The choice of an antiepileptic drug (AED) for treatment of brain tumor-related epilepsy (BTE) depends on its proof of evidence in partial epilepsies in adults and individual patient features. Recent designation of a number of AEDs prompts the use of levetiracetam followed by valproic acid as the AED monotherapy of choice in BTE. In case either one is insufficiently active as a single agent or in combination, as in the case of untoward effects, alternative AEDs are lacosamide, lamotrigine, and zonisamide.
Keywords: Glioma, Glioblastoma, Cancer, Epilepsy, Seizures, Levetiracetam, Valproic acid, Guideline, Review
Abstract
Brain tumor-related epilepsy (BTE) is common in low- and high-grade gliomas. The risk of seizures varies between 60% and 100% among low-grade gliomas and between 40% and 60% in glioblastomas. The presence of seizures in patients with brain tumors implies favorable and unfavorable factors. New-onset seizures represent an early warning sign for the presence of a brain tumor and count as a good prognostic factor for survival. Recurrence or worsening of seizures during the course of disease may signal tumor progression. Each of the modalities for tumor control (i.e., surgery, radiotherapy, chemotherapy) contributes to seizure control. Nevertheless, one third of BTE shows pharmacoresistance to antiepileptic drugs (AEDs) and may severely impair the burden of living with a brain tumor. For symptomatic therapy of BTE, seizure type and individual patient factors determine the appropriate AED. Randomized controlled trials in partial epilepsy in adults to which type BTE belongs and additional studies in gliomas indicate that levetiracetam is the agent of choice, followed by valproic acid (VPA). In the case of recurring seizures, combining these two drugs (polytherapy) seems effective and possibly synergistic. If either one is not effective or not well tolerated, lacosamide, lamotrigine, or zonisamide are additional options. A new and exciting insight is the potential contribution of VPA to prolonged survival, particularly in glioblastomas. A practice guideline on symptomatic medical management including dose schedules of AEDs is supplied.
Implications for Practice:
Seizures are common in low- and high-grade gliomas. New-onset seizures represent an early warning sign and count as a favorable prognostic factor for survival. Each of the modalities for tumor control (i.e., surgery, radiotherapy, chemotherapy) contributes to seizure control. For symptomatic management, levetiracetam followed by valproic acid are the evidence-based antiepileptic agents in low-grade gliomas and other types of brain tumors. In glioblastoma, valproic acid represents the first choice based on its extra effect on survival. With recurring seizures, combining both levetiracetam and valproic acid (polytherapy) seems synergistic. Lacosamide, lamotrigine, or zonisamide are additional options.
Introduction
Seizures are commonly seen in brain tumors, usually in the range of 40% to 60%. They often represent the first clinical sign of a brain tumor and count as a favorable prognostic factor, although reappearance or worsening of seizures may indicate tumor recurrence.
In this review, we focus on seizures in adults with low- and high-grade gliomas. Epidemiology, clinical impact, and the underlying mechanism are discussed, including the significance of isocitrate-dehydrogenase 1 mutations and changes in glutamate and GABA metabolism.
Seizure control can be achieved by both antitumor treatment and antiepileptic drugs (AEDs). Special attention is paid to the appropriate anticonvulsants among the different choices according to evidence-based criteria. This selection not only depends on the type of epilepsy, but also on individual patient characteristics. Pharmacoresistant epilepsy, adverse effects, and potential drug interactions between AEDs and chemotherapeutic drugs often complicate seizure management in brain tumors. A new development is that the use of AEDs may have a beneficial influence on survival. Recently, the activity of valproic acid (VPA) as a histone deacetylase inhibitor (HDACi) has gained attention for its antitumor effects in glioblastoma (GBM), notably in combination with systemic chemotherapy. For practical purposes, a guideline on the medical management of seizure control including dose regimens is supplied.
Literature references were identified through searches of PubMed with the search terms “glioma,” “brain tumor,” “epilepsy,” “seizure,” “antiepileptic drugs,” and “pharmacoresistance.” Articles were identified also through searches of our own files. Only articles in English were reviewed. Studies on children were excluded, as well as articles with fewer than 10 patients and studies on the traditional enzyme-inducing antiepileptic drugs (i.e., carbamazepine, phenobarbital, and phenytoin).
Molecular Biological Factors of Seizure Development
A number of molecular biological factors have been recognized in the epileptogenesis of brain tumors. The presence of mutation of codons 132 and 172 of isocitrate-dehydrogenase 1 (IDH1) and 2 (IDH2) is associated with seizures in low-grade gliomas (LGGs). The more prevalent IDH1 mutation is present in 70%–88% of LGGs and is located within the cytoplasm; IDH2 is located within mitochondria [1, 2]. IDH1 catalyzes isocitrate to α-ketoglutarate as part of the citric acid cycle. If mutated, 2-hydroxyglutarate will be formed instead. This latter product shows structural similarity to glutamate and may activate N-methyl-D-aspartate (NMDA) receptors with ensuing epileptogenesis. In LGGs, the presence of IDH1 mutations shows a strong association with seizures as the initial clinical symptom, frontal lobe tumor location, and longer survival [3, 4]. The excitatory neurotransmitter glutamate plays an important role in seizure development, in which membrane glutamate transporter proteins are involved. Abnormalities include increased expression of specific glutamate receptor subtypes, low activity of glutamine synthetase, high glutamate concentrations in glioma cells, and almost absent intracellular uptake with excessive extracellular levels. These changes correlate with higher seizure frequency and may affect tumor progression [5, 6]. Disturbances in chloride balance may play a role as well, secondary to changes in chloride cotransporters by reduced potassium chloride cotransporter 2 and increased Na-K-2Cl cotransporter expression, suggesting accompanying changes in GABA metabolism and chloride transport [7, 8]. Glutamergic stimulation of NMDA and AMPA receptors may activate intracellular mTOR, AKT, and MAPK signaling pathways, promoting both cell growth and epileptogenesis [5].
Seizures as a Presenting Sign and Their Relation to Survival
New-onset seizures often represent the first clinical symptom for each type of brain tumor and are often the only clinical sign in neuroglial tumors. More benign gliomas show a higher frequency of seizures than malignant gliomas. Neurogliomas (i.e., dysembryoplastic neuroepithelial tumors [DNETs]) and gangliogliomas have a seizure incidence of 80%–100%, oligodendrogliomas of 70%–90%, diffuse low-grade gliomas of 60%–85%, and glioblastomas (GBMs) of 40%–60% [9, 10, 11]. In GBMs, 40% of patients present with epilepsy, and 20% develop seizures later on [11–13]. Epilepsy as a presenting symptom implies a favorable prognostic factor for duration of survival in both low- and high-grade gliomas [14, 15].
In general, the seizure type in brain tumors is characterized as partial or localization-related epilepsy, and varies between simple partial in 23%–58%, complex partial in 7%–31%, and secondary generalized seizures in 10%–68% [14, 16–18]. Generalized seizures are often seen as the early warning sign of seizures in about half the cases, and partial seizures without loss of consciousness dominate in the case of persisting seizures [16].
In low-grade gliomas, favorable prognostic factors of postoperative seizure control are presence of generalized seizures, surgery within <1 year after presentation, gross tumor resection, and successful preoperative control by AEDs [14, 19, 20]. Approximately 15%–50% of patients with low-grade gliomas demonstrate pharmacoresistant seizures, often associated with insular or temporal tumor location and the presence of simple partial seizures [10, 14, 16, 17, 21, 22]. There is no clear difference in seizure outcome between grade II gliomas or other low-grade pathologies with seizures as the first clinical sign, including pilocytic astrocytomas, DNETs, gangliogliomas, and gangliocytomas. In high-grade gliomas, seizure control is more successful with better performance status and less successful with preoperatively not well-controlled seizures or parietal lobe locations [15].
