The authors report detailed safety analyses by geographic region from the phase III study CLEOPATRA with pertuzumab, trastuzumab, and docetaxel in patients with human epidermal growth factor receptor 2 (HER2)-positive first-line metastatic breast cancer. Despite more docetaxel dose reductions in patients from Asia, survival benefits were comparable between regions. The benefit-risk profile supports this regimen as the first-line therapy for patients with HER2-positive metastatic breast cancer from all geographic regions.
Keywords: Asia, Febrile neutropenia, HER2-positive metastatic breast cancer, Pertuzumab, Trastuzumab
Abstract
Introduction.
We report detailed safety analyses by geographic region from the phase III study CLEOPATRA with pertuzumab, trastuzumab, and docetaxel in patients with human epidermal growth factor receptor 2 (HER2)-positive first-line metastatic breast cancer.
Patients and Methods.
Patients received pertuzumab/placebo at 840 mg in cycle 1 and 420 mg in subsequent cycles, and trastuzumab at 8 mg/kg in cycle 1 and 6 mg/kg in subsequent cycles; docetaxel was initiated at 75 mg/m2. All study drugs were given intravenously, 3 times weekly.
Results.
Docetaxel dose reductions below 75 mg/m2 were more common in patients from Asia (47.0%) than other regions (13.4%); docetaxel dose escalations to 100 mg/m2 were less frequent in Asia (2.4%) than other regions (18.7%). Rates of edema (26.1% and 5.4% for Asia and other regions, respectively), myalgia (42.3%, 14.7%), nail disorder (39.9%, 15.1%), febrile neutropenia (18.6%, 7.1%), upper respiratory tract infection (25.7%, 10.2%), decreased appetite (47.0%, 19.1%), and rash (44.3%, 22.0%) were at least twice as high in Asia as in other regions. Adverse events did not result in a reduction in the median number of study treatment cycles administered in patients from Asia. Efficacy analyses per region showed hazard ratios similar to those of the whole intention-to-treat (ITT) population for progression-free survival (ITT: 0.63; Asia: 0.68; other regions: 0.61) and overall survival (ITT: 0.66; Asia: 0.64; other regions: 0.66).
Conclusion.
Despite a higher proportion of docetaxel dose reductions in patients from Asia, survival benefits were comparable between regions. The benefit-risk profile of pertuzumab, trastuzumab, and docetaxel supports this regimen as the first-line therapy for patients with HER2-positive metastatic breast cancer from all geographic regions.
Abstract
摘要
简介。本文按照地理区域对 III 期CLEOPATRA研究中的安全性数据进行了详细分析和报道。CLEOPATRA 研究是一项帕妥珠单抗、曲妥珠单抗和多西他赛作为一线药物治疗人表皮生长因子受体-2 (HER2) 阳性转移性乳腺癌的研究。
患者与方法。患者在周期 1 接受帕妥珠单抗/安慰剂的剂量为 840 mg,在后续周期中为 420 mg;曲妥珠单抗在周期 1 中的剂量为 8 mg/kg,在后续周期中为 6 mg/kg;多西他赛的初始剂量则为 75 mg/m2。所有研究药物均以静脉方式给药,每周 3 次。
结果。亚洲地区的患者将多西他赛的剂量减至 75 mg/m2 以下的几率 (47.0%) 高于其他地区的患者 (13.4%),将多西他赛的剂量上调至 100 mg/m2 的几率 (2.4%) 则低于其他地区的患者 (18.7%)。亚洲患者的水肿(亚洲发生率为 26.1%,其他地区发生率为 5.4%)、肌痛 (42.3%, 14.7%)、指甲异常 (39.9%, 15.1%)、发热性中性粒细胞减少症 (18.6%, 7.1%)、上呼吸道感染 (25.7%, 10.2%)、食欲下降 (47.0%, 19.1%) 和皮疹 (44.3%, 22.0%) 的发生率至少为其他地区患者的两倍之多。不良事件并未导致亚洲患者的中位研究治疗周期数发生下降。按地区进行的疗效分析显示,与整个意向性治疗人群 (ITT)相比,风险比在无进展生存期(ITT:0.63;亚洲:0.68;其他地区:0.61)和总生存期(ITT:0.66;亚洲:0.64;其他地区:0.66)方面相似。
结论。虽然多西他赛在亚洲患者中的剂量减量发生率更高,但亚洲与其他地区的生存获益是相当的。帕妥珠单抗、曲妥珠单抗和多西他赛联合用药方案所表现出的良好风险-获益比表明,这一方案有资格成为所有地区 HER2 阳性转移性乳腺癌患者的一线治疗方案。
The Oncologist 2014;19:693–701
Implications for Practice:
In the CLEOPATRA study, pertuzumab plus trastuzumab and docetaxel significantly improved overall survival over trastuzumab and docetaxel in human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer (MBC). Overall, patients from Asia experienced more toxicities than patients from other regions. The median number of treatment cycles was not reduced in patients from Asia, but the docetaxel dose was reduced in almost 50% of these patients. These dose adjustments did not affect efficacy; therefore, dose reduction in the second or subsequent cycles for patients with significant toxicity should be considered. We recommend pertuzumab plus trastuzumab and docetaxel in patients with HER2-positive MBC irrespective of geographic region.
Introduction
Approximately 20% of breast cancers are characterized by amplification and/or overexpression of human epidermal growth factor receptor 2 (HER2) [1], which has an adverse effect on prognosis compared with HER2-normal breast cancers [2]. Combining chemotherapy with agents that specifically target HER2 can significantly improve survival outcomes in patients with HER2-positive breast cancer [3–8].
In the phase III study CLEOPATRA, trastuzumab and docetaxel were combined with the novel HER2-targeted humanized monoclonal antibody pertuzumab for the treatment of patients with HER2-positive metastatic breast cancer who had not received chemotherapy or biologic therapy for metastatic disease. Compared with trastuzumab plus docetaxel, the combination of pertuzumab with trastuzumab and docetaxel resulted in statistically significant and clinically meaningful improvements in progression-free survival [9] and overall survival [10]. The combination of pertuzumab with trastuzumab and docetaxel did not raise any new safety concerns; however, the incidence of febrile neutropenia was increased in patients from Asia receiving treatment in the pertuzumab arm compared with other geographic regions. Among patients from Asia, 12% in the placebo arm and 26% in the pertuzumab arm experienced febrile neutropenia, whereas in all other geographic regions the incidence was 10% or less in either treatment arm [9]. Further analyses showed that no events of febrile neutropenia were reported after the discontinuation of docetaxel [11]. These observations led us to analyze in more detail the safety profile in patients from Asia compared with all other geographic regions and the exposure of patients to docetaxel.
