Abstract
Dental caries is initiated by demineralization of the tooth surface through acid production from sugar by plaque biofilm. Fluoride and xylitol have been used worldwide as caries-preventive reagents, based on in vitro-proven inhibitory mechanisms on bacterial acid production. We attempted to confirm the inhibitory mechanisms of fluoride and xylitol in vivo by performing metabolome analysis on the central carbon metabolism in supragingival plaque using the combination of capillary electrophoresis and a time-of-flight mass spectrometer. Fluoride (225 and 900 ppm F−) inhibited lactate production from 10% glucose by 34% and 46%, respectively, along with the increase in 3-phosphoglycerate and the decrease in phosphoenolpyruvate in the EMP pathway in supragingival plaque. These results confirmed that fluoride inhibited bacterial enolase in the EMP pathway and subsequently repressed acid production in vivo. In contrast, 10% xylitol had no effect on acid production and the metabolome profile in supragingival plaque, although xylitol 5-phosphate was produced. These results suggest that xylitol is not an inhibitor of plaque acid production but rather a non-fermentative sugar alcohol. Metabolome analyses of plaque biofilm can be applied for monitoring the efficacy of dietary components and medicines for plaque biofilm, leading to the development of effective plaque control.
Keywords: metabolome analysis, supragingival plaque, sugar metabolism, fluoride, xylitol, dental caries
Introduction
Fluoride and xylitol have been used worldwide as representative caries- preventive reagents. Fluoride inhibits demineralization and promotes remineralization of the tooth surface (ten Cate, 1999; Fejerskov, 2004). Fluoride is also known to inhibit bacterial acid production in vitro (Hamilton, 1990; Marquis, 1990; Jenkins, 1999) and plaque acid production in vivo (Tatevossian, 1990; Vogel et al., 2002). Competitive inhibition by fluoride of enolase, an enzyme in the Embden-Meyerhof-Parnas pathway (the EMP pathway) extracted from Streptococcus mutans and other plaque bacteria (Kaufmann and Bartholmes, 1992; Guha-Chowdhury et al., 1997), suggested that enolase is the target enzyme by fluoride inhibition. The addition of fluoride to a cell suspension of S. mutans, S. sobrinus, and S. sanguinis results in the intracellular accumulation of 3-phosphoglycerate and 2-phosphoglycerate (enolase substrate) and the decrease of phosphoenolpyruvate (enolase product) in the EMP pathway (Hata et al., 1990; Maehara et al., 2005), confirming the inhibition of enolase by fluoride; however, the inhibitory mechanism of fluoride has not been confirmed in plaque biofilm, consisting of multispecies of bacteria, where they may behave differently compared with in vitro behavior.
Xylitol is a non-fermentative sugar alcohol, and thus does not cause dental caries, similar to other sugar alcohols. Xylitol is known to repress acid production from glucose by S. mutans through the inhibition of glycolytic enzymes by xylitol 5-phosphate (X5P) produced from xylitol by a constitutive phosphoenolpyruvate:fructose phosphotransferase system (Assev and Rölla, 1984; Trahan et al., 1985; Trahan, 1995; Miyasawa et al., 2003). Further, X5P is dephosphorylated and returned to xylitol, resulting in the formation of a “futile cycle”, an energy-wasting cycle, and the subsequent repression of growth of S. mutans (Pihlanto-Leppälä et al., 1990; Trahan et al., 1991). Although it is true that xylitol does not cause dental caries, the inhibitory mechanism of xylitol has not been confirmed in plaque biofilm in vivo.
Metabolome analysis is the evaluation of biological systems for changes in endogenous metabolites through comprehensive metabolite profiling. Recently, the combination of capillary electrophoresis and time-of-flight mass spectrometry (CE-MS) has been developed for separation and quantification of metabolites of the central carbon metabolism, including the EMP pathway, the pentose-phosphate pathway, and the tricarbonic acid cycle (the TCA cycle) (Edwards et al., 2006; Monton and Soga, 2007; Timischl et al., 2008; Ramautar et al., 2009). In a previous study, CE-MS was used to analyze the metabolome in a small amount of supragingival plaque in vivo (Takahashi et al., 2010).
In the present study, we attempted to confirm the inhibitory mechanisms of fluoride and xylitol on the central carbon metabolism of supragingival plaque in vivo, by performing metabolome analysis after oral rinsing with fluoride and xylitol.
