Abstract
The objectives of this prospective study were to evaluate the nuclear maturation stage and the presence and location of meiotic spindles of in vivo matured oocytes from infertile women with and without endometriosis (male or tubal causes of infertility) undergoing stimulated cycles for intracytoplasmic sperm injection (ICSI). We also compared the ICSI outcomes among groups. We analyzed the meiotic spindles of oocytes from 36 patients with endometriosis I/II, 24 with endometriosis III/IV, and 60 without endometriosis (male or tubal causes of infertility). The oocytes were imaged using polarization microscopy. There were no differences in the number of oocytes in telophase I (mean [standard deviation]: 0.1 [0.5], 0.2 [0.4], and 0.2 [0.5], respectively, in the endometriosis I/II, endometriosis III/IV, and control groups), in metaphase II with visible spindles (4.2 [2.5], 3.1 [2.0], and 3.6 [2.2], respectively, in the endometriosis I/II, endometriosis III/IV, and control groups), and in spindle location among groups. We can conclude from this study that noninvasive analysis of spindles from in vivo matured oocytes of infertile patients with endometriosis did not demonstrate significant differences in terms of the nuclear maturation stage, the percentage of oocytes in metaphase II with visible spindles, and the spindle localization when compared to the control group. However, it is important to state that there are no studies evaluating the accuracy of polarization microscopy for the detection of meiotic anomalies in human oocytes, which would need to be better evaluated in future studies using an appropriate methodology.
Keywords: endometriosis, human oocyte quality, meiotic spindle, polarization microscopy, ICSI
Introduction
In the spectrum of endometriosis-related symptoms, one of the most intriguing is the association of the disease with infertility, mainly in cases where there are no mechanical alterations of the reproductive system. Although it has been a controversial issue for decades, various data have supported the concept of decreased fertility in patients with endometriosis.1,2 The finding of similar implantation rates in oocyte donation cycles for women with endometriosis and controls suggests an important role of oocyte quality in the assisted reproduction outcomes of infertile women with endometriosis.1,3,4
Some studies have suggested that oxidative stress has a potential role in explaining the etiopathogenesis of infertility associated with endometriosis,5–9 theoretically promoting an impairment of oocyte quality among these patients. Some alterations in the quality of oocytes may lead to either an impairment of embryo development10 or a total block of the process,11 when women with endometriosis are compared to infertile controls.
For the oocyte to be prepared for fertilization, it needs to be mature (at the metaphase II stage) with a morphologically functional meiotic spindle. In a recently published study from our group analyzing in vitro matured oocytes obtained from stimulated cycles of infertile patients with either endometriosis or male/tubal infertility causes, we observed that among those with the first polar body (PB) visualized, patients with endometriosis tended to have a higher percentage of oocytes in telophase I.12 This finding suggests a potential delay or impairment of meiosis I associated with endometriosis, which may contribute to decreased oocyte quality. However, we highlight the fact that the number of cases presented was small and that the data could not necessarily be extrapolated to in vivo matured oocytes. Because of these findings, we thought it would be of interest to use noninvasive and innocuous methods to analyze this important cellular structure in in vivo matured oocytes from stimulated cycles of infertile women with endometriosis, a study that has not been performed thus far. Such an approach would permit the utilization of the analyzed oocytes in subsequent assisted reproduction techniques (ARTs).
Polarization microscopy permits the observation and characterization of spindles in live oocytes without the necessity of fixation.13 It is an innocuous method that permits the utilization of the oocytes analyzed for intracytoplasmic sperm injection (ICSI) without compromising subsequent embryonic development. It also permits the identification of the nuclear maturation stage of oocytes and may have clinical utility for predicting embryo quality and fertilization after ICSI.13–15 In addition, the identification of the position of the meiotic spindle relative to the first PB may prevent embryologists from damaging this important cellular structure during ICSI.16
Thus, we evaluated the nuclear maturation stage of in vivo matured oocytes (with the first PB extruded) from women with endometriosis (minimal/mild–stage I/II and moderate/severe–stage III/IV) and male or tubal causes of infertility (controls) undergoing stimulated cycles for ICSI. We also compared the presence/location of meiotic spindles of in vivo matured oocytes (in metaphase II) from the 3 groups.
