The study objectives were to evaluate the prognostic effect of site of metastasis and M1a/b category among patients with newly diagnosed colorectal cancer and synchronous metastasis and to assess prognostic variables in subgroups of patients with resected tumors and receiving systemic therapy. The results lend support to the introduction of M1a/b colorectal cancer categories.
Keywords: Metastatic colorectal cancer, Prognostic factors, M1a, M1b
Abstract
Background.
In 2009, the American Joint Committee on Cancer version 7 staging system introduced the M1 subclassifications M1a (single metastatic site) and M1b (peritoneal or multiple metastatic sites). The study objectives were to evaluate the prognostic effect of site of metastasis and M1a/b category among patients with newly diagnosed colorectal cancer and synchronous metastasis.
Patients and Methods.
Patients with newly diagnosed pathologic or clinical category M1 colorectal cancer referred to the British Columbia Cancer Agency between 1999 and 2007 were included. Demographic, tumor, treatment, and outcome data were prospectively collected, and prognostic factors were identified. Univariate Cox models were used to assess the prognostic impact of individual sites of metastasis and to determine the effect of M1a/b category on overall survival (OS).
Results.
Among 2,049 eligible patients, 70% had M1a and 30% M1b category disease. The most common sites of common single sites of metastasis included liver (56%), lung (5.3%), and peritoneum (3.6%). Metastasis to a single organ or site, including peritoneum, was associated with improved OS compared with multiple sites of metastasis. In multivariate analysis, M1b category conferred inferior survival and hazard ratio (HR) 1.38 (95% confidence interval [CI]: 1.22, 1.55), along with age >70 and Eastern Cooperative Oncology Group performance status of 3–4. Resection of primary tumor was associated with improved survival, HR 0.46 (95% CI: 0.41, 0.52). Results were similar in subgroup analysis of patients undergoing resection of their primary tumor when histology, tumor, and node category were included.
Conclusion.
The results lend support to the introduction of M1a/b colorectal cancer categories. Consideration may be given to classifying patients with solitary peritoneal metastasis only as M1a rather than M1b category. Further refinement of category M1a to reflect resectability of metastasis at initial diagnosis may improve prognostication.
Implications for Practice:
The American Joint Committee on Cancer version 7 Staging Manual published in 2009 introduced new M1 subcategories—M1a and M1b—in metastatic colorectal cancer. Patients with single organ metastasis were assigned category M1a and had a more favorable prognosis compared with those with disease in peritoneal or multiple sites, assigned category M1b. This study analyzed a group of 2,049 M1 patients treated over an 8-year period and supports the American Joint Committee on Cancer classification scheme for prognosis, whereby metastasis to any single organ (M1a), including peritoneum, was associated with improved overall survival compared with multiple sites of disease (M1b).
Introduction
Colorectal cancer is the second most common cause of cancer deaths in North America. Twenty percent of patients are diagnosed with metastatic disease at initial diagnosis (M1 category) [1], whereas the remainder present with relapsed disease. Rapid expansion of systemic treatments and improvements in surgical techniques has increased the proportion of patients who proceed to surgical resection of metastasis and experience prolonged survival. To reflect these changes, in 2009 the American Joint Committee on Cancer (AJCC) published version 7 of its classification system for cancers and introduced new M1 subcategories in metastatic colorectal cancer, specifically M1a and M1b [2]. Patients with newly diagnosed colorectal cancer who present with metastases confined to one site such as the lung, liver, or ovary at initial diagnosis are classified as category M1a. The M1b category is assigned to patients with disease in multiple sites or those with peritoneal metastasis. Although the number of metastatic sites is an established prognostic marker among patients with advanced colorectal cancer [3–5], the prognostic impact of the newly introduced M1a and M1b categories has not yet been described.
Validated prognostic factors have been described for patients with advanced colorectal cancer and are useful in counseling patients about median life expectancies, ascertaining eligibility for therapy, and stratifying clinical trial participants. Prognostic factors for patients with relapsed colon cancer include pathological tumor stage, time to distant relapse after surgical resection, and history of adjuvant chemotherapy [6]. These variables either do not apply or are unknown among patients who present with M1 disease and who do not proceed to resection of the primary tumor. Different prognostic factors may be relevant among patients with M1 category when compared with relapsed colorectal cancer.
The objectives of the current study were to evaluate the prognostic effect of site of metastasis and M1a/b category among patients with newly diagnosed colorectal cancer and synchronous metastasis and to assess prognostic variables in subgroups of patients with resected tumors and receiving systemic therapy.
Materials and Methods
Patients
All British Columbia (BC) residents diagnosed between 1999 and 2007 and referred to the BC Cancer Agency (BCCA) with newly diagnosed pathologic or clinical category M1 colon or rectal cancer were included. During the study time period, an estimated 65% of all patients with newly diagnosed advanced colorectal cancer in BC were referred to BCCA. M1 category was defined as radiographically and/or pathologically confirmed metastatic disease diagnosed at the time, or within 4 months, of initial diagnosis of colorectal cancer.