Materials & Methods
Supragingival Plaque Sampling
After informed consent was obtained, six males and one female (age, 24.6 ± 4.6 yrs) were asked to refrain from toothbrushing and to allow dental plaque to accumulate overnight. The volunteers were periodontally healthy, with 0.14 ± 0.38 decayed teeth (DT), and had not taken any antibiotics recently or currently. After confirming that the volunteers had not consumed any food for at least 2 hrs, we collected all the available supragingival plaque, using sterilized toothpicks, mainly from marginal and interproximal areas of the right or left half of the dentition. Immediately, plaque samples were weighed and mixed with 0.80 mL ice-cold methanol containing internal standards (Internal standard-1; Human Metabolome Technologies, Tsuruoka, Japan) and sonicated for 30 sec (55W, US-1R; AS ONE Corporation, Osaka, Japan). About 10 mg wet weight of plaque was collected from half of the dentition. Internal standard-1 contains methionine sulfone and camphor-10-sulfonic acid for calibration of the quantification of MS. The volunteers were asked to rinse with 10 mL of 10 mM glucose, 10 mM xylitol, or a mixture of 10 mM xylitol plus 10 mM glucose for 60 sec, and after 10 min, supragingival plaque was collected from the other half of the dentition and treated as described above. For the fluoride trial, the volunteers were asked to rinse with 10 mL sodium fluoride (225 or 900 ppm F−) for 60 sec, and supragingival plaque was collected and treated as described above. After 10 min, the volunteers were asked again to rinse with 10 mM glucose (10 mL) for 60 sec, and after 10 min, supragingival plaque was collected and treated as described above. Each volunteer rinsed with 10 mM glucose, 10 mM xylitol, 10 mM xylitol plus 10 mM glucose solution, 225 ppm NaF, and 900 ppm NaF only once with an interval of 1 wk.
The plaque samples were mixed with 0.80 mL chloroform and 0.32 mL Milli-Q water by being vortexed for 30 sec and were then centrifuged. The aqueous layer was ultrafiltrated (Ultrafree-MC 5000NMWL UFC3 LCCNB; Millipore, Billerica, MA, USA), dried for 6 to 9 hrs, suspended in Milli-Q water containing internal standards (Internal standard-3; Human Metabolome Technologies), and stored at −80°C until analysis. Internal standard-3 contains trimesic acid and 3-hydroxynaphthalene-2,7-disulfonic acid for calibration of the retention time for CE.
CE-MS Conditions
CE-MS was performed by capillary electrophoresis (G1600AX; Agilent Technologies, Waldbronn, Germany) with time-of-flight mass spectrometry (G1969A; Agilent Technologies), as described previously (Takahashi et al., 2010). A fused silica capillary (H3305-2002), sheath liquid (H3301-1020), and electrolytes (H3302-1021) were used for analysis (Human Metabolome Technologies). The applied voltage was set at +30 kV, electrospray ionization was operated in the negative ion mode, and the capillary voltage was set at 3.5 kV. The flow rate of heated dry nitrogen gas (300oC) was monitored at 7 L/min. All standard metabolites and chemicals used were of analytical or reagent grade. The quantitative error of the CE-MS was less than 10%.
We analyzed data by calculating software (MassHunter WorkStation Software Qualitative Analysis; Agilent Technologies) using data obtained from standard metabolite solutions. All metabolites included in the central carbon metabolism were identified and quantified, except glyceraldehyde 3-phosphate, 2-phosphoglycerate (the EMP pathway), xylulose 5-phosphate (the pentose-phosphate pathway), and X5P, whose standards were not available. Lactate was also quantified, while acetate and formate were not quantified, because their mass-to-charge ratio (m/z) values are too small for the CE-MS.
