Abstract
AIM: To detect the expression of CD44 correlated with the ability of micro-metastasis in peripheral blood and bone marrow of patients with gastric cancer and to deduce its clinical significance.
METHODS: Preoperative peripheral blood and bone marrow specimens from 46 patients with gastric cancer and 6 controls were studied by semi-quantitative RT-PCR amplification of CD44v6mRNA. Preoperative and postoperative peripheral blood specimens from 40 patients with gastric cancer and 14 controls were studied by quantitative RT-PCR amplification of CD44v6mRNA in the corresponding period.
RESULTS: Semi-quantitative RT-PCR amplification showed that CD44v6mRNA expression of peripheral blood and bone marrow was positive in 39 (84.8%) and 40 (86.9%) of 46 patients with gastric cancer, respectively. In peripheral blood, CD44v6mRNA expression was positive for diffuse type in 30 (93.8%) of 32 patients and for intestinal type in 9 (64.3%) of 14 patients. On the other hand, in bone marrow, CD44v6mRNA expression was positive for diffuse type in 31 (96.9%) of 32 patients and for intestinal type in 10 (71.4%) of 14 patients. There was a significant difference between the diffuse type and intestinal type. Quantitative RT-PCR amplification demonstrated that CD44v6mRNA was not expressed in the peripheral blood of controls and CD44v6mRNA expression was positive for preoperative peripheral blood in 40 patients with gastric cancer, the expression levels being from 4.9 × 108 - 3.2× 1011 copies/g RNA. The average expression level of CD44v6mRNA in peripheral blood was 3.9 × 1010 copies/g RNA. The expression levels of CD44v6mRNA in peripheral blood in gastric cancer patients after curative operation increased from 5.5 × 106 - 7.6 × 109 copies/g RNA and the average level was 2.4×108 copies/g RNA (Figure 3B) (P = 0.00496). After curative operation, the expression level decreased markedly.
CONCLUSION: Semi-quantitative and quantitative RT-PCR amplification for CD44v6mRNA is a sensitive and specific method for the detection of micro-metastasis in peripheral blood and bone marrow, which might be used as an indicator of tumor burden and therapeutic effect.
Keywords: Gastric cancer, Micro-metastasis, Peripheral blood, Bone marrow, CD44v6
INTRODUCTION
Micro-metastasis cannot be detected by a general clinical pathological method. Gastric cancer is one of the most frequent malignant tumors. Tumor invasion, metastasis, and relapse are correlated with the prognoses of gastric cancer. Tumors metastasize through lymph and blood circulation. Twenty percent of patients have micro-metastasis[1]. Tumor cell micro-metastasis in lymphocytes, peripheral blood, bone marrow, and abdominal cavity is the main reason of metastasis and relapse[2,3]. Discovering gastric cancer in time is not only important for predicting relapse and prognoses, but also important for making decisions concerning therapy. With the development of molecular biology technology, the method of inspecting micro-metastasis has become more reliable[4]. Semi-quantitative RT-PCR can reveal gastric cancer cells in peripheral blood and bone marrow[5,6]. CD44v6, a highly glycosylated cell surface protein, is involved in cell-cell and cell-matrix interactions and takes part in cell motility, tumor growth, and invasion[7].
CD44 is an integral membrane glycoprotein with an apparent molecular mass ranging 85-250 ku. It is originally described as a lymphocyte homing receptor on circulating lymphocytes[8]. At least 20 variants (v) of CD44 have been reported[9,10] due to the alternative splicing of 10 exons (v1-v10) that encode the membrane proximal portion of the extracellular domain. It has been reported that the expression of variant 6 of CD44 is correlated with invasion and metastasis of certain types of human cancer[11]. The expression of CD44v6 and CD44v5 is correlated with tumor progression, metastasis, and prognosis of colorectal cancer, breast cancer, and gastric cancer.
In our study, we have also determined the expression of CD44v6mRNA using semi-quantitative RT-PCR in peripheral blood and bone marrow from 46 patients with gastric cancer. We have also determined the quantitative expression of CD44v6mRNA in peripheral blood specimens from 40 patients with gastric cancer and 14 controls using quantitative RT-PCR to display the role of CD44v6mRNA in clinical stage and prognosis of gastric cancer.
MATERIALS AND METHODS
Patients and serum samples
Eighty-six patients were randomly divided into gastric cancer group (52 male and 34 female patients with an average age of 58.6 years, ranging 32 - 81 years) and control group (14 male and 8 female patients with an average age of 53.8 years, ranging 40 - 65 years).
Serum samples were obtained from 86 patients with primary gastric cancer prior to surgery at the Department of Gastroenterology, the First Affiliated Hospital of Yangzhou Medical University. The diagnosis was confirmed before surgery.
