Abstract
AIM: To investigate the effect of percutaneous endoscopic gastrostomy (PEG) on gastroesophageal reflux (GER) in mechanically-ventilated patients.
METHODS : In a prospective, randomized, controlled study 36 patients with recurrent or persistent ventilator-associated pneumonia (VAP) and GER > 6% were divided into PEG group (n = 16) or non-PEG group (n = 20). Another 11 ventilated patients without reflux (GER < 3%) served as control group. Esophageal pH-metry was performed by the “pull through” method at baseline, 2 and 7 d after PEG. Patients were strictly followed up for semi-recumbent position and control of gastric nutrient residue.
RESULTS: A significant decrease of median (range) reflux was observed in PEG group from 7.8 (6.2 - 15.6) at baseline to 2.7 (0 - 10.4) on d 7 post-gastrostomy (P < 0.01), while the reflux increased from 9 (6.2 - 22) to 10.8 (6.3 - 36.6) (P < 0.01) in non-PEG group. A significant correlation between GER (%) and the stay of nasogastric tube was detected (r = 0.56, P < 0.01).
CONCLUSION: Gastrostomy when combined with semi-recumbent position and absence of nutrient gastric residue reduces the gastroesophageal reflux in ventilated patients.
Keywords: Nasogastric tube, Gastroesophageal reflux, Semi-recumbency, Gastric residue, Percutaneous endoscopic gastrostomy.
INTRODUCTION
Life threatening nosocomial pneumonia secondary to aspiration of gastric contents is frequent in intubated mechanically-ventilated patients[1,2]. A number of causes have been implicated in the development of ventilator-associat-ed pneumonia (VAP) during mechanic-al ventilation, namely the oropharyngeal colonization[2], body position[3], nasogastric tube (NGT)[4], and its size[5]. Supine position and the length of patient’s permanence in this position are other potential risk factors for aspiration[3]. Though the semi-recumbent position reduces the risk of pulmonary aspiration, gastroesophageal reflux (GER) is still possib e[6,7]. However, GER occurs with a significantly higher incidence in semi-recumbent mechanically-ventilated patients with NGT than without (74% vs 35%)[8]. According to other studies[9,10], large-bore tubes do not cause more reflux than small-bore ones.
If the duration of nasogastric intubation correlates with the degree of GER, NGT removal after gastrostomy should normally decrease both GER and aspiration pneumonia rates. Percutaneous endoscopic gastrostomy (PEG), however, is considered neither as a non-pharmacological measure for the prevention of VAP, nor as an adjunctive measure to its treatment because it elicits an increase in GER, aspiration, and incidence of pneumonia, at least in the early post-gastrostomy period[8,11]. Nevertheless, in these studies the body position was not specified and the gastric content was not controlled after gastrostomy.
The aim of the present study was to investigate if long-standing presence of NGT for feeding is associated with increased incidence of GER and if PEG combined with semi-recumbent position and avoidance of gastric nutrient retention lead to decreased incidence of GER in mechanically-ventilated patients.
MATERIALS AND METHODS
Pilot study
Simultaneous measurement of gastric and esophageal pH was performed during a 24-h period[12] in 23 adult intensive care patients, who were mechanically-ventilated and had a NGT in place for a duration of 3-90 d. Exclusion criteria from the study included unstable hemodynamic state, administration of morphine, atropine, theophylline, barbiturates, and cisapride, and a past history of GER or hiatal hernia. GER was expressed as percentage of the time when the esophageal pH was less than 4 in a given 24-h period of time GER (%). Values of GER less than 3% were considered as normal. In these 23 patients, there was a positive correlation of the duration of nasogastric intubation in mechanically-ventilated patients to the degree of GER (%) (r = 0.78, P < 0.01). GER mainly occurred after a 10-d period of NGT and mechanical ventilation. Based on these results, only patients who were on mechanical ventilation with NGT for more than 10 d were enrolled in the main study.