The study excluded patients with appendiceal cancers and those with a previous history of any malignancy (except for nonmelanoma skin cancers). Demographic, tumor, treatment, and outcome data were prospectively collected and maintained in the BCCA’s Gastrointestinal Cancer Outcomes Unit (GICOU) database. Nine predefined prognostic factors were identified with predetermined cutoff values: age (>70 or ≤70), sex (male or female), site of primary tumor (colon or rectum), tumor grade (well/moderately differentiated, poorly differentiated, or undifferentiated), tumor category (T1, T2, T3, or T4), nodal status (0 positive nodes, 1–3 positive nodes, or ≥4 positive nodes), presence of lymphovascular invasion (yes, no, or unknown), Eastern Cooperative Oncology Group (ECOG) performance status (ECOG 0, 1, 2, 3–4, or unknown), and site of first metastasis. For site of metastasis, patients were classified according to the number and location of metastatic lesions documented in their clinical record within 4 months of their initial stage IV colorectal cancer diagnosis, according to the following mutually exclusive categories: multiple sites, liver only, lung only, peritoneal/omentum only, ovarian only, distant nodal only, or other single sites/organs. Any disease limited to the omentum and/or peritoneum was defined as peritoneal/omentum only. Distant nodal metastases were defined as any nodes that were anatomically not local or regional to the primary tumor. These data were abstracted from the electronic medical record, which included investigations performed prior to and as a result of initial consultation at the BCCA.
Statistical Analysis
Overall survival (OS) was defined as the time from diagnosis to death from any cause. Univariate analysis to assess the prognostic impact of variables on OS was conducted using Kaplan-Meier methods and Cox proportional hazards models. The prognostic effects of individual sites of metastasis, M1a/b category, and other predictor variables were quantified using hazard ratio estimates from univariate Cox models. Factors found to be significant on univariate analysis were included in an initial multivariate Cox model; nonsignificant terms from this model were sequentially dropped using backward selection until only statistically significant predictors remained. The appropriateness of the proportional-hazards assumption for the Cox models was assessed using residual plots. Sensitivity analyses were conducted to examine the sensitivity of the final model interpretations to include cases with missing prognostic variables and variables with missing values in the models. Because a significant proportion of patients (22%) had an unknown ECOG status, two sets of analysis were done: one analysis included a level in the categorical predictor variable for unknown ECOG performance status, whereas the other analysis excluded patients with an unknown ECOG performance status from the model fitting.