Extraction of total RNA
Before surgery, serum samples were obtained from peripheral blood and bone marrow 1 d before surgery and 9 d after surgery. Single nucleated cells were separated and stored at -20 °C. Total RNA was extracted. After being centrifuged at 2 500 r/min for 10 min, 5 mL S-ACR was added and bathed on ice for 15 min, then the process was repeated and the samples were stored at -70 °C.
cDNA synthesis, cDNA amplification, and semi-quantitative analysis
Primers sp1 and sp2 were from the cDNA sequence[12]. Primers p1, p2, and β-actin were from the cDNA sequence[13]. Primers were separately aimed at the standard and variant CD44s, CD44v6, and β-actin[14]. The three couples of primers were synthesized by Sagon Co., Canada and stored at -20 °C (sp1: 5’-GACACATATTGCTTCAATG CTTCAGC-3’; sp2: 5’-GATGCCAAGATGATCAGCCATTCTGGAAT-3’; P1: 5’-GACAGACACCTCAGTTTTTCTGGA-3’; P2: 5’-TTCCTTCGTGTGTGGGTAATGAGA-3’; forward β-actin: 5’-CTACAATGAGCTGCGTGTGGC-3’; backward β-actin: 5’-CAGGTCCAGACGCA GGATGGC-3’). After cDNA was synthesized and amplified, the product was analyzed and the density was scanned (semi-quantitative analysis). When the value of CD44v6mRNA/β-actin was less than 0.30 and more than 0.70, it was expressed as (-), (+), and (+).
Real-time RT-PCR
The point characterized the reactions during cycling, when the PCR product was first detected. The product was accumulated after a fixed number of cycles. The higher the starting quantity of the target molecule, the earlier the significant increase in fluorescence was observed. The parameter C+ (threshold cycle) was defined as the fractional cycle number at which the fluorescence was generated. CD44v6 target message in unknown samples was quantified by measuring C+ and using a calibration curve to determine the starting target message. The precise amount of total RNA added into each reaction mix (based on absorbance) and its quality were difficult to assess. For each experimental sample, the amount of targets and endogenous reference were determined by the calibration curve. The target amount was then divided by the endogenous reference amount to obtain a normalized target value. The relative gene target expression was also normalized to healthy control serum sample. Each of the normalized target values was divided by the calibrator-normalized target value to generate the final relative expression.
Primers, probes, and PCR consumables
Primers and probes for the CD44v6 gene were chosen with the assistance of the computer programs Oligo 4.0 (National Bioscience) and Primer Express (Perkin-Elmer Applied Bio-systems). We conducted BLAST searches against dbEST and nr to confirm the total gene specificity of the nucleotide sequences chosen for the primers and probes, and the absence of DNA polymorphisms. The primer for CD44v6 was selected and compared to the sequences of the closely related CD44v6 gene. The sequences of the oligonucleotide are shown in (Table 1). The primers and probes were designated by the nucleotide position corresponding to the 5’-position, followed by the letter U for upper (sense strand) or L for lower (antisense strand). To avoid amplification of contaminating genome DNA, one of the two primers or the probe was placed at the junction between two exons or in different exons.
Table 1.
Oligonucleotide primer and probe sequences used
| Gene | Oligonucleotide | Location sequence PCR product | bp |
| CD44v | Upper primer | 5′-TCCAGGCAACTCCTAGTAGT-3′ | 740 |
| Lower primer | 5′-CAGCTGTCCCTGTTGTCGAA-3′ | ||
| β-Actin | Upper primer | 5’-CTACAATGAGCTGCGTGTGGC-3’ | 206 |
| Lower primer | 5’-CAGGTCCAGACGCAGGATGGC-3’ | ||
| Probe | 5’-TGAGATTGGGTTGAAGAAATC-3’ |
RNA extraction
Total RNA was extracted from serum specimens of gastric cancer by the acid-phenol quantum method[15]. The quality of RNA samples was determined by electrophoresis through denaturation of agarose gels and staining with thallium bromide. The 18s and 28s RNA bands were visualized under ultraviolet light.
Calibration curve
Calibration curve was constructed with four fold serial dilutions of total RNA from healthy human serum. The diluted human total RNA was liquored and stored at -80 °C until use.
cDNA synthesis
Reverse transcription of RNA was performed in a final volume of 20 µL containing 1× RT-PCR buffer [500 mmol/L each dNTP, 3 mmol/L MgCl2, 75 mmol/L KCl, 50 mmol/L Tris-HCl, pH 8.3, 10 µL of RNasinTM ribonuclease inhibitor (Promega), 10 mmol/L dithiothreitol, 50 µL of superscript RNase H– reverse transcripts (Life Technologies), 1.5 mmol/L random hexanes and 1 µg of total RNA (calibration curve points and patient samples)]. The samples were incubated at 20 °C for 10 min and at 42 °C for 30 min. Reverse transcripts were inactivated by heating at 99 °C for 5 min and cooling at 5 °C for 5 min.
