Abstract
Despite the prevalence of uterine fibroids (Fs), few studies have investigated the links between clinical features and the cellular or molecular mechanisms that drive F growth and development. Such knowledge will ultimately help to differentiate symptomatic from asymptomatic Fs and could result in the development of more effective and individualized treatments. The aim of this study was to investigate the relationship between ultrasound appearance, blood flow, and angiogenic gene expression in F, perifibroid (PM), and distant myometrial (DM) tissues. We hypothesized that angiogenic gene expression would be increased in tissues and participants that showed increased blood flow by Doppler ultrasound. The study was performed using Doppler ultrasound to measure blood flow prior to hysterectomy, with subsequent tissue samples from the F, PM, and DM being investigated for angiogenic gene expression. Overall, PM blood flow (measured as peak systolic velocity [PSV]) was higher than F blood flow, although significant heterogeneity was seen in vascularity and blood flow between different Fs and their surrounding myometrium. We did not find any correlation between PSV and any other clinical or molecular parameter in this study. We identified 19 angiogenesis pathway-related genes with significant differences in expression between F and DM, and 2 genes, matrix metalloproteinase 9 (MMP9) and Neuropilin 2 (NRP2), that were significantly different between F and PM. These results are consistent with subtle differences between PM and DM. Understanding the differences between symptomatic versus asymptomatic Fs may eventually lead to more effective treatments that directly target the source of heavy menstrual bleeding.
Keywords: leiomyoma, heavy menstrual bleeding, Doppler ultrasound, angiogenesis
Introduction
Uterine leiomyoma are benign neoplasms of the myometrium and are the most common tumors in women of reproductive age. Estimates of the prevalence of leiomyomata, or fibroids (Fs) as they are more commonly called, vary with the population sample and the detection method used. A large US-based population study found a cumulative incidence of more than 80% in black women and almost 70% in white women approaching 50 years of age using diagnostic ultrasound.1 This incidence concurs with pathological examination of surgical specimens that found that the prevalence was as high as 77%.2 It has been estimated that approximately 25% of women with Fs will experience F-related symptoms,3 most commonly heavy menstrual bleeding (HMB), but also pelvic pressure, pain, reproductive dysfunction, and pregnancy complications. Symptomatic uterine Fs pose a major public health, quality-of-life, and economic burden worldwide.4
Despite the prevalence and significance of uterine Fs and their variable clinical presentation, few studies have investigated why only a proportion of Fs are symptomatic, or have attempted to correlate clinical symptoms such as HMB with morphology and function of the F-associated vasculature. Very little is known about the cellular or molecular mechanisms that differentiate symptomatic from asymptomatic Fs.
Theories proposed to account for F-associated HMB include reduced myometrial contractility, increased endometrial surface area, and dilated vessels overlying uterine Fs.5,6 However, there is very little objective evidence to support these proposed mechanisms.7,8 Uterine Fs are associated with HMB, but there is no consistent relationship between the size and location of Fs and heavy bleeding.9 Collectively, this strongly suggests that features other than size or location account for HMB in F uteri.
The presence of intramural Fs in the uterus is likely to significantly alter blood flow within the myometrium and may also alter endometrial blood flow. It has been known for many years that the perifibroid myometrial (PM) region exhibits dilated venules and increased arterial vascularity,10 which we hypothesize might be linked to increased symptomatic blood loss. Differences in angiogenic gene expression between Fs and normal myometrium have also been demonstrated.11 However, gene expression studies involving the perifibroid region that might identify possible regulatory mechanisms for altered blood flow in this area have not been undertaken.
The aim of this study was to investigate the relationship between blood flow and angiogenic gene expression in F, PM, and distant myometrial (DM) tissues. We hypothesized that angiogenic gene expression would be increased in tissues and participants that showed increased blood flow by Doppler ultrasound. We further hypothesized that F heterogeneity, color Doppler blood flow, and spectral Doppler resistive indices would correlate with differences in gene expression profiles. The study was performed using Doppler ultrasound to measure blood flow prior to hysterectomy, with subsequent tissue samples from the F, PM, and DM being investigated for angiogenic gene expression. A better understanding of F pathophysiology with mechanistic insight into how they cause HMB may potentially lead to novel antiangiogenic treatments for symptomatic uterine Fs.
Methods
Patient Recruitment
Patients with Fs who were scheduled to undergo hysterectomy (n = 6) were recruited via Monash Medical Centre Moorabbin gynaecology outpatients. Ethical approval for the study was obtained from Southern Health Human Research and Ethics Committee B (Project 09272B) and informed consent was obtained from all patients. Clinical details and samples taken from each patient are shown in Table 1.
Table 1.
Clinical Details and Samples Used in Study.a
| ID | Age | Ethnicity | HMB | Pain | Parity | HT | Uterine vol cc | Endometrial Histopathology | Samples Analyzed | Fibroid vol cc |
|---|---|---|---|---|---|---|---|---|---|---|
| 33/10 | 34 | European | Y | N | 2 | N | 181 | Mid-secretory | Fibroid 1 | 234 |
| descent | Perifibroid 1 | |||||||||
| Distant Myo | ||||||||||
| 48/10 | 46 | European | Y | N | 4 | Y | 1184 | Inactive | Fibroid 1 | 135 |
| descent | Norethisterone | Perifibroid 1 | ||||||||
| Fibroid 2 | 373 | |||||||||
| Perifibroid 2 | ||||||||||
| Distant Myo | ||||||||||
| 73/10 | 45 | Chinese | Y | N | 2 | N | 699 | Mid-secretory | Fibroid 1 | 344 |
| descent | Perifibroid 1 | |||||||||
| Distant Myo | ||||||||||
| 77/10 | 43 | European | N | N | 1 | N | 118 | Proliferative | Fibroid 1 | 23 |
| descent | Perifibroid 1 | |||||||||
| Fibroid 2 | 28 | |||||||||
| Perifibroid 2 | ||||||||||
| Distant Myo | ||||||||||
| 85/10 | 52 | European | Y | N | 2 | Y | 112 | Exogenous hormone effect and residual simple hyperplasia, chronic endometritis | Fibroid 1 | 11 |
| descent | Progestagen | Perifibroid 1 | ||||||||
| Fibroid 2 | 3 | |||||||||
| Perifibroid 2 | ||||||||||
| Distant myo | ||||||||||
| 172/10 | 50 | European | Y | Y | 2 | Y | 449 | Inactive | Fibroid 2 | 27 |
| descent | Ceased tamoxifen 10 weeks prior | Perifibroid 2 | ||||||||
| Fibroid 3 | 20 | |||||||||
| Perifibroid 3 | ||||||||||
| Fibroid 4 | 146 | |||||||||
| Perifibroid 4 | ||||||||||
| Distant Myo |
Abbreviations: HMB, heavy menstrual bleeding (self reported); Y, yes; N, no; HT, hormone treatment within 3 months prior to hysterectomy; uterine vol. cc, uterine volume in cubic centimeters estimated from ultrasound measurement; fibroid vol cc, fibroid volume in cubic centimeters estimated from ultrasound measurement; Distant Myo, myometrial tissue taken as far as possible from any fibroid; PCR, polymerase chain reaction.
a Clinical details of 6 participants in study. Not all fibroids identified by ultrasound or found at cut up were collected, and not all tissue collected was used for molecular analysis: for example, fibroid 1 from participant 172/10 was not analyzed by PCR-array. Fibroid 1 in participant 33/10 extended beyond the serosal surface of the uterus.