Because the ECOG variable was ordinal in nature, we opted for a simplistic assessment of the effect of the missing data by (a) running an analysis on the complete case set, (b) including “unknown” as a category for the ECOG variable in the analysis, and (c) randomly imputing the value of ECOG based on the distribution of ECOG status in the complete cases (only one imputation, not multiple; additionally the imputation did not depend on other observed data). All of these approaches gave the same interpretation with respect to the M1a versus M1b question with a very stable hazard ratio (HR), and the other terms displayed similar stability across approaches, and thus we reported a single approach.
All p values presented are two-sided, and values of <.05 were considered statistically significant. All statistical analyses were performed using SAS (version 9.1.3) and the R statistical language (version 2.15.0). The study was approved by the University of British Columbia-BCCA Research Ethics Board.
Results
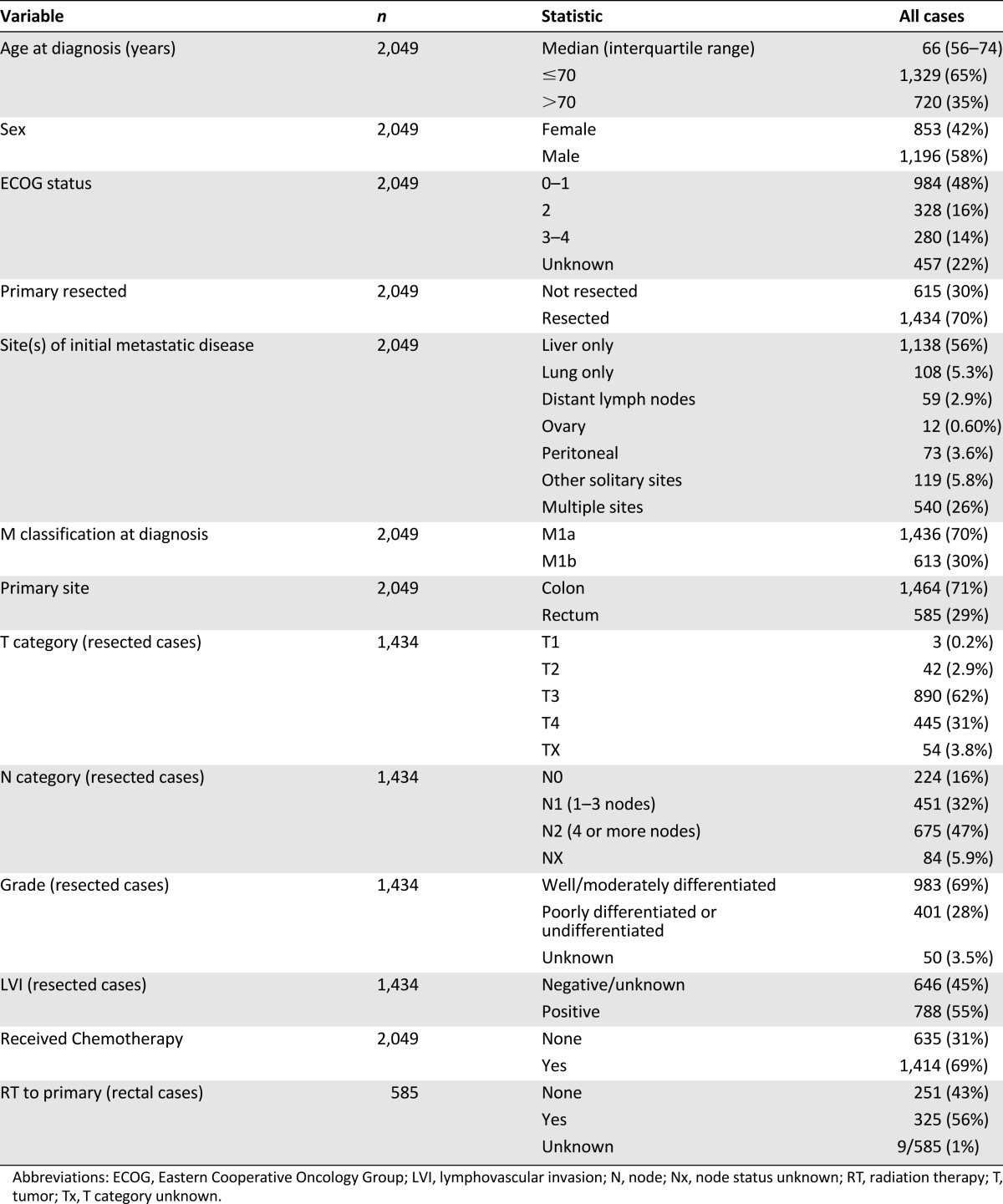
Between 1999 and 2007, a total of 2,049 patients with newly diagnosed metastatic colorectal cancer were referred to BCCA and met the eligibility requirements. At the time of the analysis, deaths had been observed in 85% of the cohort, with the surviving cases having been followed for a median of 12 months. As indicated in Table 1, the median age of patients was 66 years, and 71% had colon cancer. Although 70% of all patients had their primary tumor resected, 69% received chemotherapy, and 31% received no systemic therapy at any time during their disease course. Of 585 patients with rectal cancer, 56% received radiation to their primary tumor. ECOG status at the time of initial referral was 0 or 1 among 48% of patients, ECOG 2 among 16% of patients, and ECOG 3–4 among 14% of patients. ECOG status was not prospectively documented from the remaining 22% of cases. As defined by AJCC 7 criteria, 70% of patients had M1a category, and 30% had M1b category colorectal cancer. The sole sites of metastases at initial diagnosis were liver (55%), lung (5.3%), peritoneal (3.6%), distant lymph nodes (2.9%), ovary (0.6%), and other single sites (5.8%). The remaining 30% of patients presented with multiple sites of metastasis.
Table 1.
Patient characteristics (n = 2,049)
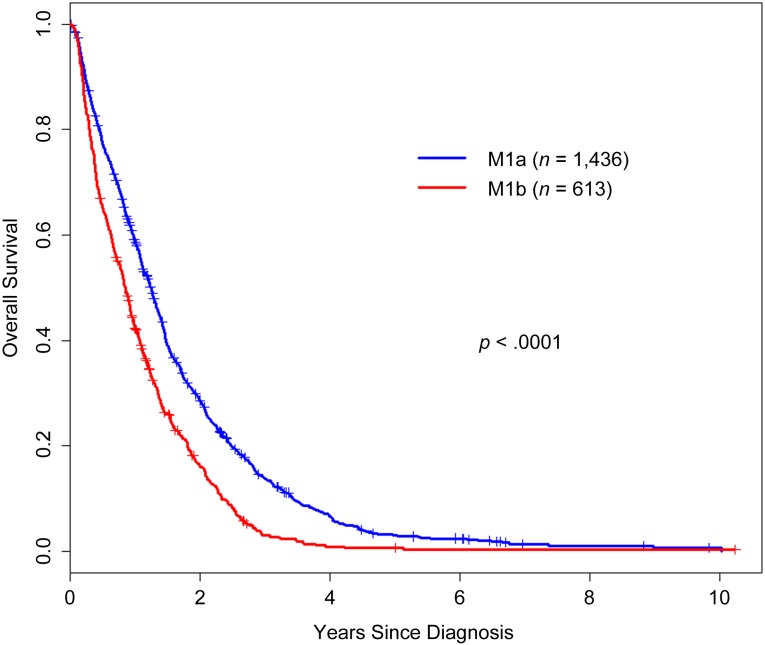