PCR amplification
All PCR reactions were performed on an ABI PRISM 7700 Sequence Detection System. For each PCR, a master mixture was prepared on ice with 1 × TaqMan buffer; 5 mmol/L MgCl2; 200 mmol/L dATP, dCTP, and dGTP; 400 mmol/L dUTP; 300 mmol/L each primer; 150 mmol/L probe; 1.25 µL of Ampli Taq gold DNA. Polymers and 10 µL of each appropriately dilated reverse transcription sample were added to 40 µL of the PCR master mixture. The thermal cycling conditions were: an initial demodulation step at 95 °C for 10 min and 50 cycles at 95 °C for 15 s, at 65 °C for 1 min. Experiments were performed in duplicate for each data point. Each PCR run included five points of the calibration curve (fourfold serially diluted human normal gastric cDNA), a non-template control, cDNA calibrator. All patient samples with a cv of the number of CD44v6mRNA copies > 10% were retested.
Statistical analysis
The association of factors was evaluated by the χ2 test. The significance of difference among the means was determined by the Student’s t test and one-way analysis of variance. SPSS 11.0 for Windows 2000 was used. P < 0.05 was considered statistically significant.
RESULTS
Semi-quantitative analysis
Proliferation of CD44s in peripheral blood and bone marrow from patients with gastric cancer and controls is shown in Figure 1A.
Figure 1.
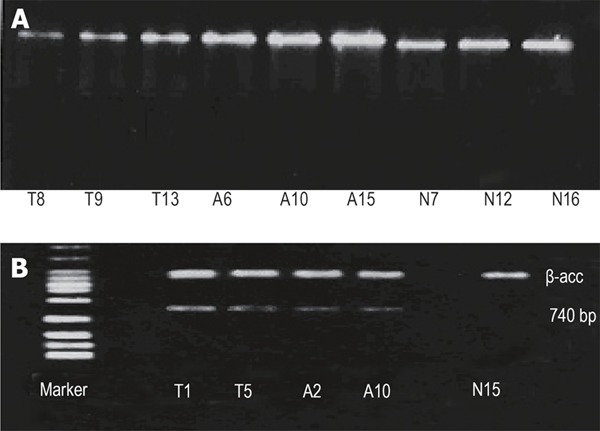
Expression of CD44s (A) and CD44v (B) in gastric mucosa.T1, T5, T8, T9, T13:bone marrow from patients with gastric cancer; A2, A6, A10, A15:peripheral blood from patients with gastric cancer; N7, N12, N15, N16:Controls.
CD44v6mRNA expression in bone marrow and peripheral blood was positive in 40 (86.9%) and 39 (84.8%) of 46 patients with gastric cancer. The positive CD44v expression was slightly higher in bone marrow than in peripheral blood (P > 0.05). The value of CD44v6mRNA expression was 64.6% ± 21.66% (Figure 1B).
In 46 patients with gastric cancer, diffuse type was found in 32 cases and intestinal type in 14 cases. Positive CD44v6mRNA expression in bone marrow was found in 31 (96.9%) of 32 diffuse type patients and 10 (71.4%) of 14 intestinal type patients (Figure 2A). On the other hand, positive CD44v6mRNA expression in peripheral blood was found in 30 (93.8%) of 32 diffuse type patients and 9 (64.3%) of 14 intestinal type patients (Figure 2B). There was a significant difference between the diffuse and intestinal types.
Figure 2.
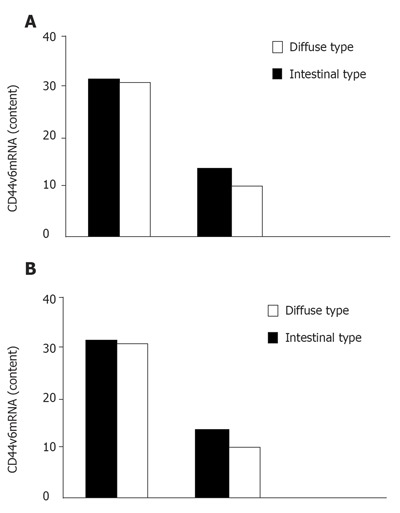
CD44v6mRNA expression in bone marrow (A) and peripheral blood (B) from patients with gastric cancer.
The relation between CD44v6mRNA expression and pathological type, lymph node metastasis, clinical pathology, and the size of tumor is presented in Tables 2 and 3.
Table 2.
Relation between CD44v6mRNA expression in bone marrow and biologic behavior of gastric cancer
| Number of cases (n) |
CD44v6mRNA/β-actin |
P value | |||||
| - | + | + | + | ||||
| Lymphnode metastasis | (+) Positive | 38 | 3 | 2 | 4 | 29 | 0.0161 |
| (-) Negative | 8 | 4 | 1 | 1 | 3 | ||
| Clinicopathology | I-II | 12 | 5 | 2 | 2 | 3 | |
| III-IV | 34 | 1 | 2 | 3 | 28 | 0.0007 | |
| Tissue type | D | 32 | 1 | 2 | 4 | 25 | |
| I | 14 | 5 | 2 | 4 | 3 | 0.0003 | |
| Tumor size | >5 cm | 24 | 3 | 2 | 6 | 13 | |
| ≤5 cm | 22 | 3 | 3 | 4 | 12 | 0.4547 | |
Table 3.