Ultrasound Assessment of Fibroid and Myometrium
Patients recruited to the study underwent B mode, color, and spectral Doppler ultrasound assessment to investigate blood flow in fibroids and PM. Ultrasounds were carried out using C5-2 (transabdominal), C8-4 (endovaginal), and 3D9-v (3D endovaginal) transducers (Philips Electronics, Germany), according to the routine clinical protocol with additional measurements to assess the Fs and surrounding myometrial blood flow. The volume of the uterus in cubic centimeters (cc) was calculated as the volume of an ellipse from 2 coronal and 1 sagittal measurement taken at the maximal diameters of the uterus using the formula 4/3π (R1·R2·R3), where R1, R2, and R3 are diameter/2 (or radius) of the 3 measurements. The number of fibroids was counted and the volume of each fibroid (cc) calculated in the same manner as the volume of the uterus. The location of each fibroid was recorded using the following descriptors: anterior/posterior, right/midline/left, fundal/body/cervix, and minimum distance from endometrium. From this information a schematic diagram was created for each patient documenting the location of the fibroids, so they could be correctly identified at tissue dissection following hysterectomy. A note was made of whether each fibroid distorted the endometrium, with all fibroids that were subsequently selected for molecular analysis falling into this category (Table 2). Using B-mode ultrasound the qualitative characteristics of each fibroid were assessed as follows: the fibroid is predominantly hypoechoic/isoechoic/hyperechoic/mixed compared to the surrounding myometrium. Using spectral Doppler ultrasound the following parameters were recorded in selected vessels: peak systolic velocity (PSV), end-diastolic velocity, and resistance index within each fibroid and in the PM (for 9 of the 11 fibroids—see Table 3). Overall vascularity in the PM region and within the fibroid was further classified as mild, moderate, or marked based on these measurements and on an overall visual assessment.
Table 2.
B-Mode and Doppler Ultrasound Data for the 6 Participants.
| ID | Sample | Echotexture of the Fibroid | Echotexture of PF Region | Arterial Flow Within Fibroid | Arterial Flow in PF Region | Flow Within Fibroid Is Therefore: | Fibroid Distorts Endometrium |
|---|---|---|---|---|---|---|---|
| Hypoechoic = 1 | No Rim = 1 | Mild = 1 | Mild = 1 | > Than Adjacent PF Region = 1 | |||
| Isoechoic = 2 | Hypoechoic Rim = 2 | Moderate = 2 | Moderate = 2 | = to Adjacent PF Region = 2 | Yes = 1 | ||
| Hyperechoic = 3 | Hyperechoic Rim = 3 | Marked = 3 | Marked = 3 | <Adjacent PF Region = 3 | No = 2 | ||
| Mixed = 4 | |||||||
| 33/10 | Fibroid 1 | 2 | 2 | 1 | 1 | ||
| Perifibroid 1 | 2 | 1 | |||||
| 48/10 | Fibroid 1 | 2 | 2 | 1 | 1 | ||
| Perifibroid 1 | 1 | 2 | |||||
| Fibroid 2 | 4 | 2 | 1 | 1 | |||
| Perifibroid 2 | 2 | 2 | |||||
| 73/10 | Fibroid 1 | 2 | 1 | 1 | 1 | ||
| Perifibroid 1 | 2 | 1 | |||||
| 77/10 | Fibroid 1 | 1 | 1 | 3 | 1 | ||
| Perifibroid 1 | 1 | 2 | |||||
| Fibroid 2 | 2 | 1 | 3 | 1 | |||
| Perifibroid 2 | 1 | 3 | |||||
| 85/10 | Fibroid 1 | 1 | 1 | 3 | 1 | ||
| Perifibroid 1 | 1 | 1 | |||||
| Fibroid 2 | 2 | 1 | 3 | 1 | |||
| Perifibroid 2 | 1 | 2 | |||||
| 172/10 | Fibroid 2 | 3 | 1 | 3 | 1 | ||
| Perifibroid 2 | 2 | 1 | |||||
| Fibroid 3 | 2 | 1 | 3 | 1 | |||
| Perifibroid 3 | 1 | 2 | |||||
| Fibroid 4 | 2 | 1 | 3 | 1 | |||
| Perifibroid 4 | 1 | 2 |
Abbreviation: PF, perifibroid.
Table 3.