Methods
Patients
This prospective case–control study was performed from March 2008 to March 2009 at the in vitro fertilization (IVF) Center of the University Hospital, Department of Gynecology and Obstetrics, Ribeirão Preto Medical School, USP, Brazil. The study was submitted to and approved by the Research Committee. All the participating couples underwent ovulation induction for purposes of ICSI, fulfilled the inclusion criteria, demonstrated a desire to participate in the project, and signed an informed consent form.
Patients aged ≤38 years, with basal follicle-stimulating hormone (FSH) levels <10 mIU/mL, and body mass index <30 kg/m2 were included. The women with endometriosis were included after diagnosis by videolaparoscopy according to the protocol of the American Society of Reproductive Medicine,17 which classifies the disease into 4 stages minimum (stage I), mild (stage II), moderate (stage III), and severe (stage IV). The women included in the control group did not have pelvic diseases associated with infertility, as demonstrated by videolaparoscopy performed as part of the routine investigation of infertility in our service.
Patients with chronic pathologies, users of tobacco or alcoholic beverages, and patients using medications that could interfere with ovarian folliculogenesis or oxidative stress (such as nonsteroidal anti-inflammatory drugs, and corticosteroids) in the 6 months prior to the beginning of ovulation induction were excluded.
A total of 120 patients met the inclusion criteria for the study. Of these, 60 presented with male and/or tubal factors and were designated as the control group, and 60 presented with infertility related to endometriosis (36 with minimal and mild endometriosis and 24 with moderate and severe endometriosis).
Controlled Ovarian Stimulation Protocol
The participating patients underwent pituitary suppression with a gonadotropin-releasing hormone analogue 10 days prior to the basal transvaginal ultrasound (long protocol), with the administration of leuprolide acetate (Lupron, Abott, Brazil). The patients received 100 to 300 IU per day of recombinant FSH (Gonal-F, Serono, Brazil; Puregon, Organon, Brazil) during the first 6 days of induction. When at least 2 follicles reached a mean diameter of 18 mm, recombinant human chorionic gonadotropin (hCG) was administered (Ovidrel, Serono). Oocytes were retrieved 34 to 36 hours after administration of recombinant hCG.
Oocyte Preparation
All the materials aspirated during oocyte retrieval were analyzed to identify and isolate the cumulus–oocyte complexes (COCs). After identification, the COCs were isolated from the follicular fluid, placed on separated plates and then carefully washed with human tubal fluid-HEPES (HTF, Irvine Scientific, Santa Ana, CA, USA) to remove blood and debris. Subsequently, they were placed on Nunc plates (4-well MultiDish, Nunclon, Delta SI, Thermo Scientific, Rochester, NY, USA) filled with HTF culture medium, covered with mineral oil, and subjected to 5% CO2 gas incubation under ideal temperature (37°C) and humidity (95%) conditions for a period of 2 to 3 hours. After this period, in order to remove the cumulus cells, the COCs were placed in microdrops of 25 µL hyaluronidase (H4272 type IV-S, Sigma, Gillingham, Medway, UK) diluted in 80 IU/mL of HTF/HEPES (Irvine Scientific) for a maximum of 30 seconds and then washed 2 to 3 times with HTF-modified medium (HTF/HEPES, Irvine Scientific) supplemented with 10% synthetic serum substitute (SSS). The mechanical removal of cellular debris was done with a stripper pipette (130 µm denuding pipette, Cook, Melbourne, Australia).
After oocyte denuding, the degree of oocyte maturation was determined using a light microscope. The immature oocytes (germinal vesicle stage or metaphase I) were discarded, and the mature oocytes (morphologically characterized by the presence of the first PB extruded) were incubated in 25 µL of HTF + 10% SSS for 1 hour (after oocyte denuding) and then analyzed by the OCTAX ICSI Guard system (Medical Technology Vertriebs-GmbH, Altdorf, Germany) immediately before ICSI.