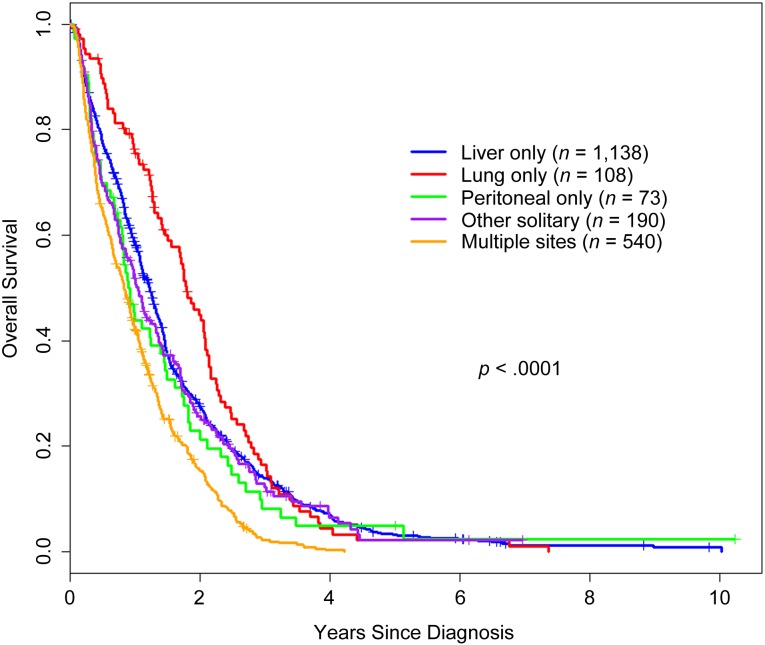
Survival by M1a/b Category and Site of Metastasis
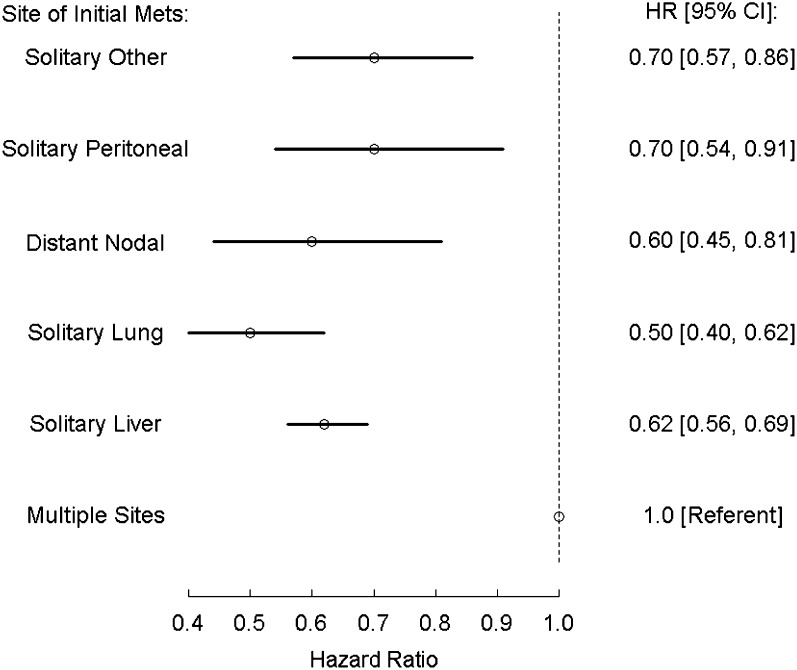
Kaplan-Meier overall survival estimates were generated for M1a/b category and for site of metastasis and are presented in Figures 1 and 2, respectively. Patients with M1a colorectal cancer survived significantly longer than those with M1b disease (p < .0001). The site of metastasis also significantly impacted survival; patients with lung, liver, peritoneal, and other single sites experienced longer overall survival than patients with multiple metastatic sites. In a Forrest plot in Figure 3, univariate hazard ratio estimates demonstrated that a metastasis to any single site, including peritoneum, was associated with significantly improved overall survival compared with patients who presented with multiple metastatic sites On multivariate analysis including other prognostic factors (resection of primary tumor, age, and ECOG status), metastasis to any single site remained a statistically significant prognostic factor (supplemental online Table 1).
Figure 1.
Overall survival from diagnosis by category M1a versus M1b. The graph shows Kaplan-Meier overall survival estimates of 2,049 patients with M1 colorectal cancer by AJCC version 7 categories M1a and M1b.
Figure 2.
Overall survival from diagnosis by site of metastatic disease at presentation. The graph shows Kaplan-Meier overall survival estimates of 2,049 patients with M1 colorectal cancer by site of metastases: liver only, lung only, peritoneal only, other solitary, and multiple sites.
Figure 3.
Forrest plot showing the effect of site of metastasis at the time of diagnosis of patients with M1 colorectal cancer.
Abbreviations: CI, confidence interval; HR, hazard ratio; Mets, metastasis.
Prognostic Factors in M1 Colorectal Cancer
Cox proportional-hazards analysis was conducted to determine variables associated with overall survival. Gender and site of primary cancer (colon versus rectum) were not significant on univariate analysis and were therefore excluded from the multivariate analysis. In analysis I (Table 2), M1b category was associated with a significantly inferior survival as compared with M1a category (HR 1.38 [95% confidence interval (CI): 1.22, 1.55]). Patients whose primary tumor was resected at the time of diagnosis of metastatic disease experienced a longer overall survival than those who did not have a primary resection (HR 0.46 [95% CI: 0.41, 0.52]). ECOG performance status was a strong prognostic factor, with the hazard ratio for overall survival lower for ECOG 2 (HR 1.64 [95% CI: 1.43, 1.88]) than for ECOG 3–4 (HR of 3.49 [95% CI: 3.02, 4.02]).
