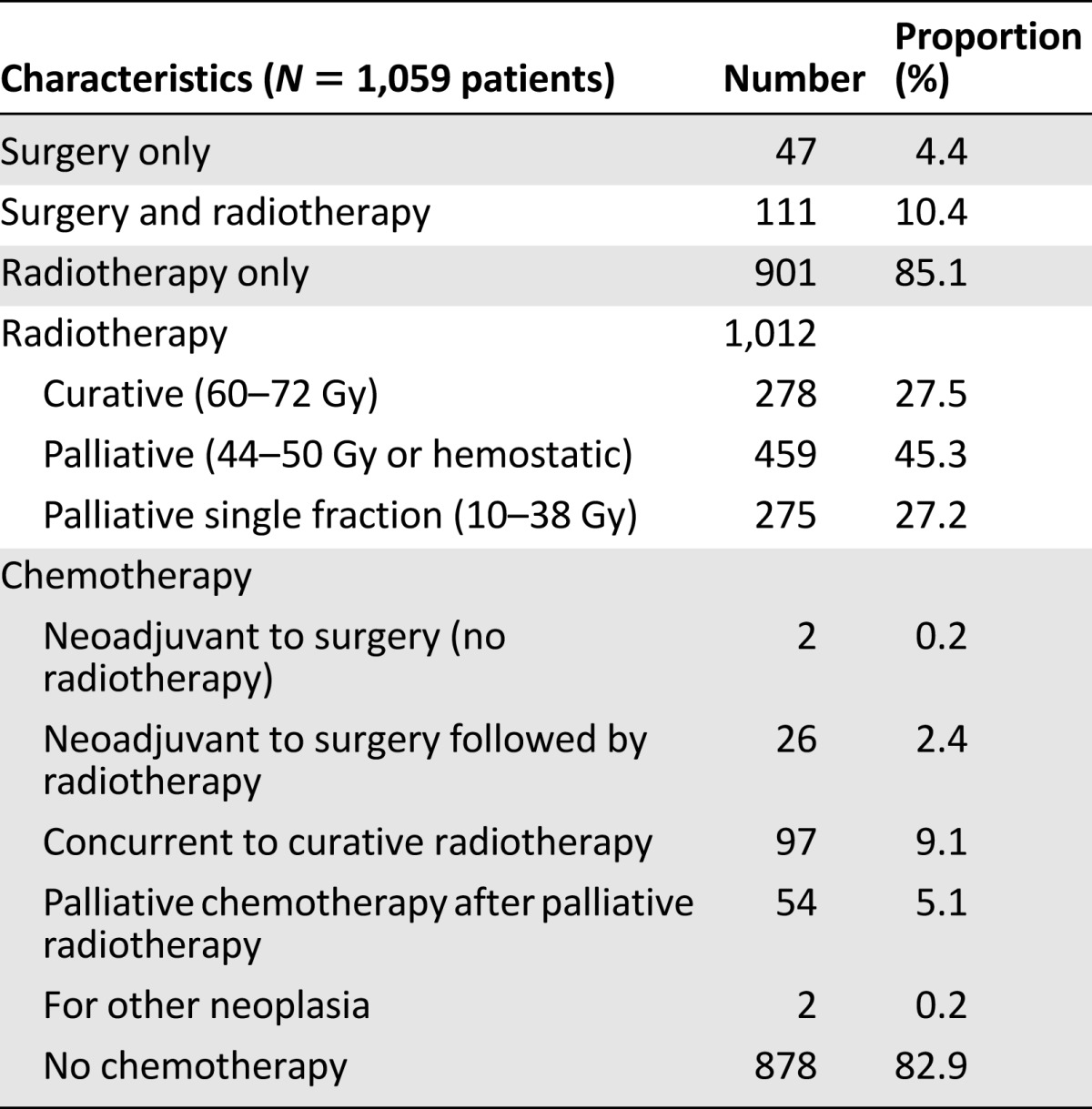
The majority of patients received radiotherapy; only 4.4% received surgery only for early stages of the disease. Out of 158 patients receiving surgery, the majority (n = 143; 70.8%) received radical hysterectomy, six received simple hysterectomy, and nine surgeries were of unknown extent. Palliative radiotherapy was administered to almost half of the patients. Because of the lack of finances or availability of the drugs, only 96 patients received simultaneous radiochemotherapy (Table 2).