X5P Quantification
X5P was synthesized from xylitol by enzymatic reaction of a phosphoenolpyruvate:fructose phosphotransferase system, with toluene-permeabilized cells of S. mutans. S. mutans NCTC 10449 was grown in complex media containing 1.7% tryptone, 0.3% yeast extract, and 0.5% glucose at pH 7 and 37°C under anaerobic conditions (10% H2, 10% CO2, 80% N2). The cells were harvested by centrifugation, washed twice with PPB solution (2 mM potassium phosphate buffer [pH 7.0] containing 150 mM KCl and 5 mM MgCl2), and stored as pellets at −20°C until use. The cell pellets were thawed, suspended in PPB solution at an optical density of 1 at 660 nm, and vortexed with 0.01 vol of toluene for 1 min. After centrifugation, the cells were suspended in the same buffer. The reaction mixture for X5P synthesis contained 60 mM xylitol, 1 mM phosphoenolpyruvate, 0.1 mM NADH, 11 U/mL lactate dehydrogenase, and the cells in PPB solution. The reaction was started with the addition of xylitol, and the decrease in NADH, which corresponds to the amount of X5P produced, was monitored photometrically at 340 nm and 37°C for 10 to 20 min. The concentration of X5P was determined by photometric calculation with the mM extinction coefficient of NADH. The photometric decrease at 340 nm was corrected by NADH oxidase activity, which oxidized NADH to NAD independently of the phosphoenolpyruvate: fructose phosphotransferase system. The reaction was terminated by the addition of 0.1 vol of 6.6 N perchloric acid and kept at 4°C overnight. The perchloric acid mixture was neutralized with 5 M potassium carbonate and used as a standard of X5P for CE-MS analysis. X5P was identified by CE-MS mass signal (m/z value) estimated from the structure formula of X5P, and was confirmed by a decrease in the signal of X5P after incubation with alkaline phosphatase, which converts X5P to xylitol.
Statistical Analysis
Differences in the amounts of metabolites between resting plaque and plaque collected after the rinses were evaluated by the paired t test. P-value was adjusted from 0.05 to 0.002 based on the Bonferroni correction for multiple comparisons. Differences in the amounts of lactate were also evaluated by the paired t test. P-value was adjusted from 0.05 to 0.017.
Results
Effects of Fluoride and Xylitol on Metabolome Profile and Lactate Production in Supragingival Plaque
In the resting supragingival plaque, most metabolites in the central carbon metabolism were detected, except for erythrose 4-phosphate in the pentose phosphate pathway and cis-acconitate and isocitrate in the TCA cycle (Fig. 1A). After the glucose rinse, pyruvate in the EMP pathway increased significantly. Glucose 6-phosphate increased, and 3-phosphoglycerate and phosphoenolpyruvate decreased, although these changes were insignificant. After the fluoride rinse, 3-phosphoglycerate in the EMP pathway increased significantly (Fig. 1B). Glucose 6-phosphate increased, while phosphoenolpyruvate and pyruvate decreased, although these changes were significant. After the glucose rinse after fluoride application, glucose 6-phosphate, fructose 6-phosphate, fructose 1,6-bisphosphate, and 3-phosphoglycerate in the EMP pathway were increased significantly (Fig. 1C). The metabolome profile after the xylitol rinse was similar to that of resting plaque except for the detection of X5P (Fig. 1D), while the metabolome profile after the rinse with the xylitol-glucose mixture was similar to that after the glucose rinse except for the detection of X5P (Fig. 1E).
Figure 1.
Effects of fluoride and xylitol on metabolome profile of supragingival plaque. (A) Glucose rinse (hatched box) and resting plaque (open box); (B) fluoride rinse (950 ppm F−) (hatched box) and resting plaque (open box); (C) fluoride-glucose rinse (hatched box) and resting plaque (open box); (D) xylitol rinse (hatched box) and resting plaque (open box); and (E) xylitol-glucose rinse (hatched box) and resting plaque (open box). Values are the mean of seven individuals. Vertical bar, standard deviation. Significant difference from resting plaque (*p < 0.002).
Lactate was produced after the glucose rinse, regardless of the presence of xylitol, while lactate production was repressed significantly by the fluoride rinse prior to glucose in a dose-dependent manner (Table). The xylitol rinse did not increase lactate, which was similar to that in the resting plaque.
Table.
The Amount of Lactate in Supragingival Plaque after Glucose Rinse, Glucose Rinse with Fluoride Application, and Xylitol-Glucose Rinse
| Oral Rinse Component | Lactate Concentration (nmol/mg wet weight of plaque) |
|---|---|
| Glucose | 45.3 ± 21.7a |
| 225 ppm Fluoride + Glucose | 29.9 ± 11.6* |
| 900 ppm Fluoride + Glucose | 24.4 ± 12.5* |
| Xylitol + Glucose | 41.1 ± 17.8 |
| Xylitol | 4.21 ± 2.90 |
| No addition (resting plaque) | 4.89 ± 6.20 |
Mean ± standard deviation.