A recurrence or worsening of seizures following first-line antitumor therapy heralds progression of GBMs in approximately two thirds of patients [12, 14, 15]. In low-grade gliomas, this association is less evident [17, 23].
A recurrence or worsening of seizures following first-line antitumor therapy heralds progression of GBMs in approximately two thirds of patients. In low-grade gliomas, this association is less evident.
Effect of Antitumor Therapy on Seizure Control
Surgery
Tumor resection is an essential part of glioma therapy, often showing inherent positive effects on seizure control. In a study of low-grade neuroepithelial tumors, 82% of 207 patients were seizure-free at 1 year following a total or subtotal resection (Table 1) [24]. A large review of 910 neuroglioma patients of whom only 8% were seizure-free before surgery showed that between 68% and 84% of patients with DNETs and between 54% and 94% of those with gangliogliomas became seizure-free following surgery [25–27]. Both a shorter interval until surgery and a gross total versus subtotal resection indicate higher rates of freedom from seizures (87% vs. 54%).
Table 1.
Effect of antitumor therapy on seizure control
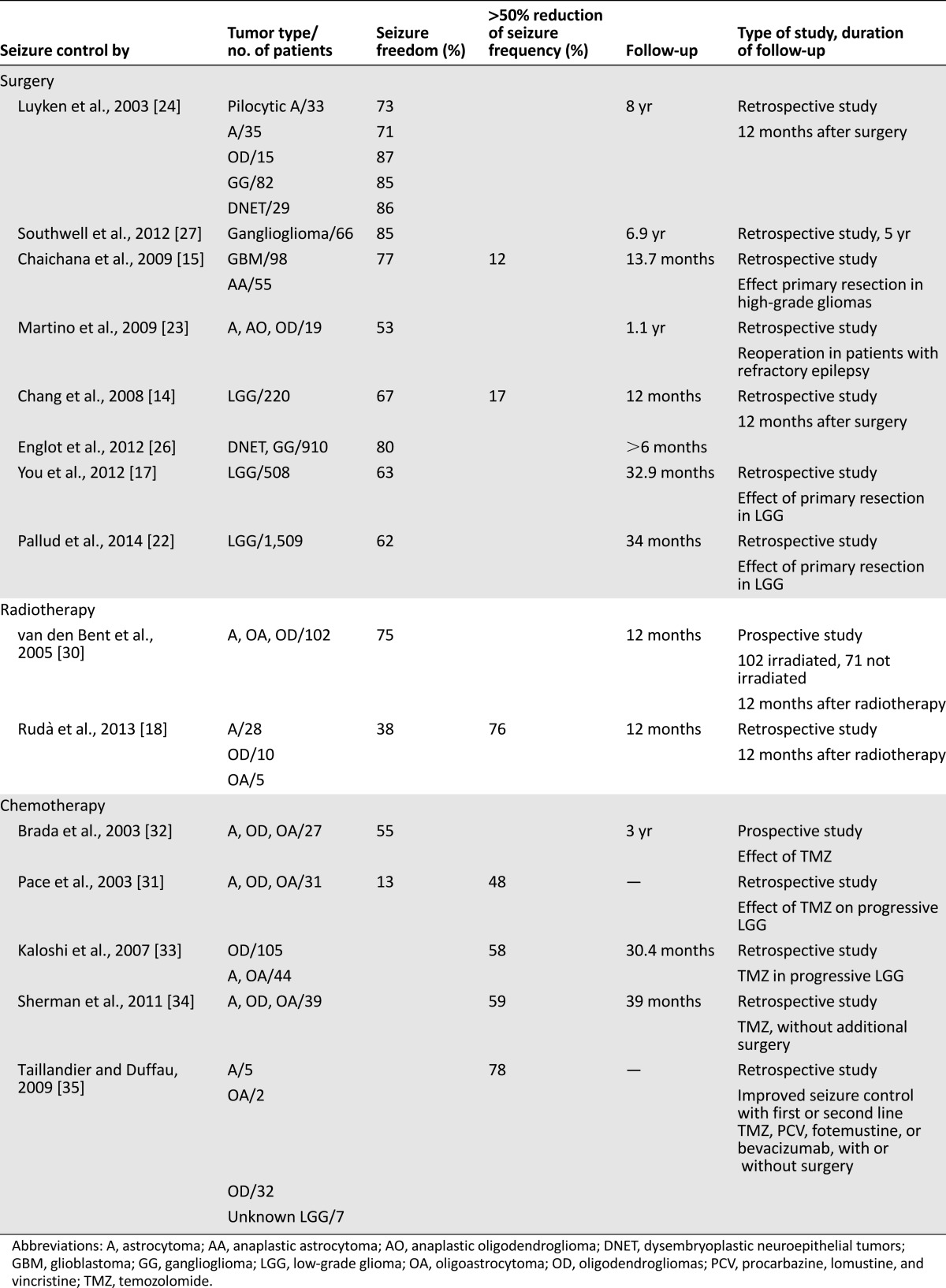
In low-grade gliomas, freedom from seizures was observed in 63%–71% of patients, and a gross total resection produced freedom from seizures in 80% as opposed to 53% by subtotal removal [14, 17, 20, 22]. In high-grade gliomas, mainly glioblastoma, 77% freedom from seizures was seen following primary tumor resection [15].
In epilepsy surgery, the use of intraoperative electrocorticography is applied for determining the location and removal of the excitatory focus. This procedure may also be valid for temporal lobe tumor locations together with performing hippocampectomy or corticectomy [28, 29].
Radio- and Chemotherapy
Radiation therapy contributes to better seizure control in low-grade gliomas and oligodendrogliomas as shown in a randomized European Organisation for Research and Treatment of Cancer phase III trial using external radiotherapy to a total dose of 65 Gy in 30 fractions; 75% of patients became seizure-free [30]. In a series of low- and high-grade gliomas, 76% of patients showed >50% seizure reduction and 38% freedom from seizures at 12 months, although no patients could discontinue AEDs [18]. Upfront temozolomide chemotherapy in low-grade gliomas resulted in >50% seizure reduction in 51%–59% of patients and freedom from seizures occurred in 13%–55% [31–34]. Likewise, by using either temozolomide, chemotherapy with procarbazine, lomustine, and vincristine, or with bevacizumab, with or without preceding surgery, >50% seizure reduction was seen in 78% of patients with recurrent low-grade gliomas (Table 1) [35].
Anticonvulsants: Guidelines on Symptomatic Management
Antiepileptic drugs can be initiated after appearance of a single seizure attributable to the presence of a brain tumor. In general, the choice of a specific AED is primarily based on the type of epilepsy. Subsequently, among the approved AEDs for a specific type of epilepsy, the choice of the most appropriate one depends on individual patient factors, particularly age, sex, weight, comorbidity, and cotherapy, including the risk of drug interactions and side effects [36, 37]. Epilepsy in patients with brain tumors belongs to the type of partial epilepsy in adults, either with or without secondary generalized seizures, and is essentially based on focal lesion or brain damage. For this type of seizure, the International League Against Epilepsy (ILAE) has recently updated the most appropriate AED choices based on a meta-analysis of a large number of randomized controlled trials [38]. As such, levetiracetam (LEV), carbamazepine, phenytoin, and zonisamide score as level A anticonvulsants. VPA represents the only level B anticonvulsant. Gabapentin, lamotrigine, oxcarbazepine, phenobarbital, topiramate, and vigabatrin are level C agents [38]. Regarding the issue of individual patient factors, consensus exists in neuro-oncology to avoid enzyme-inducing antiepileptic drugs (EIAEDs), that is, phenobarbital, carbamazepine, and phenytoin, as these accelerate the metabolism and compromise the antitumor effect of many chemotherapeutic agents [39, 40]. For zonisamide, there is as yet hardly any experience in brain tumors [41].
