Abstract
Background.
This phase II single-arm trial evaluated afatinib, an irreversible inhibitor of the ErbB receptor family as third-line treatment of Korean patients with advanced non-small cell lung cancer (NSCLC) and tumors with wild-type EGFR. Currently, no standard therapy exists for these patients.
Methods.
Eligible patients had stage IIIB/IV wild-type EGFR lung adenocarcinoma and had failed to benefit from two previous lines of chemotherapy but had not received anti-EGFR treatment. Patients received oral afatinib at 40 mg per day until disease progression or occurrence of intolerable adverse events (AEs). The primary endpoint was confirmed objective tumor response (OR) rate (confirmed complete response [CR] or partial response [PR]). Secondary endpoints included disease control rate (DCR; OR or stable disease for ≥6 weeks), progression-free survival (PFS), and safety.
Results.
Forty-two patients received afatinib treatment, and 38 of those were included in efficacy analyses. No confirmed CRs or PRs were reported. DCR was 24% (9 of 38 patients), with a median disease control duration of 19.3 weeks. Median PFS was 4.1 weeks (95% confidence interval: 3.9–8.0). Frequently reported AEs (mainly grades 1 and 2) were rash/acne (88%), diarrhea (62%), and stomatitis (57%).
Conclusion.
Heavily pretreated patients with wild-type EGFR NSCLC treated with afatinib monotherapy did not experience an objective response and only 24% had disease stabilization lasting more than 6 weeks. AEs were manageable and consistent with the expected safety profile.
Abstract
摘要
背景. 这项II期单臂试验对ErbB受体家族的不可逆抑制剂阿法替尼作为三线治疗在韩国EGFR野生型晚期非小细胞肺癌(NSCLC)患者中的应用进行了评价。目前,这类患者尚无标准治疗方案。
方法. 入选标准为:IIIB/IV期野生型EGFR肺腺癌,并且既往二线化疗治疗失败但未接受抗EGFR治疗。给予口服阿法替尼40 mg/天直至疾病进展或发生不可耐受的不良事件(AE)。主要终点是确认的肿瘤客观缓解(OR)率[确认的完全缓解(CR)或部分缓解(PR)]。次要终点包括疾病控制率(DCR;OR或疾病稳定≥ 6周)、无进展生存期(PFS)和安全性。
结果. 42例患者接受了阿法替尼治疗,其中38例纳入了有效性分析。没有报告确认的CR或PR。DCR为24%(9/38例患者),中位疾病控制时间为19.3周。中位PFS为4.1周(95%可信区间:3.9 ∼ 8.0)。经常报告的AE(主要为1级和2级)有皮疹/痤疮(88%)、腹泻(62%)和口腔炎(57%)。
结论. 阿法替尼单药治疗在多次经治的野生型EGFR NSCLC患者中未获客观缓解,且疾病控制率仅24%,持续时间超过6周。AE可管理且与预期的安全性特征相一致。The Oncologist 2014;19:702–703.
Author Summary
Discussion
Although well established for lung cancer patients with molecular targets such as activating EGFR mutations or ALK rearrangement, there is no standard targeted therapy for those patients without identified molecular drivers. In these patients, there are no standard treatment options following failure of two previous lines of standard chemotherapy.