Significant difference from the amount of lactate after glucose rinse (p < 0.017).
Changes in Metabolome Profile in the Presence of Fluoride and Xylitol
Ratios of metabolite amounts after the glucose rinse with fluoride application or the xylitol-glucose rinse to metabolite amounts after the glucose rinse (Fig. 2) show the changes in the metabolome profile more clearly. In the presence of fluoride, glucose 6-phosphate, fructose 6-phosphate, fructose 1,6-bisphosphate, and 3-phosphoglycerate in the EMP pathway and 6-phosphogluconate and sedoheptulose 7-phosphate in the pentose phosphate pathway increased, while dihydroxyacetone phosphate, phosphoenolpyruvate, and pyruvate in the EMP pathway decreased (Fig. 2A). These changes became more evident as the concentration of fluoride increased. Inhibitory steps by fluoride, expected from these results, are indicated in the metabolic pathways (Fig. 3). In contrast, there were no clear changes in the presence of xylitol (Fig. 2B). There were also no clear changes in the TCA cycle, in the presence of either fluoride or xylitol (data not shown).
Figure 2.
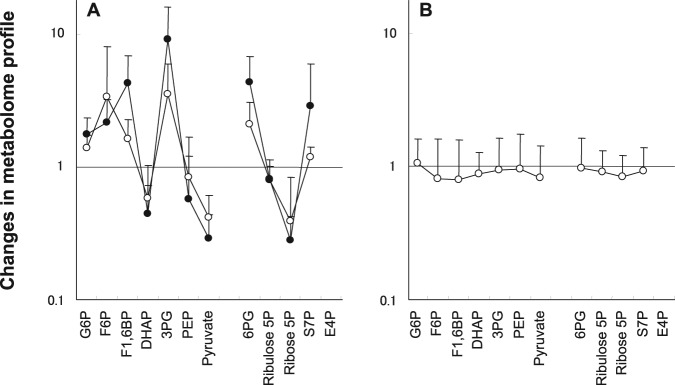
Changes in metabolome profile. (A) Fluoride-glucose rinse, 10% glucose rinse after application of fluoride containing 225 ppm F− (open circle) or 900 ppm F− (closed circle); and (B) xylitol-glucose rinse, 10% xylitol rinse with 10% glucose. The rate of change was calculated as (amount of metabolite after glucose rinse with fluoride application or xylitol-glucose rinse) / (amount of metabolite after glucose rinse). Values are the mean of seven individuals. Vertical bar, standard deviation. See Fig. 1 for abbreviations of metabolites.
Figure 3.
Expected inhibitory steps in the central carbon metabolism, including the EMP pathway, the pentose phosphate pathway, and the TCA cycle. Metabolites framed by solid lines increased in the presence of fluoride; metabolites in gray boxes decreased in the presence of fluoride; metabolites framed by broken lines showed no or low detection; and metabolites in italics were not detectable by the CE-MS system in the present study. Metabolic reactions catalyzed by (1) enolase (2PG←→PEP), (2) aldolase (F1,6BP←→DHAP + G3P), (3) glyceraldehyde 3-phosphate dehydrogenase (G3P←→1,3BPG), and (4) phosphoglycerate kinase (1,3BPG,←→.3PG). G3P, glyceraldehyde 3-phosphate; 1,3BPG, 1,3-bisphosphoglycerate; 2PG, 2-phosphoglycerate. See Fig. 1 for abbreviations of other metabolites.
Discussion
The glucose rinse resulted in lactate production in supragingival plaque, with an increase of metabolites upstream and a decrease of metabolites downstream (except pyruvate) of the EMP pathway (Fig. 1A), as reported previously (Takahashi et al., 2010).