Main study and patient selection
The institutional review board approved the study protocol and informed consent was obtained from patient’s kin in each case. Over a 28-mo period, 39 consecutive mechanically-ventilated patients with NGT in place, suffering from persistent or recurrent VAP and presenting a GER above 6% constituted the study population. The diagnosis of persistent or recurrent VAP was established according to the criteria previously described[13,14]. The exclusion criteria mentioned in the pilot study were maintained. Nineteen patients were randomly allocated to receive PEG, but three among them were excluded because of hiatal hernia (2 cases) and intestinal bloating (1 case). Finally, 16 patients received PEG (PEG group) and 20 did not (non-PEG group). In the non-PEG group patients the eventual presence of hiatal hernia was possibly missed, since no endoscopy was performed for PEG placement. Twelve patients in the PEG group and 15 patients in the non-PEG group presented persistent pneumonia. The rest of the patients in both groups had recurrent pneumonia. Eleven mechanically-ventilated patients with acute respiratory failure and NGT for more than 15 d and GER lower than 3% were used as controls. Patients enrolled in the study had comparable severity scores of VAP or acute respiratory failure, radiographic scores and ventilation parameters (data not shown). The characteristics of patients are presented in (Table 1).
Table 1.
Characteristics of patients in the study [median (range)]
| PEG (n = 16) | Non-PEG (n = 20) | Control (n = 11) | P | |
| Age | 53 (20 - 82) | 58 (25 - 85) | 56 (34 - 76) | NS |
| Sex (M/F) | 10/6 | 12/8 | 7/4 | NS |
| Weight (kg) | 75 (55 - 85) | 79 (56 - 95) | 83(68 - 90) | NS |
| APACHE II | 17 (12 - 23) | 17 (9 - 28) | 15 (5 - 26) | |
| Primary disease | ||||
| Head injury | 7 | 5 | 1 | |
| Spinal cord injury | 3 | 4 | - | |
| Multiple trauma | 5 | 8 | 3 | NS |
| Stroke | 4 | 2 | 5 | |
| COPD | 5 | 4 | 3 | |
| Post-operative | 4 | 3 | - | |
| resuscitation | ||||
| Days of MV and NGT | 25 (19 - 36) | 27 (17 - 56) | 24 (12 – 32) | NS |
| Days of VAP before study | 14 (8 - 19) | 13 (4 - 36) | - | NS |
Gastrostomy was performed using the pull through (Ponsky) technique[15] after feeding was stopped for 24 h. Patients of all groups were on continuous drip NGT or PEG-feeding at 60 mL/h with a polymeric diet of energy content of 1 000 kcal/L. Thereafter, they were placed in a semi-recumbent position (30°) and the volume of the gastric nutrient residue was measured with a syringe at 8-h intervals. If the nutrient volume exceeded 200 mL, feeding was withheld and restarted when the volume decreased. These two measures were followed for the subsequent 20-d period during which conventional treatment for pneumonia was applied.
All pH-metries were performed on a 24 h basis. Baseline pH-metries and those performed on d 7 in non-PEG patients were carried out as follows: with the patient in semi-recumbent position a single crystal antimony multi use electrode was used which disposes three sensors located at the tip, 15 and 30 cm, respectively connected to a portable recorder (Digitrapper Mk III, Synectics Medical AB, Stockholm, Sweden). The electrode had a diameter of 2.1 mm at the level of the sensors and was attached via an adhesive material to a new 14 bore NGT so that the distal pH-meter sensor corresponded at 10 cm proximally to the tip of the NGT. In this way, reflux associated with the presence of NGT could be detected. An in vitro calibration of the whole system was carried out with buffer solutions of pH 1 and pH 7 before each pH-metry.
After ordinary NGT removal, the new NGT with the sensor probe attached was introduced via the nose into the stomach until acid pH was recorded with the distal and middle sensors as previously described[12]. The electrode was then slowly withdrawn until the middle sensor channel detected a sudden pH change from acid (< 4) to above 5. The electrode was then withdrawn for further 5 cm. In this way, the distal sensor was located into the stomach and the middle and proximal sensors were located at 5 and 20 cm above the lower esophageal sphincter, respectively. The correct positioning of the electrode was verified at the end of pH-metry and before its withdrawal by a chest x-ray. The recording device measured pH values every 4 s and stored the mean of 20 values every 80 s.
Patients received sucralfate, 2 g twice daily via NGT at least 72 h before the study for gastric mucosa protection. Antacids, H-2 blocking agents or omeprazole that could interfere with pH neutralization were not used and for the same reason feeding was stopped 6 h before and during the period of pH-metry. Patients were not sedated nor paralyzed.