Doppler Ultrasound Data for Fibroid and Perifibroid Regions.a
| ID | Area of Measurement | PSV | EDV | RI |
|---|---|---|---|---|
| 33/10 | Fibroid 1 | 32.2 | 10.5 | 0.67 |
| 48/10 | Fibroid 1 | 16.8 | 7.15 | 0.57 |
| Fibroid 1 | 4.5 | 1.93 | 0.57 | |
| 73/10 | Fibroid 1 | 12.9 | 6.5 | 0.5 |
| Fibroid 1 | 11.3 | 4.1 | 0.64 | |
| Fibroid 1 | 10.8 | 4.1 | 0.63 | |
| Perifibroid 1 | 9.9 | 4.8 | 0.52 | |
| Perifibroid 1 | 25.5 | 5.8 | 0.77 | |
| Perifibroid 1 | 47.6 | 12.6 | 0.74 | |
| 77/10 | Fibroid 1 | 6.3 | 0.6 | 0.9 |
| Fibroid 1 | 16.8 | 4.2 | 0.75 | |
| Fibroid 1 | 12.2 | 3.6 | 0.7 | |
| Perifibroid 1 | 13.2 | 3.3 | 0.75 | |
| Perifibroid 1 | 36.7 | 10.5 | 0.71 | |
| Perifibroid 1 | 27.1 | 8.1 | 0.7 | |
| Fibroid 2 | 37.6 | 5.7 | 0.85 | |
| Fibroid 2 | 6.6 | 4.5 | 0.32 | |
| Fibroid 2 | 11.4 | 3 | 0.74 | |
| Fibroid 2 | 12.6 | 5.4 | 0.57 | |
| Perifibroid 2 | 48.1 | 10.5 | 0.78 | |
| Perifibroid 2 | 36.1 | 8.1 | 0.78 | |
| Perifibroid 2 | 13.5 | 4.2 | 0.69 | |
| 85/10 | Fibroid 1 | 7.6 | 2.1 | 0.72 |
| Fibroid 1 | 9.2 | 2.7 | 0.7 | |
| Fibroid 1 | 6.4 | 0 | 1 | |
| Perifibroid 1 | 21.2 | 4.8 | 0.77 | |
| Perifibroid 1 | 29.5 | 7.5 | 0.74 | |
| Perifibroid 1 | 15.7 | 4.9 | 0.69 | |
| Fibroid 2 | 15.6 | 5.4 | 0.65 | |
| Fibroid 2 | 8.9 | 3.5 | 0.61 | |
| Fibroid 2 | 12.6 | 6.3 | 0.5 | |
| Perifibroid 2 | 36.1 | 13.5 | 0.63 | |
| Perifibroid 2 | 32.6 | 10 | 0.63 | |
| Perifibroid 2 | 23.6 | 6 | 0.74 | |
| 172/10 | Fibroid 2 | 8.7 | 4.2 | 0.52 |
| Perifibroid 2 | 26.5 | 11.2 | 0.58 | |
| Perifibroid 2 | 12.0 | 6 | 0.5 | |
| Fibroid 3 | 4.5 | 1.8 | 0.6 | |
| Fibroid 3 | 4.7 | 2.1 | 0.56 | |
| Fibroid 3 | 9.6 | 3.2 | 0.67 | |
| Fibroid 3 | 12.6 | 3.2 | 0.75 | |
| Perifibroid 3 | 14.9 | 4.4 | 0.71 | |
| Fibroid 4 | 12.0 | 6 | 0.5 | |
| Fibroid 4 | 7.1 | 3.9 | 0.45 | |
| Fibroid 4 | 12.8 | 7.1 | 0.45 | |
| Perifibroid 4 | 45.8 | 19.3 | 0.58 | |
| Perifibroid 4 | 65.1 | 32.8 | 0.5 | |
| Perifibroid 4 | 53.9 | 22.9 | 0.57 |
Abbreviations: PSV, peak systolic velocity; EDV, end diastolic velocity; RI, resistance index.
a Doppler ultrasound data for 6 participants in study. No perifibroid data were recorded for the first 2 participants in the study.
Sample Collection
Three hysterectomy specimens were from premenopausal women who had not received exogenous hormones for the previous 3 months (patients 33/10, 73/10, 77/10). Of the 3 women who had received exogenous hormones in the 3 months prior to hysterectomy, 2 were premenopausal (patients 85/10, 172/10) and 1 was perimenopausal (patient 48/10). Five women were having hysterectomy for fibroid-related symptoms (HMB) and 1 had an atypical polyp and incidental fibroids (see Table 1 for details).
Immediately following hysterectomy, tissue samples were dissected from 3 areas within each uterus: fibroid F (n = 11), PM (n = 11), and DM (n = 6). Distant myometrium was defined as tissue taken as far as possible from any F. Perifibroid myometrium was defined as directly adjacent to the F. Care was taken to match pathology findings of F size and location with prior ultrasound findings. Samples were collected into RNAlater for transport and storage prior to RNA extraction.
RNA Extraction and Complementary DNA Synthesis
RNA was extracted from tissues using Trizol (Invitrogen, Carlsbad, California) according to the manufacturer’s instructions. RNA solutions were treated with 4 U of Turbo DNase (Life Technologies, Carlsbad, California) for 30 minutes at 37°C, ethanol precipitated, resuspended in RNase-free water quantified using a Nano Drop spectrophotometer. RNA was stored at −80°C until required for use. RNA was converted to complementary DNA (cDNA) using a Transcriptor High-Fidelity cDNA synthesis Kit (Roche, Castle Hill, NSW, Australia) in accordance with the manufacturer’s instructions.
The RT2 Profiler PCR Angiogenesis Array, Data Analysis, and Bioinformatics
Complementary DNA samples were used to perform reverse transcriptase–qualitative polymerase chain reaction (RT-qPCR) using a commercially available Human Angiogenesis RT2 Profiler PCR array. This custom array covers 84 genes involved in modulating the biological processes related to angiogenesis. The RT-qPCR was run according to the manufacturer’s instructions on an ABI PCR system.
The PCR array data were analyzed using the Bioconductor limma package with fitted linear model, moderated t statistics and Benjamini-Hochberg multiple testing correction. Each of the 11 F and 11 PM samples was first normalized by subtracting the Cts of the corresponding DM samples and then normalized again by subtracting the average results for the control genes Beta-2-microglobulin (B2M); Hypoxanthine phosphoribosyltransferase 1 (HPRT1); Ribosomal protein L13a (RPL13A); Glyceraldehyde-3-phosphate dehydrogenase (GAPDH); and Actin, beta (ACTB). For simplicity, all genes which had missing values were excluded from the analysis. Genes were ranked according to adjusted P values.
Results
Ultrasound Findings
The majority of Fs (10 of the 11 Fs measured) showed equal or increased vascularity in the perifibroid region when compared to vascularity within the F (Table 2 and Figure 1). The majority of flow measured in the F was considered to be mild, while the majority of flow measured in the perifibroid region was considered moderate or marked. Fibroids displayed variable echogenicity by B-mode ultrasound between patients (Table 2 and Figure 2). There was also variability between individual Fs from the same participant.
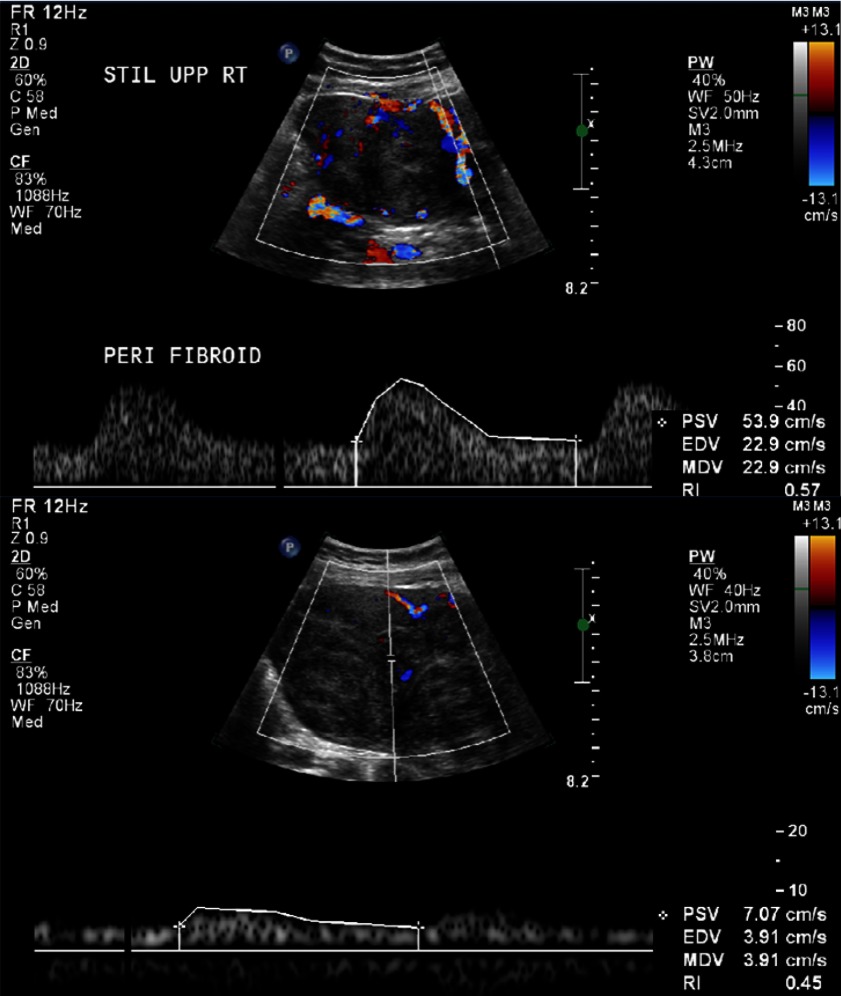
Figure 1.