Patients and Methods
Study Design and Treatment
Study details have been described previously [9, 10]. CLEOPATRA was a randomized, double-blind, placebo-controlled, international phase III trial designed with two treatment arms: placebo, trastuzumab (Herceptin; F. Hoffmann-La Roche, Basel, Switzerland, http://www.roche.com), and docetaxel (Taxotere; Sanofi-Aventis, Paris, France, http://en.sanofi.com) (referred to as placebo arm); and pertuzumab (Perjeta; F. Hoffmann-La Roche), trastuzumab, and docetaxel (referred to as pertuzumab arm). Treatment allocation was stratified by geographic region (Asia, Europe, North America, and South America) and prior treatment status (neoadjuvant and/or adjuvant treatment received or not). Primary endpoint was independently assessed progression-free survival. Secondary endpoints included overall survival, progression-free survival by investigator assessment, objective response rate, and safety.
Study drugs were administered intravenously on a 3-week schedule. Pertuzumab/placebo was given at 840 mg in cycle 1, followed by 420 mg in subsequent cycles. Trastuzumab was given at 8 mg/kg in cycle 1 and at 6 mg/kg in subsequent cycles. Dose modifications of pertuzumab and trastuzumab were not permitted. Pertuzumab/placebo and trastuzumab were administered until investigator-assessed disease progression or unmanageable toxicity. Docetaxel was initiated at 75 mg/m2. At the discretion of the treating physician, the docetaxel dose could be increased to 100 mg/m2 for patients who had tolerated at least one cycle of 75 mg/m2. Per protocol, the treating physician could reduce the docetaxel dose by 25% from 75 to 55 mg/m2 or from 100 to 75 mg/m2 to manage febrile neutropenia or neutrophil counts <500/mm3 for >1 week, platelet count <100,000/mm3, or severe or cumulative cutaneous reactions. Re-escalation of the docetaxel dose was not permitted. Use of granulocyte-colony-stimulating factors (G-CSFs) was allowed as primary prophylaxis and for the treatment of febrile neutropenia, according to local practice and guidelines from the American Society of Clinical Oncology [12]. Docetaxel dose reductions were the preferred measure for prevention of febrile neutropenia in subsequent cycles; however, secondary prophylaxis with G-CSFs was also allowed. The administration of docetaxel could be delayed because of toxicities. If docetaxel was delayed for more than 3 weeks without recovery, the patient was withdrawn from docetaxel treatment. On or before cycle 6, docetaxel was discontinued only for disease progression or unmanageable toxicity. After cycle 6, continuation of docetaxel was at the discretion of the patient and treating physician.
The study was conducted according to Good Clinical Practice and the Declaration of Helsinki. Protocol approval was obtained from independent ethics committees at each participating site. All patients provided written informed consent.
Eligibility Criteria
Patients with HER2-positive locally recurrent, unresectable, or metastatic breast cancer who had not received prior chemotherapy or biologic therapy for their advanced disease were eligible. Further inclusion criteria were measurable and/or nonmeasurable disease, a baseline left ventricular ejection fraction (LVEF) of at least 50%, an Eastern Cooperative Oncology Group performance status of 0 or 1, and a disease-free interval of at least 12 months. Among the exclusion criteria were decline in LVEF to below 50% during or after prior therapy with trastuzumab, prior exposure to a cumulative dose of doxorubicin (or its equivalent) of more than 360 mg/m2, and history of congestive heart failure.
Assessments
Adverse events were monitored continuously and graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 3.0. During the treatment period until the treatment discontinuation visit, all adverse events were reported and followed until resolution, improvement to baseline, confirmation by the investigator that no further improvement could be expected, or death. Nonserious adverse events that were ongoing at the time of study treatment discontinuation were not followed up at the post-treatment discontinuation visit. Serious adverse events that were ongoing at the time of treatment discontinuation were followed until resolution, stabilization, or death up to 1 year after the final study dose. Treatment-related serious adverse events that started after the treatment discontinuation visit were reported at any time and followed until resolution, stabilization, or death up to 1 year after start of the event. LVEF measurements took place at baseline, every 9 weeks during the treatment period, at the treatment discontinuation visit, every 6 months in the first year of follow-up, and then annually for up to 3 years. Tumor assessments were performed every 9 weeks.
Statistical Methods
The primary analysis was defined to take place after 381 independently assessed progression-free survival events had occurred. An unstratified log-rank test was used to compare independently assessed progression-free survival and overall survival between treatment arms by geographic regions. A Cox proportional hazards model was used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs). Adverse events were reported and analyzed using descriptive methods. To investigate a potential effect of body weight and height on the incidence of febrile neutropenia, diarrhea, and mucosal inflammation, we grouped patients into quartiles according to weight, body surface area (BSA), and body mass index (BMI), and the incidence of adverse events was reported descriptively. Efficacy analyses were performed according to treatment allocation (intention-to-treat [ITT] population), and safety analyses were performed as per treatment received (safety population). The trial is registered with ClinicalTrials.gov, NCT00567190.
Results
Study Population
Between February 2008 and July 2010, 808 patients were enrolled into CLEOPATRA from 204 sites in 25 countries from Asia, Europe, North America, and South America (Europe, North America, and South America are referred to as “other regions”). Participating countries from Asia were the People’s Republic of China (including Hong Kong; n = 18), Japan (n = 53), Korea (n = 94), the Philippines (n = 30), Singapore (n = 20), and Thailand (n = 38). The data cutoff date for the analyses presented here was in May 2011; the only exception is the analysis of overall survival for which the data cutoff was in May 2012. The ITT population comprised 406 patients in the placebo arm and 402 patients in the pertuzumab arm. Two patients in each arm did not receive any study treatment. Eight patients randomized to the placebo arm received at least one dose of pertuzumab and one patient randomized to the pertuzumab arm received treatment allocated to the placebo arm only. The safety population therefore comprised 397 patients in the placebo arm and 407 patients in the pertuzumab arm (supplemental online Fig. 1). The median time on study treatment was 11.8 months for patients in the placebo arm and 18.1 months for patients in the pertuzumab arm. According to race, there were 133 Asian patients randomized to the placebo arm and 128 Asian patients randomized to the pertuzumab arm. Split by region, 128 and 125 patients in the placebo and pertuzumab arms, respectively, were from Asia. We present analyses according to region. The median age of patients from Asia was 52.0 years (28−81) in the placebo arm and 54.0 years (29−76) in the pertuzumab arm. Patients from all other regions had a median age of 54.5 years (27−89) and 54.0 years (22−82) in the placebo and pertuzumab arms, respectively. Previous exposure to docetaxel was generally similar for patients from Asia and for patients from all other regions: 7.0% and 8.0% of patients from Asia in the placebo and pertuzumab arms, respectively, received docetaxel for early breast cancer compared with 10.4% (placebo arm) and 9.6% (pertuzumab arm) of patients from all other regions.