For LEV, numerous studies in brain tumor-related epilepsy (BTE) have been carried out either as monotherapy or add-on therapy, resulting in freedom from seizures between 76% and 91%, a 50% seizure reduction up to 100%, and it counts as one of the best-tolerated AEDs [42–50]. These results are summarized in Table 2. For that reason, together with its designation as a level A agent for partial epilepsy, LEV is the preferred monotherapy choice in patients with brain tumors. LEV may also be effective with seizures in brain metastasis and meningiomas [13, 51, 52]. Nevertheless, one has to realize that these excellent figures probably include the beneficial effects of preceding surgery or other concomitant antitumor therapy.
Table 2.
Efficacy of antiepileptic drugs in low- and high-grade gliomas
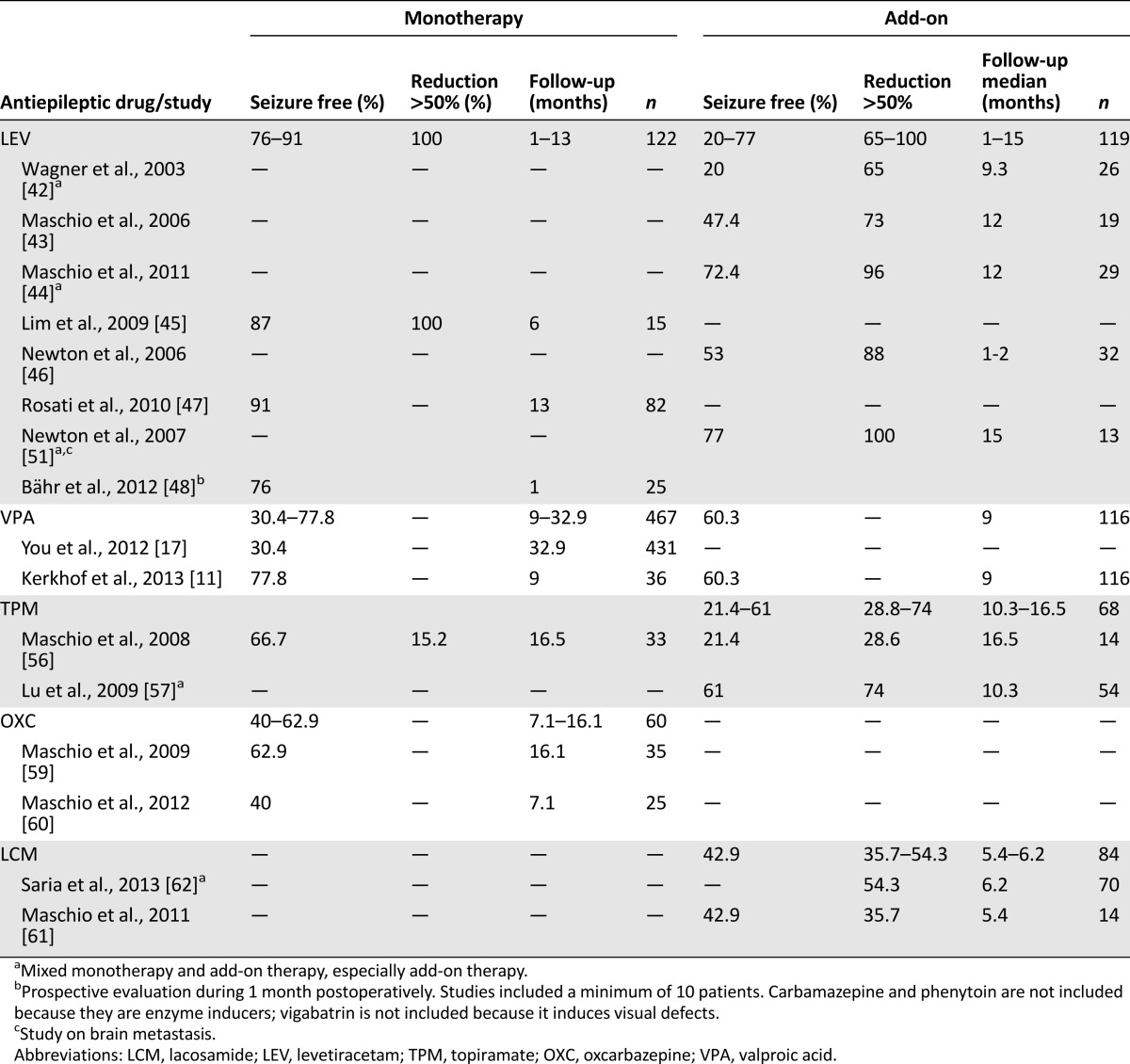
For VPA monotherapy, the rationale in BTE is based on its designation as a level B agent for partial epilepsy in adults, and is supported by a large experience and efficacy profile in BTE, showing freedom from seizures in 30%–78% of patients with low-grade gliomas or GBMs and good tolerability [11, 12, 16, 17, 50]. VPA may induce or aggravate thrombopenia, particularly in combination with chemotherapy, although multifactorial analysis in GBMs has indicated temozolomide as the only significant factor [53, 54]. For GBMs, we prefer VPA as the anticonvulsant of choice based on its favorable interaction with survival (see Effect of AEDs on Survival section). If seizure control is insufficient with monotherapy of LEV or VPA, polytherapy with both drugs combined is preferred over sequential trials of AED monotherapy [11, 55].
With regard to level C AEDs, there is limited information on gabapentin and lamotrigine in BTE, although both are well tolerated, with lamotrigine showing better efficacy. Topiramate has been studied especially as an add-on therapy, although it may produce considerable cognitive dysfunction [56, 57] (Table 2). Oxcarbazepine as monotherapy in BTE produces freedom from seizures in 40%–62.9% of patients, but it often is not without side effects [58–60]. Lacosamide has been approved as an add-on therapy and has shown 42.9% freedom from seizures in low- and high-grade gliomas (Table 2) [61, 62]. Recently, pregabalin has shown a 49% retention rate as compared with 58% LEV in a randomized comparison [63].
In our opinion, if either LEV or VPA or its combination is less effective, it is our choice for the present time to use either lacosamide as an add-on AED based on its activity and tolerability in BTE, lamotrigine for its good tolerability and indications of synergistic activity with VPA, or zonisamide considering its efficacy, tolerability, low degree of interactions, and recent designation as a level A agent for the partial epilepsies [38, 61, 62].
Table 3 provides details of the preferred choices of AEDs in tumoral epilepsy, including dosing, titrating, and tapering regimens. These guidelines extend to the perioperative period, even in the absence of preceding seizures. However, ongoing prophylactic use of AEDs in BTE has never indicated clear efficacy, and studies on this issue often have shown methodological shortcomings [40].
Table 3.