In PEG group patients, two additional pH-metries were carried out: the first at an early (48 h) post-PEG period and the second at a late (7 d) post-PEG period. The first pH-metry investigated the described increased incidence of reflux and/or aspiration at that period[8]. The second pH-metry also performed in the non-PEG group of patients was carried out in order to estimate and evaluate the effectiveness of PEG on reflux. These additional pH-metries were performed in the same manner with the following modifications: a two-sensor probe was used and positioned in such a way that the distal and proximal sensors were located at 5 and 20 cm over the lower esophageal sphincter, respectively. With the two-sensor probe the lower esophageal sphincter was free from the presence of any catheter. Patients were fed through PEG and the degree of reflux was assessed in the absence of NGT.
PEG was arbitrarily considered effective if within a 7-d post PEG period the GER (%) decreased by more than 60% compared to the pre-PEG value PEG and non-PEG patients were followed up for 20 d for pneumonia healing and all patients for weaning from mechanical ventilation and intensive care discharge.
Statistical analysis
To evaluate the differences in GER (%) between the three study periods (pre-PEG vs early and late post-PEG) in each group, Wilcoxon’s signed-rank test was used. To assess the changes in the number of days of mechanical ventilation and NGT standing, pneumonia occurrence before PEG, weaning after PEG, days from PEG to discharge between the two groups, Wilcoxon’s rank sum test was performed. Spearman’s r was used to detect if there was any correlation between the standing-period of NGT and GER (%). Statistical significance was defined as a P value of 0.05 or less.
RESULTS
The correlation between GER (%) and duration of NGT permanence in the 23 patients from the pilot study along with the values obtained from the first pH-metry in the 47 patients of the main study is shown in Figure 1. After 20 d of NGT in situ, 38 out of 50 patients (76%) presented a reflux rate of above 6% while all the 14 patients with NGT in situ for less than 15 d had a reflux rate of less than 6%.
Figure 1.
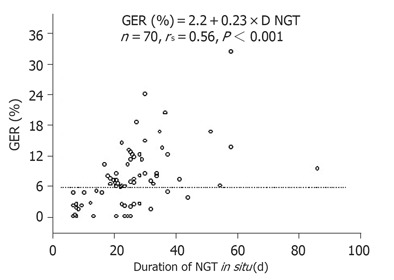
Correlation between duration of NGT and mechanical ventilation standing period to the GER rate measured in 70 patients.
The pH-metry from the lower esophageal sensor consistently recorded 15 - 20% higher GER than that from the upper sensor. However, since the importance of reflux detection in the upper part of esophagus is greater in relation to aspiration, only data from the upper sensor were presented. The median (range) GER (%) in the PEG group was 7.8 (6.2 - 15.6) at baseline. Forty-eight hours post-PEG, though there was no significant change in the median value [8.7 (0.1 - 19)], an increase in GER (%) was observed in 5 out of 16 patients. On d 7, post-PEG, GER (%) decreased to 2.7 (0 - 10.4) (P < 0.01). In contrast, in the non-PEG group, GER (%) increased from 9 (6.2 - 22) at baseline to 10.8 (6.3 - 36.6) on d 7 (P < 0.01) (Figure 2).
Figure 2.
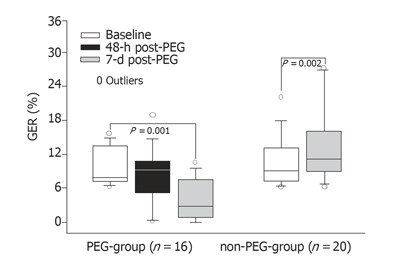
Variation of the median GER (%) together with 10%, 25%, 75%, and 90% from pH-metries originated from the sensor located at 20 cm proximally to the lower esophageal sphincter. The symbol in the 48-h period is lacking in patients of non-PEG-group since no pH-metry was performed in that period in this group of patients.
In the following 20-d period, the weaned, discharged, and died patients were respectively 11 and 4 (P = 0.006), 10 and 2 (P < 0.01), and 3 and 5 in PEG and non-PEG groups. The respective values for patients in the control group were 8, 8, and 1. The outcome of the PEG-group was similar to that of the control group.
DISCUSSION
This study showed that the degree of GER correlated with the duration of NGT in situ. Removal of NGT and feeding through PEG with the patients in semi-recumbent position along with the volume control of the nutrient gastric residue resulted in a decrease or even elimination of the reflux in almost two-thirds of the patients. Additionally, PEG seemed to exert a favorable effect on pneumonia healing rate, weaning period, and patient discharge from ICU.
