Abstract
We recently demonstrated that co-administration of mouse zona pellucida 3 (mZP3) DNA and protein vaccine enhanced the contraception of mice by increasing humoral immune responses. In this study, we try to use granulocyte–macrophage colony-stimulating factor (GM-CSF) to further improve the humoral immune responses induced by mZP3 DNA and protein co-administration. BALB/c mice were intranasally pre-injected with GM-CSF 4 days before co-administration. Compared to DNA and protein coadministration without GM-CSF, the combination of GM-CSF and coadministration significantly enhances humoral immune responses, especially the level of secretory immunoglobulin A (sIgA) in vaginal washes. The enhanced antibody responses are correlated with the upregulated level of interleukin 4 (IL-4) and enhanced maturation of dendritic cells (DCs). Thus, GM-CSF is a potential candidate adjuvant to be used for the development of a safe and effective contraceptive vaccine.
Keywords: mZP3, GM-CSF, humoral responses, DCs
Introduction
Many efforts were focused on the development of a safe and effective contraceptive vaccine for pest animals or human beings. The zona pellucida (ZP) is composed of 3 sulfated glycoproteins (ZP1, ZP2, and ZP3) that have been proposed as candidates for developing contraceptive vaccines,1–4 especially ZP3 served as the primary ligand for sperm binding. However, inflammatory T cells induced by ZP vaccinations against ovary always accompanies with the deterioration of ovarian functions.5–9 Different approaches were used to develop a vaccine that could avoid cellular responses without impairing humoral responses.10–14 Most recently, we demonstrated that mouse ZP3 (mZP3) DNA and protein coadministration enhanced the contraception without abnormal follicular development, which is correlated with high antibody (Ab) titers.15,16
Here, we try to use granulocyte–macrophage colony-stimulating factor (GM-CSF) to further enhance the humoral immune responses induced by mZP3 DNA and protein coadministration. granulocyte–macrophage CSF is a safe adjuvant and promotes the activation and recruitment of macrophages and dendritic cells (DCs) to the reaction site, which enhances antigen presentation to improve the immunity of different vaccines.17–21 Previous study showed that the timing of GM-CSF expression plasmid administration is critical for the induction of T-helper type (Th1/Th2) response.22 In order to induce high level of Ab responses and avoid Th1 response, GM-CSF plasmid was predelivered 4 days prior to mZP3 DNA and protein coadministration in this study. Since the intranasal coadministration with mZP3 DNA and protein induced both systemic immunoglobulin G (IgG) and mucosal IgA responses,16 the intranasal route was chosen to deliver antigens. To enhance the efficiency of mucosal delivery, a mucolytic agent lysophosphatidylcholine (LPC) and chitosan (as delivery vehicle) were used for mZP3 DNA and protein vaccines.16,23–25 We demonstrated that the combination of GM-CSF and coadministration enhanced both systemic and mucosal humoral immune responses and inhibited cellular immune responses.
Materials and Methods
Recombinant Plasmid Construction and Expression in Vivo
The pcDNA3-mZP3 (pcD-mZP3) and pGEX-4T-1-mZP3 plasmids were constructed according to previous description.15,16 The pcD-mZP3 was digested by restriction enzymes EcoRI and XhoI (TaKaRa, Japan) and mZP3 gene fragments were purified, then subcloned into pVAX1 vector, and designed as p-mZP3. Mouse GM-CSF sequence (coding region of nucleic acids: 1-399; GenBank No.NM_009969) was amplified by reverse transcription–polymerase chain reaction (RT-PCR) from total RNA isolated from the spleen of BALB/c mice using a TRIzol RNA Isolation Kit (Invitrogen, Grand Island, New York). The GM-CSF gene fragments were constructed into pVAX1 vector at EcoRI and BamHI sites by digestions and named as p-GM-CSF.
The expression levels of p-GM-CSF and p-mZP3 were tested by hydrodynamic injection technique.26 Briefly, 25 μg (10 μg/mL) of plasmid was intravenously administered into mouse within 10 seconds with a 21-gauge needle; pVAX1 was used as a negative control. Total RNA was extracted from the liver 7 hours after the injection and the expressions of GM-CSF and mZP3 genes were detected by RT-PCR. RNAs from livers of mice injected with p-GM-CSF or p-mZP3 were used as the templates for PCR to exclude the contamination of DNA. p-GM-CSF and p-mZP3 as the templates for PCR were used as positive controls. The primers used for GM-CSF are sense, 5′-CGGGATCCAGGATGTGGCTGCAGAATTTACTT-3′; antisense, 5′-CGGAATTCTCA AAGGGGATATCAGTCAGAA-3′; the expected fragment is 400 bp. The primers used for mZP3 are sense, 5′-GTTGCCTTGTGGATGGTC-3′; antisense, 5′-GGTGATGTAGA GCGTATTGC-3′; the expected fragment is 130 bp.