Exposure to Docetaxel
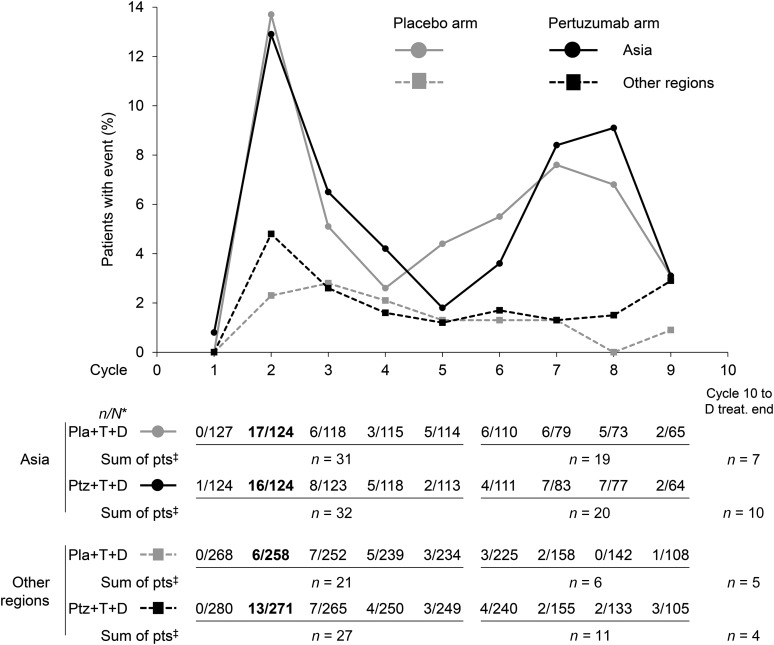
During the study, the median number of docetaxel cycles administered in patients from Asia was nine in both treatment arms, and thus slightly higher than that in patients from all other geographic regions (eight and seven) (Table 1). The proportion of patients who underwent a docetaxel dose escalation to 100 mg/m2 was lower in patients from Asia compared with all other regions (2.4% vs. 18.7%). Docetaxel dose reductions below 75 mg/m2 were carried out in 47.0% of patients from Asia compared with 13.4% of patients from other regions. Overall, docetaxel dose reductions below 75 mg/m2 were most commonly performed at cycle 2 (Fig. 1). The majority of docetaxel dose reductions were per protocol, with a minimal number of off-protocol dose reductions: five patients in the pertuzumab arm (two from Asia, three from all other regions) and two patients in the placebo arm (both from regions other than Asia). Figure 1 presents data up until cycle 9, which was the median number of docetaxel cycles in patients from Asia. The number of patients receiving study treatment with docetaxel decreased at later cycles (Fig. 1). The proportion of patients who permanently discontinued treatment with docetaxel was comparable between patients from Asia and those from other regions receiving the same treatment regimen (Table 1). In all subgroups, at least 95% of patients who permanently discontinued docetaxel had completed the minimum recommended number of six docetaxel cycles. The majority of patients from Asia who discontinued docetaxel treatment did so because of an adverse event, whereas the majority of patients from other regions discontinued treatment with docetaxel as a result of standard practice (Table 1).
Table 1.
Exposure to docetaxel
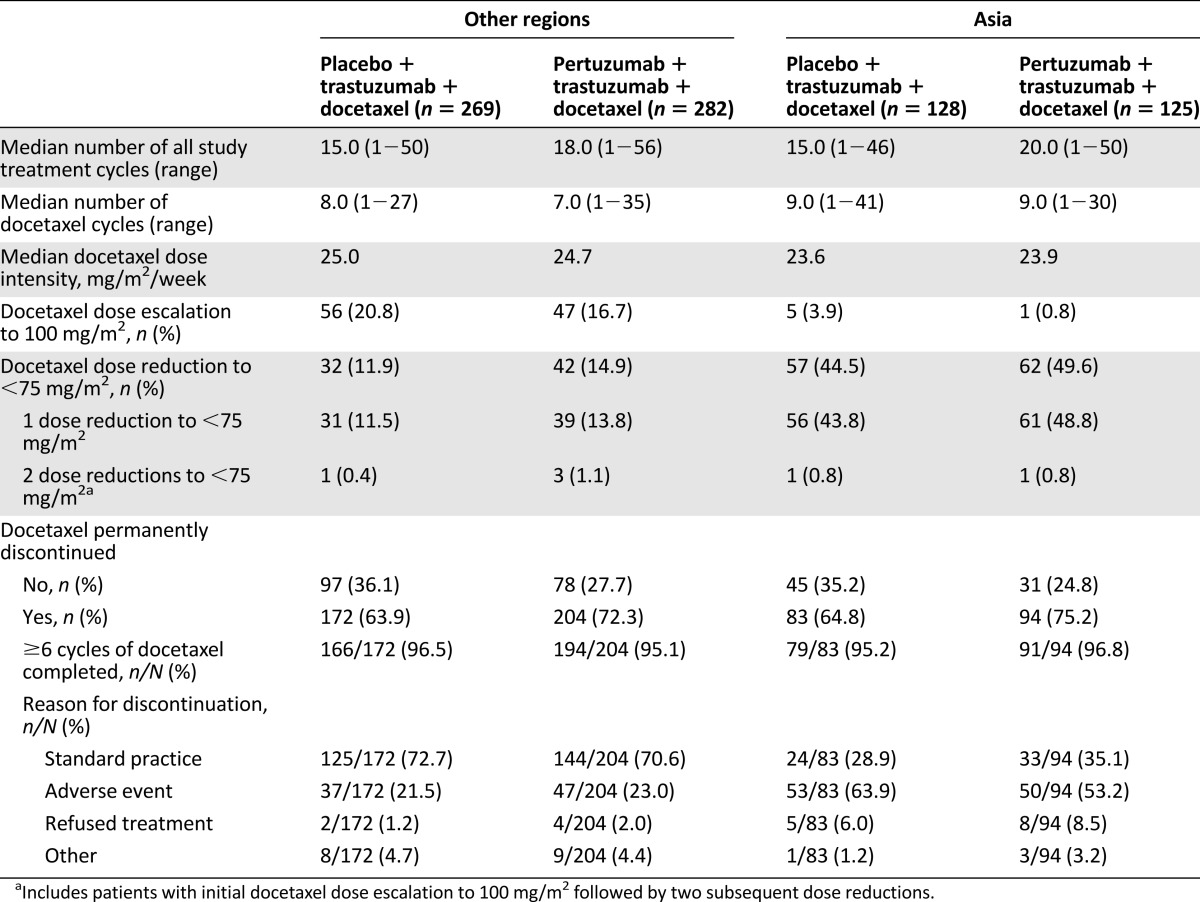
Figure 1.
First occurrence of docetaxel dose reduction <75 mg/m2 by study treatment cycle in the safety population according to treatment received and geographic region: Asia versus other regions (Europe, North America, and South America). Overall, docetaxel dose reductions below 75 mg/m2 were most commonly performed at cycle 2 (data in bold). Data are presented up until cycle 9, the median number of docetaxel cycles in patients from Asia. ∗, n, number of patients with first occurrence of docetaxel dose reduction <75 mg/m2; N, number of patients receiving any docetaxel dose at a given cycle. ‡, Sum of patients with first occurrence of docetaxel dose reduction <75 mg/m2 in cycles 1−5, cycles 6−9, and cycle 10 until the end of docetaxel treatment.