Table 2.
Cox proportional hazards analysis of variables associated with overall survival in 1,592 patients with known ECOG status and M1 colorectal cancer
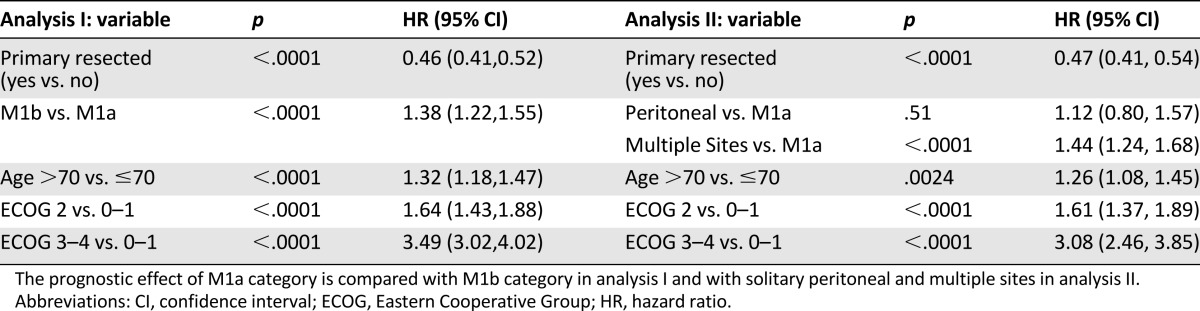
A second analysis (analysis II, Table 2) was conducted to evaluate the prognostic effect of peritoneal metastasis only and multiple sites separately in a multivariate model. Multiple sites of metastasis, but not solitary peritoneal metastasis, were associated with inferior overall survival compared with M1a category.
The analyses were repeated separately among patients who received chemotherapy for M1 disease (n = 1,414) versus those that did not (n = 635). The prognostic effect of M1a/b classification was similar regardless of receipt of chemotherapy (supplemental online Table 2). Because the availability of chemotherapy substantially changed during the era of the study, further analysis was done in subgroups diagnosed in 1999–2003 (n = 1,014) and 2004–2007 (n = 1,035). The effect of M1a/b was similar in 1999–2003, 1.48 (1.29, 1.71), and in 2004–2007, 1.39 (1.20, 1.61). Other prognostic factors also performed similarly (supplemental online Table 3).
Prognostic Factors Among Patients With Resected Primary Tumors
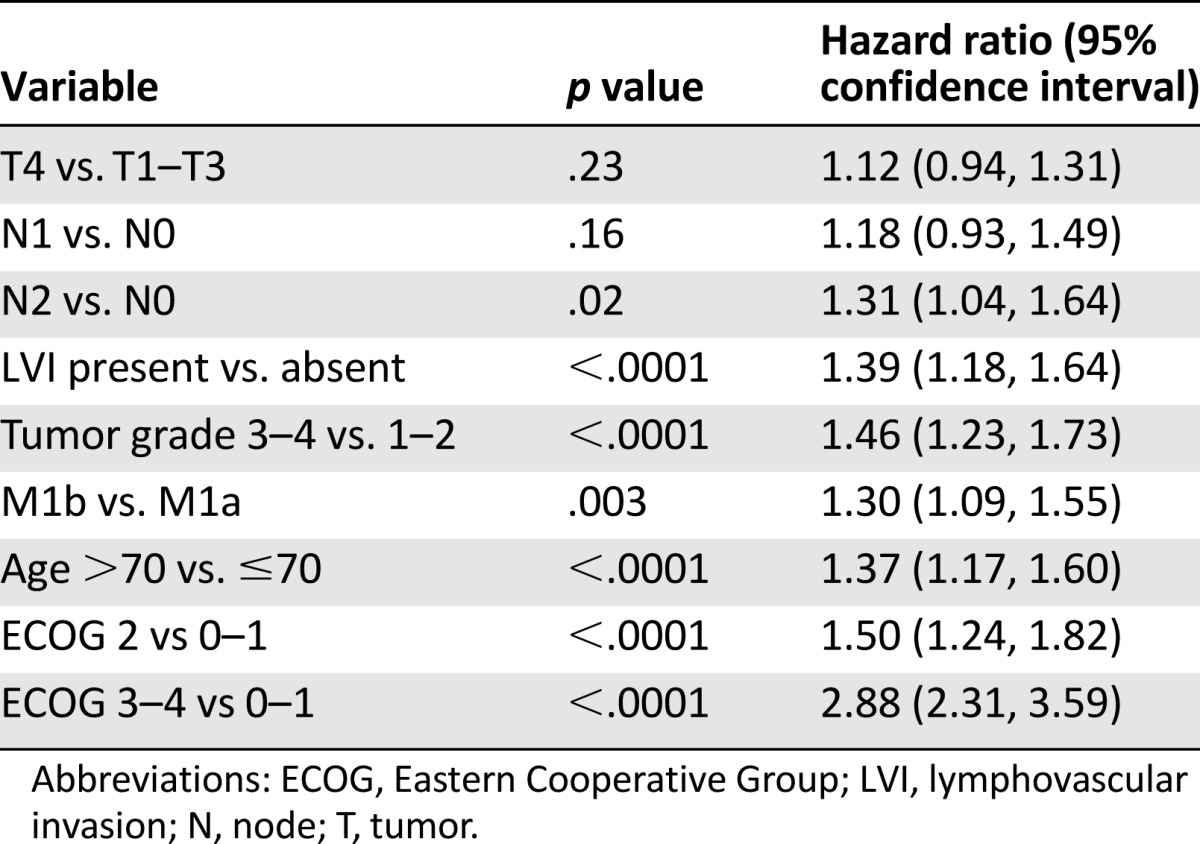
To compare the prognostic value of the M1a and M1b subcategories against the established pathologic prognostic factors, an analysis was conducted of patients who had a resection of their primary tumor (n = 1,434) (Table 3). M1b remained significantly associated with inferior overall survival compared with M1a status (HR 1.30 [95% CI: 1.09, 1.55]). Patients with a high nodal burden of disease as defined by pN2 category had inferior outcomes than those with pN0 category (HR 1.31 [95% CI: 1.04, 1.64]), but N1 and T categories did not affect outcomes. High tumor grade and the presence of lymphatic and/or vascular invasion were associated with inferior survival, whereas poor ECOG status and age >70 remained independently prognostic at levels similar to those demonstrated in the full cohort.