Immunization of Mice and Sample Collection
The mZP3 protein were purified as described previously.15 The vaccine formulations were prepared by complexing chitosan (Dalian Xindie Chitin Co, Ltd, Dalian, China) with the plasmid DNA or protein according to the previous formula description.16 These complexes were designated as Chi-pVAX, Chi-p-GM-CSF, and Chi-(p-mZP3 + mZP3), respectively.
Adult female BALB/c mice, 6 to 8 weeks old, purchased from Center for Disease Control and Prevention (Xinjiang, China), were housed under controlled temperature and humidity conditions. All experiments were conducted in compliance with the guidelines of the Chinese Animal Care for Laboratory Animals. All mice were randomly divided into 4 groups (n = 10). Each mouse was pretreated with 10 μL of 0.1% LPC for 1 hour prior to vaccine administration. Mice were intranasally preinjected with Chi-p-GM-CSF 4 days before Chi-(p-mZP3 + mZP3) coadministration as the experimental group. Mice intranasally administered with Chi-pVAX, Chi-p-GM-CSF, and Chi-(p-mZP3 + mZP3) served as the control group (Figure 1). Each mouse received DNA or protein vaccines at 100 μg, respectively, by holding them in an inverted position and pipetted 40 μL encapsulated mixes into nasal cavity and allowed to inhale on day 0 and boosted at 2-week interval for 3 times.
Figure 1.
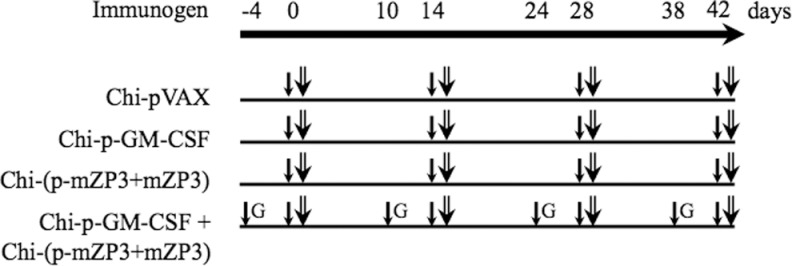
Immunogens and immunization schedules. All mice were pretreated with LPC (↓) for 1 h before each immunization. Mice were immunized with different immunogens (⇐) at 2-week intervals for 4 times. For Chi-p-GM-CSF + Chi-(p-mZP3 + mZP3) group, mice were pre-immunized with Chi-pGM-CSF (G) 4 days before Chi-(p-mZP3 + mZP3) immunization. LPC indicates lysophosphatidylcholine; mZP3, mouse zona pellucida 3; GM-CSF, granulocyte–macrophage colony-stimulating factor.
Serum samples were collected from mice prior to each administration. Vaginal wash samples were collected by washing the vaginal cavities of mice with 150 μL of sterile phosphate-buffered sodium (PBS), and all samples were stored at −20°C.
Assay of Antibodies
The mZP3-specific immunoglobulin G (IgG, IgG1, and IgG2a) and secretory immunoglobulin A (sIgA) in serum and vaginal washes were determined by enzyme-linked immunosorbent assay (ELISA), respectively, according to previous description with some revision.16 Briefly, 96-well plates were coated with mZP3 protein at 2.5 μg/mL in 100 μL/well of 0.05 mol/L bicarbonate buffer and incubated overnight at 4°C, then blocked with 3% bovine serum albumin (BSA). Individual serum sample was diluted at 1:100 and mixture of vaginal washes of all mice in 1 group was diluted at 1:2 with the 1% BSA. Secondary goat anti-mouse IgG and IgA Abs conjugated with horseradish peroxidase (SouthernBiotech, Birmingham, Alabama) diluted at 1:10 000 with the 1% BSA were added into each well. 3,3′,5,5′-tetramethylbenzidine (TMB; Sigma, St Louis, Illinois) was added for the color development. The optical density (OD) values of 450 nm were read by a spectrophotometer (Benchmark, Bio-Rad, Hayward, California) and represented as the Ab responses.
T-Cell Proliferation
Single lymphocyte suspensions were prepared from spleens of the mice on 7 days after the final administration as described previously.16 The splenocytes were cultured in 96-well plate at 3 × 106 cell/well and stimulated with 10 μg/mL mZP3 in vitro for 48 hours and then 20 μL of 5 mg/mL 3-(4, 5-dimethylthiazol-2-yi)-2, 5-diphenyltetrazolium (MTT, Sigma) solution was added. The plate was read at 490 nm by a spectrophotometer (Benchmark, Bio-Rad). Data were expressed as stimulation index (SI), calculated as the mean reading of triplicate wells stimulated with an antigen, and divided by the mean reading of triplicate wells stimulated with the medium. Each group included at least 3 mice. Experiments were carried out 2 or 3 times.
Flow Cytometric Analysis
The following fluorescently labeled anti-mouse monoclonal antibodies (mAbs) were used in this study: anti–interferon (IFN)-γ-phycoerythrin (PE), anti-interleukin (IL)-4-PE, anti–major histocompatibility complex (MHC)-II-PE, anti-CD4-fluoroscein isothiocyanate (FITC), anti-CD11c-FITC, isotype controls, monensin, and Fc-Block were purchased from BD PharMingen (BD Bioscience, California).