Abbreviations: D, docetaxel; Pla, placebo; pts, patients; Ptz, pertuzumab; T, trastuzumab; treat., treatment.
Adverse Events
Adverse events (all grades) that were reported with an incidence of at least 25% in patients from Asia and the incidences of febrile neutropenia and left ventricular systolic dysfunction (LVSD) are presented in Table 2. As reported previously, the incidence of febrile neutropenia was 11.7% in the placebo arm versus 25.6% in the pertuzumab arm in patients from Asia. Similarly, the incidence of mucosal inflammation was doubled in the pertuzumab arm (36.8%) compared with the placebo arm (18.0%) in patients from Asia. For both treatment arms combined, an increased incidence of at least twofold was reported for edema, myalgia, nail disorder, febrile neutropenia, upper respiratory tract infection, decreased appetite, and rash in patients from Asia compared with patients from other regions. For the pertuzumab arms only, an increased incidence of at least twofold in patients from Asia compared with patients from other regions was reported for edema, myalgia, febrile neutropenia, decreased appetite, upper respiratory tract infection, nail disorder, and constipation. The frequencies of grade ≥3 adverse events and serious adverse events were higher in patients from Asia compared with patients from other regions; however, the incidence of adverse events resulting in discontinuation of all study treatment (pertuzumab/placebo plus trastuzumab plus docetaxel or pertuzumab/placebo plus trastuzumab) was similar for patients from Asia and other regions.
Table 2.
Adverse events (all grades) reported in ≥25% of patients from Asia plus adverse events of special interest (incidence reported overall and after docetaxel discontinuation)a
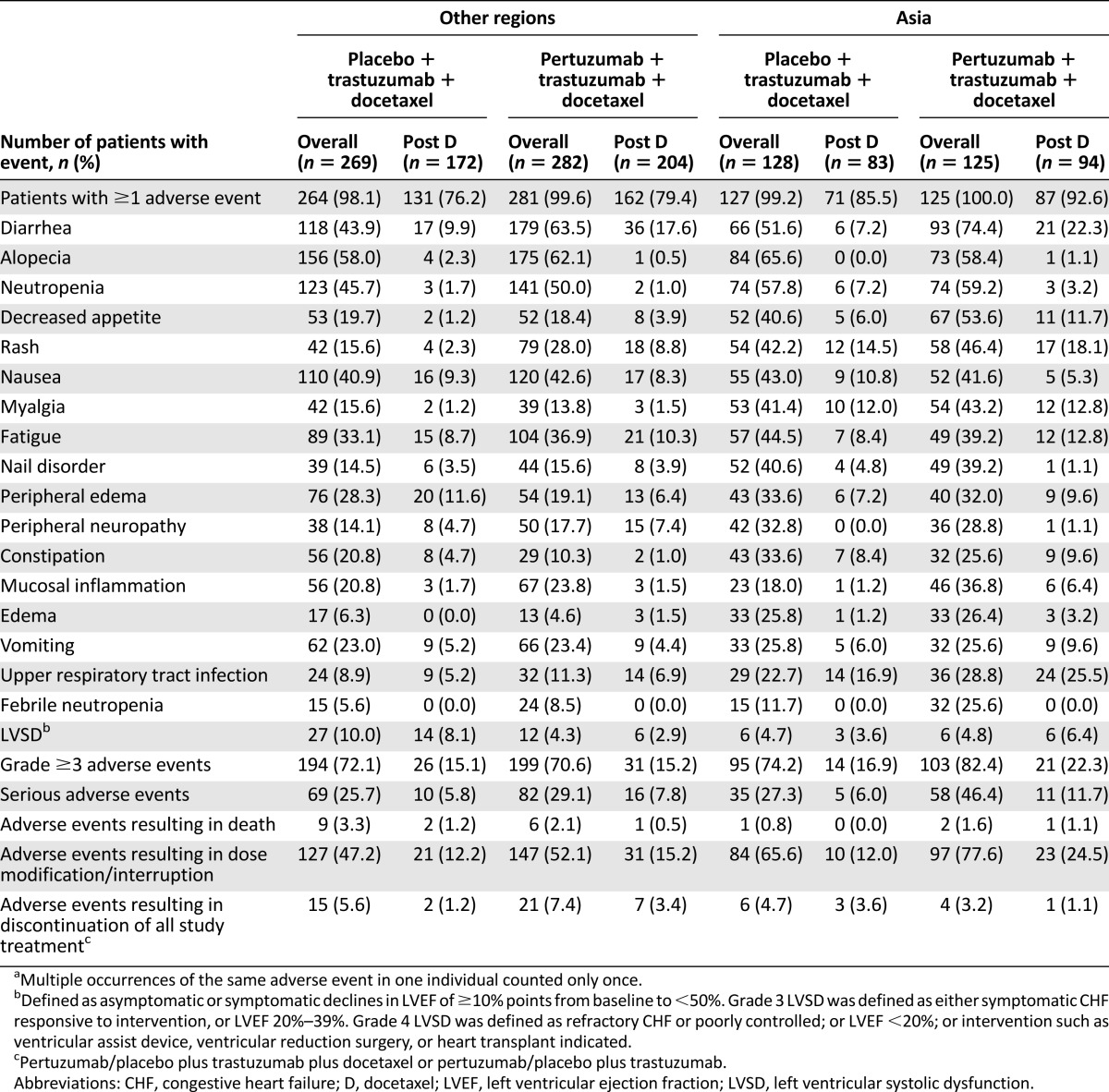
Following the discontinuation of docetaxel, the incidence of adverse events decreased considerably with the exception of LVSD (Table 2). No further events of febrile neutropenia were reported in any arm or geographic region after the discontinuation of docetaxel. Although diarrhea and mucosal inflammation were reported less frequently after docetaxel treatment had been discontinued, the incidence of diarrhea continued to be higher in patients treated in the pertuzumab arm than in the placebo arm in all regions, and the incidence of mucosal inflammation in patients from Asia remained higher in the pertuzumab arm compared with the placebo arm.