The fluoride-glucose rinse promoted the intracellular accumulation of 3-phosphoglycerate (Fig. 1C) along with decreased lactate production (Table), and the amount of 3-phosphoglycerate was higher than that after the glucose rinse (Fig. 2A). These observations indicate the existence of an inhibitory step at enolase in vivo, which catalyzes the conversion of 2-phosphoglycerate to phosphoenolpyruvate [(1) in Fig. 3], as reported in vitro in Streptococcus mutans and Streptococcus sanguinis (Hata et al., 1990; Maehara et al., 2005). In addition, the oral rinse with only fluoride resulted in a similar metabolome profile, except for pyruvate (Fig. 1B). This suggests that supragingival plaque metabolized carbohydrates derived from saliva and bacteria-stored polysaccharides slowly and constantly, and that fluoride inhibited the metabolism and subsequently provided a metabolome profile similar to that after the fluoride-glucose rinse (Fig. 1C). This inhibition by fluoride on the glycolysis may repress bacterial growth over the long term.
In the presence of fluoride, an increase in glucose 6-phosphate, fructose 6-phosphate, and fructose 1,6-bisphosphate and a slight decrease in dihydroxyacetone phosphate were observed (Fig. 2A), suggesting the inhibition of aldolase, glyceraldehyde 3-phosphate dehydrogenase, and/or phosphoglycerate kinase in the EMP pathway [(2)-(4) in Fig. 3], although there is no report on this type of inhibition in Streptococcus mutans and Streptococcus sanguinis (Hata et al., 1990; Maehara et al., 2005). Further study is needed to clarify this phenomenon by investigating other plaque bacteria. The increase in 6-phosphogluconate and the decrease in ribulose 5-phosphate and ribose 5-phosphate in the presence of fluoride (Fig. 2A) suggest the inhibition of 6-phosphogluconate dehydrogenase, an enzyme in the pentose phosphate pathway (Fig. 3), which may influence bacterial growth, because this pathway is essential to supply NADPH for fatty acid synthesis and pentose phosphates for nucleotide synthesis.
In the present study, glucose was rinsed 10 min after fluoride application, and acid production was inhibited significantly. The effectiveness of the fluoride rinse on acid production of supragingival plaque is still controversial (Giertsen et al., 1999), since the preservation and release of fluoride in supragingival plaque and on the tooth surface have not been elucidated well in vivo. However, it is possible that fluoroapatite can release fluoride in supragingival plaque (Harper and Loesche, 1986), and a recent study revealed that fluoride is preserved in supragingival plaque, and the preservation can be enhanced by pre-rinsing with calcium ions (Vogel et al., 2008), suggesting that a fluoride rinse might be more effective on acid production from supragingival plaque by pre-rinsing with calcium compounds.
Xylitol had no effect on the metabolome profile and lactate production from glucose of supragingival plaque in vivo (Figs. 1E, 2B, and Table). X5P was detected at a significant level only after a rinse with xylitol or a xylitol-glucose mixture, as previously reported (Assev et al., 1996), supporting that X5P is produced via a bacterial phosphoenolpyruvate-dependent phosphotransferase system (Assev and Rölla, 1984; Trahan et al., 1985; Trahan, 1995). The decrease of 3-phosphoglycerate and phosphoenoly-pyruvate (substrates of the phosphoenolpyruvate-dependent phosph-otransferase system) after the xylitol rinse (Fig. 1D) suggests the involvement of this system in X5P formation. However, the presence of X5P does not seem to influence glucose fermentation by supragingival plaque in vivo. This might be because dental plaque covering the clinically healthy tooth surface contains only a small number of mutans streptococci (Bowden et al., 1975; Nyvad and Killian, 1990), whose acid production and growth are inhibited by X5P. Another possibility is that X5P is not as effective in vivo as in vitro. Thus, it seems that the role of xylitol in caries prevention is as a non-fermentative sugar substitute, since no lactate production from xylitol and no effect of xylitol on the metabolome profile (Fig. 1D) were observed in the present study.
The present study revealed that metabolome analyses can detect metabolic regulation in supragingival plaque in vivo. In vivo effects of fluoride on supragingival plaque sugar metabolism and acid production were basically consistent with those previously reported as in vitro data obtained from representative oral bacteria. However, the metabolome analyses in the present study suggest an additional inhibitory mechanism of fluoride on plaque bacteria, and that xylitol is not an inhibitor of plaque acid production but rather is a non-fermentative sugar alcohol. Metabolome analyses of plaque biofilm can be applied for monitoring the efficacy of dietary components and medicines on plaque biofilm, leading to the development of effective plaque control.