Symptomatic management of seizures in brain tumors

Pharmacoresistance
Pharmacoresistant epilepsy is commonly defined as a failure of adequate attempts of two or more appropriately dosed antiepileptic and tolerated drug regimens to control seizures. In LGGs, refractory epilepsy before initial surgery is seen in 50% of patients and is seen following surgery in 15%–35% of cases despite anticonvulsant therapy [17, 66].
One hypothesis to explain treatment resistance is based on alterations in drug targets affecting antiepileptic drug binding. Another one is overexpression of multidrug resistant proteins (MRPs) belonging to the ATP-binding cassette transporter family (i.e., P-glycoprotein, MRP1, MRP5, and breast-cancer resistance protein) [67, 68]. This mechanism results in impaired parenchymal brain access and decreased intracellular drug transport of AEDs that function as substrates of these transporter proteins. This process may likewise contribute to the chemotherapy resistance of gliomas and other cancers [69]. Phenytoin, phenobarbital, carbamazepine, and lamotrigine probably represent substrates for P-glycoprotein, whereas LEV and VPA seem to be less affected [46, 70]. One option to diminish pharmacoresistance is to apply AEDs that are not or are less dependent on multidrug transporter proteins. Nevertheless, our current understanding is limited as it is mainly based on in vitro or animal studies with inconclusive data.
Synergistic Activity of AEDs
Another option to diminish pharmacoresistance is to combine AEDs with synergistic antiepileptic activity. The general approach in epilepsy therapy is to use at least two subsequential monotherapies trials of anticonvulsant therapy before two AEDs are combined. However, recently there has been a tendency toward earlier antiepileptic polytherapy because of potential synergisms [64, 65]. One such combination is the addition of lamotrigine to VPA, although its drawback is the need to slowly uptitrate the dose of lamotrigine because of enzyme inhibition by VPA of UDP-glucuronosyltransferase glucuronidation of lamotrigine [71, 72]. In addition, the use of LEV in combination with a range of AEDs including VPA and lacosamide may produce enhanced antiepileptic activity [55, 62]. In GBMs, we found indications that combined LEV/VPA produces better seizure control [11]. These effects of LEV seem particularly apparent if combined with AEDs that enhance GABAergic activity or reduce glutaminergic neurotransmitter activity, such as VPA or benzodiazepines [73–76]. A major advantage of synergism is that lower dosages of AEDs would be sufficient for similar or better antiseizure effects. This implies smaller cumulative doses and thus reduced risks of drug toxicity given that antiepileptic therapy in BTE is prone to neurotoxicity [77].
A major advantage of synergism is that lower dosages of AEDs would be sufficient for similar or better antiseizure effects. This implies smaller cumulative doses and thus reduced risks of drug toxicity given that antiepileptic therapy in BTE is prone to neurotoxicity.
Effect of AEDs on Survival
The use of VPA in patients with GBMs has recently drawn attention because of its potential beneficial antitumor activity. First indications were observations that cotherapy with VPA produced a 3-month longer survival as compared with carbamazepine in patients with GBMs [78]. Several studies have appeared on the effect of VPA on survival in both children and adults [11, 53, 79–81]. A post hoc analysis of the pivotal European Organisation for Research and Treatment of Cancer/National Cancer Institute of Canada study on temozolomide chemoradiation in GBMs, in which 97 patients received VPA, showed a 3-month longer survival [53]. Another study of GBMs in 108 patients on VPA using the same combination produced similar results (Table 4) [11].
Table 4.
Effect of valproic acid on survival in glioblastoma
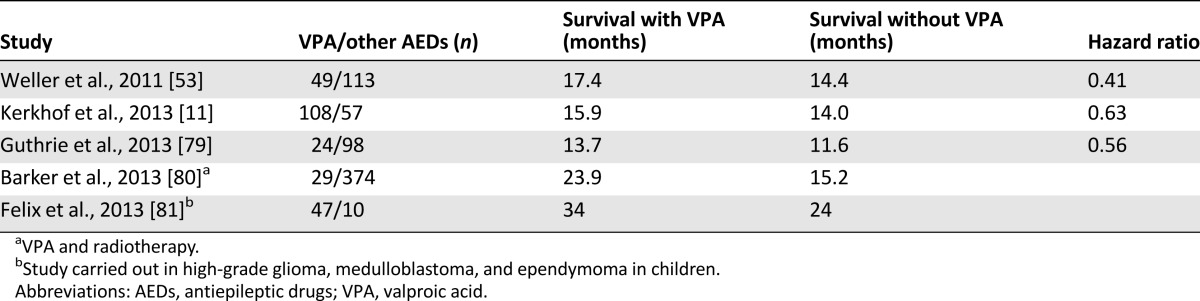
The mechanism as an HDACi of VPA probably explains these observations. By virtue of this activity, VPA or other HDACis may stimulate histone protein acetylation together with demethylation of parts of the DNA genome, leading to at least partially restored expression of upregulated oncogene or downregulated tumor suppressor genes with ensuing normalization of cell cycle control function. This would affect cell growth, angiogenesis, and tumor invasion, as well as apoptosis and autophagy [82, 83]. Although other HDACis such as trichostatin, vorinostat, and sodium butyrate have shown antitumor effects [84, 85], randomized and phase II studies in a number of hematological and solid cancers have indicated efficacy of VPA given in combination with chemotherapeutic agents [86–88]. An important aspect of these observations is that administration of HDACis together with chemotherapeutic or DNA-demethylating agents such as 5-azacytidine seems to act synergistically. This may be explained by the epigenetic modulation by HDACis, which would lead to better accessibility of chemotherapy secondary to lesser condensation of chromatin by acetylation of histone proteins. In this way, greater accessibility of chemotherapy to unfolded parts of DNA would facilitate the efficacy of restored cell functions such as apoptosis and autophagy [83, 89–91]. It is uncertain whether other anticonvulsants may also affect tumor growth. LEV may inhibit transcription of the O-6 methylguanine-DNA methyltransferase repair protein gene and may interact with temozolomide [92]. Some EIAEDs, notably carbamazepine, might prolong survival in GBM, although the numbers studied are small [79, 93].
Need for Future Studies
In general, it is desirable that once an AED is accepted for partial epilepsy in adults, additional good-quality studies on efficacy and tolerability are carried out in BTE to further assess its appropriateness. Ideally, this again would be based on randomized prospective studies, although once a drug has been registered, it is doubtful that such studies will be performed considering time and costs involved. Rather, analysis of collected data from studies carried out in well-defined subgroups and application of pharmacotherapeutical characteristics in relation to individual patient factors will provide sufficient and reliable information. One example of such an approach is a consensus paper of the ILAE on preferred AED drug choices in HIV-positive patients, based on pharmacotherapeutic understanding of antiretroviral agents and AEDs, including potential drug-drug interactions and the outcome of studies on plasma concentrations (therapeutic drug monitoring) with both agents combined [94]. Therefore, phase II studies or postapproval studies on AEDs that have recently been registered, such as zonisamide monotherapy or add-on lacosamide, are necessary before general acceptance in the clinical practice of BTE [95, 96]. Ongoing randomized trials and registration studies in partial epilepsy in adults may well enlarge the number of AEDs that qualify for application in BTE.