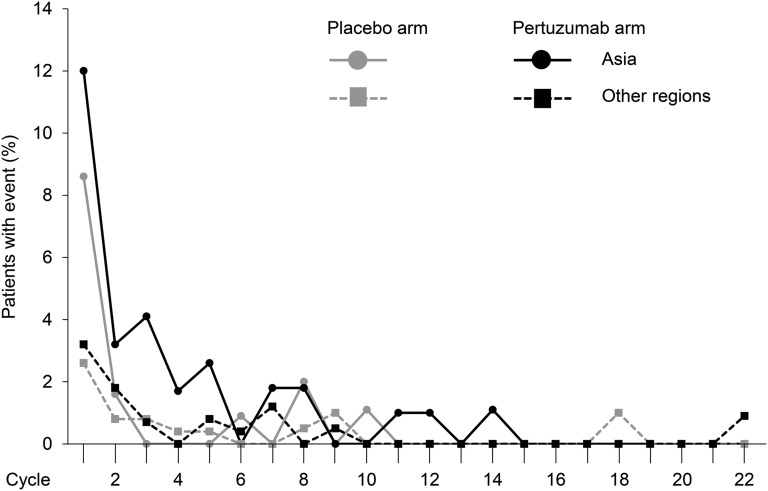
We analyzed the rate of febrile neutropenia per treatment cycle (Fig. 2). The incidence of febrile neutropenia was notably high during the first treatment cycle in all regions compared with subsequent cycles, but it was particularly high in patients from Asia. From cycle 1 to cycle 2, the incidence of febrile neutropenia decreased between approximately two- and fivefold in all subgroups and was low during later treatment cycles.
Figure 2.
Incidence of febrile neutropenia by study treatment cycle in the safety population according to treatment received and geographic region: Asia versus other regions (Europe, North America, and South America).
Four patients, all treated in the pertuzumab arm, discontinued treatment with docetaxel because of febrile neutropenia. Two of these patients were from Asia and two were from other regions. One additional patient from other regions discontinued pertuzumab, trastuzumab, and docetaxel because of febrile neutropenia. The docetaxel dose was reduced in 73.3% (11 of 15) and 65.6% (21 of 32) of patients from Asia experiencing febrile neutropenia in the placebo and pertuzumab arms, respectively, and in 46.7% (7 of 15) and 41.7% (10 of 24) of patients from other regions. In patients who experienced febrile neutropenia, G-CSFs were used to treat the event in 80.0% (12 of 15) and 93.8% (30 of 32) of patients from Asia and in 53.3% (8 of 15) and 45.8% (11 of 24) of patients from other regions in the placebo and pertuzumab arms, respectively. In patients who had experienced febrile neutropenia, G-CSFs were used prophylactically at subsequent cycles in 6.7% (1 of 15) and 34.4% (11 of 32) of patients from Asia and in 40.0% (6 of 15) and 12.5% (3 of 24) of patients from other regions in the placebo and pertuzumab arms, respectively. Of all patients who experienced febrile neutropenia, mucosal inflammation and diarrhea were reported concomitantly in 46.7% (7 of 15) versus 59.4% (19 of 32) of patients from Asia in the placebo and pertuzumab arms and in 26.7% (4 of 15) versus 45.8% (11 of 24) of patients from other regions in the placebo and pertuzumab arms.
More than 50% of patients from Asia belonged to the lowest weight and BSA quartile (≤55.8 kg and ≤1.549 m2, respectively). To analyze whether the incidences of febrile neutropenia, diarrhea, and mucosal inflammation were associated with patient weight and height, we compared the rates of these adverse events per weight, BSA, and BMI quartiles in patients from Asia and other geographic regions per treatment arm (supplemental online Tables 1–9). There was no trend for patients in the lower quartiles, irrespective of geographic region and treatment received, to be more likely to experience febrile neutropenia, diarrhea, or mucosal inflammation than patients in the upper quartiles.
Efficacy
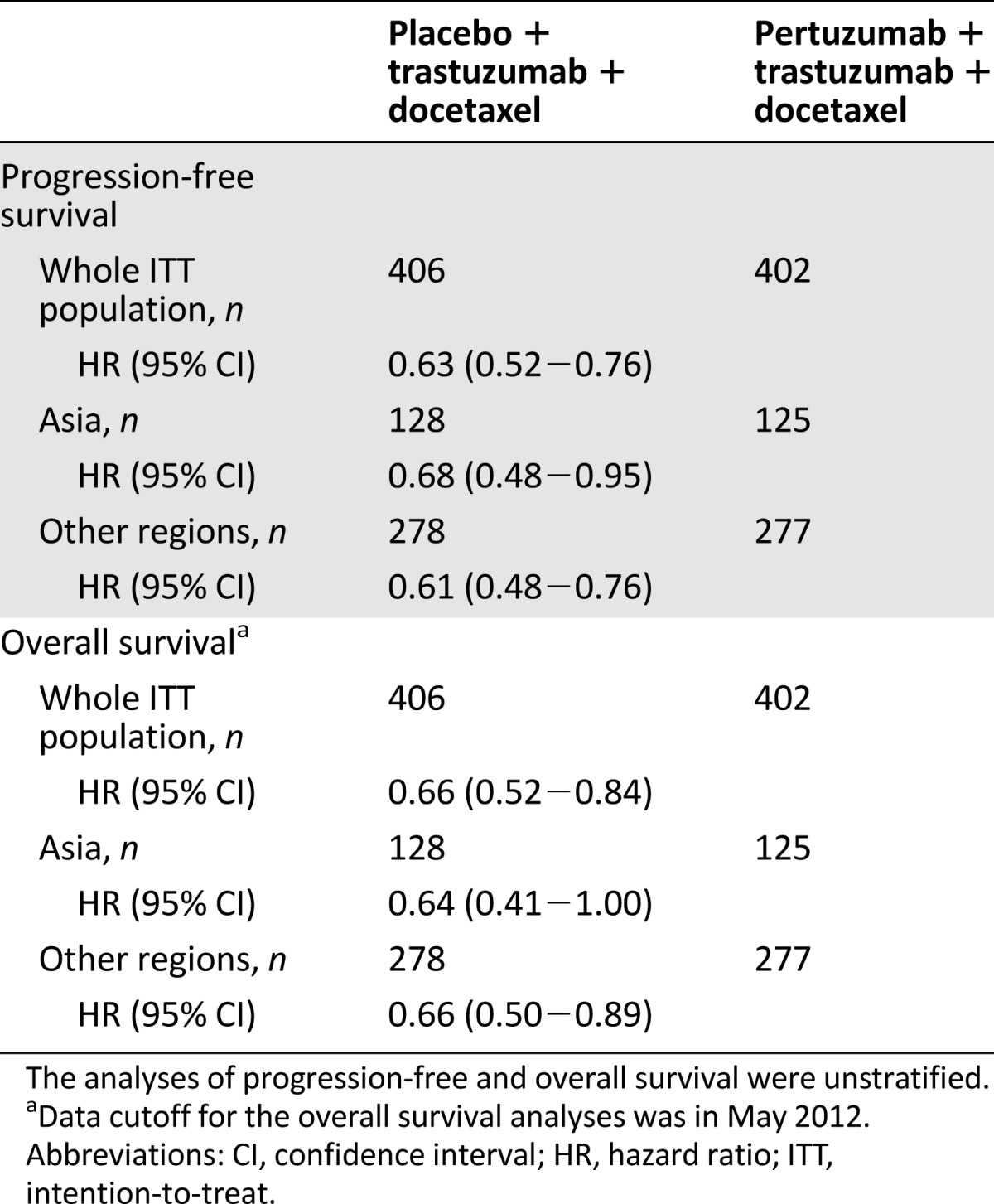
In the whole ITT population, independently assessed progression-free survival (HR = 0.63; data cutoff in May 2011) and overall survival (HR = 0.66; data cutoff in May 2012) were significantly improved with pertuzumab plus trastuzumab plus docetaxel compared with placebo plus trastuzumab plus docetaxel (unstratified analyses) [9, 10]. Exploratory analyses of progression-free and overall survival performed per region (Asia vs. all other regions combined) were similar to the results in the whole ITT population: independently assessed progression-free survival, HR = 0.68 (95% CI, 0.48−0.95) for Asia, HR = 0.61 (95% CI, 0.48−0.76) for other regions; overall survival, HR = 0.64 (95% CI, 0.41−1.00) for Asia, HR = 0.66 (95% CI, 0.50−0.89) for other regions (Table 3).