Upper panel shows an example of moderate flow in the perifibroid region by Doppler ultrasound, while the lower panel is an example of mild flow within the fibroid. Both images are from participant 172/10, fibroid 4.
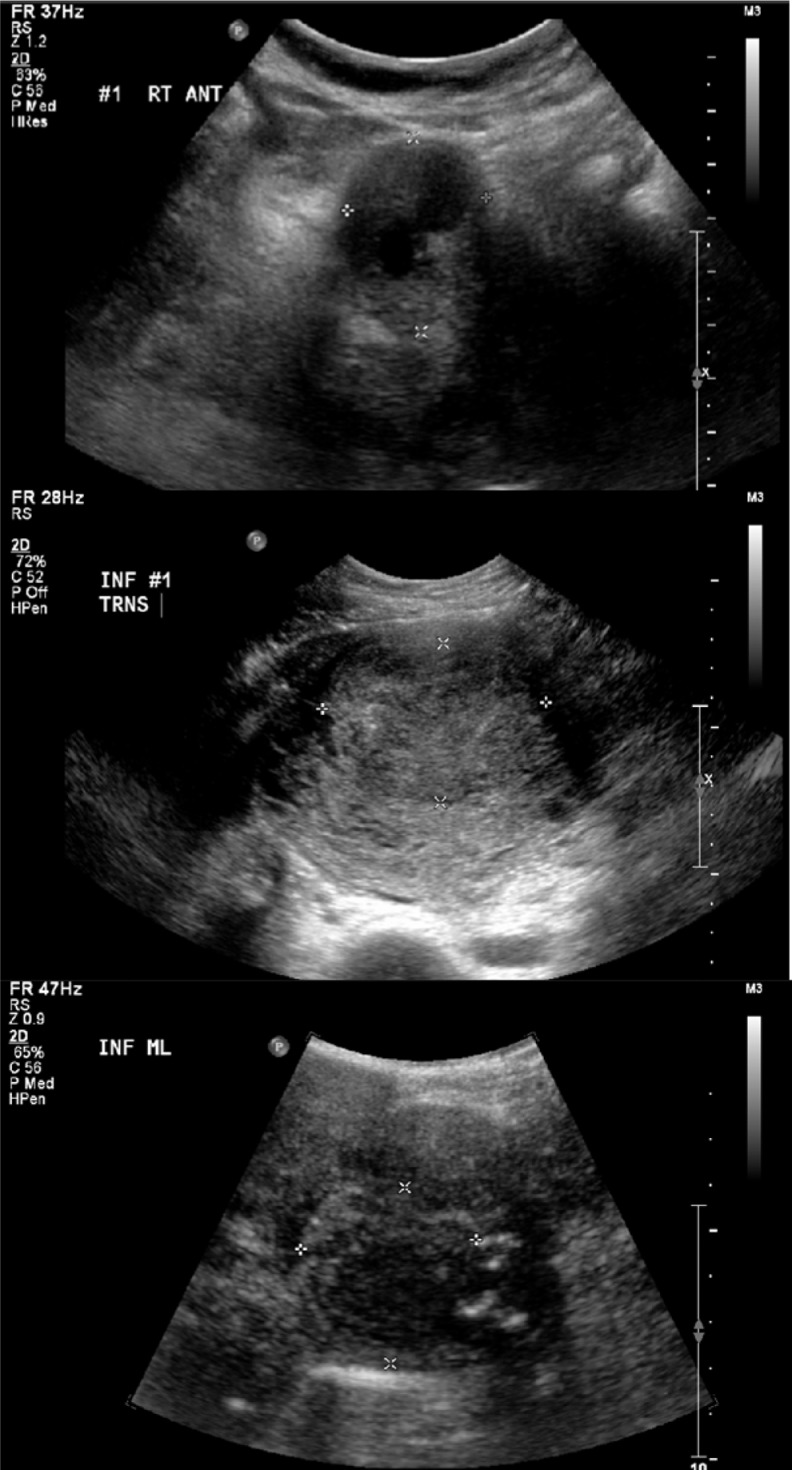
Figure 2.
Upper panel (participant 85/10, fibroid 1), middle panel (participant 48/10, fibroid 1), and lower panel (participant 172/10, fibroid 2) demonstrate hypoechoic, isoechoic, and mixed fibroid echogenicity, respectively.
Peak systolic velocity was highly variable when measured in selected vessels in each F and perifibroid region (Table 3). Overall, the mean PSV was significantly higher in the perifibroid region than within the F (30.2 vs 12.0 cm/s, P < .001).
For the bioinformatics analysis of the gene expression data, 3 sets of comparisons were undertaken: relative gene expression in F versus PM samples, F versus DM samples, and PM versus DM samples (Table 4). Of the 84 angiogenesis-related genes on the PCR array, 19 showed statistically significant differences in expression between F and DM (Table 4). Of these, 13 were up in F and 6 were up in DM. A further 13 genes were identified as “of interest” with a P value <.2. Of these, 4 were down in F and 8 were up. There were no genes identified with significant differences in expression between PM and DM, although 4 genes (placental growth factor [PGF], Platelet/endothelial cell adhesion molecule 1 [PECAM1], TIMP1[TIMP metallopeptidase inhibitor 1], and STAB1[Stabilin 1]) were up in PM with a P value of .1 to .2. Two genes were significantly up in F compared to PM, with a further 9 genes (6 up and 3 down), showing differences with a P value of .05 to .2.
Table 4.