Polarization Microscopy
The spindles of oocytes with the first PB extruded were evaluated using an inverted microscope equipped with a video camera and with the hardware of a polarization microscope, which consisted of electric crystals and an optic-electric controller (OCTAX ICSI Guard system). The electric crystal groups were controlled by the computer through the OCTAX EyeWare software (Medical Technology Vertriebs-GmbH). The oocytes were analyzed at 37°C in 5 µL drops of HTF/Hepes + 10% SSS on coated glass bottom Petri dishes (MatTek Corp, Ashland, Massachusetts) and placed on a surface heated to 37°C.
In order to control any potential methodological biases due to the nonvisualization of the spindle after oocyte denuding, the procedure was done 2 to 3 hours after retrieval.18 After denuding, the oocytes were placed on the previously balanced culture plates in the incubator for an additional hour, followed by imaging and ICSI. It is important to state that those oocytes in which the spindle was not observed were rotated to 3 different positions before being marked as lacking a spindle. The oocytes were imaged only once, and rigorous temperature control was maintained in order to minimize environmental changes outside the incubator. A maximum of 7 oocytes were analyzed on each plate because the total time, including the time necessary to analyze the cell spindle by polarization microscopy and ICSI, was 7 minutes or less as recommended by the manufacturer of the OCTAX ICSI Guard system.
The oocytes analyzed by polarization microscopy were characterized according to the nuclear maturation stage (telophase I or metaphase II), the presence or absence of a visible spindle, and the location of the cell spindle relative to the first PB. The oocytes that presented with an elongated cellular spindle perpendicular to the oocyte membrane and extending to the first PB were considered to be in telophase I. The cellular spindles in metaphase II were characterized by the presence of radially distributed birefringent fibers in the shape of a barrel and oriented parallel to the cortical membrane. Because chromosomes are minimally birefringent, they were not properly analyzed by this methodology. The cellular spindle localization was based on the angle formed between this structure and the first PB; the oocytes were divided into 6 groups with spindles, respectively, forming angles of 0° to 30°, 30° to 60°, 60° to 90°, 90° to 120°, 120° to 150°, and 150° to 180° relative to the first PB.
At 18 to 19 hours after ICSI, fertilization was analyzed and characterized by the presence of 2 pronuclei and 2 polar bodies (the fertilization rate was calculated for each patient as the number of fertilized oocytes divided by the number of injected oocytes). Cleavage was verified about 24 hours after fertilization with the observation of cellular division (the cleavage rate was calculated for each patient as the number of cleaved embryos divided by the number of fertilized oocytes). At about 44 hours after ICSI (D2), the embryonic quality was analyzed and characterized by the symmetry and number of blastomeres, the percentage of fragmentation, and the presence or absence of multinucleation. Top quality embryos in D2 were defined as having 4 symmetric blastomeres, without fragmentation or multinucleation. We analyzed the percentage of cycles with at least one good quality embryo produced among the groups analyzed.
Statistical Analysis
Data were analyzed considering the patient as the experimental unit of this study. The endometriosis (stages I/II and III/IV) and control groups were analyzed comparatively. Each variable was adjusted to a regression following a particular distribution, according to its characteristics.
The variable oocytes in metaphase II, oocytes in metaphase II with visible spindle, oocytes in metaphase II with spindle in a position between 0° and 30°, number of mature oocytes, number of retrieved oocytes, number of fertilized oocytes, and number of cleaved embryos were characterized as count data and adjusted using a regular Poisson distribution for the comparisons between groups. In contrast, the variable oocytes in telophase I, oocytes in metaphase II with no visible spindle, and oocytes in metaphase II with a visible spindle between 30° and 60°, 60° and 90°, and 90° and 120°, after being characterized as count data, showed values equal to zero in most observations. Thus, these variables were adjusted following a Poisson distribution with an excess of zero (zero-inflated Poisson) and comparisons between groups were then made. Due to the fact that oocytes in metaphase II with the spindle located between 90° and 120° present only 1 observation with nonzero value, the model did not achieve convergence and, therefore, could not be adjusted.
Quantitative continuous variables (age, total FSH dose, length of ovarian stimulation, number of follicles 14-17 mm, number of follicles ≥18 mm, and endometrium) were analyzed statistically by analysis of variance followed by the Tukey test. The fertilization rate and cleavage rate of the variables were analyzed by binomial regression. All analyses were performed using the PROC GENMOD procedure of the SAS 9.2 software. The level of significance was set at 5% (P < .05) in all analyses.