Table 3.
Independently assessed progression-free survival and overall survival
Discussion
During the primary analysis of results from CLEOPATRA in 2011, it was noted that the incidence of febrile neutropenia was almost twice as high in the pertuzumab arm (13.8%) as in the placebo arm (7.6%) [9]. These rates, however, were lower than those of 23% [8] and 37% [13] for febrile neutropenia reported in previous studies of trastuzumab with docetaxel for HER2-positive metastatic breast cancer with a docetaxel starting dose of 100 mg/m2. A further analysis of data from CLEOPATRA revealed that the incidence of febrile neutropenia was 10% or less in either arm in patients from Europe, North America, and South America, whereas it was reported in 12% and 26% of patients in the placebo and pertuzumab arms, respectively, in patients from Asia [9]. Similarly, the rate of mucosal inflammation was doubled in the pertuzumab arm (37%) compared with the placebo arm (18%) in patients from Asia. The increased incidence of febrile neutropenia in patients from Asia could be a result of the higher incidences of mucosal inflammation and diarrhea in this population.
An apparent difference between patients from Asia and patients from other regions was the proportion with docetaxel dose escalation and reduction, as well as the reason for permanent docetaxel discontinuation. The proportion of patients in Asia with a docetaxel dose escalation to 100 mg/m2 was small (2.4%), whereas 47.0% in this region had a reduction below 75 mg/m2. The main reason for permanent discontinuation of docetaxel in Asia was adverse events, but discontinuation was due to standard practice in the majority of patients from other regions. However, the incidence of adverse events resulting in discontinuation of all study treatment was not higher in patients from Asia compared with other regions, suggesting that dosing guidelines and support measures were successfully applied. Despite the higher rate of docetaxel dose reductions and of adverse events overall in patients from Asia, the median number of docetaxel cycles was slightly higher than that in patients from other regions. This observation, together with the finding that docetaxel was discontinued because of an adverse event rather than standard practice, in the majority of patients from Asia suggest that after cycle 6 patients from Asia were more often treated until unmanageable toxicity occurred, in contrast to patients from other regions who more often discontinued treatment with docetaxel according to local practice. Importantly, at least 95% of patients who permanently discontinued docetaxel had received the minimum per-protocol recommendation of six docetaxel cycles. These results suggest that adverse events were mainly addressed by an adjustment of the docetaxel dose rather than early discontinuation of treatment.
To investigate whether the higher incidences of febrile neutropenia, diarrhea, and mucosal inflammation in patients from Asia were associated with a lower body weight and height, we grouped patients according to weight, BSA, and BMI quartile. More than 50% of patients from Asia belonged to the lowest weight and BSA quartile compared with 12%−13% of patients from other regions. Our descriptive analyses gave no evidence of an association between weight, BSA, or BMI and the incidences of febrile neutropenia, diarrhea, or mucosal inflammation in patients from any region. Within the same weight, BSA, or BMI quartile, patients from Asia appeared to be at greater risk for experiencing febrile neutropenia than patients from other regions. It should be noted, however, that the number of patients experiencing the event per quartile was particularly low for febrile neutropenia, which constitutes a limitation to the interpretation of our findings.
Docetaxel has a narrow therapeutic index, which makes the interindividual variability in docetaxel clearance particularly problematic [14–17]. Docetaxel is metabolized mainly by the hepatic cytochrome P450 isoenzyme CYP3A4 and to a lesser extent by CYP3A5 [18]. Several polymorphisms have been described for cytochrome P450 enzymes [19] and CYP3A4 in particular [17, 20–24]. Although interindividual variability in CYP3A4 enzyme activity has frequently been described [15, 25, 26], with some studies suggesting that Asians have a lower CYP3A4 activity than Caucasians [27, 28], research into the correlation of polymorphisms with phenotypes has been inconclusive. As CYP3A4 enzyme activity is positively correlated with docetaxel clearance, variability in CYP3A4 activity may cause an increase in toxicity in some patients [14, 15] but underexposure to the detriment of efficacy in others. Consistent with studies suggesting lower CYP3A4 activity in Asians, some retrospective studies have reported a higher incidence of chemotherapy-related grade ≥3 hematologic toxicities in Asian patients with early breast cancer compared with other races [29, 30].
In addition to the interindividual variability in CYP3A4 enzyme activity, another mechanism that can potentially affect drug clearance is drug-drug interactions. Cortés et al. have reported that, for the regimen used in CLEOPATRA, there is no evidence of a pharmacokinetic (PK) drug-drug interaction of pertuzumab with docetaxel or with trastuzumab [31]. This finding was expected given that pertuzumab and docetaxel have different clearance pathways [32, 33] and because pertuzumab and trastuzumab bind noncompetitively [34] to distinct epitopes on HER2 [35, 36]. With a sample size of 37 patients, the PK substudy of CLEOPATRA was small. Four patients in the placebo arm and five patients in the pertuzumab arm were Asian [31]. One of these patients in each arm experienced febrile neutropenia. Although the sample size is too small to draw any conclusions, individual docetaxel PK parameters were similar in Asian patients and patients from other races in this substudy (unpublished data).
The correlation of drug clearance with BSA has been challenged [37] and individualized docetaxel dosing based on CYP3A4 activity/docetaxel clearance may be more appropriate to reduce interindividual variability and toxicity [25, 38–40] while maintaining efficacy. Yamamoto et al. have investigated the effect of individualized docetaxel dosing based on CYP3A4 activity in patients with advanced non-small cell lung cancer. They found that interpatient PK variability of docetaxel was reduced with individualized dosing compared with BSA-based dosing, whereas response rates were similar between both arms [25]. The individualized dosing had, however, no effect on the toxicity profile of docetaxel, and it should be noted that the study was powered to detect variability in PK but was not powered for safety or efficacy endpoints [25]. Several CYP3A4 phenotyping tests are currently available [41–45], and their potential applicability in routine clinical practice merits further investigation. Carboplatin is an established example of a chemotherapeutic agent whose dose is calculated based on target exposure and glomerular filtration rate [46].