Table 2.
Treatment modalities
Survival
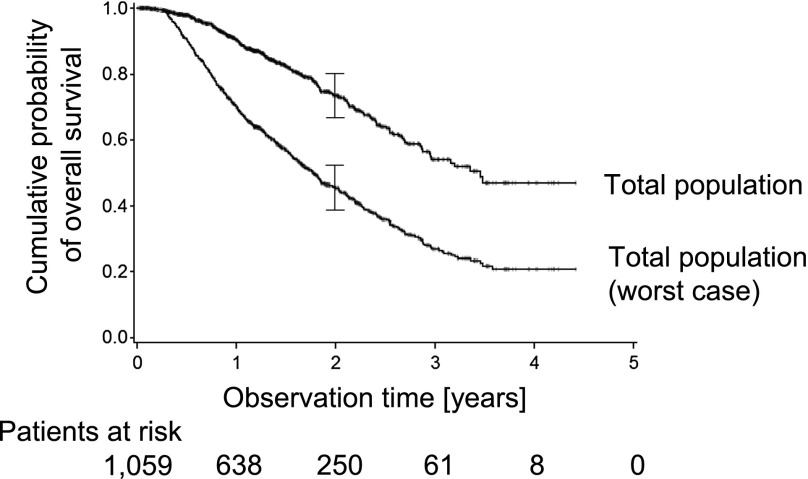
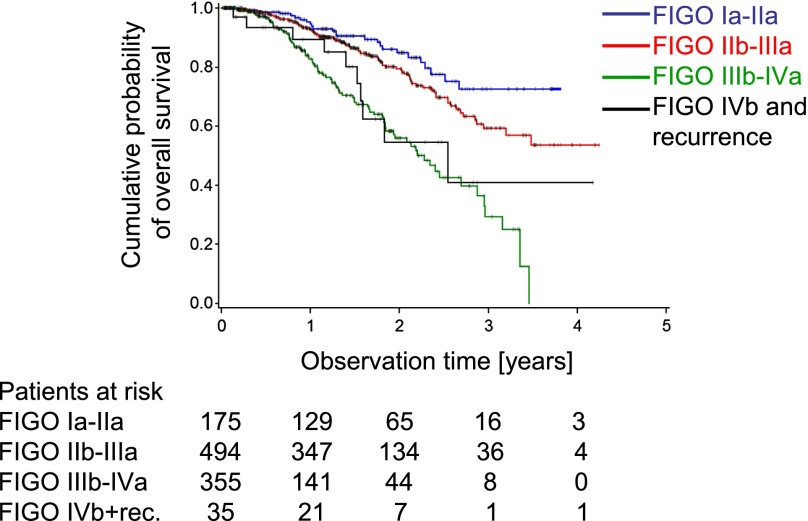
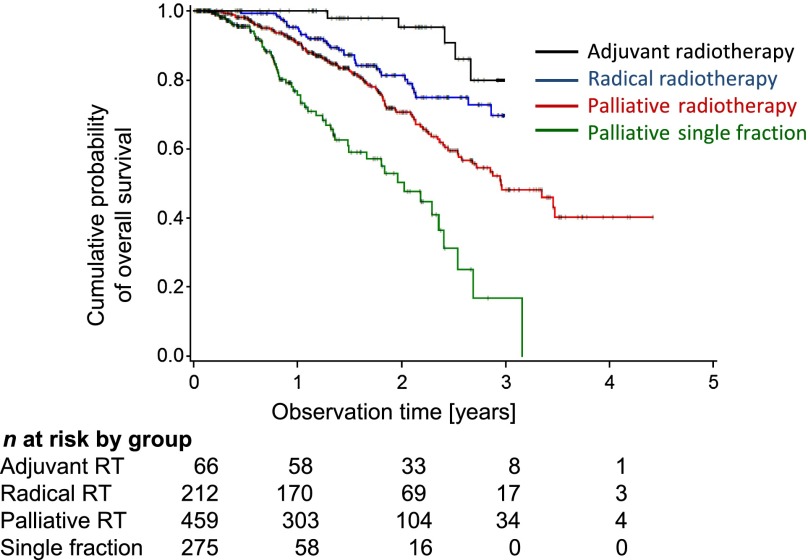
Within the whole cohort, a total of 212 deaths were registered. Another 37 patients were reported dead at the time of telephone interview, but the date of death remained unknown. Therefore, these patients were entered as alive and censored at the time of last personal appointment documented in the files. A total of 378 patients were lost to follow-up; therefore a worst-case analysis was performed (see Materials and Methods). The estimated overall survival is shown for the total patient cohort. The estimated 1- and 2-year survival probabilities were 90.4% and 73.6%, respectively (note at the end of year 3, the number of patients under observation declined to 8). In the worst-case analysis the estimated 2-year overall survival declined to 45.40% (Fig. 3). The median survival time was 40.6 months, declining to 21.5 months for the worst-case analysis. The estimated 2-year overall survival was most favorable for patients with FIGO stage Ia–IIa disease at the time of first presentation (84.8%, or worst-case 66.7%). Patients with FIGO stage IIb–IIIa had lower overall survival probabilities (79.5%, or worst-case 53.1%), and patients with FIGO stage IIIb–IVa the lowest (55.8%, or worst-case 25.3%) estimated overall survival probabilities after 2 years. There were only a few patients with FIGO stage IVb disease (n = 7). Twenty-eight patients experienced recurrence. These cases together had an estimated 2-year overall survival probability of 54.6%, or worst-case 32.7%. Those patients represent a heterogeneous group with a rather unfavorable estimated survival probability (Fig. 4). Comparison of the FIGO stages showed relevant differences in survival probability between stages (p < .001). Comparing patients’ outcome according to the dose of radiation showed better outcome for higher doses: 2-year survival probabilities were 80.8%, 72.3%, and 46.6% for 52 Gy and above, 32–50 Gy, and 4–30 Gy, respectively (supplemental online Fig. 2). Outcome by 2-year survival probabilities according to intention of treatment was best in the postoperative adjuvant situation (90.6%), radical radiotherapy (79.9%), followed by palliative radiotherapy (68.6%), and single fraction approach (50.3%) (Fig. 5).