There are several reasons for frequent GER occurrence in the critically ill patients. Drugs depressing the function of lower esophageal sphincter and/or delaying gastric emptying, such as morphine, atropine, theophylline, and barbiturates, are frequently administered in the critically ill patients[16]. The presence of NGT is an important cause of reflux, since it may induce lower esophageal sphincter relaxation[17]. In patients undergoing elective laparotomy and nasogastric intubation, a significant increase in the mean number of reflux episodes has been observed during the perioperative period in contrast to those without NGT (137 vs 8, P < 0.01)[18]. The mean lower esophageal sphincter pressures are also lower in the same patients, and reflux occurs within 24 h after NGT insertion at the induction of anesthesia. Similarly, in patients undergoing cardiac surgery with simultaneous esophageal and tracheal pH-metry, reflux is seen more frequently in those with a NGT in place than in those without (P < 0.001)[19].
The above observations lead to the notion that NGT removal after PEG should theoretically eliminate or decrease the incidence of GER. However, data in the literature paradoxically support the opposite. There is evidence that PEG or percutaneous endoscopic jejunostomy provides no benefit in terms of reflux and/or incidence of aspiration pneumonia[8,11,19-23]. In a study of 64 patients, 9 developed aspiration pneumonia within 3 d of the procedure and four died[8]. Aspiration occurred in 11% of 79 patients with either neurologic disorders or cancer, whose PEG or percutaneo-us endoscopic jejunostomy was performed[11]. In another study comprising 20 malnourished patients, aspiration was the most common adverse event following percutaneous endoscopic jejunostomy, accounting for 50% of deaths[20]. Similarly, GER seems to be a frequent disorder following PEG in children[21-23]. Yet, in 58 neurologically disabled patients who had clinical evidence of aspiration pneumonia, 17 demonstrated pneumonia after gastrostomy[24]. As a result of the negative outcomes, the use of PEG has declined during recent years. However, in all these studies the body position following PEG was not reported and the volume of gastric nutrient residue was not controlled.
The positive results found in our PEG group compared to those reported in the literature have to be attributed to the volume control of the nutrient gastric residue and the semi-recumbent position during the whole 20-dobservation period. The semi-recumbent position has been previously shown to reduce aspiration of gastric contents[6]. Gastric distension is also an important cause of lower esophageal sphincter transient relaxation, thus permitting GER[25]. It seems that owing to gravity, semi-recumbency, and avoidance of gastric retention prevent reflux of gastric juice into the esophagus.
The results of the present study describe the pathoph-ysiological sequence of PEG-induced lower esophageal sphincter restoration. In 11/16 patients in the PEG group, the reflux rate did not decrease during the first 48 post-PEG hours. Moreover, in 5 of them GER (%) increased in this period. These results indicate that “PEG was arbitrarily considered effective if within a 7-day post PEG period the GER (%) decreased by more than 60% compared to the pre-PEG value”. Thus, a period of at least 7 d seems to be required after NGT removal before lower esophageal sphincter returns to its normal function. The same findings also provide an explanation for the high incidence of aspiration pneumonia encountered during the early post-PEG period[7,8,17,18].
The role of GER in the pathogen-esis of VAP is still controversial[26-28]. All patients in the present study suffering from persistent or recurrent pneumonia were severely ill and failed to respond to the usual therapeutic measures. However, reflux was eliminated in 10 out of 16 patients undergoing PEG who weaned from the respirator and discharged from ICU within the 20-d period of observation. In contrast, among the 20 non-PEG patients reflux increased through time and only 4 and 2 patients were weaned and discharged, respectively. The curative rate of PEG-group was similar to that of control group with the GER being lower than 3%. Among the 11 patients with GER < 3% only one presented VAP. Therefore, it is concluded that GER is implicated in the pathogenesis of VAP and the elimination of reflux results in a more favorable outcome, possibly because the repetitive instillation of infective materials to the trachea that occurs during reflux, is halted.
In conclusion, NGT presence seems to promote reflux of gastric contents, resulting in aspiration and/or pneumonia. The NGT replacement by PEG combined with semi-recumbent position and control of gastric residue can decrease GER in the majority of patients. However, there is persistence of GER and aspiration in some patients, which may be due to the functional alteration of the lower esophageal sphincter rather than ineffectiveness of PEG per se. Gastrostomy combined with semi-recumbency and control of gastric residue should be taken into consideration for the effective management and prevention of VAP.
ACKNOWLEDGMENT
Dr J. Milic-Emili for his editorial assistance and useful criticism as well as L. Haziroglou, BSc, for language review.