Conclusion
In our study, patients with HER2-positive metastatic breast cancer from Asia experienced more toxicities from treatment with pertuzumab, trastuzumab, and docetaxel than patients from other regions. This outcome did not, however, result in a reduction in the median number of study treatment cycles administered in these patients compared with patients from other regions. But given that in almost 50% of patients from Asia the docetaxel dose was reduced below 75 mg/m2 without a decrease in efficacy, a reduction of the docetaxel dose in the second or subsequent cycles for patients who experience significant toxicity with administration of 75 mg/m2 should be considered to improve the safety profile of the study combination. Our findings here provide an example of the importance of assessing the actual treatment experience of novel agents within subgroups of patients given that reports of results from clinical trials often lack the specific details about therapeutic administration necessary for the translation of trial results to clinical practice [47]. We believe that our data from patients in Asia are an important contribution for practicing clinicians as they provide a valuable insight into the safety profile and into adverse event management without compromising efficacy.
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
The study was funded by F. Hoffmann-La Roche Ltd. (Basel, Switzerland) and Genentech, a member of the Roche Group (South San Francisco, CA). Targos Molecular Pathology (Kassel, Germany) conducted central HER2 testing. Support for third-party writing assistance for this manuscript, furnished by Vilma Graupner, Ph.D., CMPP (Health Interactions, London, U.K.), was provided by F. Hoffmann-La Roche Ltd.
Footnotes
For Further Reading: Sandra M. Swain, Michael S. Ewer, Javier Cortés et al. Cardiac Tolerability of Pertuzumab Plus Trastuzumab Plus Docetaxel in Patients With HER2-Positive Metastatic Breast Cancer in CLEOPATRA: A Randomized, Double-Blind, Placebo-Controlled Phase III Study. The Oncologist 2013;18:257–264.
Implications for Practice: CLEOPATRA was the first phase III trial in which the combination of pertuzumab with trastuzumab and docetaxel was studied in patients with HER2-positive metastatic breast cancer in the first line. As therapy with trastuzumab, especially in combination with anthracyclines, has been associated with cardiac dysfunction, it was important to investigate the cardiac tolerability of the study combination of two HER2-targeted antibodies, trastuzumab and pertuzumab, with docetaxel. Our analyses showed that the combination of pertuzumab, trastuzumab, and docetaxel was not associated with an increase in cardiac dysfunction, especially LVSD, compared with placebo, trastuzumab and docetaxel. Cardiac adverse events were largely reversible and clinically manageable. Despite our encouraging findings, we recommend the regular cardiac monitoring of patients while long-term safety data with pertuzumab-trastuzumab-based treatment are still being accrued in clinical practice.
Author Contributions
Conception/design: Sandra M. Swain, Adam Knott, Graham Ross, José Baselga
Provision of study material or patients: Sandra M. Swain, Young-Hyuck Im, Seock-Ah Im, Valorie Chan, David Miles, José Baselga
Collection and/or assembly of data: Sandra M. Swain, Seock-Ah Im, David Miles, Adam Knott, Graham Ross, José Baselga
Data analysis and interpretation: Sandra M. Swain, Young-Hyuck Im, Seock-Ah Im, Valorie Chan, David Miles, Adam Knott, Emma Clark, Graham Ross, José Baselga
Manuscript writing: Sandra M. Swain, Young-Hyuck Im, Seock-Ah Im, Valorie Chan, David Miles, Adam Knott, Emma Clark, Graham Ross, José Baselga
Final approval of manuscript: Sandra M. Swain, Young-Hyuck Im, Seock-Ah Im, Valorie Chan, David Miles, Adam Knott, Emma Clark, Graham Ross, José Baselga
Disclosures
Sandra M. Swain: Genentech/Roche (C/A, uncompensated), Genentech/Roche, Pfizer (Puma), Sanofi-Aventis, Bristol-Myers Squibb (RF); David Miles: Roche/Genentech (C/A); Graham Ross: Roche Products Ltd. (E, IP, OI); Adam Knott: Roche (E); Emma Clark: Roche (E), AstraZeneca (OI); José Baselga: Roche (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18–43. doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]
- 2.Ross JS, Slodkowska EA, Symmans WF, et al. The HER-2 receptor and breast cancer: Ten years of targeted anti-HER-2 therapy and personalized medicine. The Oncologist. 2009;14:320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 3.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 4.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 5.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 6.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 8.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 9.Baselga J, Cortés J, Kim S-B, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swain SM, Kim SB, Cortés J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): Overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baselga J, Cortés J, Im S-A et al. Adverse events with pertuzumab and trastuzumab: Evolution during treatment with and without docetaxel in CLEOPATRA. Poster presented at: 48th Annual Meeting of the American Society of Clinical Oncology; June 1–5, 2012; Chicago, IL. [Google Scholar]
- 12.Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: An evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 13.Andersson M, Lidbrink E, Bjerre K, et al. Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: The HERNATA study. J Clin Oncol. 2011;29:264–271. doi: 10.1200/JCO.2010.30.8213. [DOI] [PubMed] [Google Scholar]
- 14.Bruno R, Hille D, Riva A, et al. Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol. 1998;16:187–196. doi: 10.1200/JCO.1998.16.1.187. [DOI] [PubMed] [Google Scholar]
- 15.Hirth J, Watkins PB, Strawderman M, et al. The effect of an individual’s cytochrome CYP3A4 activity on docetaxel clearance. Clin Cancer Res. 2000;6:1255–1258. [PubMed] [Google Scholar]
- 16.Yamamoto N, Tamura T, Kamiya Y, et al. Correlation between docetaxel clearance and estimated cytochrome P450 activity by urinary metabolite of exogenous cortisol. J Clin Oncol. 2000;18:2301–2308. doi: 10.1200/JCO.2000.18.11.2301. [DOI] [PubMed] [Google Scholar]
- 17.Tran A, Jullien V, Alexandre J, et al. Pharmacokinetics and toxicity of docetaxel: Role of CYP3A, MDR1, and GST polymorphisms. Clin Pharmacol Ther. 2006;79:570–580. doi: 10.1016/j.clpt.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Shou M, Martinet M, Korzekwa KR, et al. Role of human cytochrome P450 3A4 and 3A5 in the metabolism of Taxotere and its derivatives: Enzyme specificity, interindividual distribution and metabolic contribution in human liver. Pharmacogenetics. 1998;8:391–401. doi: 10.1097/00008571-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Guengerich FP. Polymorphism of cytochrome P-450 in humans. Trends Pharmacol Sci. 1989;10:107–109. doi: 10.1016/0165-6147(89)90207-1. [DOI] [PubMed] [Google Scholar]