T-cells were isolated from mice 7 days after the final immunization and stimulated with mZP3 protein (10 μg/mL) for 5 hours, followed by the addition of the monensin at 2 µg/mL for 8 hours and then blocked with 1 µL of Fc-Block (0.5 µg/mL) for 30 minutes at 4°C. Cell surface staining was performed using CD4-FITC before fixing with 4% paraformaldehyde and the cell membranes were ruptured with 0.1% saponin. The cells were intracellularly stained with IL-4-PE or IFN-γ-PE for 30 minutes at 4°C and analyzed with a FACSCalibur using the Cell Quest Pro Software (BD Bioscience).
For DCs staining, the splenocytes were isolated from spleen of mice on day 5 after the final immunization. Cell surface staining was performed using CD11c-FITC combined with MHC-II-PE.
Statistical Analysis
The experiment data were analyzed using student t test and 1-way analysis of variance (ANOVA) using GraphPad prism 4 software. A value of P < .05 was considered to be statistically significant.
Results
Expression of Recombinant Plasmids in Vivo
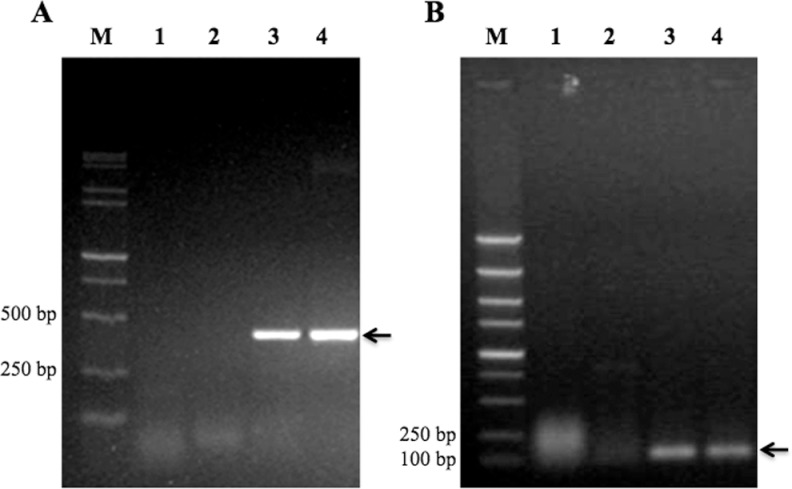
To test whether p-GM-CSF and p-mZP3 can be properly expressed in mice, plasmids were injected into mice by hydrodynamic method. The total RNA was extracted from liver 7 hours after the injection and the expressions of GM-CSF and mZP3 genes were detected by RT-PCR. Results showed that the specific bands for GM-CSF (400 bp) and mZP3 (130 bp) were obtained using complementary DNA (cDNA) from liver of mice injected with p-GM-CSF and p-mZP3, respectively, but not from the liver of mice injected with pVAX1 vector (Figure 2). The results revealed that GM-CSF and mZP3 genes were correctly expressed in the eukaryotic system.
Figure 2.
Analysis of eukaryotic expression of plasmids. Total RNA was extracted from the liver 7 hours after the injection and the expressions of GM-CSF and mZP3 were detected by RT-PCR. A, lane M, 15 000 + 2000 bp DNA marker; lane 1, p-GM-CSF RNA; lane 2, pVAX cDNA; lane 3, p-GM-CSF cDNA; lane 4, p-GM-CSF plasmid. B, lane M, 5000 bp DNA marker; lane 1, p-mZP3 RNA; lane 2, pVAX cDNA; lane 3, p-mZP3 cDNA; lane 4, p-mZP3 plasmid. RT-PCR indicates reverse transcription–polymerase chain reaction; GM-CSF, granulocyte–macrophage colony-stimulating factor; mZP3, mouse zona pellucida 3; cDNA, complementary DNA.