Relation between CD44v6mRNA expression in bone marrow and biologic behavior of gastric cancer
| Number of cases (n) |
CD44v6mRNA/β-actin |
P value | |||||
| - | + | + | + | ||||
| Lymphnode metastasis | (+) Positive | 38 | 3 | 2 | 4 | 29 | 0.0131 |
| (-) Negative | 8 | 4 | 1 | 1 | 2 | ||
| Clinicopathology | I-II | 12 | 5 | 2 | 2 | 3 | |
| III-IV | 34 | 2 | 2 | 3 | 27 | 0.0005 | |
| Tissue type | D | 32 | 2 | 2 | 4 | 25 | |
| I | 14 | 5 | 2 | 4 | 3 | 0.0003 | |
| Tumor size | >5 cm | 24 | 3 | 2 | 6 | 13 | |
| ≤5 cm | 22 | 4 | 3 | 4 | 11 | 0.4240 | |
Calibration curve and dynamic range of real-time RT-PCR
The calibration curve was constructed from the total RNA extracted from healthy human gastric serum diluted fourfold in mouse total RNA. The primer chosen to analyze the CD44v6 gene did not amplify human genomic DNA or mouse cDNA. The dynamic range was at least three orders of magnitude in samples containing 50 µg or 0.2 ng equivalent to total cDNA. A strong linear relation between Ct and log of the starting copy number was demonstrated (r2 ≥ 0.99). The efficiency of the reaction (E) was 90 % - 100% calculated by the formula: E = 101/[m] - 1, where m is the slope of calibration curve.
To determine the cut-off value for altered CD44v6 gene expression at the RNA level in gastric cancer serum, the CD44v6 value (ratio of CD44v6mRNA to β-actin) was determined for four normal gastric serum RNAs. Because this value fluctuated between 0.5 and 1.7, values of 3 or more were considered as overexpression of the CD44v6 gene in tumor RNA samples.
CD44v6mRNA status and clinical and pathological factors
We sought for links between CD44v6mRNA status and standard clinical, pathological and biological factors in gastric cancer. Significant association was found between the overexpression of CD44v6mRNA gene and standard histopathological grade (P < 0.05) and negative progesterone receptor status (P < 0.001). A trend toward a link between the overexpression of CD44v6 gene and estrogen receptor negativity was also observed (P = 0.09).
CD44v6mRNA amplification (patients for CD44v6mRNA expression)
As shown in Table 4, all the 20 peripheral blood samples of gastric cancer had the expression of CD44v6mRNA. The expression level ranged 4.9×108 - 3.2×1011 copies/g RNA and the average levels of peripheral blood was 3.9×1010 copies/g RNA (Figure 3A). The expression level of gastric cancer was 5.5 × 106 - 7.6×109 copies/g RNA and the average level was 2.4×108 copies/g RNA (Figure 3B) (P = 0.00496) after curative surgery.
Table 4.
Quantitative expression of CD44v6mRNA in peripheral blood of patients with gastric cancer before and after surgery
| Id | Sex | Yr | Path | Metastasis | >5cm | Histopathology | Real-time RT-PCR before surgery | (×102 copies/µg RNA) after surgery |
| 1 | M | 56 | D | + | T | III | 3100 | 300 |
| 2 | M | 52 | I | - | F | II | 4.9 | 2.0 |
| 3 | W | 60 | D | + | T | IV | 580 | 66 |
| 4 | W | 69 | D | + | T | III | 696 | 72 |
| 5 | W | 41 | D | + | T | IV | 3200 | 460 |
| 6 | M | 39 | D | + | T | IV | 2650 | 244 |
| 7 | M | 61 | I | - | F | II | 26 | 9.0 |
| 8 | W | 42 | D | + | T | IV | 360 | 48 |
| 9 | M | 71 | I | - | F | II | 590 | 72 |
| 10 | M | 61 | I | - | F | II | 430 | 58 |
| 11 | M | 41 | D | + | T | II | 1230 | 142 |
| 12 | W | 47 | D | + | T | IV | 1670 | 186 |
| 13 | M | 32 | D | + | T | IV | 1340 | 166 |
| 14 | M | 49 | I | + | T | III | 280 | 36 |
| 15 | W | 61 | D | + | T | II | 1080 | 128 |
| 16 | M | 76 | I | + | F | I | 480 | 68 |
| 17 | M | 81 | I | - | F | II | 36 | 6.9 |
| 18 | W | 60 | I | + | F | III | 290 | 42 |
| 19 | M | 37 | D | + | T | III | 980 | 112 |
| 20 | W | 40 | D | + | T | IV | 1880 | 218 |
| 21 | W | 46 | D | + | T | III | 1896 | 122 |
| 22 | W | 68 | I | - | T | IV | 2208 | 342 |
| 23 | M | 72 | I | - | T | III | 1960 | 140 |
| 24 | M | 64 | I | + | F | II | 868 | 22 |
| 25 | W | 51 | D | + | T | III | 1020 | 100 |
| 26 | M | 32 | D | + | T | IV | 4100 | 289 |
| 27 | W | 26 | D | + | T | IV | 3908 | 432 |
| 28 | M | 72 | I | - | F | II | 680 | 107 |
| 29 | W | 74 | I | - | T | III | 1024 | 210 |
| 30 | M | 61 | D | + | T | IV | 1468 | 134 |
| 31 | M | 61 | I | F | II | 430 | 101 | |
| 32 | M | 41 | D | + | T | II | 1230 | 11 |
| 33 | W | 47 | D | + | T | III | 1670 | 25 |
| 34 | M | 32 | D | + | T | IV | 1340 | 40 |
| 35 | M | 49 | I | + | T | I | 280 | 11 |
| 36 | W | 61 | D | + | T | II | 1080 | 120 |
| 37 | M | 76 | I | + | F | I | 480 | 91 |
| 38 | M | 81 | I | - | F | I | 36 | 137 |
| 39 | W | 60 | I | + | F | II | 290 | 43 |
| 40 | M | 37 | D | + | T | III | 980 | 69 |
Figure 3.