- 20.Ball SE, Scatina J, Kao J, et al. Population distribution and effects on drug metabolism of a genetic variant in the 5′ promoter region of CYP3A4. Clin Pharmacol Ther. 1999;66:288–294. doi: 10.1016/S0009-9236(99)70037-8. [DOI] [PubMed] [Google Scholar]
- 21.Lamba JK, Lin YS, Thummel K, et al. Common allelic variants of cytochrome P4503A4 and their prevalence in different populations. Pharmacogenetics. 2002;12:121–132. doi: 10.1097/00008571-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Lamba JK, Lin YS, Schuetz EG, et al. Genetic contribution to variable human CYP3A-mediated metabolism. Adv Drug Deliv Rev. 2002;54:1271–1294. doi: 10.1016/s0169-409x(02)00066-2. [DOI] [PubMed] [Google Scholar]
- 23.Maekawa K, Harakawa N, Yoshimura T, et al. CYP3A4*16 and CYP3A4*18 alleles found in East Asians exhibit differential catalytic activities for seven CYP3A4 substrate drugs. Drug Metab Dispos. 2010;38:2100–2104. doi: 10.1124/dmd.110.034140. [DOI] [PubMed] [Google Scholar]
- 24.van Schaik RH. CYP450 pharmacogenetics for personalizing cancer therapy. Drug Resist Updat. 2008;11:77–98. doi: 10.1016/j.drup.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto N, Tamura T, Murakami H, et al. Randomized pharmacokinetic and pharmacodynamic study of docetaxel: Dosing based on body-surface area compared with individualized dosing based on cytochrome P450 activity estimated using a urinary metabolite of exogenous cortisol. J Clin Oncol. 2005;23:1061–1069. doi: 10.1200/JCO.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 26.Watkins PB. Noninvasive tests of CYP3A enzymes. Pharmacogenetics. 1994;4:171–184. doi: 10.1097/00008571-199408000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Lin Y, Anderson GD, Kantor E, et al. Differences in the urinary excretion of 6-beta-hydroxycortisol/cortisol between Asian and Caucasian women. J Clin Pharmacol. 1999;39:578–582. doi: 10.1177/00912709922008182. [DOI] [PubMed] [Google Scholar]
- 28.Ahsan CH, Renwick AG, Waller DG, et al. The influence of dose and ethnic origins on the pharmacokinetics of nifedipine. Clin Pharmacol Ther. 1993;54:329–338. doi: 10.1038/clpt.1993.155. [DOI] [PubMed] [Google Scholar]
- 29.Ma B, Yeo W, Hui P, et al. Acute toxicity of adjuvant doxorubicin and cyclophosphamide for early breast cancer—A retrospective review of Chinese patients and comparison with an historic Western series. Radiother Oncol. 2002;62:185–189. doi: 10.1016/s0167-8140(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 30.Han HS, Reis IM, Zhao W, et al. Racial differences in acute toxicities of neoadjuvant or adjuvant chemotherapy in patients with early-stage breast cancer. Eur J Cancer. 2011;47:2537–2545. doi: 10.1016/j.ejca.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 31.Cortés J, Swain SM, Kudaba I, et al. Absence of pharmacokinetic drug-drug interaction of pertuzumab with trastuzumab and docetaxel. Anticancer Drugs. 2013;24:1084–1092. doi: 10.1097/CAD.0000000000000016. [DOI] [PubMed] [Google Scholar]
- 32.Royer I, Monsarrat B, Sonnier M, et al. Metabolism of docetaxel by human cytochromes P450: Interactions with paclitaxel and other antineoplastic drugs. Cancer Res. 1996;56:58–65. [PubMed] [Google Scholar]
- 33.Keizer RJ, Huitema AD, Schellens JH, et al. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:493–507. doi: 10.2165/11531280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 34.Scheuer W, Friess T, Burtscher H, et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69:9330–9336. doi: 10.1158/0008-5472.CAN-08-4597. [DOI] [PubMed] [Google Scholar]
- 35.Franklin MC, Carey KD, Vajdos FF, et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 36.Cho HS, Mason K, Ramyar KX, et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756–760. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 37.Sawyer M, Ratain MJ. Body surface area as a determinant of pharmacokinetics and drug dosing. Invest New Drugs. 2001;19:171–177. doi: 10.1023/a:1010639201787. [DOI] [PubMed] [Google Scholar]
- 38.Rivory LP, Slaviero K, Seale JP, et al. Optimizing the erythromycin breath test for use in cancer patients. Clin Cancer Res. 2000;6:3480–3485. [PubMed] [Google Scholar]
- 39.Goh BC, Lee SC, Wang LZ, et al. Explaining interindividual variability of docetaxel pharmacokinetics and pharmacodynamics in Asians through phenotyping and genotyping strategies. J Clin Oncol. 2002;20:3683–3690. doi: 10.1200/JCO.2002.01.025. [DOI] [PubMed] [Google Scholar]
- 40.Puisset F, Chatelut E, Dalenc F, et al. Dexamethasone as a probe for docetaxel clearance. Cancer Chemother Pharmacol. 2004;54:265–272. doi: 10.1007/s00280-004-0823-0. [DOI] [PubMed] [Google Scholar]
- 41.Rivory LP, Slaviero KA, Hoskins JM, et al. The erythromycin breath test for the prediction of drug clearance. Clin Pharmacokinet. 2001;40:151–158. doi: 10.2165/00003088-200140030-00001. [DOI] [PubMed] [Google Scholar]
- 42.Farrell GC, Zaluzny L. Accuracy and clinical utility of simplified tests of antipyrine metabolism. Br J Clin Pharmacol. 1984;18:559–565. doi: 10.1111/j.1365-2125.1984.tb02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watkins PB, Turgeon DK, Saenger P, et al. Comparison of urinary 6-beta-cortisol and the erythromycin breath test as measures of hepatic P450IIIA (CYP3A) activity. Clin Pharmacol Ther. 1992;52:265–273. doi: 10.1038/clpt.1992.140. [DOI] [PubMed] [Google Scholar]
- 44.Thummel KE, Shen DD, Podoll TD, et al. Use of midazolam as a human cytochrome P450 3A probe: I. In vitro-in vivo correlations in liver transplant patients. J Pharmacol Exp Ther. 1994;271:549–556. [PubMed] [Google Scholar]
- 45.Thummel KE, Shen DD, Podoll TD, et al. Use of midazolam as a human cytochrome P450 3A probe: II. Characterization of inter- and intraindividual hepatic CYP3A variability after liver transplantation. J Pharmacol Exp Ther. 1994;271:557–566. [PubMed] [Google Scholar]
- 46.Calvert AH. Dose optimisation of carboplatin in adults. Anticancer Res. 1994;14:2273–2278. [PubMed] [Google Scholar]
- 47.Duff JM, Leather H, Walden EO, et al. Adequacy of published oncology randomized controlled trials to provide therapeutic details needed for clinical application. J Natl Cancer Inst. 2010;102:702–705. doi: 10.1093/jnci/djq117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.