Genes Identified by RT-qPCR Using a Commercially Available Human Angiogenesis RT2 Profiler PCR Array That Are Differentially Expressed Between Fibroid (F), Perifibroid Myometrium (PM), and Distant Myometrium (DM) After Multiple Testing Correction.
| Symbol | F vs DM | P Value | F vs PM | P Value | PM vs DM | P Value | Unigene | Ref seq | Description |
|---|---|---|---|---|---|---|---|---|---|
| CXCL9 | Down in F | <.01 | Hs.77367 | NM_002416 | Chemokine (C-X-C motif) ligand 9 | ||||
| KDR | Down in F | <.01 | Hs.479756 | NM_002253 | Kinase insert domain receptor (a type III receptor tyrosine kinase) | ||||
| S1PR1 | Down in F | .01-.05 | Down in F | >.05−.1 | Hs.154210 | NM_001400 | Sphingosine-1-phosphate receptor 1 | ||
| SERPINF1 | Down in F | <.01 | Down in F | >.05−.1 | Hs.532768 | NM_002615 | Serpin peptidase inhibitor, clade F (alpha-2 antiplasmin, pigment epithelium derived factor), member 1 | ||
| TEK | Down in F | <.01 | Hs.89640 | NM_000459 | TEK tyrosine kinase, endothelial | ||||
| THBS1 | Down in F | .01−.05 | Hs.164226 | NM_003246 | Thrombospondin 1 | ||||
| EFNA3 | Down in F | <.01 | Up in F | >.1−.2 | Hs.516656 | NM_004952 | Ephrin-A3 | ||
| EGF | Up in F | .01−.05 | Up in F | >.05−.1 | Hs.419815 | NM_001963 | Epidermal growth factor (beta-urogastrone) | ||
| EPHB4 | Up in F | .01−.05 | Hs.437008 | NM_004444 | EPH receptor B4 | ||||
| FGF1 | Up in F | <.01 | Up in F | >.05−.1 | Hs.483635 | NM_000800 | Fibroblast growth factor 1 (acidic) | ||
| HAND2 | Up in F | .01−.05 | Hs.388245 | NM_021973 | Heart and neural crest derivatives expressed 2 | ||||
| ITGAV | Up in F | <.01 | Hs.436873 | NM_002210 | Integrin, alpha V (vitronectin receptor, alpha polypeptide, antigen CD51) | ||||
| MMP2 | Up in F | <.01 | Up in F | >.05−.1 | Hs.513617 | NM_004530 | Matrix metalloproteinase 2 (gelatinase A, 72 kDa gelatinase, 72 kDa type IV collagenase) | ||
| MMP9 | Up in F | <.01 | Up in F | .01−.05 | Hs.297413 | NM_004994 | Matrix metallopeptidase 9 (gelatinase B, 92 kDa gelatinase, 92 kDa type IV collagenase) | ||
| NRP2 | Up in F | <.01 | Up in F | .01−.05 | Hs.471200 | NM_003872 | Neuropilin 2 | ||
| PDGFA | Up in F | .01−.05 | Hs.535898 | NM_002607 | Platelet-derived growth factor alpha polypeptide | ||||
| PGF | Up in F | <.01 | Up in PM | >.1−.2 | Hs.252820 | NM_002632 | Placental growth factor | ||
| TGFB2 | Up in F | <.01 | Up in F | >.05−.1 | Hs.133379 | NM_003238 | Transforming growth factor, beta 2 | ||
| THBS2 | Up in F | .01−.05 | Hs.371147 | NM_003247 | Thrombospondin 2 | ||||
| CCL2 | Down in F | >.1−.2 | Hs.303649 | NM_002982 | Chemokine (C-C motif) ligand 2 | ||||
| COL4A3 | Down in F | >.1−.2 | Hs.570065 | NM_000091 | Collagen, type IV, alpha 3 (Goodpasture antigen) | ||||
| FLT1 | Down in F | >.05−.1 | Hs.654360 | NM_002019 | Fms−related tyrosine kinase 1 (vascular endothelial growth factor/vascular permeability factor receptor) | ||||
| PECAM1 | Down in F | >.05−.1 | Up in PM | >.1−.2 | Hs.514412 | NM_000442 | Platelet/endothelial cell adhesion molecule | ||
| COL18A1 | Up in F | >.1−.2 | Hs.517356 | NM_030582 | Collagen, type XVIII, alpha 1 | ||||
| EFNB2 | Up in F | >.1−.2 | Hs.149239 | NM_004093 | Ephrin-B2 | ||||
| HIF1A | Up in F | >.1−.2 | Hs.597216 | NM_001530 | Hypoxia inducible factor 1, alpha subunit (basic helix-loop-helix transcription factor) | ||||
| LAMA5 | Up in F | >.1−.2 | Hs.473256 | NM_005560 | Laminin, alpha 5 | ||||
| NOTCH4 | Up in F | >.1−.2 | Hs.436100 | NM_004557 | Notch homolog 4 (Drosophila) | ||||
| PLG | Up in F | >.1−.2 | Hs.143436 | NM_000301 | Plasminogen | ||||
| TIMP1 | Up in F | >.1−.2 | Up in PM | >.1−.2 | Hs.522632 | NM_003254 | TIMP metallopeptidase inhibitor 1 | ||
| TYMP | Up in F | >.1−.2 | Hs.592212 | NM_001953 | Thymidine phosphorylase | ||||
| STAB1 | Up in PM | >.1−.2 | Hs.301989 | NM_015136 | Stabilin 1 |
Abbreviation: RT−qPCR, reverse transcriptase–polymerase chain reaction.
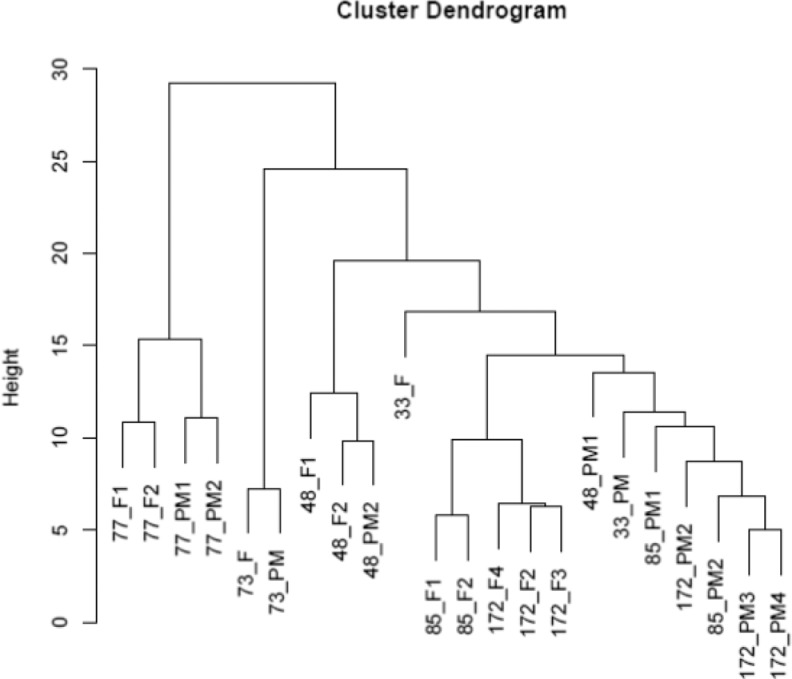
A cluster dendrogram was created to help visualize degrees of similarity in gene expression between the F and PM samples (Figure 3). This showed that the majority of the PM samples clustered together (7 of 11), although there was a tendency for PM and F samples from the same participant to cluster together in some cases (eg, participants 73/10 and 77/10). We also performed an analysis incorporating the complete data set for all 6 patients (clinical, ultrasound, and gene expression data) but did not identify any other statistically significant correlations.