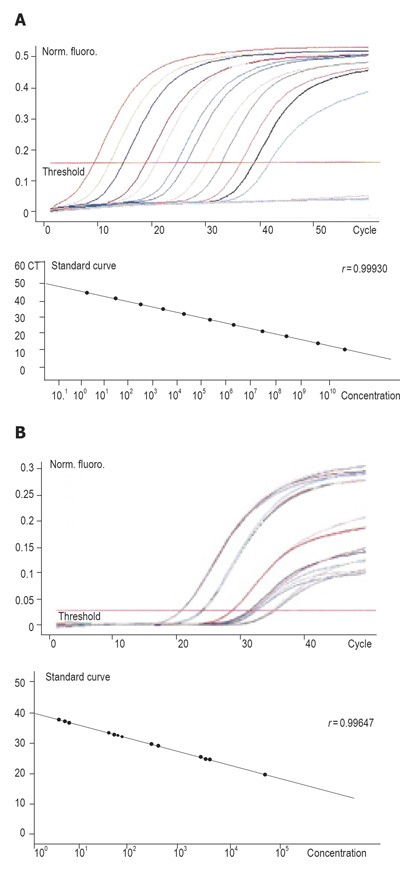
CD44v6mRNA expression in 20 peripheral blood samples from gastric cancer patients before (A) and after (B) surgery.
DISCUSSION
Gastric cancer is one of the most frequent malignant tumors. The survival rate of patients after radical surgery of gastric cancer is 40% and patients always die because of metastasis and relapse[16]. Some patients have already existed micro-metastasis which is not detectable by general clinical pathology during the treatment. The detection of micro-metastasis is correlated with the prognosis of gastric cancer patients[17,18]. It was reported that micro-metastasis could be detected in lymph nodes of patients with intestinal cancer with negative pathology[19]. Micro-metastasis has been considered as an indicator of prognosis and the value of micro-metastasis is superior to the Duke’s stage and tumor grade. Zhang et al.[20] demonstrated that tumor cells in metastatic lymph nodes of colorectal carcinoma possess cell proliferation activity and metastatic ability of tumor cells. Series slice examination has been used in inspecting tumor micro-metastasis since 1920s, but it is difficult to popularize[21]. RT-PCR is a sensitive and specific method which can find a tumor cell from 1×106 peripheral blood monocytes[22]. Positive CD44v6mRNA expression was found in 39 of 46 patients with gastric cancer and negative CD44v6mRNA expression in patients with remote metastasis. The latter may be caused by sampling error[23].
In our research, the peripheral blood and bone marrow specimens from 46 patients with gastric cancer and 6 controls were compared. The positive CD44v6mRNA expression rate was 84.4% and 86.9%, respectively in patients with gastric cancer. The CD44v6mRNA expression rate of diffuse type cancers was higher than that of intestinal type cancers, suggesting that the expression of CD44v6mRNA is correlated with the malignant phenotype of gastric cancer and CD44v6mRNA can be used as an indicator of the degree of tumor invasion and lymph node metastasis[24]. The positive rate of CD44v6 is high in gastric cancer and may serve as a marker for diagnosing gastric cancer[25-27].
The CD44v6mRNA expression rate in patients with lymph node or remote metastasis was higher than that in those without lymph node metastasis. The positive micro-metastasis rate of grades III-IV gastric cancer was significantly higher than that of grades I-II gastric cancer, suggesting that the expression of CD44v6mRNA can be used as an indicator of relapse and metastasis. CD44v6 expression is a significant risk factor for lymph node metastasis in patients with advanced carcinoma. Expression of CD44v6 plays an important role in tumor progression and may be a useful predictor of lymph node metastasis[28-32].