Effect of GM-CSF on Humoral Immune Responses
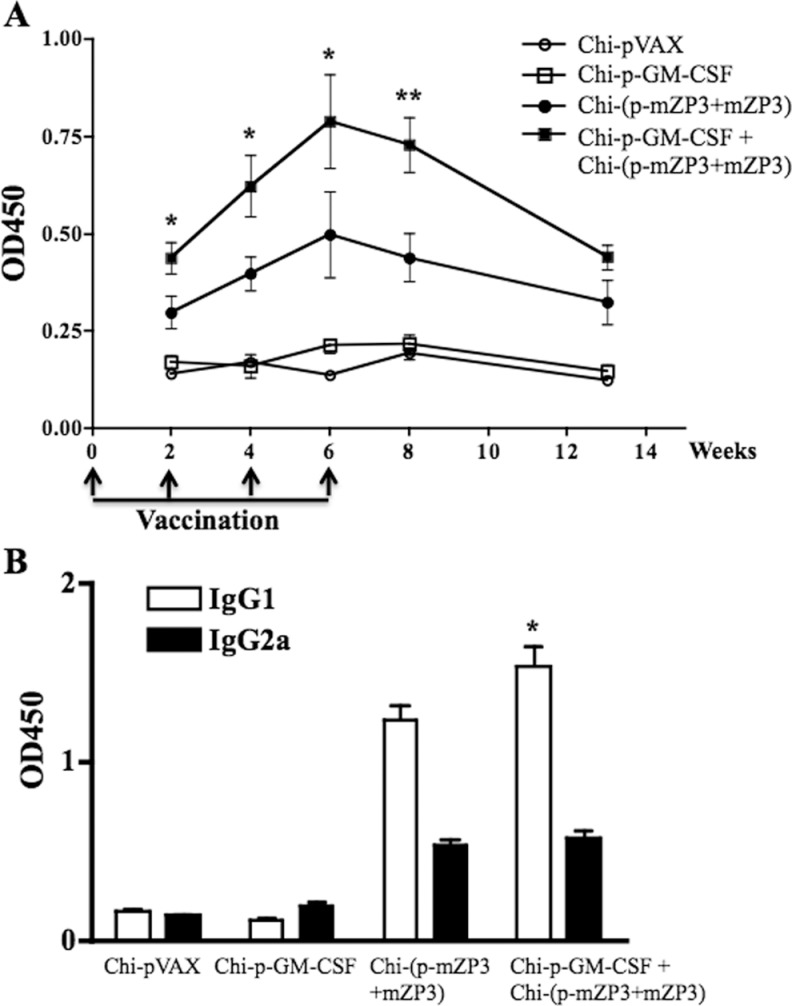
It has been demonstrated that high level of ZP3-specific antibodies (Abs) correlated with the infertility.8,15,16,27 Enzyme-linked immunosorbent assay (ELISA) was used to evaluate the effect of GM-CSF on Ab responses induced by p-mZP3 + mZP3 coadministration. The mZP3-specific IgG in Chi-(p-mZP3 + mZP3) coadministered group was induced after first injection and boosted by the following immunization. The level was significantly higher than Chi-p-GM-CSF and Chi-pVAX groups and reached the highest 2 weeks after third immunization (Figure 3A). The combination of Chi-p-GM-CSF and Chi-(p-mZP3 + mZP3) further enhanced Ab responses compared to Chi-(p-mZP3 + mZP3) coadministered group (Figure 3A). The Ab responses lasted at least 5 weeks after final immunization. The results showed that GM-CSF could enhance humoral immune responses induced by p-mZP3 + mZP3 coadministration.
Figure 3.
Serum IgG antibody responses in immunized mice. Absorbance values are shown for sera diluted at 1:100. A, IgG Ab responses were assessed by enzyme-linked immunosorbent assay (ELISA) in serum collected on 14, 28, 42, 56, and 91 days after first administration. B, The IgG1 and IgG2a Ab isotypes were detected by ELISA in serum collected on day 42 after first administration. Data are reported as mean + SEM. There was a significant difference (*P < .05; **P < .01) at IgG or IgG1 Ab level among Chi-p-GM-CSF + Chi-(p-mZP3 + mZP3) and other groups.IgG indicates immunoglobulin G; GM-CSF, granulocyte–macrophage colony-stimulating factor; Ab, antibody; mZP3, mouse zona pellucida 3; cDNA, complementary DNA; SEM, standard error of the mean.
The subtype of IgG was further analyzed by ELISA. Chi-(p-mZP3 + mZP3) coadministration elicited both IgG1 and IgG2a, but the level of IgG1 was much higher than IgG2a. The combination of Chi-p-GM-CSF and Chi-(p-mZP3 + mZP3) further enhanced IgG1 response induced by Chi-(p-mZP3 + mZP3) coadministration but not for IgG2a (Figure 3B). The result suggested that preinjection of GM-CSF triggered Th2-biased immune response that is potentially advantageous for humoral immunity.
Effect of GM-CSF on Mucosal Immune Responses
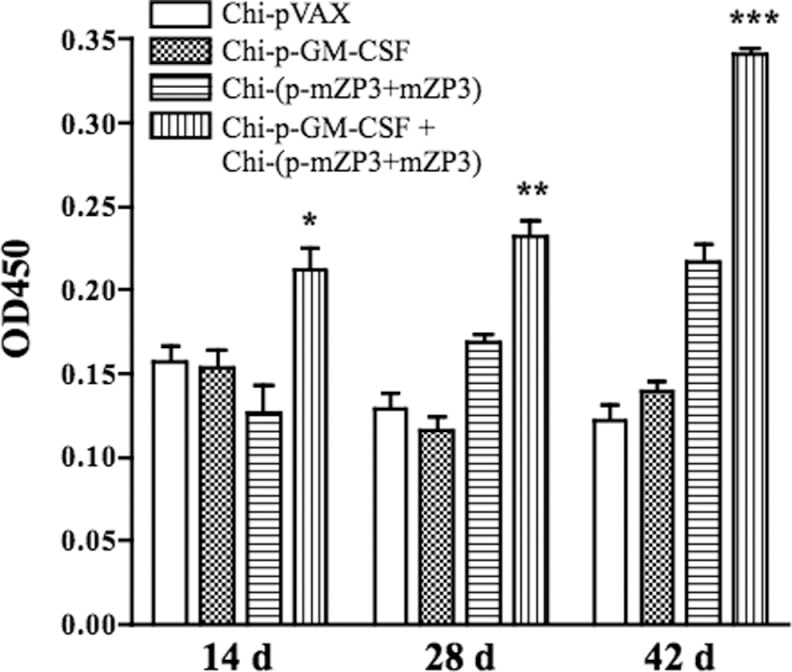
To investigate the adjuvant effect of GM-CSF on the mucosal immune responses, sIgA Ab in vaginal washes was analyzed by ELISA. The sIgA level in the combination of Chi-p-GM-CSF and Chi-(p-mZP3 + mZP3) group was significantly higher than other groups at all time points after first immunization (Figure 4). The results showed that GM-CSF enhanced both systemic and mucosal Ab responses induced by mZP3 DNA and protein coadministration.