Figure 3.
Cumulative overall survival probability. The uncorrected estimated overall survival time of the total cohort is shown (upper curve). The worst-case scenario assumes that patients who did not come to appointments for more than 6 months had died after the last appointment (lower curve).
Figure 4.
The estimated cumulative overall survival time according to FIGO stage at the first presentation to the radiation oncologist is shown. Group 1 is FIGO stage Ia–IIa, group 2 is FIGO stage IIb–IIIa, group 3 is FIGO stage IIIb–IVa, and group 4 is FIGO stage IVb and recurrences.
Abbreviations: FIGO, International Federation of Gynecology and Obstetrics; rec., recurrences.
Figure 5.
The estimated cumulative overall survival time according to intent of radiation is shown.
Abbreviation: RT, radiotherapy.
Discussion
This is the first study from Ethiopia and also sub-Saharan Africa to report on the outcome of a large cohort of patients who received oncologic therapy for cancer of the cervix uteri. With a median follow-up time of 16.5 months (for surviving patients), the overall survival was 90.4% and 73.6% at 1 and 2 years, respectively. In total, 55.8% and 34.6% of the patients presented with stages IIb–IIIa and stage IIIb, respectively. We found extremely long waiting times until the start of radiotherapy (median 3.8 months) leading to even higher proportions of patients with late-stage disease by the time radiotherapy was started.
Cancer survival data from Africa are mainly based on cohorts from national or regional population-based registries. Therefore, we discuss our data with respect to these published results. It should be noted that the registry cohorts aim at including all cases irrespective of stage of disease or whether treatment was received. Our cohort lacks late-stage patients who died before the start of therapy and poor patients unable to pay for therapy (n = 900; Fig. 1). Therefore, because of the nature of our cohort, survival was expected to be more favorable compared with population-based data.