Conclusion
In recent years, considerable gains in knowledge have been achieved in the field of tumor-related epilepsy. The great advantage of the introduction of second- and third-generation antiepileptic drug therapy has been its overall good tolerability and absence or weak tendency of interactions. AED application in BTE is primarily based on the outcome of randomized trials designed for the common type of partial or localization-dependent epilepsy in adults to which BTE belongs. Subsequently, the particular choice for an approved or registered AED in clinical practice depends on its pharmacotherapeutical properties in relation to individual patient features including cotherapy and comorbidity. For that reason, it is better to avoid EIAEDs in BTE because they compromise concurrent chemotherapy. The recent designation of a number of AEDs that qualify as level A or B agents for partial epilepsy in adults together with pharmacotherapeutical understanding indicate the use of LEV followed by VPA as the AED monotherapy of choice in BTE. In case either one is insufficiently active as a single agent or—as a next therapeutic step—in combination, as in the case of untoward effects, subsequent AEDs that represent justifiable choices are lacosamide, lamotrigine, and zonisamide, based on their therapeutic profile.
Despite these options, the occurrence of pharmacoresistance is not rare and is seen in 10%–35% of BTE. Indications that LEV works synergistically with other anticonvulsants including VPA seems one approach to tackle pharmacoresistance. In addition, as BTE seems prone for a high incidence of side effects of AEDs, a great advantage of synergistic activity would be that smaller doses of AEDs are sufficient to achieve similar results, implying fewer risks of neurotoxicity. Synergistic activity of AEDs in neuro-oncology with the aim of improving both pharmacoresistance and cognitive functioning warrants attention and study.
Another reason for the reappearance or worsening of seizures following a long period of seizure control is progression of low-grade glioma or GBM. This necessitates another round of symptomatic management by AEDs and re-evaluation of the tumor status. In that case, it is relevant to realize that surgical resection, radiotherapy, and chemotherapy contribute to seizure control in BTE. These observations probably extend to recurrent tumor and deserve further investigation.
Although VPA belongs to the group of classical AEDs in use for decades, it is only recently that there have been strong indications that this broad-spectrum AED may improve survival in cancer. This action is most probably based on its activity as an HDACi influencing the epigenetic mechanisms that determine the status of acetylation of histone proteins and of demethylation of DNA. In this way, a cascade of transcription processes is triggered that control cell cycle pathways. An intriguing aspect of these observations includes the efficacy of valproate in combination with chemotherapeutic agents including temozolomide, resulting in improved survival of GBM. Together, these observations on valproate as well as of other HDACis open new and exciting avenues for phase II and III studies in combination with different types of chemoradiation. It is unclear to what extent other AEDs may offer similar advantages.
Author Contributions
Conception/design: Charles J. Vecht
Collection and/or assembly of data: Melissa Kerkhof, Alberto Duran-Pena
Data analysis and interpretation: Charles J. Vecht
Manuscript writing: Charles J. Vecht, Melissa Kerkhof, Alberto Duran-Pena
Final approval of manuscript: Charles J. Vecht, Melissa Kerkhof, Alberto Duran-Pena
Disclosures
Charles J. Vecht: UCB Pharma (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Houillier C, Wang X, Kaloshi G, et al. IDH1 or IDH2 mutations predict longer survival and response to temozolomide in low-grade gliomas. Neurology. 2010;75:1560–1566. doi: 10.1212/WNL.0b013e3181f96282. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe T, Nobusawa S, Kleihues P, et al. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. 2009;174:1149–1153. doi: 10.2353/ajpath.2009.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubbink HJ, Taal W, van Marion R, et al. IDH1 mutations in low-grade astrocytomas predict survival but not response to temozolomide. Neurology. 2009;73:1792–1795. doi: 10.1212/WNL.0b013e3181c34ace. [DOI] [PubMed] [Google Scholar]
- 4.Stockhammer F, Misch M, Helms HJ, et al. IDH1/2 mutations in WHO grade II astrocytomas associated with localization and seizure as the initial symptom. Seizure. 2012;21:194–197. doi: 10.1016/j.seizure.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Yuen TI, Morokoff AP, Bjorksten A, et al. Glutamate is associated with a higher risk of seizures in patients with gliomas. Neurology. 2012;79:883–889. doi: 10.1212/WNL.0b013e318266fa89. [DOI] [PubMed] [Google Scholar]
- 6.Rosati A, Poliani PL, Todeschini A, et al. Glutamine synthetase expression as a valuable marker of epilepsy and longer survival in newly diagnosed glioblastoma multiforme. Neuro Oncol . 2013;15:618–625. doi: 10.1093/neuonc/nos338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huberfeld G, Wittner L, Clemenceau S, et al. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci. 2007;27:9866–9873. doi: 10.1523/JNEUROSCI.2761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pallud J, Capelle L, Huberfeld G. Tumoral epileptogenicity: How does it happen? Epilepsia. 2013;54(suppl 9):30–34. doi: 10.1111/epi.12440. [DOI] [PubMed] [Google Scholar]
- 9.Kahlenberg CA, Fadul CE, Roberts DW, et al. Seizure prognosis of patients with low-grade tumors. Seizure. 2012;21:540–545. doi: 10.1016/j.seizure.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Rudà R, Bello L, Duffau H, et al. Seizures in low-grade gliomas: Natural history, pathogenesis, and outcome after treatments. Neuro Oncol. 2012;14(suppl 4):iv55–iv64. doi: 10.1093/neuonc/nos199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerkhof M, Dielemans JC, van Breemen MS, et al. Effect of valproic acid on seizure control and on survival in patients with glioblastoma multiforme. Neuro Oncol. 2013;15:961–967. doi: 10.1093/neuonc/not057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wick W, Menn O, Meisner C, et al. Pharmacotherapy of epileptic seizures in glioma patients: Who, when, why and how long? Onkologie. 2005;28:391–396. doi: 10.1159/000086375. [DOI] [PubMed] [Google Scholar]
- 13.van Breemen MS, Rijsman RM, Taphoorn MJ, et al. Efficacy of anti-epileptic drugs in patients with gliomas and seizures. J Neurol. 2009;256:1519–1526. doi: 10.1007/s00415-009-5156-9. [DOI] [PubMed] [Google Scholar]