The quantitative expression of CD44v6mRNA in 40 patients with gastric cancer before and after surgery showed that CD44v6mRNA was an indicator of tumor burden and therapeutic effect. The results showed that the expression level of CD44v6mRNA in peripheral blood of terminal gastric cancer patients (grades III-IV) was obviously higher than that in early gastric cancer patients (grades I-II). The expression level of CD44v6mRNA in gastric cancer patients with remote metastasis was obviously higher than that in those without metastasis. The expression level of CD44v6mRNA obviously decreased after curative surgery. The findings indicate that the expression level of CD44v6mRNA in peripheral blood is correlated with tumor burden.
Animal experiments indicate that 0.01% of tumor cells in circulation may lead to positive metastasis[31,33,34]. Gulmann et al[35] showed that CD44v6 expression is a late phenomenon in the transformation of intestinal metaplasia to dysplasia/cancer. Gene expression of severe gastric mucosal dysplasia displays an obviously latent malignant tendency and gastric carcinoma with the expression of CD44v6 protein has a stronger ability to infiltrate and to metastasize via lymph nodes[36]. Serum level of sCD44v6 could be taken as the criterion for evaluating the development and prognosis of gastric cancer, as well as the therapeutic target for anti-metastasis[37]. Therefore, micro-metastasis should be routinely detected to afford evidence for establishing individual therapy scheme[38]. Quantitative RT-PCR can improve the diagnostic sensitivity and specificity. Semi-quantitative RT-PCR is restricted because it cannot generate accurate gene quantification[15]. In our study, we have validated a RT-PCR method recently developed for the quantification of gene expression based on real-time analysis of PCR amplification and TaqMan methodology, which has several advantages over other RT-PCR-based quantitative assays such as competitive quantitative RT-PCR[39].
Xin et al[40,41] discovered that patients with positive CD44v6 have a lower 3- and 5-year survival rate (P = 0.0002). Immunohistochemical detection of CD44v6 could now be used as an indicator of tumor progression in patients with gastric carcinoma. Tumor cells in the bone marrow indicate that patients have blood micro-metastasis[42]. The probability of relapse and remote metastasis is great for patients who have postoperative bone marrow micro-metastasis and dynamic observation of micro-metastasis in these patients can predict the therapeutic effect and establish individual therapy scheme. Yamaguchi et al.[43] found that CD44v6-positive cancers are more frequently associated with hematogenous metastasis. Therefore, blood is another metastatic route of gastric cancer. Detecting peripheral blood micro-metastasis of early gastric cancer patients is of great importance in predicting the prognosis and deciding the rational therapy of gastric cancer patients.
Footnotes
Supported by the grant from Science and Technology Committee of Jiangsu Province, No. 457-99064
S- Editor Wang XL and Guo SY L- Editor Elsevier HK E- Editor Wu M
References
- 1.Bustin SA, Gyselman VG, Williams NS, Dorudi S. Detection of cytokeratins 19/20 and guanylyl cyclase C in peripheral blood of colorectal cancer patients. Br J Cancer. 1999;79:1813–1820. doi: 10.1038/sj.bjc.6990289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan HY, Cheng FL, Wei ZZ, Yang GL, Chen JK. [Clinical significance of detecting lymph node micrometastasis of colorectal cancer by reverse transcriptase-polymerase chain reaction (RT-PCR)] Ai Zheng. 2004;23:1069–1073. [PubMed] [Google Scholar]
- 3.Takayama O, Yamamoto H, Ikeda K, Ishida H, Kato T, Okuyama M, Kanou T, Fukunaga M, Tominaga S, Morita S, et al. Application of RT-PCR to clinical diagnosis of micrometastasis of colorectal cancer: A translational research study. Int J Oncol. 2004;25:597–604. [PubMed] [Google Scholar]
- 4.Xia JZ, Zhu ZG, Liu BY, Yan M, Yin HR. Significance of immunohistochemically demonstrated micrometastases to lymph nodes in gastric carcinomas. Shijie Huaren Xiaohua Zazhi. 2000;8:1113–1116. [Google Scholar]
- 5.Peck K, Sher YP, Shih JY, Roffler SR, Wu CW, Yang PC. Detection and quantitation of circulating cancer cells in the peripheral blood of lung cancer patients. Cancer Res. 1998;58:2761–2765. [PubMed] [Google Scholar]