Figure 4.
Secretory immunoglobulin A (sIgA) in vaginal washes from immunized mice. Vaginal washes were collected on 14, 28, and 42 days after first administration and mZP3-specific sIgA Ab was determined by ELISA. Secretory IgA level of group coadministered with Chi-p-GM-CSF + Chi-(p-mZP3 + mZP3) was significantly higher than other groups (*P < .05; **P < .01; ***P < .001). ELISA indicates enzyme-linked immunosorbent assay; GM-CSF, granulocyte–macrophage colony-stimulating factor; Ab, antibody; mZP3, mouse zona pellucida 3.
Cellular Immune Responses
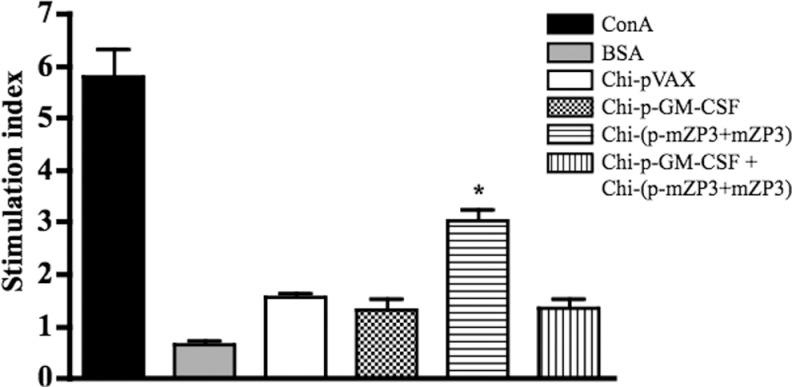
To detect mZP3-specific lymphocyte proliferative responses, splenocytes were isolated from mice 7 days after the final administration and stimulated with mZP3 protein for 48 hours. The mZP3-specific T-cell proliferation was observed in the Chi-(p-mZP3 + mZP3) coadministered group but not in Chi-p-GM-CSF + Chi-(p-mZP3 + mZP3) coadministered group that was similar with other 2 control groups (Figure 5). The results indicated that preinjected GM-CSF inhibited T-cell response induced by p-mZP3 + mZP3 coadministration.
Figure 5.
T-cell proliferation in immunized mice. T cells were isolated from mice on day 7 after the final administration and restimulated in vitro with mZP3 protein as a specific antigen and the proliferation activity was determined by the MTT method. Data were expressed as stimulation index. There was a significant difference (*P < .05) among Chi-(p-mZP3 + mZP3) and other groups. MTT indicates 3-(4, 5-dimethylthiazol-2-yi)-2, 5-diphenyltetrazolium; mZP3, mouse zona pellucida 3.
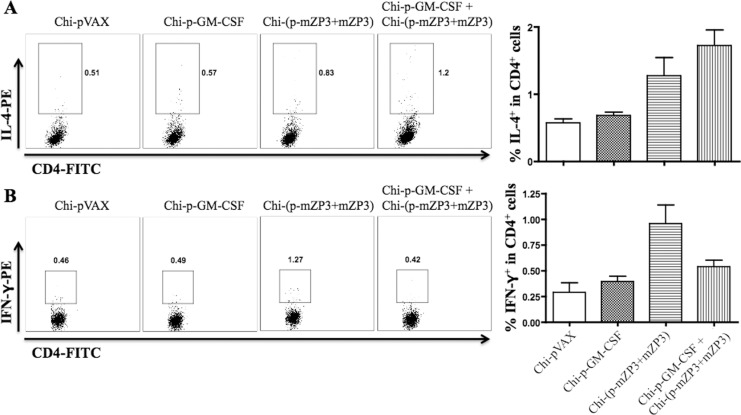
Effect of GM-CSF on Cytokine Production
Antibody analysis showed that preinjected GM-CSF triggered Th2-biased immune responses. To further prove it, the cytokine profiles, such as IFN-γ and IL-4, indicators of Th1- and Th2-like immune response, were detected on day 7 after the final immunization. Percentage of IL-4 in CD4+ T-cells generated by Chi-p-GM-CSF + Chi-(p-mZP3 + mZP3) coadministered group is higher than the other groups (Figure 6A). However, the level of IFN-γ in Chi-p-GM-CSF + Chi-(p-mZP3 + mZP3) group is lower than Chi-(p-mZP3 + mZP3) group (Figure 6B), although they did not reach the statistical significance. The results were consistent with the Ab analysis and T-cell proliferative responses, suggesting that pre-injected GM-CSF triggered Th2-biased responses.