Data from the population-based cancer registry of Kampala/Uganda show a 5-year age-standardized survival of 19% in 1993–1997 [19]. The Harare/Zimbabwe cancer registry (n = 284, half treated with radiotherapy) found a 3-year overall survival of 44.2% [20]. Sankaranarayanan et al. [21] report 5-year overall survival rates of 22% and 13% in the Gambia and Uganda, respectively. In our study, we found a 2-year overall survival of 74%, which is higher than the data from Zimbabwe and considerably better than the data from the Gambia and Uganda. This higher survival may point to a positive radiotherapy treatment effect beyond the lack of late-stage patients in our cohort.
Looking at high-income countries, Petereit et al. [22] reviewed treatment results in 5,619 patients receiving external beam radiotherapy followed by high-dose brachytherapy as reported in original publications between 1985 and 1997. The FIGO stages in their cohort were comparable to our cohort. The overall 5-year survival probabilities were higher than our findings: 85%, 68%, and 47% for FIGO stages I, II, and III, respectively [22]. The lack of brachytherapy in our cohort may explain the lower survival probabilities observed.
Looking at medium income countries, overall 5-year survival probabilities from Asia and Central America (population-based registries, tumor stages unknown) were higher than our cohort: for example, China 82%, India, 46% and Costa Rica, 53% [21]. The African American population from the SEER database showed an overall 5-year survival of 58% [23]. The lack of early detection activities, as well as the lack of therapeutic options in our cohort, may explain the lower survival probabilities we observed.
Late stage at presentation is a major prognostic factor for decreased survival. In our cohort, 34.2% of the patients were diagnosed at FIGO stage IIIb and above. This is comparable with data reported from Cape Town/South Africa, where screening was not available between 1984 and 2000 [24]. As an example of a country with high screening activity, data from Germany showed that 66% and 22% of the women presented with T1 and T2 tumors, respectively [25]. Because of the lack of screening opportunities in Ethiopia, patients usually present late with symptomatic disease.
Prevention, screening, and early detection have been proven useful also in low-resource settings. In Africa, screening with visual inspection and acetic acid combined with immediate cryotherapy of suspicious lesions has been implemented regionally. Recently, the role of human papillomavirus (HPV) testing in screening programs is under discussion. Additionally, HPV vaccination has become a possible option for primary prevention [26–29]. In Ethiopia, there is little awareness of HPV and CC within society, and symptoms of vaginal bleeding are socially stigmatized. The benefits of modern therapy are still perceived as low [30]. Vaccination, screening, or early detection are not available for most of the population [15]. Studies from different African countries (e.g., Nigeria and Uganda) show positive uptake of screening service after community-based advocacy [31, 32]. Rwanda has now become the first country in Africa to implement a nationwide program, including vaccination, early detection, and screening [33]. These options could eventually change the burden of cervical cancer disease in Africa [4, 34].
As expected, we found a high proportion of HIV-positive patients (9.4%). The true rate may have been even higher, because general screening of all new patients was conducted only in years 2–4 of the study. The Ethiopia Demographic and Health Survey from 2010 reported that 1.5% of women aged 15–49 were HIV-positive [14]. Ongoing efforts in Ethiopia to screen HIV-positive women for cervical cancer seem justified [15].
There are certain factors that limit our results. First, there is no clinical information on patients who registered but never saw a physician (estimated n = 463). Economic reasons, logistic reasons, and progression of the disease are possible explanations. Other patients (estimated n = 437) saw a physician but never started therapy—the relevant files could not be retrieved. We assume that a number of very advanced cases were not included in this study, leading to more favorable characteristics than in the overall cohort of patients presenting at the referral hospital. Second, radiotherapy did not include the application of brachytherapy. This is known to be a suboptimal standard of treatment. Because half of all radiotherapy departments in Africa are not able to offer brachytherapy, we still consider these findings of interest for countries with limited resources. Third, we were unable to retrieve almost 400 files (27%) belonging to patients who received therapy. The files are handwritten and manually stored, and names also vary in spelling. Misplacing is common. We are not aware of any other reason for missing files, and therefore we do not suspect any associated selection bias. Despite these limitations, our study is the first to thoroughly characterize and follow >1,000 patients in the clinical setting and therefore adds information about cervical cancer in Africa to the literature.