- 14.Chang EF, Potts MB, Keles GE, et al. Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg. 2008;108:227–235. doi: 10.3171/JNS/2008/108/2/0227. [DOI] [PubMed] [Google Scholar]
- 15.Chaichana KL, Parker SL, Olivi A, et al. Long-term seizure outcomes in adult patients undergoing primary resection of malignant brain astrocytomas. Clinical article. J Neurosurg. 2009;111:282–292. doi: 10.3171/2009.2.JNS081132. [DOI] [PubMed] [Google Scholar]
- 16.Hildebrand J, Lecaille C, Perennes J, et al. Epileptic seizures during follow-up of patients treated for primary brain tumors. Neurology. 2005;65:212–215. doi: 10.1212/01.wnl.0000168903.09277.8f. [DOI] [PubMed] [Google Scholar]
- 17.You G, Sha ZY, Yan W, et al. Seizure characteristics and outcomes in 508 Chinese adult patients undergoing primary resection of low-grade gliomas: A clinicopathological study. Neuro Oncol. 2012;14:230–241. doi: 10.1093/neuonc/nor205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudà R, Magliola U, Bertero L, et al. Seizure control following radiotherapy in patients with diffuse gliomas: A retrospective study. Neuro Oncol . 2013;15:1739–1749. doi: 10.1093/neuonc/not109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pignatti F, van den Bent M, Curran D, et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20:2076–2084. doi: 10.1200/JCO.2002.08.121. [DOI] [PubMed] [Google Scholar]
- 20.Englot DJ, Han SJ, Berger MS, et al. Extent of surgical resection predicts seizure freedom in low-grade temporal lobe brain tumors. Neurosurgery. 2012;70:921–928; discussion 928. doi: 10.1227/NEU.0b013e31823c3a30. [DOI] [PubMed] [Google Scholar]
- 21.Duffau H, Capelle L, Lopes M, et al. Medically intractable epilepsy from insular low-grade gliomas: Improvement after an extended lesionectomy. Acta Neurochir (Wien) 2002;144:563–572; discussion 572–573. doi: 10.1007/s00701-002-0941-6. [DOI] [PubMed] [Google Scholar]
- 22.Pallud J, Audureau E, Blonski M, et al. Epileptic seizures in diffuse low-grade gliomas in adults. Brain. 2014;137:449–462. doi: 10.1093/brain/awt345. [DOI] [PubMed] [Google Scholar]
- 23.Martino J, Taillandier L, Moritz-Gasser S, et al. Re-operation is a safe and effective therapeutic strategy in recurrent WHO grade II gliomas within eloquent areas. Acta Neurochir (Wien) 2009;151:427–436; discussion 436. doi: 10.1007/s00701-009-0232-6. [DOI] [PubMed] [Google Scholar]
- 24.Luyken C, Blümcke I, Fimmers R, et al. The spectrum of long-term epilepsy-associated tumors: Long-term seizure and tumor outcome and neurosurgical aspects. Epilepsia. 2003;44:822–830. doi: 10.1046/j.1528-1157.2003.56102.x. [DOI] [PubMed] [Google Scholar]
- 25.Thom M, Blümcke I, Aronica E. Long-term epilepsy-associated tumors. Brain Pathol. 2012;22:350–379. doi: 10.1111/j.1750-3639.2012.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Englot DJ, Berger MS, Barbaro NM, et al. Factors associated with seizure freedom in the surgical resection of glioneuronal tumors. Epilepsia. 2012;53:51–57. doi: 10.1111/j.1528-1167.2011.03269.x. [DOI] [PubMed] [Google Scholar]
- 27.Southwell DG, Garcia PA, Berger MS, et al. Long-term seizure control outcomes after resection of gangliogliomas. Neurosurgery. 2012;70:1406–1413; discussion 1413–1414. doi: 10.1227/NEU.0b013e3182500a4c. [DOI] [PubMed] [Google Scholar]
- 28.Morioka T, Hashiguchi K, Nagata S, et al. Additional hippocampectomy in the surgical management of intractable temporal lobe epilepsy associated with glioneuronal tumor. Neurol Res. 2007;29:807–815. doi: 10.1179/016164107X223566. [DOI] [PubMed] [Google Scholar]
- 29.Hu WH, Ge M, Zhang K, et al. Seizure outcome with surgical management of epileptogenic ganglioglioma: A study of 55 patients. Acta Neurochir (Wien) 2012;154:855–861. doi: 10.1007/s00701-011-1259-z. [DOI] [PubMed] [Google Scholar]
- 30.van den Bent MJ, Afra D, de Witte O, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: The EORTC 22845 randomised trial. Lancet. 2005;366:985–990. doi: 10.1016/S0140-6736(05)67070-5. [DOI] [PubMed] [Google Scholar]
- 31.Pace A, Vidiri A, Galiè E, et al. Temozolomide chemotherapy for progressive low-grade glioma: Clinical benefits and radiological response. Ann Oncol. 2003;14:1722–1726. doi: 10.1093/annonc/mdg502. [DOI] [PubMed] [Google Scholar]
- 32.Brada M, Viviers L, Abson C, et al. Phase II study of primary temozolomide chemotherapy in patients with WHO grade II gliomas. Ann Oncol. 2003;14:1715–1721. doi: 10.1093/annonc/mdg371. [DOI] [PubMed] [Google Scholar]
- 33.Kaloshi G, Benouaich-Amiel A, Diakite F, et al. Temozolomide for low-grade gliomas: Predictive impact of 1p/19q loss on response and outcome. Neurology. 2007;68:1831–1836. doi: 10.1212/01.wnl.0000262034.26310.a2. [DOI] [PubMed] [Google Scholar]
- 34.Sherman JH, Moldovan K, Yeoh HK, et al. Impact of temozolomide chemotherapy on seizure frequency in patients with low-grade gliomas. J Neurosurg. 2011;114:1617–1621. doi: 10.3171/2010.12.JNS101602. [DOI] [PubMed] [Google Scholar]
- 35.Taillandier L, Duffau H. Epilepsy and insular grade II gliomas: An interdisciplinary point of view from a retrospective monocentric series of 46 cases. Neurosurg Focus. 2009;27:E8. doi: 10.3171/2009.6.FOCUS09102. [DOI] [PubMed] [Google Scholar]
- 36.Vecht CJ, Wagner GL, Wilms EB. Interactions between antiepileptic and chemotherapeutic drugs. Lancet Neurol. 2003;2:404–409. doi: 10.1016/s1474-4422(03)00435-6. [DOI] [PubMed] [Google Scholar]
- 37.Bénit C, Vecht CJ. Spectrum of side effects of anticonvulsants in patients with brain tumours. EANO Magazine. 2012;2:15–24. [Google Scholar]
- 38.Glauser T, Ben-Menachem E, Bourgeois B, et al. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2013;54:551–563. doi: 10.1111/epi.12074. [DOI] [PubMed] [Google Scholar]
- 39.Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: Anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Neurology. 2000;54:1886–1893. doi: 10.1212/wnl.54.10.1886. [DOI] [PubMed] [Google Scholar]
- 40.Soffietti R, Baumert BG, Bello L, et al. Guidelines on management of low-grade gliomas: Report of an EFNS-EANO Task Force. Eur J Neurol. 2010;17:1124–1133. doi: 10.1111/j.1468-1331.2010.03151.x. [DOI] [PubMed] [Google Scholar]
- 41.Maschio M, Dinapoli L, Saveriano F, et al. Efficacy and tolerability of zonisamide as add-on in brain tumor-related epilepsy: Preliminary report. Acta Neurol Scand. 2009;120:210–212. doi: 10.1111/j.1600-0404.2009.01226.x. [DOI] [PubMed] [Google Scholar]
- 42.Wagner GL, Wilms EB, Van Donselaar CA, et al. Levetiracetam: Preliminary experience in patients with primary brain tumours. Seizure. 2003;12:585–586. doi: 10.1016/s1059-1311(03)00096-7. [DOI] [PubMed] [Google Scholar]
- 43.Maschio M, Albani F, Baruzzi A, et al. Levetiracetam therapy in patients with brain tumour and epilepsy. J Neurooncol. 2006;80:97–100. doi: 10.1007/s11060-006-9162-9. [DOI] [PubMed] [Google Scholar]