Table 3.
Cox proportional hazards analysis of variables associated with overall survival among 1,434 patients with M1 colorectal cancer and resected primary tumors
Discussion
In 2009, version 7 of the AJCC cancer staging system introduced M1a and M1b subclassification for patients with newly diagnosed M1 colorectal cancer. Results of the current study demonstrate that M1a/b classification has a significant prognostic impact independent of tumor histology, stage, ECOG status, and resection status. Results validate the newly introduced M1a/b classification in a population-based setting and confirm its prognostic effect in a heterogeneous population regardless of resection status and receipt of systemic therapy. Metastasis to one organ or site, including peritoneum/omentum, was associated with improved survival compared with patients with multiple metastatic sites at presentation.
The study represents a population-based cohort during the period of 1999–2007, and subgroup analysis in patients diagnosed in 1999–2003 versus 2004–2007 showed no difference in the effect of M1a/b classification in spite of significant changes in the availability of systemic therapy that occurred between these eras. One-third of patients were not candidates for chemotherapy or declined any chemotherapy for metastatic disease, and patients with ECOG 0–4 were included. The prognostic impact of M1a/b was similar regardless of receipt of palliative chemotherapy as presented in supplemental online Table 2. This suggests that results can be generalized to a broad population of patients with advanced colorectal cancer.
Although previous studies have documented inferior outcomes of patients with multiple sites of metastasis, not all studies have differentiated between patients presenting with newly diagnosed stage IV colorectal cancer versus those with relapsed disease [3–5]. Validated prognostic factors used among patients with relapsed disease include stage at initial presentation (stage I/II versus III), receipt of adjuvant chemotherapy, and interval between initial disease and relapse [6]. Because these factors are not applicable to patients who present with M1 disease, this study excluded patients presenting with relapsed disease.
Oligometastatic disease has been established as an important prognostic and therapeutic factor and has been described as a state between purely localized and widely metastatic disease [7, 8]. The extent to which oligometastatic disease is a positive prognostic factor may be dependent on whether the patient is eligible for and proceeds to curative resection or ablation of metastatic lesions. Numerous retrospective studies have documented prolonged survival durations among patients with resected colorectal metastases [9–11]. A limitation of our analysis is that information regarding resection of distant metastasis was not available for all patients included in the study. Previously published studies of similar BCCA cohorts have documented hepatic resection rates of 8%–9% between 2003 and 2006, typical of resection rates in other population-based series [12, 13]. Thus, the more favorable outcomes seen with M1a category may be due to both surgical resection of oligometastatic disease and the lower burden of disease. The observation of improved outcomes regardless of site of solitary metastasis lends support to the current practice of surgical resection of solitary liver and nonliver metastasis.
The majority of patients in this study had resection of their primary tumor at time of initial diagnosis. Although the benefit of routine resection of the primary tumor in the setting of synchronous distant disease is not established [14], studies have shown resection status to be a consistent and relevant prognostic factor [15, 16]. It should be noted that there is a substantial possibility of confounding present in such correlative analyses of resection of the primary based both on tumor presentation and patient factors, such that a causal interpretation is impossible. In a study of 810 patients with synchronous metastasis enrolled in clinical trials, 59% had resection of their primary tumor and experienced significantly longer survival (HR 0.63 [95% CI: 0.53, 0.75]). The findings were validated in this study in which resection of primary tumor was associated with a substantial reduction in risk of death (HR 0.46 [95% CI: 0.41, 0.52]); however, this study was published in abstract format only [16].
A subgroup analysis was conducted of patients with resected primary tumors to determine the effect of tumor histology and pathologic T and N category in relation to M1a/b category. Results demonstrated that T category and N1 versus N0 category do not have a prognostic effect. Only cases with an N2 category conferred a negative prognostic effect similar in magnitude to M1a/b. High histological grade and the presence of lymphovascular invasion were also associated with a significant detrimental effect similar in magnitude to previous studies of early category and advanced colorectal cancer [3, 5, 17, 18].