Results
We analyzed a total of 120 patients who underwent ovarian stimulation for ICSI, 60 with infertility due to male and/or tubal factors (control group), and 60 with infertility related to endometriosis (36 with minimal and mild endometriosis, and 24 with moderate and severe endometriosis). No significant differences were found among the 3 groups analyzed (control, endometriosis I/II, and endometriosis III/IV), regarding median age, length of ovarian stimulation, number of follicles between 14 and 17 mm, number of follicles ≥18 mm, endometrium, and percentage of cycles producing at least 1 good quality embryo transferred on D2. A significant increase in the total dose of FSH used for ovarian stimulation was observed in patients with advanced endometriosis (stages III/IV; Table 1).
Table 1.
Comparison of Clinical Variables Between Infertile Women With and Without Endometriosis (Tubal and/or Male Infertility Factors).a
| Variables | Tubal and/or Male Infertility Factors | Endometriosis | P | |
|---|---|---|---|---|
| Mean (SD) | I to II | III to IV | ||
| Mean (SD) | Mean (SD) | |||
| Number of patients | 60 | 36 | 24 | – |
| Age, years | 32.8 (3.8) | 33.1 (3.2) | 33.1 (3.6) | .88 |
| Total FSH dose, UI | 1973 (654.6)b | 1709 (554.9)b | 2263 (671.7)c | .04 |
| Length of ovarian stimulation, days | 9.1 (1.6) | 8.7 (1.3) | 9.3 (1.6) | .69 |
| Number of follicles 14 to 17 mm | 4.6 (2.4) | 4.2 (2.7) | 3.6 (3.2) | .57 |
| Number of follicles ≥18 mm | 4 (2.8) | 3.2 (1.8) | 2 (2.2) | .54 |
| Endometrium, mm | 11 (2.4) | 10.1 (1.5) | 10.5 (2) | .13 |
| Number of cycles with oocyte retrieval | 60 | 36 | 24 | – |
| Number of cycles with transfer | 57 | 35 | 22 | – |
| Number of cycles with transfer in D2 | 23 | 15 | 9 | – |
| Number of cycles with transfer in D3 | 34 | 20 | 13 | – |
| % of cycles with at least one good quality embryo transferred in D2 | 26 (06/23) | 27 (04/15) | 22 (02/09) | .51 |
Abbreviation: FSH, follicle-stimulating hormone.
a Analysis of variance (ANOVA) followed by the Tukey posttest. Data presented as mean (standard deviation [SD]). The level of significance was set at 5% (P < .05). Different superscripts b and c on the same line indicate a statistically significant difference (P < .05).
No significant differences in the median number of oocytes in telophase I (Figure 1A) were observed in the endometriosis III/IV group (median [standard deviation]: 0.2 [0.4]) compared to the endometriosis I/II group (0.1 [0.5]) and the control group (0.2 [0.5]; Table 2). We did not observe a significant difference in the number of oocytes in metaphase II with visible (Figure 1B) and nonvisible (Figure 1C) spindles among the groups (4.2 [2.5], 3.1 [2.0], and 3.6 [2.2] of patients who had oocytes with visible spindles in the endometriosis I/II, endometriosis III/IV, and control groups; Table 2). All patients presented oocytes with a cellular spindle localized between 0° and 120° in relation to the first PB. We did not observe any significant differences in the number of oocytes with cellular spindles localized between 0° and 30° (Figure 1D), 30° and 60° (Figure 1E), and 60° and 90° (Figure 1F) among the groups analyzed (Table 2).
Figure 1.