- 6.Noguchi S, Hiratsuka M, Furukawa H, Aihara T, Kasugai T, Tamura S, Imaoka S, Koyama H, Iwanaga T. Detection of gastric cancer micrometastases in lymph nodes by amplification of keratin 19 mRNA with reverse transcriptase-polymerase chain reaction. Jpn J Cancer Res. 1996;87:650–654. doi: 10.1111/j.1349-7006.1996.tb00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jothy S. CD44 and its partners in metastasis. Clin Exp Metastasis. 2003;20:195–201. doi: 10.1023/a:1022931016285. [DOI] [PubMed] [Google Scholar]
- 8.Picker LJ, Nakache M, Butcher EC. Monoclonal antibodies to human lymphocyte homing receptors define a novel class of adhesion molecules on diverse cell types. J Cell Biol. 1989;109:927–937. doi: 10.1083/jcb.109.2.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Screaton GR, Bell MV, Jackson DG, Cornelis FB, Gerth U, Bell JI. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci U S A. 1992;89:12160–12164. doi: 10.1073/pnas.89.24.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harn HJ, Isola N, Cooper DL. The multispecific cell adhesion molecule CD44 is represented in reticulocyte cDNA. Biochem Biophys Res Commun. 1991;178:1127–1134. doi: 10.1016/0006-291x(91)91009-2. [DOI] [PubMed] [Google Scholar]
- 11.Mayer B, Jauch KW, Günthert U, Figdor CG, Schildberg FW, Funke I, Johnson JP. De-novo expression of CD44 and survival in gastric cancer. Lancet. 1993;342:1019–1022. doi: 10.1016/0140-6736(93)92879-x. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Zhang JH, Kuang G, Yang JQ, Zhao Q, Wang XL, Jiao ZK, Zhang ZD, Wang LL. Expresson of MUC,CD44v6,nm23 in gastric carcinomas and regional lymph node tissues and their association with invasion, metastasis, and prognosis the tumor. Zhonghua Aizheng Zazhi. 2003;22:985–989. [PubMed] [Google Scholar]
- 13.Jiang HG, Tang WP, Liao XB, Cao J, Wu DM, Lin H. Significance and expression of CD44v6 and E-cadherin in gastric carcinomas and their lymph-node metastases. Zhonghua Linchuang Weichangbingxue Zazhi. 2001;13:60–63. [Google Scholar]
- 14.Wang DR, Chen GY, Liu XL, Miao Y, Pan SY, Xia JG, Wu ZY. The serum concentration of soluble CD44v6 and the expression of CD44v6 in patients with gastric carcinoma. Nanjing Daxue Xuebao (Yixueban) 2002;22:365–367. [Google Scholar]
- 15.Gibson UE, Heid CA, Williams PM. A novel method for real time quantitative RT-PCR. Genome Res. 1996;6:995–1001. doi: 10.1101/gr.6.10.995. [DOI] [PubMed] [Google Scholar]
- 16.Yang L, Xia JG, Zheng SZ, Zhang XW, Chen GY, Xu ZQ, Yang HY, Zhou HB. The radioimmunoimaging of131I labelled CD44v6 monoclonal antibody in nude mice with metastatic focus of gastric cancer in vivo. Nanjing Daxue Xuebao (Yixueban) 2002;22:282–283. [Google Scholar]
- 17.Liu ZH, Li CM, Wang XJ, Long PB. Clinical application of gene detectionin micrometastases of gastric cancer. Shijie Huaren Xiaohua Zazhi. 2004;12:2033–2035. [Google Scholar]
- 18.Chen XM, Chen GY, Wang ZR, Zhu FS, Wang XL, Zhang X. Detection of micrometastasis of gastric carcinoma in peripheral blood circulation. World J Gastroenterol. 2004;10:804–808. doi: 10.3748/wjg.v10.i6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi N, Ito I, Yanagisawa A, Kato Y, Nakamori S, Imaoka S, Watanabe H, Ogawa M, Nakamura Y. Genetic diagnosis of lymph-node metastasis in colorectal cancer. Lancet. 1995;345:1257–1259. doi: 10.1016/s0140-6736(95)90922-2. [DOI] [PubMed] [Google Scholar]
- 20.Zhang JC, Wang ZR, Cheng YJ, Yang DZ, Shi JS, Liang AL, Liu NN, Wang XM. Expression of proliferating cell nuclear antigen and CD44 variant exon 6 in primary tumors and corresponding lymph node metastases of colorectal carcinoma with Dukes' stage C or D. World J Gastroenterol. 2003;9:1482–1486. doi: 10.3748/wjg.v9.i7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun XW, Shen BZ, Shi MS, Dai XD. Relationship between CD44v6 expression and risk factors in gastric carcinoma patients. Shijie Huaren Xiaohua Zazhi. 2002;10:1129–1132. [Google Scholar]
- 22.Zhou Y, Zhu WQ, Shi YQ, Lu HF, Shi DR. Expression and prognostic significance of CD44v6 in primary gastric carcinoma. Zhongguo Zhongliu. 2002;12:391–394. [Google Scholar]
- 23.Chen Y, Feng LM, Zhong BY, Xu Q, Xu GY, Wang L, Zhou DH. Detection of serum soluble CD44v6 and its clinical application in the patients with gastric carcinoma. Zhongguo Shiyan Zhenduan. 2001;5:169–170. [Google Scholar]