Figure 3.
Cluster dendrogram showing relative similarity in the overall pattern of gene expression between all fibroid (F) and perifibroid myometrium (PM) samples.
Discussion
This is the first study which has attempted to correlate F-associated ultrasonographic blood flow and other clinical parameters with gene expression. This is also the first study to consider the PM region as a potentially distinct tissue type in the F uterus and to analyze the angiogenic gene expression levels in this area.
The significantly higher mean PSV in the PM region compared to the F demonstrated by this study is consistent with early dye and corrosion casting studies showing a perifibroid “shell” of increased vascularity in this region.10,12 The PSV is however only 1 component of vascularity, and while every PM region measured had vessels with higher average PSV than the adjacent F, overall differences in vascularity were not always so clear-cut. The observation that blood velocity is typically higher in PM vessels suggests a fundamental difference in the vasculature compared to within the F. We did not find any correlation between PSV and any other clinical or molecular parameter in this study, although numbers are probably too small to permit meaningful conclusions. What these ultrasonographic findings do confirm is that while perifibroid blood flow is generally higher than F blood flow, significant heterogeneity exists in vascularity and blood flow between Fs and their surrounding myometrium. We also observed significant heterogeneity in F and perifibroid echogenicity by B-mode ultrasound. As with the blood flow data, we were unable to identify any significant correlation between echogenicity and any of the other parameters we measured.
This study has identified 19 angiogenesis pathway-related genes with significant differences in expression between F and DM. By comparison only 2 genes, matrix metalloproteinase 9 (MMP9) and Neuropilin 2 (NRP2), were significantly different between F and PM. Thus, despite the fact that direct comparison between DM and PM failed to identify any significant difference in gene expression with the small numbers of participants in our study, our results are consistent with subtle differences between these regions as evidenced by the different gene lists when each of DM and PM were compared to F.
Of the 19 genes that we identified as significantly different between F and DM, most have been linked to F pathophysiology previously, and at least 7 (tyrosine kinase [TEK], thrombospondin 1 [THBS1], ephrin type-B receptor 4 [EPHB4], THBS2, MMP9, MMP2, and PGF) have been reported with the same direction of up- or downregulation between F and myometrium in previous array studies13–20 This degree of concurrence with the existing literature provides considerable assurance over the validity of our findings. We showed THBS1 was down in Fs compared to myometrium, and THBS2 was up. These results are in broad agreement with earlier studies.13,19,21–23 It has been suggested that Fs are caused by an abnormal fibrotic process that is initiated by altered tissue repair in response to injury.24,25 This is reflected in the aberrant expression of genes that play a role in fibrosis, including thrombospondins, transforming growth factor beta,16 and extracellular matrix proteins.22 Of the other genes from this previously reported list, MMP2, EPHB4, and PGF have been shown to be upregulated in Fs,26–28 but there is less convincing evidence for MMP9.
Both sympathetic nerve fibers and the vascular compartment are reduced in Fs compared to myometrium.29,30 It is therefore not unexpected that genes such as TEK (also known as Tie-2) and KDR (also known as VEGF-R2) are significantly down in F compared to myometrium, since these 2 tyrosine kinase receptors are expressed primarily in endothelial cells. Defects in TEK are associated with inherited venous malformations and the TEK-signalling pathway plays a critical role in endothelial cell–smooth muscle cell communication during venous morphogenesis.31 It is thus possible that one or both of these receptors plays a role in the increased vascularity and blood flow seen in the perifibroid region by Doppler ultrasound in the current study. In contrast to TEK and KDR, NRP2 and EFNA3 expression was elevated in Fs compared to myometrium. The neuropilin receptors act as semaphorin coreceptors with plexins to regulate nervous system development as well as receptors for vascular endothelial growth factor family members.32 The ephrins and ephrin-related receptors comprise the largest subfamily of receptor protein tyrosine kinases and have been implicated in mediating developmental events, especially in the nervous system and in erythropoiesis. As for NRP2, elevated EFNA3 in Fs is thus counterintuitive, given that nerves and blood vessels are reduced in this tissue. The EFNA3 receptor family also plays a major role in tumor remodeling,33 and hence higher levels in Fs may contribute to F growth.
Other genes that we found to have elevated expression in F compared to myometrium were Epidermal growth factor (EGF), Fibroblast growth factor 1 (FGF1), and Platelet-derived growth factor alpha polypeptide (PDGFA). There is evidence implicating EGF as having a central role in mediating the effects of estrogen and progesterone on F growth.34,35 It has been postulated that both FGF1 and FGF2 play important roles in transformation of normal myometrium into leiomyoma and further growth of this tumor.36 Expression of PDGFA and PDGFB is higher in leiomyoma tissue than in matched myometrial tissue and the expression of PDGF and its receptors in leiomyoma and myometrial tissue varied during the menstrual cycle.37 The same authors speculated that PDGF may play a role in the pathogenesis of leiomyomas through mechanisms that include proliferation and increased expression of extracellular matrix.