Footnotes
S- Editor Wang XL and Guo SY L- Editor Elsevier HK E- Editor Wu M and Li HY
References
- 1.Kingston GW, Phang PT, Leathley MJ. Increased incidence of nosocomial pneumonia in mechanically ventilated patients with subclinical aspiration. Am J Surg. 1991;161:589–592. doi: 10.1016/0002-9610(91)90906-t. [DOI] [PubMed] [Google Scholar]
- 2.Pingleton SK, Hinthorn DR, Liu C. Enteral nutrition in patients receiving mechanical ventilation. Multiple sources of tracheal colonization include the stomach. Am J Med. 1986;80:827–832. doi: 10.1016/0002-9343(86)90623-6. [DOI] [PubMed] [Google Scholar]
- 3.Torres A, Serra-Batlles J, Ros E, Piera C, Puig de la Bellacasa J, Cobos A, Lomeña F, Rodríguez-Roisin R. Pulmonary aspiration of gastric contents in patients receiving mechanical ventilation: the effect of body position. Ann Intern Med. 1992;116:540–543. doi: 10.7326/0003-4819-116-7-540. [DOI] [PubMed] [Google Scholar]
- 4.Bullock TK, Waltrip TJ, Price SA, Galandiuk S. A retrospective study of nosocomial pneumonia in postoperative patients shows a higher mortality rate in patients receiving nasogastric tube feeding. Am Surg. 2004;70:822–826. [PubMed] [Google Scholar]
- 5.Ibáñez J, Peñafiel A, Marsé P, Jordá R, Raurich JM, Mata F. Incidence of gastroesophageal reflux and aspiration in mechanically ventilated patients using small-bore nasogastric tubes. JPEN J Parenter Enteral Nutr. 2000;24:103–106. doi: 10.1177/0148607100024002103. [DOI] [PubMed] [Google Scholar]
- 6.Orozco-Levi M, Torres A, Ferrer M, Piera C, el-Ebiary M, de la Bellacasa JP, Rodriguez-Roisin R. Semirecumbent position protects from pulmonary aspiration but not completely from gastroesophageal reflux in mechanically ventilated patients. Am J Respir Crit Care Med. 1995;152:1387–1390. doi: 10.1164/ajrccm.152.4.7551400. [DOI] [PubMed] [Google Scholar]
- 7.Ibáñez J, Peñafiel A, Raurich JM, Marse P, Jordá R, Mata F. Gastroesophageal reflux in intubated patients receiving enteral nutrition: effect of supine and semirecumbent positions. JPEN J Parenter Enteral Nutr. 1992;16:419–422. doi: 10.1177/0148607192016005419. [DOI] [PubMed] [Google Scholar]
- 8.Jarnagin WR, Duh QY, Mulvihill SJ, Ridge JA, Schrock TR, Way LW. The efficacy and limitations of percutaneous endoscopic gastrostomy. Arch Surg. 1992;127:261–264. doi: 10.1001/archsurg.1992.01420030023003. [DOI] [PubMed] [Google Scholar]
- 9.Dotson RG, Robinson RG, Pingleton SK. Gastroesophageal reflux with nasogastric tubes. Effect of nasogastric tube size. Am J Respir Crit Care Med. 1994;149:1659–1662. doi: 10.1164/ajrccm.149.6.8004326. [DOI] [PubMed] [Google Scholar]
- 10.Ferrer M, Bauer TT, Torres A, Hernández C, Piera C. Effect of nasogastric tube size on gastroesophageal reflux and microaspiration in intubated patients. Ann Intern Med. 1999;130:991–994. doi: 10.7326/0003-4819-130-12-199906150-00007. [DOI] [PubMed] [Google Scholar]
- 11.Kadakia SC, Sullivan HO, Starnes E. Percutaneous endoscopic gastrostomy or jejunostomy and the incidence of aspiration in 79 patients. Am J Surg. 1992;164:114–118. doi: 10.1016/s0002-9610(05)80367-8. [DOI] [PubMed] [Google Scholar]
- 12.Schindlbeck NE, Heinrich C, König A, Dendorfer A, Pace F, Müller-Lissner SA. Optimal thresholds, sensitivity, and specificity of long-term pH-metry for the detection of gastroesophageal reflux disease. Gastroenterology. 1987;93:85–90. doi: 10.1016/0016-5085(87)90318-0. [DOI] [PubMed] [Google Scholar]