Multivariate analysis demonstrated that metastasis to a single site was associated with improved outcomes. Patients with lung metastasis only experienced particularly favorable outcomes, which are consistent with multiple studies documenting high 5-year survival rates among patients with resected lung metastasis [19–21]. It is noteworthy that peritoneal metastasis alone are associated with improved overall survival when compared with patients with multiple sites of metastasis. Peritoneal carcinomatosis has been described as a poor prognostic factor [22, 23]; however, some studies have included patients with synchronous metastasis [24]. Patients with peritoneal carcinomatosis alone are increasingly offered cytoreductive surgery and perioperative chemotherapy similar to other patients with single organ or site of metastasis. In a large, multi-institutional study of 543 patients treated with surgery and, in the majority of cases, heated intraperitoneal chemotherapy, median overall survival was 30 months, and 1-, 3-, and 5-year overall survival rates were high [25]. Patients with peritoneal metastasis only in the current study had improved survival compared with patients with multiple sites, and their survival duration was similar to patients with single-site metastasis. A more detailed chart review of the 73 patients with peritoneal/omental only metastasis included in this study revealed that 66% had a resection of their primary tumor, 7% proceeded to resection of metastatic disease in the form of peritoneal stripping/debulking (with or without intraperitoneal chemotherapy), and 63% received palliative chemotherapy. These treatment frequencies are numerically similar to those described for the cohort as a whole and suggest that the more favorable outcomes among patients with only peritoneal metastasis may relate to a disease biology that is similar to other single sites of metastasis. Results call into question whether this subgroup should be included with patients with M1b category and instead classified as M1a disease. In clinical terms, peritoneal metastasis only will likely be viewed as an increasingly distinct therapeutic group in which the extent of peritoneal disease may influence decisions regarding eligibility for debulking and intraperitoneal chemotherapy. This parameter may inform prognosis more than the current AJCC staging.
The overall strength of the prognostic effect of M1a/b category was modest regardless of treatment era and receipt of systemic chemotherapy. Its effect was similar to N category and histologic factors but less than ECOG status. It is notable that 70% of patients presented with M1a category, whereas only 30% were classified as M1b. This may point to significant heterogeneity particularly within the M1a group. The revised staging system does not differentiate between patients with innumerable liver/lung-only metastases versus those with solitary liver or lung lesions who are resectable even without the use of systemic therapy. Previous authors have suggested classification systems that consider not only site of metastasis but also resectability status at the time of initial diagnosis, in which patients are classified as resectable, initially unresectable, and unresectable [26]. Although this can be difficult to accurately define at the time of initial diagnosis, it may further strengthen M1 subclassification and contribute to early multidisciplinary review and decision making. Such a staging system may better reflect the impressive advances in both surgical techniques [27] and systemic therapy options currently available for patients with advanced colorectal cancer.
Conclusion
The results of this population-based study lend further support to the introduction of the M1a/b colorectal cancer subclassification in version 7 of the AJCC classification system [2]. Consideration may be given to classify patients with peritoneal/omental metastasis only as M1a rather than M1b category, but further data from other series/analyses are needed to justify the change. Further refinement of M1 category to reflect resectability of metastasis at initial diagnosis may increase the prognostic value of M1 category and encourage early, multidisciplinary workup in which all treatment strategies are considered.
This article is available for continuing medical education credit at CME.TheOncologist.com.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
Research support for this study was provided by the British Columbia Cancer Agency. Some of this work was presented in abstract form at the American Society of Clinical Oncology annual meeting in June 2013.
Author Contributions
Conception/Design: Hagen Kennecke, Jason Yu, Sharlene Gill, Winson Y. Cheung, Charles D. Blanke, Caroline Speers, Ryan Woods
Provision of study material or patients: Hagen Kennecke, Jason Yu, Sharlene Gill, Winson Y. Cheung, Charles D. Blanke, Caroline Speers, Ryan Woods
Collection and/or Assembly of data: Hagen Kennecke, Jason Yu, Sharlene Gill, Winson Y. Cheung, Charles D. Blanke, Caroline Speers, Ryan Woods
Data analysis and interpretation: Hagen Kennecke, Jason Yu, Sharlene Gill, Winson Y. Cheung, Charles D. Blanke, Caroline Speers, Ryan Woods
Manuscript writing: Hagen Kennecke, Jason Yu, Sharlene Gill, Winson Y. Cheung, Charles D. Blanke, Caroline Speers, Ryan Woods
Final approval of manuscript: Hagen Kennecke, Jason Yu, Sharlene Gill, Winson Y. Cheung, Charles D. Blanke, Caroline Speers, Ryan Woods
Disclosures