Conclusion
In this study, we present a description and follow-up of 1,059 patients diagnosed with cancer of the cervix uteri between September 2008 and 2012 at Addis Ababa Radiotherapy Center. We found a relatively high estimated 2-year overall survival of 73.6% (declining to 45.5% in the worst-case analysis). This survival is higher than previously reported from African cancer registries. It probably reflects a positive effect of the treatment, as well as selection bias through lack of untreated late-stage patients in our hospital cohort. The survival is lower than that of treated patients in high-income settings, which probably reflects the lack of brachytherapy in Addis Ababa. Stage at presentation in Addis Ababa is late, and the disease progresses during waiting times. Later stages showed lower survival probabilities compared with earlier stages, and higher radiation doses showed higher survival probabilities compared with lower doses. There is an urgent need for increased awareness, primary prevention by vaccination, and early detection programs to increase early stages at presentation. To further improve survival, radiotherapy, including brachytherapy, should be made available sooner.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
This work was done at Addis Ababa University. We thank the staff of Tikur Anbessa University Radiotherapy Center, who treated all of the cervical cancer patients. We also thank the nursing staff for obtaining the follow-up data and the record keeping staff for collecting the patient files.
Footnotes
For Further Reading: Julie S. Townsend, Analía Romina Stormo, Katherine B. Roland et al. Current Cervical Cancer Screening Knowledge, Awareness, and Practices Among U.S. Affiliated Pacific Island Providers: Opportunities and Challenges. The Oncologist 2014;19:383–393.
Implications for Practice: The U.S. Affiliated Pacific Island Jurisdictions (USAPIJ) are located in a geographically disparate region with a high burden of cervical cancer. Although cervical cancer screening providers in the USAPIJ stated that screening is a priority in clinical practice, costs associated with screening and varying levels of support for alternative screening tests pose barriers to screening throughout the USAPIJ. Use of alternative screening tests and routine monitoring and quality assurance to ensure all eligible women are reached may be needed to reduce the cervical cancer burden in the USAPIJ and to ensure effective use of limited resources.
Author Contributions
Conception/Design: Eva Johanna Kantelhardt, Ulrike Moelle, Matthias Begoihn, Adamu Addissie, Andreas Stang, Christoph Thomssen, Dirk Vordermark, Yirgu Gebrehiwot, Abreha Aynalem, Assefa Mathewos
Provision of study material or patients: Bekuretsion Yonas, Petros Hezkiel, Tufa Gemechu, Yirgu Gebrehiwot, Tigeneh Wondemagegnehu, Abreha Aynalem, Assefa Mathewos
Collection and/or assembly of data: Eva Johanna Kantelhardt, Ulrike Moelle, Matthias Begoihn, Adamu Addissie, Bekuretsion Yonas, Petros Hezkiel, Tufa Gemechu, Tigeneh Wondemagegnehu, Abreha Aynalem, Assefa Mathewos
Data analysis and interpretation: Eva Johanna Kantelhardt, Ulrike Moelle, Matthias Begoihn, Adamu Addissie, Pietro Trocchi, Bekuretsion Yonas, Petros Hezkiel, Andreas Stang, Christoph Thomssen, Dirk Vordermark, Tufa Gemechu, Yirgu Gebrehiwot, Tigeneh Wondemagegnehu, Abreha Aynalem, Assefa Mathewos
Manuscript writing: Eva Johanna Kantelhardt, Ulrike Moelle, Matthias Begoihn, Pietro Trocchi, Andreas Stang, Christoph Thomssen, Yirgu Gebrehiwot, Assefa Mathewos
Final approval of manuscript: Eva Johanna Kantelhardt, Ulrike Moelle, Matthias Begoihn, Adamu Addissie, Pietro Trocchi, Bekuretsion Yonas, Petros Hezkiel, Andreas Stang, Christoph Thomssen, Dirk Vordermark, Tufa Gemechu, Yirgu Gebrehiwot, Tigeneh Wondemagegnehu, Abreha Aynalem, Assefa Mathewos
Disclosures
Dirk Vordermark: Roche, Bristol-Myers Squibb, Lilly, Astra Zeneca (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Forouzanfar MH, Foreman KJ, Delossantos AM, et al. Breast and cervical cancer in 187 countries between 1980 and 2010: a systematic analysis. Lancet. 2011;378:1461–1484. doi: 10.1016/S0140-6736(11)61351-2. [DOI] [PubMed] [Google Scholar]
- 2. Cancer Incidence and Mortality Worldwide. International Agency for Research on Cancer. Available at: http://globocan.iarc.fr. Accessed March 30, 2014.