- 13.Malangoni MA. Single versus combination antimicrobial therapy for ventilator-associated pneumonia. Am J Surg. 2000;179:58S–62S. [PubMed] [Google Scholar]
- 14.Bonten MJ, Bergmans DC, Ambergen AW, de Leeuw PW, van der Geest S, Stobberingh EE, Gaillard CA. Risk factors for pneumonia, and colonization of respiratory tract and stomach in mechanically ventilated ICU patients. Am J Respir Crit Care Med. 1996;154:1339–1346. doi: 10.1164/ajrccm.154.5.8912745. [DOI] [PubMed] [Google Scholar]
- 15.Ponsky JL, Gauderer MW, Stellato TA. Percutaneous endoscopic gastrostomy. Review of 150 cases. Arch Surg. 1983;118:913–914. doi: 10.1001/archsurg.1983.01390080021006. [DOI] [PubMed] [Google Scholar]
- 16.Ogorek CP, Cohen S. Gastroesophageal reflux disease: new concepts in pathophysiology. Gastroenterol Clin North Am. 1989;18:275–292. [PubMed] [Google Scholar]
- 17.NAGLER R, SPIRO HM. PERSISTENT GASTROESOPHAGEAL REFLUX INDUCED DURING PROLONGED GASTRIC INTUBATION. N Engl J Med. 1963;269:495–500. doi: 10.1056/NEJM196309052691003. [DOI] [PubMed] [Google Scholar]
- 18.Manning BJ, Winter DC, McGreal G, Kirwan WO, Redmond HP. Nasogastric intubation causes gastroesophageal reflux in patients undergoing elective laparotomy. Surgery. 2001;130:788–791. doi: 10.1067/msy.2001.116029. [DOI] [PubMed] [Google Scholar]
- 19.Russell GN, Yam PC, Tran J, Innes P, Thomas SD, Berry PD, Fox MA, Fabri BM, Jackson M, Weir WI. Gastroesophageal reflux and tracheobronchial contamination after cardiac surgery: should a nasogastric tube be routine. Anesth Analg. 1996;83:228–232. doi: 10.1097/00000539-199608000-00005. [DOI] [PubMed] [Google Scholar]
- 20.DiSario JA, Foutch PG, Sanowski RA. Poor results with percutaneous endoscopic jejunostomy. Gastrointest Endosc. 1990;36:257–260. doi: 10.1016/s0016-5107(90)71018-8. [DOI] [PubMed] [Google Scholar]
- 21.Grunow JE, al-Hafidh A, Tunell WP. Gastroesophageal reflux following percutaneous endoscopic gastrostomy in children. J Pediatr Surg. 1989;24:42–4; Discussion 44-5. doi: 10.1016/s0022-3468(89)80298-2. [DOI] [PubMed] [Google Scholar]
- 22.Mollitt DL, Golladay ES, Seibert JJ. Symptomatic gastroesophageal reflux following gastrostomy in neurologically impaired patients. Pediatrics. 1985;75:1124–1126. [PubMed] [Google Scholar]
- 23.Berezin S, Schwarz SM, Halata MS, Newman LJ. Gastroesophageal reflux secondary to gastrostomy tube placement. Am J Dis Child. 1986;140:699–701. doi: 10.1001/archpedi.1986.02140210097034. [DOI] [PubMed] [Google Scholar]
- 24.Hassett JM, Sunby C, Flint LM. No elimination of aspiration pneumonia in neurologically disabled patients with feeding gastrostomy. Surg Gynecol Obstet. 1988;167:383–388. [PubMed] [Google Scholar]
- 25.Coben RM, Weintraub A, DiMarino AJ, Cohen S. Gastroesophageal reflux during gastrostomy feeding. Gastroenterology. 1994;106:13–18. doi: 10.1016/s0016-5085(94)93969-1. [DOI] [PubMed] [Google Scholar]
- 26.Ewig S, Torres A. Prevention and management of ventilator-associated pneumonia. Curr Opin Crit Care. 2002;8:58–69. doi: 10.1097/00075198-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Kollef MH. The prevention of ventilator-associated pneumonia. N Engl J Med. 1999;340:627–634. doi: 10.1056/NEJM199902253400807. [DOI] [PubMed] [Google Scholar]
- 28.Fiddian-Green RG, Baker S. Nosocomial pneumonia in the critically ill: product of aspiration or translocation. Crit Care Med. 1991;19:763–769. [PubMed] [Google Scholar]