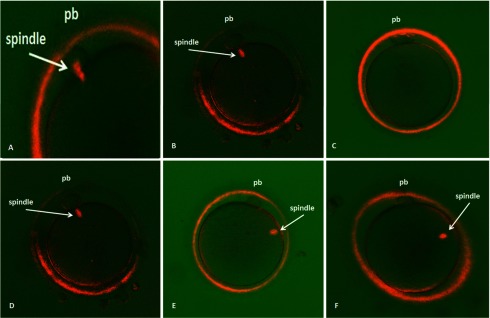
Birefringent spindles in living human oocytes imaged with the Polscope just before ICSI. A, Birefringent spindles at telophase I. B, Oocytes had visible spindles at metaphase II. C, Oocytes had no visible spindles at metaphase II. D, Oocytes with the spindle located between 0° and 30° relative to the polar body. E, Oocytes with the spindle located between 30° and 60° relative to the polar body. F, Oocytes with the spindle located between 60° and 90° relative to the polar body.
Table 2.
Comparison of Noninvasive Analysis of Oocytes With the First Polar Body Extruded Obtained From Stimulated Cycles of Infertile Patients With and Without Endometriosis (Tubal and/or Male Infertility Factors).a
| Variables | Tubal and/or Male Infertility Factors | Endometriosis | |
|---|---|---|---|
| I to II | III to IV | ||
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Nuclear maturation | |||
| Telophase Ib | 0.2 (0.5) | 0.1 (0.5) | 0.2 (0.4) |
| Metaphase IIc | 4.1 (2.1) | 4.7 (2.4) | 3.5 (2.3) |
| Spindle visualization | |||
| Nonvisibleb | 0.5 (0.9) | 0.5 (1.0) | 0.4 (0.8) |
| Visiblec | 3.6 (2.2) | 4.2 (2.5) | 3.1 (2.0) |
| Spindle localization | |||
| 0° to 30°c | 3.1 (2.0) | 3.4 (2.0) | 2.5 (1.6) |
| 30° to 60°b | 0.4 (0.7) | 0.7 (1.0) | 0.5 (0.7) |
| 60° to 90°b | 0.1 (0.5) | 0.1 (0.4) | 0.1 (0.3) |
a Data presented as mean (standard deviation [SD]). The level of significance was set at 5% (P < .05). There were no significant differences (P ≥ .05) among groups regarding any of the variables analyzed.
b Data analyzed by zero-inflated Poisson.
c Data analyzed by a Poisson Model.
We observed a significant decrease in the number of fertilized oocytes and produced embryos in patients with endometriosis III/IV compared to patients with endometriosis I/II and controls. We also observed lower fertilization rates in this group of patients compared to control and to patients with endometriosis I/II. There was no significant difference in cleavage rate among groups (Table 3).
Table 3.
Comparison of the Parameters of Controlled Ovarian Stimulation of Infertile Women With and Without Endometriosis (Tubal and/or Male Infertility Factors).
| Variables | Tubal and/or Male Infertility Factors | Endometriosis | |
|---|---|---|---|
| Mean (SD) | I to II | III to IV | |
| Mean (SD) | Mean (SD) | ||
| Number of retrieved oocytesw | 6.8 (3.9)a | 7.6 (5.3)b | 5.8 (3.9)c |
| Number of mature oocytesw | 5.2 (3.4)d | 5.5 (3.4)e | 4.2 (2.9)f |
| Number of fertilized oocytesw | 3.4 (1.8)g | 3.8 (2.1)h | 2.4 (1.6)i |
| Number of cleaved embryosw | 2.9 (1.5)j | 3.2 (1.7)l | 2.2 (1.5)m |
| Number of embryos producedw | 2.7 (1.4)n | 2.8 (1.6)o | 1.8 (1.1)p |
| Fertilization rate (%)x | 79.5 (23.1)q | 81.2 (20.4)r | 69.5 (24.6)s |
| Cleavage rate (%)x | 88.4 (19.6)t | 88.1 (17.3)u | 90.3 (23.6)v |
a,b and a,c (P > .05); b,c (P < .05).
d,e and d,f (P > .05); e,f (P < .05).
g,h (P > .05); g,i and h,i (P < .05).
j,l and j,m (P > .05); l,m (P < .05).
n,o (P > .05); n,p and o,p (P < .05).
q,r (P > .05); q,s and r,s (P < .05).
t,u,v (P > .05).
wData analyzed by a Poisson model.
xData analyzed by binomial Regression. Data presented as mean (standard deviation [SD]). Fertilization rate: number of fertilized oocytes/number of injected oocytes. Cleavage rate: number of cleaved embryos/number of fertilized oocytes. The level of significance was set at 5% (P < .05).