Our gene expression results are not in complete concurrence with the literature. While we found integrin, alpha V (ITGAV) to be up in Fs, others have recently reported that this gene and related survival-signalling pathways were downregulated in Fs.38
We identified several genes not previously linked to Fs as differentially expressed compared to DM. The protein from HAND2 is a basic helix-loop-helix transcription factor that plays an essential role in cardiac morphogenesis as well as a role in limb and branchial arch development. More recently a role for HAND2 has been identified in decidualization of human and mouse uterine fibroblast cells.39 The same study identified progesterone as a likely regulator of HAND2 expression during uterine sensitization of the mouse uterus. Elevated expression of a transcription factor in Fs that may be regulated by progesterone and with a role in smooth muscle cell morphogenesis is intriguing and worthy of further study. S1PR1, which was down in Fs, is a receptor for sphingosine-1-phosphate, a bioactive lipid that increases cell survival, proliferation, and angiogenesis. The sphingosine-1-phosphate pathway has established roles in late pregnancy decidua and myometrial contraction,40 leading to the possibility that reduced levels of S1PR1 may be linked to failure of normal contraction in F smooth muscle cells. The SERPINF1 (also known as alpha-2 antiplasmin or pigment epithelium–derived factor) is a secreted member of the serpin family that strongly inhibits angiogenesis. In addition, this protein is a neurotrophic factor involved in neuronal differentiation in retinoblastoma cells. Although no role has previously been postulated for SERPINF1 in Fs, there is a recent report of reduced SERPINF1 in peritoneal fluid of women with endometriosis.41
There are a number of limitations to this study. Assessment of blood flow by ultrasound was problematic with attenuation of signal as the tissue depth increased and wide variability between individual vessels. As a consequence, assessment criteria were usually made as relative comparisons between adjacent tissues rather than absolute values. The limitations of ultrasound are well documented;42 however, reproducibility did increase as the study progressed and as the ultrasonographer gained experience with the study protocol. While magnetic resonance imaging is not routinely performed for Fs in a typical clinical setting, it is probable that this imaging modality may be more informative for a similar study in the future.
Most of the women included in this study had multiple Fs and significant anatomical distortion of the uterus and endometrium. It was thus not possible to determine which Fs were responsible for clinical symptoms. This is a common clinical finding and is a significant impediment to linking clinical symptoms with F molecular or cell biology, particularly since Fs often differ in location, size, and appearance within the same uterus. Fibroid heterogeneity was demonstrated with each investigative technique in this study, and while we were unable to produce evidence in the current setting, it seems likely that differences between Fs probably contribute to overall differences in symptomatology.
In this study, participants were recruited from women scheduled for hysterectomy some of whom were taking hormonal medication to reduce symptoms associated with HMB. This medication may have altered gene expression, although the cluster dendrogram analysis (Figure 3) did not show any separation of participants based on hormonal therapy.
Additionally, within the hospital operating lists we were not able to schedule participants at predetermined stages of the menstrual cycle, and with only 6 participants it was not possible to divide into statistically valid cycle stages for subgroup analysis. It is highly likely that with larger numbers and appropriate subgroup analysis for these and other variables, many more genes with significant differences in expression between F, PM, and DM would be identified.
A major limitation of the study was low patient numbers relative to the numbers required for statistically strong bioinformatics analysis of gene array data. In order to better correlate molecular profiles with clinical and ultrasonographic data and to be able to predict features that are associated with HMB, larger groups of both symptomatic and asymptomatic patients are needed. This would be a significant clinical and financial undertaking.
Conclusion
This is the first study that attempts to correlate F-associated clinical features with ultrasound and gene expression data. Despite the small sample size, we were able to show significant differences in angiogenic gene expression between Fs and DM, as well as to a lesser extent PM. While the primary hypothesis that angiogenic gene expression would be increased in tissues and participants that showed increased blood flow by Doppler ultrasound was not proven, we were able to show significant changes in angiogenic gene expression between F and surrounding tissues, with DM and PM having different profiles relative to F. Future studies that correlate clinical symptoms with radiological, cellular, and molecular results are required to allow a better understanding of F heterogeneity, and why some may cause HMB while others do not. The clinical implications of understanding differences between asymptomatic versus symptomatic Fs are significant and may eventually lead to effective treatments that target the source of HMB.
Acknowledgments
Thanks are due to various gynecological surgeons affiliated with Monash Medical Centre who helped with the study, and the Ultrasound Department, Monash Medical Centre, for provision of ultrasound scans.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Health and Medical Research Council (606613 to P.A.W.R and B.V.).
References
- 1.Day Baird D, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–107. [DOI] [PubMed] [Google Scholar]
- 2.Cramer SF, Patel A. The frequency of uterine leiomyomas. Am J Clin Pathol. 1990;94(4):435–438. [DOI] [PubMed] [Google Scholar]
- 3.Buttram VC, Jr, Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981;36(4):433–445. [DOI] [PubMed] [Google Scholar]
- 4.Hartmann KE, Birnbaum H, Ben-Hamadi R, et al. Annual costs associated with diagnosis of uterine leiomyomata. Obstet Gynecol. 2006;108(4):930–937. [DOI] [PubMed] [Google Scholar]
- 5.Lefebvre G, Vilos G, Allaire C, et al. The management of uterine leiomyomas. J Obstet Gynaecol Can. 2003;25(5):396–418. [PubMed] [Google Scholar]
- 6.Farrer-Brown G, Beilby JO, Rowles PM. Microvasculature of the uterus. An injection method of study. Obstet Gynecol. 1970;35(1):21–30. [PubMed] [Google Scholar]
- 7.Makhija D, Mathai AM, Naik R, et al. Morphometric evaluation of endometrial blood vessels. Indian J Pathol Microbiol. 2008;51(3):346–350. [DOI] [PubMed] [Google Scholar]
- 8.Hickey M, Fraser I. Human uterine vascular structures in normal and diseased states. Microsc Res Tech. 2003;60(4):377–389. [DOI] [PubMed] [Google Scholar]
- 9.Parker WH. Uterine myomas: management. Fertil Steril. 2007;88(2):255–271. [DOI] [PubMed] [Google Scholar]