- 44.Maschio M, Dinapoli L, Sperati F, et al. Levetiracetam monotherapy in patients with brain tumor-related epilepsy: Seizure control, safety, and quality of life. J Neurooncol. 2011;104:205–214. doi: 10.1007/s11060-010-0460-x. [DOI] [PubMed] [Google Scholar]
- 45.Lim DA, Tarapore P, Chang E, et al. Safety and feasibility of switching from phenytoin to levetiracetam monotherapy for glioma-related seizure control following craniotomy: A randomized phase II pilot study. J Neurooncol. 2009;93:349–354. doi: 10.1007/s11060-008-9781-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newton HB, Goldlust SA, Pearl D. Retrospective analysis of the efficacy and tolerability of levetiracetam in brain tumor patients. J Neurooncol. 2006;78:99–102. doi: 10.1007/s11060-005-9070-4. [DOI] [PubMed] [Google Scholar]
- 47.Rosati A, Buttolo L, Stefini R, et al. Efficacy and safety of levetiracetam in patients with glioma: A clinical prospective study. Arch Neurol. 2010;67:343–346. doi: 10.1001/archneurol.2009.335. [DOI] [PubMed] [Google Scholar]
- 48.Bähr O, Hermisson M, Rona S, et al. Intravenous and oral levetiracetam in patients with a suspected primary brain tumor and symptomatic seizures undergoing neurosurgery: The HELLO trial. Acta Neurochir (Wien) 2012;154:229–235; discussion 235. doi: 10.1007/s00701-011-1144-9. [DOI] [PubMed] [Google Scholar]
- 49.de Groot M, Douw L, Sizoo EM, et al. Levetiracetam improves verbal memory in high-grade glioma patients. Neuro Oncol . 2013;15:216–223. doi: 10.1093/neuonc/nos288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bodalia PN, Grosso AM, Sofat R, et al. Comparative efficacy and tolerability of anti-epileptic drugs for refractory focal epilepsy: Systematic review and network meta-analysis reveals the need for long term comparator trials. Br J Clin Pharmacol. 2013;76:649–667. doi: 10.1111/bcp.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Newton HB, Dalton J, Goldlust S, et al. Retrospective analysis of the efficacy and tolerability of levetiracetam in patients with metastatic brain tumors. J Neurooncol. 2007;84:293–296. doi: 10.1007/s11060-007-9373-8. [DOI] [PubMed] [Google Scholar]
- 52.Maschio M, Dinapoli L, Gomellini S, et al. Antiepileptics in brain metastases: Safety, efficacy and impact on life expectancy. J Neurooncol. 2010;98:109–116. doi: 10.1007/s11060-009-0069-0. [DOI] [PubMed] [Google Scholar]
- 53.Weller M, Gorlia T, Cairncross JG, et al. Prolonged survival with valproic acid use in the EORTC/NCIC temozolomide trial for glioblastoma. Neurology. 2011;77:1156–1164. doi: 10.1212/WNL.0b013e31822f02e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simó M, Velasco R, Graus F, et al. Impact of antiepileptic drugs on thrombocytopenia in glioblastoma patients treated with standard chemoradiotherapy. J Neurooncol. 2012;108:451–458. doi: 10.1007/s11060-012-0836-1. [DOI] [PubMed] [Google Scholar]
- 55.Otoul C, Arrigo C, van Rijckevorsel K, et al. Meta-analysis and indirect comparisons of levetiracetam with other second-generation antiepileptic drugs in partial epilepsy. Clin Neuropharmacol. 2005;28:72–78. doi: 10.1097/01.wnf.0000159956.87511.67. [DOI] [PubMed] [Google Scholar]
- 56.Maschio M, Dinapoli L, Zarabla A, et al. Outcome and tolerability of topiramate in brain tumor associated epilepsy. J Neurooncol. 2008;86:61–70. doi: 10.1007/s11060-007-9430-3. [DOI] [PubMed] [Google Scholar]
- 57.Lu Y, Yu W, Wang X. Efficacy of topiramate in adult patients with symptomatic epilepsy: An open-label, long-term, retrospective observation. CNS Drugs. 2009;23:351–359. doi: 10.2165/00023210-200923040-00006. [DOI] [PubMed] [Google Scholar]
- 58.Mauro AM, Bomprezzi C, Morresi S, et al. Prevention of early postoperative seizures in patients with primary brain tumors: Preliminary experience with oxcarbazepine. J Neurooncol. 2007;81:279–285. doi: 10.1007/s11060-006-9229-7. [DOI] [PubMed] [Google Scholar]
- 59.Maschio M, Dinapoli L, Vidiri A, et al. The role side effects play in the choice of antiepileptic therapy in brain tumor-related epilepsy: A comparative study on traditional antiepileptic drugs versus oxcarbazepine. J Exp Clin Cancer Res. 2009;28:60. doi: 10.1186/1756-9966-28-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maschio M, Dinapoli L, Sperati F, et al. Oxcarbazepine monotherapy in patients with brain tumor-related epilepsy: Open-label pilot study for assessing the efficacy, tolerability and impact on quality of life. J Neurooncol. 2012;106:651–656. doi: 10.1007/s11060-011-0689-z. [DOI] [PubMed] [Google Scholar]
- 61.Maschio M, Dinapoli L, Mingoia M, et al. Lacosamide as add-on in brain tumor-related epilepsy: Preliminary report on efficacy and tolerability. J Neurol. 2011;258:2100–2104. doi: 10.1007/s00415-011-6132-8. [DOI] [PubMed] [Google Scholar]
- 62.Saria MG, Corle C, Hu J, et al. Retrospective analysis of the tolerability and activity of lacosamide in patients with brain tumors: Clinical article. J Neurosurg. 2013;118:1183–1187. doi: 10.3171/2013.1.JNS12397. [DOI] [PubMed] [Google Scholar]
- 63.Rossetti AO, Jeckelmann S, Novy J, et al. Levetiracetam and pregabalin for antiepileptic monotherapy in patients with primary brain tumors. A phase II randomized study. Neuro Oncol. 2014;16:584–588. doi: 10.1093/neuonc/not170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.French JA, Faught E. Rational polytherapy. Epilepsia. 2009;50(suppl 8):63–68. doi: 10.1111/j.1528-1167.2009.02238.x. [DOI] [PubMed] [Google Scholar]
- 65.Brodie MJ, Sills GJ. Combining antiepileptic drugs—Rational polytherapy? Seizure. 2011;20:369–375. doi: 10.1016/j.seizure.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 66.Smits A, Duffau H. Seizures and the natural history of World Health Organization Grade II gliomas: A review. Neurosurgery. 2011;68:1326–1333. doi: 10.1227/NEU.0b013e31820c3419. [DOI] [PubMed] [Google Scholar]
- 67.Aronica E, Gorter JA, Jansen GH, et al. Expression and cellular distribution of multidrug transporter proteins in two major causes of medically intractable epilepsy: Focal cortical dysplasia and glioneuronal tumors. Neuroscience. 2003;118:417–429. doi: 10.1016/s0306-4522(02)00992-2. [DOI] [PubMed] [Google Scholar]
- 68.Calatozzolo C, Pollo B, Botturi A, et al. Multidrug resistance proteins expression in glioma patients with epilepsy. J Neurooncol. 2012;110:129–135. doi: 10.1007/s11060-012-0946-9. [DOI] [PubMed] [Google Scholar]
- 69.Loscher W. Mechanisms of drug resistance. Epileptic Disord. 2005;7:S3–S9. [PubMed] [Google Scholar]
- 70.Zhang C, Zuo Z, Kwan P, et al. In vitro transport profile of carbamazepine, oxcarbazepine, eslicarbazepine acetate, and their active metabolites by human P-glycoprotein. Epilepsia. 2011;52:1894–1904. doi: 10.1111/j.1528-1167.2011.03140.x. [DOI] [PubMed] [Google Scholar]