- 3.Bray F, Jemal A, Grey N, et al. Global cancer transitions according to the Human Development Index (2008–2030): A population-based study. Lancet Oncol. 2012;13:790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Bray F, Forman D, et al. Cancer burden in Africa and opportunities for prevention. Cancer. 2012;118:4372–4384. doi: 10.1002/cncr.27410. [DOI] [PubMed] [Google Scholar]
- 5.Bray F, Ren JS, Masuyer E, et al. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132:1133–1145. doi: 10.1002/ijc.27711. [DOI] [PubMed] [Google Scholar]
- 6.Cancer in Africa: Epidemiology and Prevention. Parkin DM, Ferlay M, Hamdi-Cherif M, Sitas F, Thomas J, Wabinga H, Whelan SL, editors. IARC Scientific Publications No. 153. IARC Press Lyon, France 2003. [Google Scholar]
- 7.Abdel-Wahab M, Bourque JM, Pynda Y, et al. Status of radiotherapy resources in Africa: An International Atomic Energy Agency analysis. Lancet Oncol. 2013;14:e168–e175. doi: 10.1016/S1470-2045(12)70532-6. [DOI] [PubMed] [Google Scholar]
- 8.Parkin DM, Sitas F, Chirenje M, et al. Part I: Cancer in indigenous Africans: Burden, distribution, and trends. Lancet Oncol. 2008;9:683–692. doi: 10.1016/S1470-2045(08)70175-X. [DOI] [PubMed] [Google Scholar]
- 9.Curado MP, Edwards B, Shin HR, et al. Cancer Incidence in Five Continents, Vol. IX: IARC Scientific Publications No. 160. Lyon, France: International Agency for Research on Cancer; 2009. [Google Scholar]
- 10.Dey S, Hablas A, Seifeldin IA, et al. Urban-rural differences of gynaecological malignancies in Egypt (1999–2002) BJOG. 2010;117:348–355. doi: 10.1111/j.1471-0528.2009.02447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sighoko D, Bah E, Haukka J, et al. Population-based breast (female) and cervix cancer rates in the Gambia: Evidence of ethnicity-related variations. Int J Cancer. 2010;127:2248–2256. doi: 10.1002/ijc.25244. [DOI] [PubMed] [Google Scholar]
- 12.Braun G, Fuhrer A, Breitenstein E, et al. Cancer in Africa: AORTIC 8th International Cancer Conference 'Entering the 21st Century for Cancer Control in Africa' 30.11.-2.12.2011. Breast Care (Basel) 2012;7:177–179. doi: 10.1159/000188335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. United Nations Population Estimates, “Total Population - Female.” Available at http://esa.un.org/unpd/wpp/Excel-Data/population.htm. Accessed March 6, 2013.
- 14. Central Statistical Agency [Ethiopia] and ICF International. Ethiopia Demographic and HealthSurvey 2011. 2012. Addis Ababa, Ethiopia and Calverton, Maryland, USA: Central Statistical Agency and ICF International. [Google Scholar]
- 15.Population Fact sheets Ethiopia. Available at http://globocan.iarc.fr/Pages/fact_sheets_population.aspx. Accessed May 15, 2014.