The authors indicated no financial relationships.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Washington K. 7th edition of the AJCC Cancer Staging Manual: Stomach. Ann Surg Oncol. 2010;17:3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 3.Forti L, Miraglia S, Bertona E, et al. Prognostic factors in advanced colorectal carcinoma: A multifactorial analysis. J Clin Oncol. 2005;23(suppl 16):a3732. [Google Scholar]
- 4.Hsu CW, King TM, Wang HT, et al. Factors that influence survival in unresectable metastatic or locally advanced colorectal cancer. Int J Colorectal Dis. 2011;26:1559–1566. doi: 10.1007/s00384-011-1231-7. [DOI] [PubMed] [Google Scholar]
- 5.Stangl R, Altendorf-Hofmann A, Charnley RM, et al. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343:1405–1410. doi: 10.1016/s0140-6736(94)92529-1. [DOI] [PubMed] [Google Scholar]
- 6.O’Connell MJ, Campbell ME, Goldberg RM, et al. Survival following recurrence in stage II and III colon cancer: Findings from the ACCENT data set. J Clin Oncol. 2008;26:2336–2341. doi: 10.1200/JCO.2007.15.8261. [DOI] [PubMed] [Google Scholar]
- 7.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 8.Quiet CA, Ferguson DJ, Weichselbaum RR, et al. Natural history of node-negative breast cancer: A study of 826 patients with long-term follow-up. J Clin Oncol. 1995;13:1144–1151. doi: 10.1200/JCO.1995.13.5.1144. [DOI] [PubMed] [Google Scholar]
- 9.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver: A prognostic scoring system to improve case selection, based on 1568 patients. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 10.de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: An international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440–448. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- 11.Hughes KS, Simon R, Songhorabodi S, et al. Resection of the liver for colorectal carcinoma metastases: A multi-institutional study of patterns of recurrence. Surgery. 1986;100:278–284. [PubMed] [Google Scholar]
- 12.Renouf DJ, Lim HJ, Speers C, et al. Survival for metastatic colorectal cancer in the bevacizumab era: A population-based analysis. Clin Colorectal Cancer. 2011;10:97–101. doi: 10.1016/j.clcc.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poultsides GA, Servais EL, Saltz LB, et al. Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J Clin Oncol. 2009;27:3379–3384. doi: 10.1200/JCO.2008.20.9817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruo L, Gougoutas C, Paty PB, et al. Elective bowel resection for incurable stage IV colorectal cancer: Prognostic variables for asymptomatic patients. J Am Coll Surg. 2003;196:722–728. doi: 10.1016/S1072-7515(03)00136-4. [DOI] [PubMed] [Google Scholar]
- 16.Faron M, Bourredjem A, Pignon JP et al. Impact on survival of primary tumor resection in patients with colorectal cancer and unresectable metastasis: Pooled analysis of individual patients’ data from four randomized trials. J Clin Oncol 2012;30(suppl):a3507. [DOI] [PubMed] [Google Scholar]
- 17.Takebayashi Y, Aklyama S, Yamada K, et al. Angiogenesis as an unfavorable prognostic factor in human colorectal carcinoma. Cancer. 1996;78:226–231. doi: 10.1002/(SICI)1097-0142(19960715)78:2<226::AID-CNCR6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 18.Betge J, Pollheimer MJ, Lindtner RA, et al. Intramural and extramural vascular invasion in colorectal cancer: Prognostic significance and quality of pathology reporting. Cancer. 2012;118:628–638. doi: 10.1002/cncr.26310. [DOI] [PubMed] [Google Scholar]
- 19.Goya T, Miyazawa N, Kondo H, et al. Surgical resection of pulmonary metastases from colorectal cancer: 10-year follow-up. Cancer. 1989;64:1418–1421. doi: 10.1002/1097-0142(19891001)64:7<1418::aid-cncr2820640709>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 20.Tampellini M, Ottone A, Bellini E, et al. The role of lung metastasis resection in improving outcome of colorectal cancer patients: Results from a large retrospective study. The Oncologist. 2012;17:1430–1438. doi: 10.1634/theoncologist.2012-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiorentino F, Hunt I, Teoh K, et al. Pulmonary metastasectomy in colorectal cancer: A systematic review and quantitative synthesis. J R Soc Med. 2010;103:60–66. doi: 10.1258/jrsm.2009.090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jayne DG, Fook S, Loi C, et al. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89:1545–1550. doi: 10.1046/j.1365-2168.2002.02274.x. [DOI] [PubMed] [Google Scholar]
- 23.Kesikli SA, Eren MF, Babacan NA, Kilickap S. Prognosis of colorectal cancer with peritoneal carcinomatosis: Is a new staging necessary? J Clin Oncol. 2012;30:2287–2288. doi: 10.1200/JCO.2012.42.1701. [DOI] [PubMed] [Google Scholar]
- 24.Franko J, Sargent D, Grothey A. Reply to S.A. Kesikli et al. J Clin Oncol . 2012;30:2288–2289. [Google Scholar]
- 25.Elias D, Gilly F, Boutitie F, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: Retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28:63–68. doi: 10.1200/JCO.2009.23.9285. [DOI] [PubMed] [Google Scholar]
- 26.Poston GJ, Figueras J, Giuliante F, et al. Urgent need for a new staging system in advanced colorectal cancer. J Clin Oncol. 2008;26:4828–4833. doi: 10.1200/JCO.2008.17.6453. [DOI] [PubMed] [Google Scholar]
- 27.Simmonds PC, Primrose JN, Colquitt JL, et al. Surgical resection of hepatic metastases from colorectal cancer: A systematic review of published studies. Br J Cancer. 2006;94:982–999. doi: 10.1038/sj.bjc.6603033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.