- 71.Brodie MJ, Yuen AW. Lamotrigine substitution study: Evidence for synergism with sodium valproate? 105 Study Group. Epilepsy Res. 1997;26:423–432. doi: 10.1016/s0920-1211(96)01007-8. [DOI] [PubMed] [Google Scholar]
- 72.Poolos NP, Warner LN, Humphreys SZ, et al. Comparative efficacy of combination drug therapy in refractory epilepsy. Neurology. 2012;78:62–68. doi: 10.1212/WNL.0b013e31823ed0dd. [DOI] [PubMed] [Google Scholar]
- 73.Kinirons P, McCarthy M, Doherty CP, et al. Predicting drug-resistant patients who respond to add-on therapy with levetiracetam. Seizure. 2006;15:387–392. doi: 10.1016/j.seizure.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 74.Surges R, Volynski KE, Walker MC. Is levetiracetam different from other antiepileptic drugs? Levetiracetam and its cellular mechanism of action in epilepsy revisited. Ther Adv Neurol Disord. 2008;1:13–24. doi: 10.1177/1756285608094212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dudra-Jastrzebska M, Andres-Mach MM, Ratnaraj N, et al. Isobolographic characterization of the anticonvulsant interaction profiles of levetiracetam in combination with clonazepam, ethosuximide, phenobarbital and valproate in the mouse pentylenetetrazole-induced seizure model. Seizure. 2009;18:607–614. doi: 10.1016/j.seizure.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 76.Wojda E, Wlaz A, Patsalos PN, et al. Isobolographic characterization of interactions of levetiracetam with the various antiepileptic drugs in the mouse 6 Hz psychomotor seizure model. Epilepsy Res. 2009;86:163–174. doi: 10.1016/j.eplepsyres.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 77.Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3:159–168. doi: 10.1016/S1474-4422(04)00680-5. [DOI] [PubMed] [Google Scholar]
- 78.Oberndorfer S, Piribauer M, Marosi C, et al. P450 enzyme inducing and non-enzyme inducing antiepileptics in glioblastoma patients treated with standard chemotherapy. J Neurooncol. 2005;72:255–260. doi: 10.1007/s11060-004-2338-2. [DOI] [PubMed] [Google Scholar]
- 79.Guthrie GD, Eljamel S. Impact of particular antiepileptic drugs on the survival of patients with glioblastoma multiforme. J Neurosurg. 2013;118:859–865. doi: 10.3171/2012.10.JNS12169. [DOI] [PubMed] [Google Scholar]
- 80.Barker CA, Bishop AJ, Chang M, et al. Valproic acid use during radiation therapy for glioblastoma associated with improved survival. Int J Radiat Oncol Biol Phys. 2013;86:504–509. doi: 10.1016/j.ijrobp.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Felix FH, de Araujo OL, da Trindade KM, et al. Survival of children with malignant brain tumors receiving valproate: A retrospective study. Childs Nerv Syst. 2013;29:195–197. doi: 10.1007/s00381-012-1997-0. [DOI] [PubMed] [Google Scholar]
- 82.Das CM, Aguilera D, Vasquez H, et al. Valproic acid induces p21 and topoisomerase-II (alpha/beta) expression and synergistically enhances etoposide cytotoxicity in human glioblastoma cell lines. J Neurooncol. 2007;85:159–170. doi: 10.1007/s11060-007-9402-7. [DOI] [PubMed] [Google Scholar]
- 83.Roy Choudhury S, Karmakar S, Banik NL, et al. Valproic acid induced differentiation and potentiated efficacy of taxol and nanotaxol for controlling growth of human glioblastoma LN18 and T98G cells. Neurochem Res. 2011;36:2292–2305. doi: 10.1007/s11064-011-0554-7. [DOI] [PubMed] [Google Scholar]
- 84.Fujiwara Y, Yamamoto N, Yamada Y, et al. Phase I and pharmacokinetic study of vorinostat (suberoylanilide hydroxamic acid) in Japanese patients with solid tumors. Cancer Sci. 2009;100:1728–1734. doi: 10.1111/j.1349-7006.2009.01237.x. [DOI] [PubMed] [Google Scholar]
- 85.Kavanaugh SM, White LA, Kolesar JM. Vorinostat: A novel therapy for the treatment of cutaneous T-cell lymphoma. Am J Health Syst Pharm. 2010;67:793–797. doi: 10.2146/ajhp090247. [DOI] [PubMed] [Google Scholar]
- 86.Scherpereel A, Berghmans T, Lafitte JJ, et al. Valproate-doxorubicin: Promising therapy for progressing mesothelioma. A phase II study. Eur Respir J. 2011;37:129–135. doi: 10.1183/09031936.00037310. [DOI] [PubMed] [Google Scholar]
- 87.Corsetti MT, Salvi F, Perticone S, et al. Hematologic improvement and response in elderly AML/RAEB patients treated with valproic acid and low-dose Ara-C. Leuk Res. 2011;35:991–997. doi: 10.1016/j.leukres.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 88.Coronel J, Cetina L, Pacheco I, et al. A double-blind, placebo-controlled, randomized phase III trial of chemotherapy plus epigenetic therapy with hydralazine valproate for advanced cervical cancer. Preliminary results. Med Oncol. 2011;28:S540–S546. doi: 10.1007/s12032-010-9700-3. [DOI] [PubMed] [Google Scholar]
- 89.Chinnaiyan P, Cerna D, Burgan WE, et al. Postradiation sensitization of the histone deacetylase inhibitor valproic acid. Clin Cancer Res. 2008;14:5410–5415. doi: 10.1158/1078-0432.CCR-08-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bangert A, Cristofanon S, Eckhardt I, et al. Histone deacetylase inhibitors sensitize glioblastoma cells to TRAIL-induced apoptosis by c-myc-mediated downregulation of cFLIP. Oncogene. 2012;31:4677–4688. doi: 10.1038/onc.2011.614. [DOI] [PubMed] [Google Scholar]
- 91.Khalil MA, Hrabeta J, Cipro S, et al. Neuroblastoma stem cells—Mechanisms of chemoresistance and histone deacetylase inhibitors. Neoplasma. 2012;59:737–746. doi: 10.4149/neo_2012_093. [DOI] [PubMed] [Google Scholar]
- 92.Bobustuc GC, Baker CH, Limaye A, et al. Levetiracetam enhances p53-mediated MGMT inhibition and sensitizes glioblastoma cells to temozolomide. Neuro Oncol. 2010;12:917–927. doi: 10.1093/neuonc/noq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jaeckle KA, Ballman K, Furth A, et al. Correlation of enzyme-inducing anticonvulsant use with outcome of patients with glioblastoma. Neurology. 2009;73:1207–1213. doi: 10.1212/WNL.0b013e3181bbfeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Birbeck GL, French JA, Perucca E, et al. Antiepileptic drug selection for people with HIV/AIDS: Evidence-based guidelines from the ILAE and AAN. Epilepsia. 2012;53:207–214. doi: 10.1111/j.1528-1167.2011.03335.x. [DOI] [PubMed] [Google Scholar]
- 95.Chung S, Sperling MR, Biton V, et al. Lacosamide as adjunctive therapy for partial-onset seizures: A randomized controlled trial. Epilepsia. 2010;51:958–967. doi: 10.1111/j.1528-1167.2009.02496.x. [DOI] [PubMed] [Google Scholar]
- 96.Baulac M, Brodie MJ, Patten A, et al. Efficacy and tolerability of zonisamide versus controlled-release carbamazepine for newly diagnosed partial epilepsy: A phase 3, randomised, double-blind, non-inferiority trial. Lancet Neurol. 2012;11:579–588. doi: 10.1016/S1474-4422(12)70105-9. [DOI] [PubMed] [Google Scholar]