- 16.Holmes MD, Dalal S, Volmink J, et al. Non-communicable diseases in sub-Saharan Africa: The case for cohort studies. PLoS Med. 2010;7:e1000244. doi: 10.1371/journal.pmed.1000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olsen J, Bertollini R, Victora C, et al. Global response to non-communicable diseases: The role of epidemiologists. Int J Epidemiol. 2012;41:1219–1220. doi: 10.1093/ije/dys145. [DOI] [PubMed] [Google Scholar]
- 18.Pecorelli S, Zigliani L, Odicino F. Revised FIGO staging for carcinoma of the cervix. Int J Gynaecol Obstet. 2009;105:107–108. doi: 10.1016/j.ijgo.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Wabinga H, Parkin DM, Nambooze S, et al. Cancer survival in Kampala, Uganda, 1993–1997. IARC Sci Publ. 2011:243–247. [PubMed] [Google Scholar]
- 20.Chokunonga E, Ramanakumar AV, Nyakabau AM, et al. Survival of cervix cancer patients in Harare, Zimbabwe, 1995–1997. Int J Cancer. 2004;109:274–277. doi: 10.1002/ijc.11670. [DOI] [PubMed] [Google Scholar]
- 21.Sankaranarayanan R, Swaminathan R, Brenner H, et al. Cancer survival in Africa, Asia, and Central America: A population-based study. Lancet Oncol. 2010;11:165–173. doi: 10.1016/S1470-2045(09)70335-3. [DOI] [PubMed] [Google Scholar]
- 22.Petereit DG, Pearcey R. Literature analysis of high dose rate brachytherapy fractionation schedules in the treatment of cervical cancer: Is there an optimal fractionation schedule? Int J Radiat Oncol Biol Phys. 1999;43:359–366. doi: 10.1016/s0360-3016(98)00387-3. [DOI] [PubMed] [Google Scholar]
- 23.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 24.Denny L, Anorlu R. Cervical cancer in Africa. Cancer Epidemiol Biomarkers Prev. 2012;21:1434–1438. doi: 10.1158/1055-9965.EPI-12-0334. [DOI] [PubMed] [Google Scholar]
- 25. Robert Koch-Institut (Hrsg) und die Gesellschaft der Epidemiologischen Krebsregister in Deutschland e.V. (Hrsg). Krebs in Deutschland 2007/2008: 8. Ausgabe. Berlin, Germany: Agentur Consalis-Media, 2012. [Google Scholar]
- 26.Denny L. Cervical cancer prevention: New opportunities for primary and secondary prevention in the 21st century. Int J Gynaecol Obstet. 2012;119(suppl 1):S80–S84. doi: 10.1016/j.ijgo.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 27.Denny L, Kuhn L, Hu CC, et al. Human papillomavirus-based cervical cancer prevention: Long-term results of a randomized screening trial. J Natl Cancer Inst. 2010;102:1557–1567. doi: 10.1093/jnci/djq342. [DOI] [PubMed] [Google Scholar]
- 28.Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009;360:1385–1394. doi: 10.1056/NEJMoa0808516. [DOI] [PubMed] [Google Scholar]
- 29.Qiao YL, Sellors JW, Eder PS, et al. A new HPV-DNA test for cervical-cancer screening in developing regions: A cross-sectional study of clinical accuracy in rural China. Lancet Oncol. 2008;9:929–936. doi: 10.1016/S1470-2045(08)70210-9. [DOI] [PubMed] [Google Scholar]
- 30.Birhanu Z, Abdissa A, Belachew T, et al. Health seeking behavior for cervical cancer in Ethiopia: A qualitative study. Int J Equity Health. 2012;11:83–91. doi: 10.1186/1475-9276-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chigbu CO, Onyebuchi AK, Ajah LO, et al. Motivations and preferences of rural Nigerian women undergoing cervical cancer screening via visual inspection with acetic acid. Int J Gynaecol Obstet. 2013;120:262–265. doi: 10.1016/j.ijgo.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Busingye P, Nakimuli A, Nabunya E, et al. Acceptability of cervical cancer screening via visual inspection with acetic acid or Lugol’s iodine at Mulago Hospital, Uganda. Int J Gynaecol Obstet. 2012;119:262–265. doi: 10.1016/j.ijgo.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 33.Binagwaho A, Ngabo F, Wagner CM, et al. Integration of comprehensive women’s health programmes into health systems: Cervical cancer prevention, care and control in Rwanda. Bull World Health Organ. 2013;91:697–703. doi: 10.2471/BLT.12.116087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arbyn M, de Sanjosé S, Saraiya M, et al. EUROGIN 2011 roadmap on prevention and treatment of HPV-related disease. Int J Cancer. 2012;131:1969–1982. doi: 10.1002/ijc.27650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.