- 24.Lu PL, JI F, Peng KR, Cui JH, Yu CH, Li YM. The expression of CD44v6 mRNA in human gastric cancer. Zhejiang Yixue Zazhi. 2004;26:184–186. [Google Scholar]
- 25.He Y, Zhang L, Zhang PQ, Zhong ZJ, Jin XM. The expression and prognosis of tumor metastasis associated genes CD44v6 and p53 in the gastric carcinomas. Beihua Daxue Xuebao (Nat Sci) 2002;3:399–401. [Google Scholar]
- 26.Zhou Y, Xu H, Wang ZZ. Expression of Cd44v6 and PCNA in gastric cancer. Zhengzhou Daxue Xuebao (Med Sci) 2002;37:308–310. [Google Scholar]
- 27.Peng AB, Shi W, Hu SH, Zhao Q. [Expression of CD44v6 in gastric cancer and its correlation with Helicobacter pylori infection] Ai Zheng. 2003;22:1184–1187. [PubMed] [Google Scholar]
- 28.Li Y, Zhang JH, Kuang G, Yang JQ, Zhao Q, Wang XL, Jiao ZK, Zhang ZD, Wang LL. [Expression of MUC1, CD44v6, nm23 in gastric carcinomas and regional lymph node tissues and their association with invasion, metastasis, and prognosis of the tumor] Ai Zheng. 2003;22:985–989. [PubMed] [Google Scholar]
- 29.Joo M, Lee HK, Kang YK. Expression of E-cadherin, beta-catenin, CD44s and CD44v6 in gastric adenocarcinoma: relationship with lymph node metastasis. Anticancer Res. 2003;23:1581–1588. [PubMed] [Google Scholar]
- 30.Zhang S, Li L, Lin H. [A multianalysis study on clinicopathologic factors related to lymph node metastasis in gastric cancer] Zhonghua Zhongliu Zazhi. 2001;23:399–402. [PubMed] [Google Scholar]
- 31.Zhang JH, Li Y, Wang XL, Cao YM, Kuang G, Wang LL. Relation between expressions of CD44v6,nm23-H1 in gastric carcinoma and lymph node tissues and clincal biological behavior. Zhongguo Linchuang Zhongliu Zazhi. 2004;31:256–258. [Google Scholar]
- 32.Wang DR, Chen GY, Liu XL, Miao Y, Pan SY, Xia JG, Wu ZY. The serum content of soluble CD44v6 and the expression of CD44v6 in patients with gastric carcinoma. Zhonghua Shiyan Waike Zazhi. 2002;19:308–309. [Google Scholar]
- 33.Zhou Y, Zong H, Xu H. Expression of CD44v6 in gastric cancer and its adjacent cancer tissues. Zhengzhou Daxue Xuebao (Med Sci) 2002;37:593–595. [Google Scholar]
- 34.Fan KX, Zhong WX, Sun LP, Li WM. Study on the expression of p16 and CD44v6 in gastric cancer. Shiyong Zhongliuxue Zazhi. 2000;15:391–393. [Google Scholar]
- 35.Gulmann C, Grace A, Leader M, Butler D, Patchett S, Kay E. CD44v6: a potential marker of malignant transformation in intestinal metaplasia of the stomach An immunohistochemical study using tissue microarrays. Eur J Gastroenterol Hepatol. 2003;15:981–986. doi: 10.1097/01.meg.0000085462.12407.fc. [DOI] [PubMed] [Google Scholar]
- 36.Mi JQ, Zhang ZH, Shen MC. Significance of CD44v6 protein expression in gastric carcinoma and precancerous lesions. Shijie Huaren Xiaohua Zazhi. 2000;8:156–158. [Google Scholar]
- 37.Chen X, Ouyang XN, Dai XH, Chen MH, Lin QC. [Relationship between sCD44v6 expression and TCM differentiation type of gastric carcinoma patients and influence of weitai capsule on the expression] Zhongguo Zhongxiyi Jiehe Zazhi. 2005;25:12–15. [PubMed] [Google Scholar]
- 38.Qiu WC, Chen G, Ding YT. The relationship between micrometastasis of gastric carcinoma and its prognosis. Zhonghua Putong Waike Zazhi. 2003;12:535–537. [Google Scholar]
- 39.Shi Y, Zhao QZ. Advance in Adenovirus EIA Gene Research. Zhongguo Zhongliu Shengwu Zhiliao Zazhi. 1999;6:158–160. [Google Scholar]
- 40.Xin Y, Grace A, Gallagher MM, Curran BT, Leader MB, Kay EW. CD44V6 in gastric carcinoma: a marker of tumor progression. Appl Immunohistochem Mol Morphol. 2001;9:138–142. doi: 10.1097/00129039-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Xin Y, Zhao FK, Zhang SM, Wu DY, Wang YP, Xu L. Relationship between Cd44v6 expression and prognosis in gastric carcinoma patients. Shijie Huaren Xiaohua Zazhi. 1999;7:210–214. [Google Scholar]
- 42.Shang XW, Xu YF. The expression and chinical significance of CD44 splice variant V6 in human gastric cancer. Zhongliu Yanjiu Yu Linchuang. 2001;13:17–18. [Google Scholar]
- 43.Yamaguchi A, Goi T, Yu J, Hirono Y, Ishida M, Iida A, Kimura T, Takeuchi K, Katayama K, Hirose K. Expression of CD44v6 in advanced gastric cancer and its relationship to hematogenous metastasis and long-term prognosis. J Surg Oncol. 2002;79:230–235. doi: 10.1002/jso.10082. [DOI] [PubMed] [Google Scholar]