Figure 6.
Analysis of antigen-specific cytokine productions in T-cells by flow cytometric analysis. Intracellular staining for IL-4 (A) and IFN-γ (B) in CD4+ T-cells of mice on day 7 after the final administration was analyzed. The percentage of cytokine-positive cells was showed in each dot plot and the gate was set on CD4+ T-cells. Data shown is a representative (left panel) or summary (right panel) of 3 independent experiments. IL indicates interleukin; IFN-γ, interferon-γ.
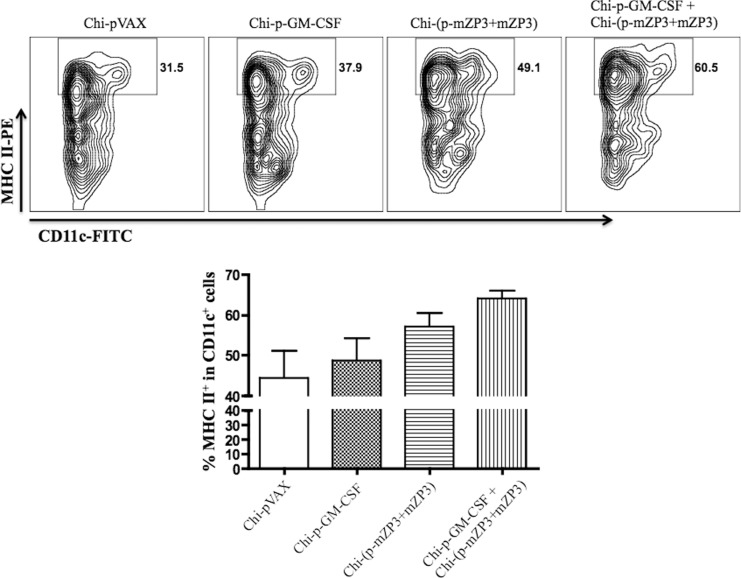
Effect of GM-CSF on DC Maturation
The GM-CSF is a pleiotropic cytokine that can enhance the recruitment of monocytes/macrophages and DCs to inflammatory sites and the activation of antigen-presenting cells (APCs) including upregulation of MHC and CD80/CD86 molecules.19,28 To investigate whether preinjection of GM-CSF can affect the maturation of DCs, splenocytes were isolated on day 5 after final immunization and the maturation of DCs was analyzed by flow cytometric analysis. The levels of MHC-II expression in CD11c+ cells from mice immunized with Chi-p-GM-CSF + Chi-(p-mZP3 + mZP3) or Chi-(p-mZP3 + mZP3) were significantly higher than Chi-pVAX and Chi-p-GM-CSF groups (Figure 7). The combination of Chi-p-GM-CSF and Chi-(p-mZP3 + mZP3) increased the level of MHC-II expression compared with Chi-(p-mZP3 + mZP3) coadministered group, although they did not reach statistical significance (Figure 7). The levels of CD86 expression in all groups were similar (data not shown). The results suggested that GM-CSF, to some extent, enhanced the maturation of DCs to promote the presentation of mZP3 antigen and improve the humoral immune responses.
Figure 7.
Detection of MHC II expression in DCs by flow cytometric analysis. Three mice of each group were sacrificed on day 5 after the final immunization and the expression of MHC II in the CD11c+ cells were analyzed. The percentage of MHC II+ cells was shown in each contour plot and the gate was set on CD11c+ cells. Data shown is a representative (upper panel) or summary (bottom panel) of 3 independent experiments. MHC indicates major histocompatibility complex; DCs, dendritic cells.
Discussion
Recently, we developed a safe and effective contraceptive vaccine using coadministration of mZP3 DNA and protein-based vaccines and the contraception was correlated with high Ab titers.15,16 In this study, we have shown that GM-CSF further enhanced the systemic and mucosal Ab responses induced by mZP3 DNA and protein coadministration. The enhanced humoral responses might be due to the upregulated level of IL-4 and enhanced maturation of DCs. We have also shown that GM-CSF mainly enhanced Th2-biased responses.