Discussion
The mechanisms involved in the etiopathogenesis of infertility related to endometriosis have not been fully elucidated. Lower fertilization and implantation rates in women with endometriosis undergoing ovarian stimulation for ART1,3,4 might result from decreased oocyte quality.12 However, we could not find any studies that objectively evaluated markers of oocyte quality in infertile women with endometriosis using noninvasive methods that permit the clinical use of oocytes in ART.
Some studies have shown significant DNA damage and increased microtubule and chromosomal anomalies in mouse oocytes incubated with peritoneal fluid from patients with endometriosis.19 These results suggest that meiotic anomalies may be related to impaired oocyte quality in patients with endometriosis. It is well established that meiotic abnormalities can contribute to developmental failure through several pathways, ranging from the inability of the oocyte to complete the maturation process, which makes it incapable of being normally fertilized, to the appearance of variable errors in the meiotic maturation process that do not make fertilization impossible but can compromise embryonic development pre- and/or postimplantation, as well as the future viability of the embryo.20–22 On this basis, first of all, we intended to evaluate the nuclear maturation stage of in vivo matured oocytes obtained from stimulated cycles of infertile women with and without endometriosis.
In clinical practice, embryologists perform oocyte denudation before ICSI, and the visualization of the first PB is used as the criterion to classify the oocyte as mature and able to be injected. However, oocytes with the first PB extruded may not have completed meiosis I, being in telophase I instead of metaphase II.15 We did not detect a higher percentage of telophase I in live in vivo matured oocytes from infertile women with endometriosis compared to control. These data do not corroborate the recent findings from our group, indicating a higher proportion of telophase I in in vitro matured oocytes obtained from stimulated cycles of patients with endometriosis.12 Thus, the delay or impairment of meiosis I does not seem to be involved in the potential worsening of oocyte quality in in vivo matured oocytes obtained from stimulated cycles of patients with infertility related to endometriosis.
Some authors15 have shown that apparently mature oocytes (with the first PB visible) that were actually in telophase I according to analysis by polarization microscopy presented significantly lower fertilization rates when compared to oocytes that had completed meiosis I (15.8% vs 80%, respectively). Although in the present study we observed a significant reduction in the number of fertilized oocytes and produced embryos, and lower fertilization rates in patients with endometriosis III/IV compared to patients with endometriosis I/II and to controls, these did not seem to be related to the stage of oocyte maturation since we did not detect a significant difference in the percentage of oocytes in telophase I between the groups studied.
Another objective of the present study was to compare the visualization and localization of metaphase II meiotic spindles between infertile women with endometriosis (stages I/II and III/IV) and controls. In this study, the meiotic spindle was visualized in almost 90% of the total number of oocytes examined, in agreement with literature data, where the visualization of the cellular spindle ranged from 62.8% to 91%.23,24 The high percentage of oocytes with visible cellular spindles observed in the 3 groups analyzed in this study can be attributed to rigorous methodological control, including incubating oocytes for 1 hour after denuding and adequate control of ambient conditions (maintenance of ideal temperature and pH, short time during which oocytes were exposed to light or left outside incubation during the analyses).25 We did not observe a significant difference in the percentage of oocytes in metaphase II with visible and nonvisible spindles between patients with endometriosis I/II, those with endometriosis III/IV, and controls. We also did not observe a significant difference in the localization of the cell spindle between groups. All the oocytes with visible spindles presented this structure localized between 0° and 120° in relation to the first PB, with almost 80% localized between 0° and 30°. Studies have evaluated the angle formed between the PB and the cellular spindle and have concluded that when the angle does not exceed 90°, there are no effects on the fertilization rates of these oocytes.24,26 According to our results, we suggest that the localization of the oocyte spindle in anomalous positions in patients with endometriosis may not be the responsible for the poor oocyte quality.