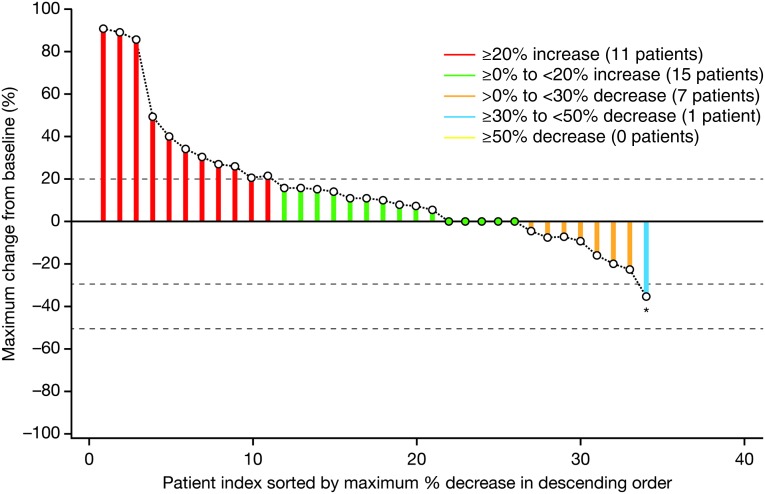
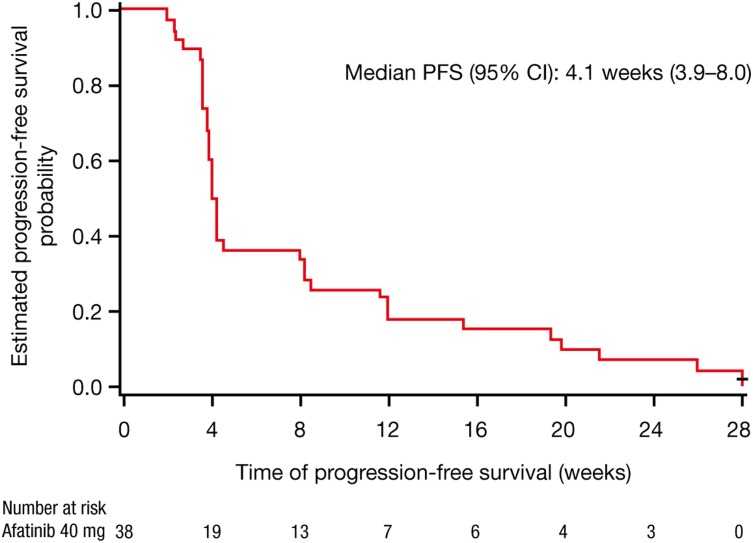
Preclinical studies demonstrated that afatinib conferred greater antitumor activity than reversible EGFR tyrosine kinase inhibitors in wild-type EGFR cancer models. Hence, this study was designed to investigate the efficacy of afatinib monotherapy in heavily pretreated NSCLC patients whose tumors have wild-type EGFR. Overall, 9 of 38 evaluable patients (24%) experienced stabilization of their disease for ≥6 weeks with afatinib monotherapy for a median duration of 19.3 weeks (range: 11.6‒28.0 weeks), whereas no patients achieved an objective tumor response (Fig. 1). Median progression-free survival, a secondary endpoint, was 4.1 weeks (95% confidence interval: 3.9–8.0) (Fig. 2). These limited efficacies in this population are consistent with existing evidence.
Figure 1.
Maximum change in tumor size (target lesions) from baseline in patients with wild-type epidermal growth factor receptor (n = 34). The maximum mean change from baseline is +15% (range: −35 to 91). *This patient had a partial response after 3.7 weeks; however, the response was not confirmed at the subsequent scan (week 8), which showed progressive disease.
Figure 2.
Progression-free survival.
Abbreviations: CI, confidence interval; PFS, progression-free survival.
All treated patients had at least one AE, the majority of which were mild (grade 1) or moderate (grade 2). The most common AEs were rash/acne (37 patients, 88%), followed by diarrhea (26 patients, 62%) and stomatitis (24 patients, 57%)—all known characteristics of EGFR inhibition and consistent with the known safety profile of afatinib. Seventeen patients (40%) had at least one serious AE (SAE); nine had fatal events, but none of the SAEs was considered to be treatment related. There were no events of interstitial lung disease or pneumonitis.
The absence of a comparator arm restricts, to some extent, the conclusions that can be drawn from this study. Because tissue samples were available from only 19 patients, the central laboratory was unable to confirm wild-type EGFR status for the total population, although eligible patients should have been identified as wild-type EGFR by a local laboratory. Four patients were excluded from the efficacy analysis because they tested positive for EGFR mutations by the central laboratory; three of these derived benefit (two with partial response and one with stable disease).
In conclusion, 24% of heavily pretreated NSCLC patients with wild-type EGFR experienced disease stabilization for ≥6 weeks with afatinib. Third-line afatinib was tolerable, and AEs were manageable.
Supplementary Material
Footnotes
Access the full results at: Park-13-419.theoncologist.com
ClinicalTrials.gov Identifier: NCT01003899
Sponsor(s): Boehringer Ingelheim
Principal Investigator: Keunchil Park
IRB Approved: Yes
Author disclosures available online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.