Granulocyte–macrophage CSF as an adjuvant promotes the activation and recruitment of APCs to reaction site to enhance antigen presentation and improve the immunity of different vaccines.17–21 Kusakabe et al have shown that the timing of GM-CSF administration is critical for the induction of Th1/Th2 response.22 Considering Ab responses play important roles in contraception,7,9,15,16,29 we preadministered GM-CSF 4 days before mZP3 DNA and protein coadministration, and the timing of GM-CSF administration induced Th2-biased responses, which was consistent with previous results.22 Previous study showed that IgA Ab reactivity in the vaginal tract played a pivotal role in the decrease of pups.30 Both Chi-(p-mZP3 + mZP3) and Chi-p-GM-CSF + Chi-(p-mZP3 + mZP3) administration in mice not only induced high levels of systemic IgG Ab but also produced high levels of local sIgA Ab, but the level of the latter is higher than the former. Granulocyte–macrophage CSF enhanced both systemic and local Ab responses that might cause lower fertility based on the results of our previous studies.15,16
ZP3 contained T-cell epitopes and could induce inflammatory T-cell responses that were correlated with the deterioration of ovarian functions.7,9,15,16,31 Our previous studies showed that coadministration of mZP3 DNA and protein induced humoral and Th2-biased responses, whereas Th1 responses were inhibited.15,16 This strategy caused lower fertility without inflammatory T-cell infiltration into ovary. Here, we showed GM-CSF enhanced humoral and Th2 responses induced by mZP3 DNA and protein coadministration but not for Th1 responses through enhancing the maturation of DCs.
In conclusion, our study demonstrated that the systemic and local Ab responses induced by mZP3 DNA and protein coadministration were enhanced by preadministration of GM-SCF. Further, preadministration of GM-SCF generated Th2 responses and inhibited the inflammatory T-cell responses. The results suggested that GM-CSF might be used as an adjuvant to develop a safe and effective immunocontraceptive vaccine.
Acknowledgments
We would like to thank Dr Ailian Zhang, Nabijan Mattursun, Xinping Li, and Yakupjan Haxim for their assistance in this work.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Natural Science Foundation of China (No 30760136) and animal science Foundation of key discipline of Autonomous Region (No 20101102)
References
- 1.Aitken RJ, Paterson M, van Duin M. The potential of the zona pellucida as a target for immunocontraception. Am J Reprod Immunol. 1996;35(3):175–180. [DOI] [PubMed] [Google Scholar]
- 2.Greenhouse S, Castle PE, Dean J. Antibodies to human ZP3 induce reversible contraception in transgenic mice with ‘humanized’ zonae pellucidae. Hum Reprod. 1999;14(3):593–600. [DOI] [PubMed] [Google Scholar]
- 3.Bleil JD, Wassarman PM. Structure and function of the zona pellucida: identification and characterization of the proteins of the mouse oocyte's zona pellucida. Dev Biol. 1980;76(1):185–202. [DOI] [PubMed] [Google Scholar]
- 4.Shimizu S, Tsuji M, Dean J. In vitro biosynthesis of three sulfated glycoproteins of murine zonae pellucidae by oocytes grown in follicle culture. J Biol Chem. 1983;258(9):5858–5863. [PubMed] [Google Scholar]
- 5.Paterson M, Koothan PT, Morris KD, et al. Analysis of the contraceptive potential of antibodies against native and deglycosylated porcine ZP3 in vivo and in vitro. Biol Reprod. 1992;46(4):523–534. [DOI] [PubMed] [Google Scholar]
- 6.Paterson M, Wilson MR, Morris KD, van Duin M, Aitken RJ. Evaluation of the contraceptive potential of recombinant human ZP3 and human ZP3 peptides in a primate model: their safety and efficacy. Am J Reprod Immunol. 1998;40(3):198–209. [DOI] [PubMed] [Google Scholar]
- 7.Clydesdale G, Pekin J, Beaton S, Jackson RJ, Vignarajan S, Hardy CM. Contraception in mice immunized with recombinant zona pellucida subunit 3 proteins correlates with Th2 responses and the levels of interleukin 4 expressed by CD4+ cells. Reproduction. 2004;128(6):737–745. [DOI] [PubMed] [Google Scholar]
- 8.Jackson RJ, Maguire DJ, Hinds LA, Ramshaw IA. Infertility in mice induced by a recombinant ectromelia virus expressing mouse zona pellucida glycoprotein 3. Biol Reprod. 1998;58(1):152–159. [DOI] [PubMed] [Google Scholar]
- 9.Lloyd ML, Shellam GR, Papadimitriou JM, Lawson MA. Immunocontraception is induced in BALB/c mice inoculated with murine cytomegalovirus expressing mouse zona pellucida 3. Biol Reprod. 2003;68(6):2024–2032. [DOI] [PubMed] [Google Scholar]
- 10.Hasegawa A, Hamada Y, Shigeta M, Koyama K. Contraceptive potential of synthetic peptides of zona pellucida protein (ZPA). J Reprod Immunol. 2002;53(1-2):91–98. [DOI] [PubMed] [Google Scholar]