We can conclude from this study that the noninvasive analysis of spindles of in vivo matured oocytes from infertile patients with mild (stages I and II) and advanced (stages III and IV) endometriosis, and without endometriosis undergoing stimulated cycles for ICSI did not demonstrate significant differences between groups in terms of the nuclear maturation stage, the percentage of oocytes in metaphase II with visible spindles, and the spindle localization. However, it is important to state that there are no studies evaluating the accuracy of polarization microscopy for the detection of meiotic anomalies in human oocytes (by comparing noninvasive analyses with invasive analyses after immunostaining for morphological visualization of both microtubules and chromatin by high-performance confocal microscopy). Thus, although no differences were observed in the percentage of oocytes in metaphase II with visible spindles between infertile women with and without endometriosis, we cannot conclude that the percentage of meiotic anomalies in in vivo matured oocytes was similar for the 2 groups.
Acknowledgments
The authors wish to thank the following employees of the Laboratory of Assisted Reproduction, Department of Gynecology and Obstetrics, University Hospital, Faculty of Medicine of Ribeirão Preto, University of São Paulo, for technical support: Maria Aparecida Carneiro Vasconcelos, Marilda Yamada Dantas, Maria Auxiliadora Pádua Rosa, and Sandra Vianna.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Luciana A. Dib was supported by a scholarship granted by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil. Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) - Edital MCT/CNPq 15/2007 - Processo 478396/2007-4. Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) - 2008/58197-6.
References
- 1.Garrido N, Navarro J, Remohi J, Simon C, Pellicer A. Follicular hormonal environment and embryo quality in women with endometriosis. Hum Reprod Update. 2000;6(1):67–74. [DOI] [PubMed] [Google Scholar]
- 2.Garrido N, Navarro J, Garcia-Velasco J, Remoh J, Pellice A, Simon C. The endometrium versus embryonic quality in endometriosis-related infertility. Hum Reprod Update. 2002;8(1):95–103. [DOI] [PubMed] [Google Scholar]
- 3.Barnhart K, Dunsmoor-Su R, Coutifaris C. Effect of endometriosis on in vitro fertilization. Fertil Steril. 2002;77(6):1148–1155. [DOI] [PubMed] [Google Scholar]
- 4.Al-Fadhli R, Kelly SM, Tulandi T, Tanr SL. Effects of different stages of endometriosis on the outcome of in vitro fertilization. J Obstet Gynaecol Can. 2006;28(10):888–891. [DOI] [PubMed] [Google Scholar]
- 5.Jackson LW, Schisterman EF, Dey-Rao R, Browne R, Armstrong D. Oxidative stress and endometriosis. Hum Reprod. 2005;20(7):2014–2020. [DOI] [PubMed] [Google Scholar]
- 6.Gupta S, Agarwal A, Krajcir N, Alvarez JG. Role of oxidative stress in endometriosis. Reprod Biomed Online. 2006;13(1):126–134. [DOI] [PubMed] [Google Scholar]
- 7.Mansour G, Abdelrazik H, Sharma RK, Radwan E, Falcone T, Agarwal A. L-carnitine supplementation reduces oocyte cytoskeleton damage and embryo apoptosis induced by incubation in peritoneal fluid from patients with endometriosis. Fertil Steril. 2009;91(5 suppl):2079–2086. [DOI] [PubMed] [Google Scholar]
- 8.Andrade AZ, Rodrigues JK, Dib LA, et al. [Serum markers of oxidative stress in infertile women with endometriosis] [Article in Portuguese]. Rev Bras Ginecol Obstet. 2010;32(6):279–285. [DOI] [PubMed] [Google Scholar]
- 9.Prieto L, Quesada JF, Cambero O, et al. Analysis of follicular fluid and serum markers of oxidative stress in women with infertility related to endometriosis. Fertil Steril. 2012;98(1):126–130. [DOI] [PubMed] [Google Scholar]