- 10.Faulkner RL. The blood vessels of the myomatous uterus. Am J Obstet Gynaecol. 1944;47:185–197. [Google Scholar]
- 11.Weston G, Trajstman AC, Gargett CE, Manuelpillai U, Vollenhoven BJ, Rogers PA. Fibroids display an anti-angiogenic gene expression profile when compared with adjacent myometrium. Mol Hum Reprod. 2003;9(9):541–549. [DOI] [PubMed] [Google Scholar]
- 12.Sampson J. The influence of myomata on the blood supply of the uterus, with special reference to abnormal uterine bleeding. Surg Gynecol Obstet. 1912;16:144–180. [Google Scholar]
- 13.Tsibris JC, Segars J, Coppola D, et al. Insights from gene arrays on the development and growth regulation of uterine leiomyomata. Fertil Steril. 2002;78(1):114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahn WS, Kim KW, Bae SM, et al. Targeted cellular process profiling approach for uterine leiomyoma using cDNA microarray, proteomics and gene ontology analysis. Int J Exp Pathol. 2003;84(6):267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quade BJ, Wang TY, Sornberger K, Dal Cin P, Mutter GL, Morton CC. Molecular pathogenesis of uterine smooth muscle tumors from transcriptional profiling. Genes Chromosomes Cancer. 2004;40(2):97–108. [DOI] [PubMed] [Google Scholar]
- 16.Arslan AA, Gold LI, Mittal K, et al. Gene expression studies provide clues to the pathogenesis of uterine leiomyoma: new evidence and a systematic review. Hum Reprod. 2005;20(4):852–863. [DOI] [PubMed] [Google Scholar]
- 17.Lee EJ, Kong G, Lee SH, et al. Profiling of differentially expressed genes in human uterine leiomyomas. Int J Gynecol Cancer. 2005;15(1):146–154. [DOI] [PubMed] [Google Scholar]
- 18.Vanharanta S, Wortham NC, Laiho P, et al. 7q deletion mapping and expression profiling in uterine fibroids. Oncogene. 2005;24(43):6545–6554. [DOI] [PubMed] [Google Scholar]
- 19.Zaitseva M, Vollenhoven BJ, Rogers PA. In vitro culture significantly alters gene expression profiles and reduces differences between myometrial and fibroid smooth muscle cells. Mol Hum Reprod. 2006;12(3):187–207. [DOI] [PubMed] [Google Scholar]
- 20.Zaitseva M, Vollenhoven BJ, Rogers PA. Retinoids regulate genes involved in retinoic acid synthesis and transport in human myometrial and fibroid smooth muscle cells. Hum Reprod. 2008;23(5):1076–1086. [DOI] [PubMed] [Google Scholar]
- 21.Bodner-Adler B, Nather A, Bodner K, et al. Expression of thrombospondin 1 (TSP 1) in patients with uterine smooth muscle tumors: an immunohistochemical study. Gynecol Oncol. 2006;103(1):186–189. [DOI] [PubMed] [Google Scholar]
- 22.Behera MA, Feng L, Yonish B, Catherino W, Jung SH, Leppert P. Thrombospondin-1 and thrombospondin-2 mRNA and TSP-1 and TSP-2 protein expression in uterine fibroids and correlation to the genes COL1A1 and COL3A1 and to the collagen cross-link hydroxyproline. Reprod Sci. 2007;14(8 suppl):63–76. [DOI] [PubMed] [Google Scholar]
- 23.Iwahashi M, Muragaki Y. Increased type I and V collagen expression in uterine leiomyomas during the menstrual cycle. Fertil Steril. 2011;95(6):2137–2139. [DOI] [PubMed] [Google Scholar]
- 24.Leppert PC, Catherino WH, Segars JH. A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am J Obstet Gynecol. 2006;195(2):415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chegini N. Proinflammatory and profibrotic mediators: principal effectors of leiomyoma development as a fibrotic disorder. Semin Reprod Med. 2010;28(3):180–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolanska M, Sobolewski K, Bankowski E, Jaworski S. Matrix metalloproteinases of human leiomyoma in various stages of tumor growth. Gynecol Obstet Invest. 2004;58(1):14–18. [DOI] [PubMed] [Google Scholar]
- 27.Bogusiewicz M, Stryjecka-Zimmer M, Postawski K, Jakimiuk AJ, Rechberger T. Activity of matrix metalloproteinase-2 and -9 and contents of their tissue inhibitors in uterine leiomyoma and corresponding myometrium. Gynecol Endocrinol. 2007;23(9):541–546. [DOI] [PubMed] [Google Scholar]
- 28.Jiang Y, Suo G, Sadarangani A, Cowan B, Wang JY. Expression profiling of protein tyrosine kinases and their ligand activators in leiomyoma uteri. Syst Biol Reprod Med. 2010;56(4):318–326. [DOI] [PubMed] [Google Scholar]
- 29.Casey R, Rogers PA, Vollenhoven BJ. An immunohistochemical analysis of fibroid vasculature. Hum Reprod. 2000;15(7):1469–1475. [DOI] [PubMed] [Google Scholar]
- 30.Brauer MM. Cellular and molecular mechanisms underlying plasticity in uterine sympathetic nerves. Auton Neurosci. 2008;140(1-2):1–16. [DOI] [PubMed] [Google Scholar]
- 31.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10(3):165–177. [DOI] [PubMed] [Google Scholar]
- 32.Bagri A, Tessier-Lavigne M, Watts RJ. Neuropilins in tumor biology. Clin Cancer Res. 2009;15(6):1860–1864. [DOI] [PubMed] [Google Scholar]
- 33.Bhuvaneswari R, Gan YY, Lucky SS, et al. Molecular profiling of angiogenesis in hypericin mediated photodynamic therapy. Mol Cancer. 2008;7:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimomura Y, Matsuo H, Samoto T, Maruo T. Up-regulation by progesterone of proliferating cell nuclear antigen and epidermal growth factor expression in human uterine leiomyoma. J Clin Endocrinol Metab. 1998;83(6):2192–2198. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Ohara N, Wang Z, et al. A novel selective progesterone receptor modulator asoprisnil (J867) down-regulates the expression of EGF, IGF-I, TGFbeta3 and their receptors in cultured uterine leiomyoma cells. Hum Reprod. 2006;21(7):1869–1877. [DOI] [PubMed] [Google Scholar]
- 36.Wolanska M, Bankowski E. Fibroblast growth factors (FGF) in human myometrium and uterine leiomyomas in various stages of tumour growth. Biochimie. 2006;88(2):141–146. [DOI] [PubMed] [Google Scholar]
- 37.Liang M, Wang H, Zhang Y, Lu S, Wang Z. Expression and functional analysis of platelet-derived growth factor in uterine leiomyomata. Cancer Biol Ther. 2006;5(1):28–33. [DOI] [PubMed] [Google Scholar]
- 38.Tsai FC, Liu WM, Pai MH, Hsieh MS, Lin JY, Chou CM. Downregulation of the integrin alpha(v) signaling pathway in uterine leiomyomas. Gynecol Obstet Invest. 2011;71(2):129–135. [DOI] [PubMed] [Google Scholar]
- 39.Huyen DV, Bany BM. Evidence for a conserved function of heart and neural crest derivatives expressed transcript 2 in mouse and human decidualization. Reproduction. 2011;142(2):353–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto Y, Olson DM, van Bennekom M, Brindley DN, Hemmings DG. Increased expression of enzymes for sphingosine 1-phosphate turnover and signaling in human decidua during late pregnancy. Biol Reprod. 2010;82(3):628–635. [DOI] [PubMed] [Google Scholar]
- 41.Chen L, Fan R, Huang X, Xu H, Zhang X. Decreased concentrations of pigment epithelium-derived factor in peritoneal fluid of patients with endometriosis. Fertil Steril. 2011;95(5):1798–1800. [DOI] [PubMed] [Google Scholar]
- 42.Dueholm M, Lundorf E, Sorensen JS, Ledertoug S, Olesen F, Laursen H. Reproducibility of evaluation of the uterus by transvaginal sonography, hysterosonographic examination, hysteroscopy and magnetic resonance imaging. Hum Reprod. 2002;17(1):195–200. [DOI] [PubMed] [Google Scholar]