- 11.Sivapurapu N, Upadhyay A, Hasegawa A, Koyama K, Gupta SK. Native zona pellucida reactivity and in-vitro effect on human sperm-egg binding with antisera against bonnet monkey ZP1 and ZP3 synthetic peptides. J Reprod Immunol. 2002;56(1-2):77–91. [DOI] [PubMed] [Google Scholar]
- 12.Ringleb J, Rohleder M, Jewgenow K. Impact of feline zona pellucida glycoprotein B-derived synthetic peptides on in vitro fertilization of cat oocytes. Reproduction. 2004;127(2):179–186. [DOI] [PubMed] [Google Scholar]
- 13.Borillo J, Coonrod SA, Wu J, Zhou C, Lou Y. Antibodies to two ZP3 B cell epitopes affect zona pellucida assembly. J Reprod Immunol. 2008;78(2):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy CM, Beaton S, Hinds LA. Immunocontraception in mice using repeated, multi-antigen peptides: immunization with purified recombinant antigens. Mol Reprod Dev. 2008;75(1):126–135. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Jin H, Zhang A, Li Y, Wang B, Zhang F. Enhanced contraceptive response by co-immunization of DNA and protein vaccines encoding the mouse zona pellucida 3 with minimal oophoritis in mouse ovary. J Gene Med. 2007;9(12):1095–1103. [DOI] [PubMed] [Google Scholar]
- 16.Zhang A, Li J, Zhao G, et al. Intranasal co-administration with the mouse zona pellucida 3 expressing construct and its coding protein induces contraception in mice. Vaccine. 2011;29(39):6785–6792. [DOI] [PubMed] [Google Scholar]
- 17.Perales MA, Yuan J, Powel S, et al. Phase I/II study of GM-CSF DNA as an adjuvant for a multipeptide cancer vaccine in patients with advanced melanoma. Mol Ther. 2008;16(12):2022–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qing Y, Chen M, Zhao J, et al. Construction of an HBV DNA vaccine by fusion of the GM-CSF gene to the HBV-S gene and examination of its immune effects in normal and HBV-transgenic mice. Vaccine. 2010;28(26):4301–4307. [DOI] [PubMed] [Google Scholar]
- 19.Hirata Y, Egea L, Dann SM, Eckmann L, Kagnoff MF. GM-CSF-facilitated dendritic cell recruitment and survival govern the intestinal mucosal response to a mouse enteric bacterial pathogen. Cell Host Microbe. 2010;7(2):151–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones T, Stern A, Lin R. Potential role of granulocyte-macrophage colony-stimulating factor as vaccine adjuvant. Eur J Clin Microbiol Infect Dis. 1994;13 (suppl 2):S47–S53. [DOI] [PubMed] [Google Scholar]
- 21.Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176(6):1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kusakabe K, Xin KQ, Katoh H, et al. The timing of GM-CSF expression plasmid administration influences the Th1/Th2 response induced by an HIV-1-specific DNA vaccine. J Immunol. 2000;164(6):3102–3111. [DOI] [PubMed] [Google Scholar]
- 23.Illum L, Jabbal-Gill I, Hinchcliffe M, Fisher AN, Davis SS. Chitosan as a novel nasal delivery system for vaccines. Adv Drug Deliv Rev. 2001;51(1-3):81–96. [DOI] [PubMed] [Google Scholar]
- 24.Nishi E, Kume N, Ueno Y, Ochi H, Moriwaki H, Kita T. Lysophosphatidylcholine enhances cytokine-induced interferon gamma expression in human T lymphocytes. Circ Res. 1998;83(5):508–515. [DOI] [PubMed] [Google Scholar]
- 25.Perrin-Cocon L, Agaugue S, Coutant F, et al. Lysophosphatidylcholine is a natural adjuvant that initiates cellular immune responses. Vaccine. 2006;24(9):1254–1263. [DOI] [PubMed] [Google Scholar]
- 26.Sung CM, Yeh CT, Shiau SS, Liang CK, Chang ML. Hydrodynamics-based transfection of the combination of betacellulin and neurogenic differentiation 1 DNA ameliorates hyperglycemia in mice with streptozotocin-induced diabetes. Diabetes Technol Ther. 2011;13:519–525. [DOI] [PubMed] [Google Scholar]
- 27.Hardy CM, ten Have JF, Mobbs KJ, Hinds LA. Assessment of the immunocontraceptive effect of a zona pellucida 3 peptide antigen in wild mice. Reprod Fertil Dev. 2002;14(3-4):151–155. [DOI] [PubMed] [Google Scholar]
- 28.Mellstedt H, Fagerberg J, Osterborg A. Local low-dose of soluble GM-CSF significantly augments an immune response against tumour antigens in man. Eur J Cancer. 1999;35 (suppl 3):S29–S32. [DOI] [PubMed] [Google Scholar]
- 29.Hardy CM, ten Have JF, Pekin J, Beaton S, Jackson RJ, Clydesdale G. Contraceptive responses of mice immunized with purified recombinant mouse zona pellucida subunit 3 (mZP3) proteins. Reproduction. 2003;126(1):49–59. [DOI] [PubMed] [Google Scholar]
- 30.Naz RK, Chauhan SC. Human sperm-specific peptide vaccine that causes long-term reversible contraception. Biol Reprod. 2002;67(2):674–680. [DOI] [PubMed] [Google Scholar]
- 31.Lou YH, Park KK, Agersborg S, Alard P, Tung KS. Retargeting T cell-mediated inflammation: a new perspective on autoantibody action. J Immunol. 2000;164(10):5251–5257. [DOI] [PubMed] [Google Scholar]