- 10.Brizek CL, Schlaff S, Pellegrini VA, Frank JB, Worrilow KC. Increased incidence of aberrant morphological phenotypes in human embryogenesis--an association with endometriosis. J Assist Reprod Genet. 1995;12(2):106–112. [DOI] [PubMed] [Google Scholar]
- 11.Pellicer A, Oliveira N, Ruiz A, Remohi J, Simon C. Exploring the mechanism(s) of endometriosis-related infertility: an analysis of embryo development and implantation in assisted reproduction. Hum Reprod. 1995;10(suppl 2):91–97. [DOI] [PubMed] [Google Scholar]
- 12.Barcelos ID, Vieira RC, Ferreira EM, Martins WP, Ferriani RA, Navarro PA. Comparative analysis of the spindle and chromosome configurations of in vitro-matured oocytes from patients with endometriosis and from control subjects: a pilot study. Fertil Steril. 2009;92(5):1749–1752. [DOI] [PubMed] [Google Scholar]
- 13.Petersen CG, Oliveira JB, Mauri AL, et al. Relationship between visualization of meiotic spindle in human oocytes and ICSI outcomes: a meta-analysis. Reprod Biomed Online. 2009;18(2):235–243. [DOI] [PubMed] [Google Scholar]
- 14.Wang WH, Keefe DL. Prediction of chromosome misalignment among in vitro matured human oocytes by spindle imaging with the PolScope. Fertil Steril. 2002;78(5):1077–1081. [DOI] [PubMed] [Google Scholar]
- 15.Hyun CS, Cha JH, Son WY, Yoon SH, Kim KA, Lim JH. Optimal ICSI timing after the first polar body extrusion in in vitro matured human oocytes. Hum Reprod. 2007;22(7):1991–1995. [DOI] [PubMed] [Google Scholar]
- 16.Moon JH, Hyun CS, Lee SW, Son WY, Yoon SH, Lim JH. Visualization of the metaphase II meiotic spindle in living human oocytes using the Polscope enables the prediction of embryonic developmental competence after ICSI. Hum Reprod. 2003;18(4):817–820. [DOI] [PubMed] [Google Scholar]
- 17.Revised American Society for reproductive medicine classification of endometriosis: 1996. Fertil Steril. 1997;67(5):817–821. [DOI] [PubMed] [Google Scholar]
- 18.Cohen Y, Malcov M, Schwartz T, et al. Spindle imaging: a new marker for optimal timing of ICSI? Hum Reprod. 2004;19(3):649–654. [DOI] [PubMed] [Google Scholar]
- 19.Mansour G, Radwan E, Sharma R, Agarwal A, Falcone T, Goldberg J. DNA damage to embryos incubated in the peritoneal fluid of patients with endometriosis: role in infertility. Fertil Steril. 2007;88:S311. [Google Scholar]
- 20.Pavlok A, Lucas-Hahn A, Niemann H. Fertilization and developmental competence of bovine oocytes derived from different categories of antral follicles. Mol Reprod Dev. 1992;31(1):63–67. [DOI] [PubMed] [Google Scholar]
- 21.Lonergan P, Monaghan P, Rizos D, Boland MP, Gordon I. Effect of follicle size on bovine oocyte quality and developmental competence following maturation, fertilization, and culture in vitro. Mol Reprod Dev. 1994;37(1):48–53. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong DT. Effects of maternal age on oocyte developmental competence. Theriogenology. 2001;55(6):1303–1322. [DOI] [PubMed] [Google Scholar]
- 23.Madaschi C, de Souza Bonetti TC, de Almeida Ferreira Braga DP, Pasqualotto FF, Iaconelli A, Jr, Borges E., Jr. Spindle imaging: a marker for embryo development and implantation. Fertil Steril. 2008;90(1):194–198. [DOI] [PubMed] [Google Scholar]
- 24.Rienzi L, Ubaldi F, Martinez F, et al. Relationship between meiotic spindle location with regard to the polar body position and oocyte developmental potential after ICSI. Hum Reprod. 2003;18(6):1289–1293. [DOI] [PubMed] [Google Scholar]
- 25.Roberts R, Franks S, Hardy K. Culture environment modulates maturation and metabolism of human oocytes. Hum Reprod. 2002;17(11):2950–2956. [DOI] [PubMed] [Google Scholar]
- 26.Fang C, Tang M, Li T, et al. Visualization of meiotic spindle and subsequent embryonic development in in vitro and in vivo matured human oocytes. J Assist Reprod Genet. 2007;24(11):547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]