Footnotes
This study was supported by Grants-in-Aid for Scientific Research B (19390539 and 22390399), JSPS, Japan, and by Research and Education Funding for the Inter-University Research Project (2007-2011), MEXT, Japan.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Assev S, Rölla G. (1984). Evidence for presence of a xylitol phosphotransferase system in Streptococcus mutans OMZ 176. Acta Pathol Microbiol Immunol Scand B 92:89-92 [DOI] [PubMed] [Google Scholar]
- Assev S, Wåler SM, Rølla G. (1996). Xylitol fermentation by human dental plaque. Eur J Oral Sci 104(Pt 1):359-362 [DOI] [PubMed] [Google Scholar]
- Bowden GH, Hardie JM, Slack GL. (1975). Microbial variations in approximal dental plaque. Caries Res 9:253-277 [DOI] [PubMed] [Google Scholar]
- Edwards JL, Chisolm CN, Shackman JG, Kennedy RT. (2006). Negative mode sheathless capillary electrophoresis electrospray ionization-mass spectrometry for metabolite analysis of prokaryotes. J Chromatogr A 1106:80-88 [DOI] [PubMed] [Google Scholar]
- Fejerskov O. (2004). Changing paradigms in concepts on dental caries: consequences for oral health care. Caries Res 38:182-191 [DOI] [PubMed] [Google Scholar]
- Giertsen E, Emberland H, Scheie AA. (1999). Effects of mouth rinses with xylitol and fluoride on dental plaque and saliva. Caries Res 33:23-31 [DOI] [PubMed] [Google Scholar]
- Guha-Chowdhury N, Clark AG, Sissons CH. (1997). Inhibition of purified enolases from oral bacteria by fluoride. Oral Microbiol Immunol 12:91-97 [DOI] [PubMed] [Google Scholar]
- Hamilton IR. (1990). Biochemical effects of fluoride on oral bacteria. J Dent Res 69(Spec Iss):660-667 [DOI] [PubMed] [Google Scholar]
- Harper DS, Loesche WJ. (1986). Inhibition of acid production from oral bacteria by fluorapatite-derived fluoride. J Dent Res 65:30-33 [DOI] [PubMed] [Google Scholar]
- Hata S, Iwami Y, Kamiyama K, Yamada T. (1990). Biochemical mechanisms of enhanced inhibition of fluoride on the anaerobic sugar metabolism by Streptococcus sanguis. J Dent Res 69:1244-1247 [DOI] [PubMed] [Google Scholar]
- Jenkins GN. (1999). Review of fluoride research since 1959. Arch Oral Biol 44:985-992 [DOI] [PubMed] [Google Scholar]
- Kaufmann M, Bartholmes P. (1992). Purification, characterization and inhibition by fluoride of enolase from Streptococcus mutans DSM 320523. Caries Res 26:110-116 [DOI] [PubMed] [Google Scholar]
- Maehara H, Iwami Y, Mayanagi H, Takahashi N. (2005). Synergistic inhibition by combination of fluoride and xylitol on glycolysis by mutans streptococci and its biochemical mechanism. Caries Res 39:521-528 [DOI] [PubMed] [Google Scholar]
- Marquis RE. (1990). Diminished acid tolerance of plaque bacteria caused by fluoride. J Dent Res 69(Spec Iss):672-675 [DOI] [PubMed] [Google Scholar]
- Miyasawa H, Iwami Y, Mayanagi H, Takahashi N. (2003). Xylitol inhibition of anaerobic acid production by Streptococcus mutans at various pH levels. Oral Microbiol Immunol 18:215-219 [DOI] [PubMed] [Google Scholar]
- Monton MR, Soga T. (2007). Metabolome analysis by capillary electrophoresis-mass spectrometry. J Chromatogr A 1168:237-246 [DOI] [PubMed] [Google Scholar]
- Nyvad B, Kilian M. (1990). Comparison of the initial streptococcal microflora on dental enamel in caries-active and in caries-inactive individuals. Caries Res 24:267-272 [DOI] [PubMed] [Google Scholar]
- Pihlanto-Leppälä A, Söderling E, Mäkinen KK. (1990). Expulsion mechanism of xylitol 5-phosphate in Streptococcus mutans. Scand J Dent Res 98:112-119 [DOI] [PubMed] [Google Scholar]
- Ramautar R, Somsen GW, de Jong GJ. (2009). CE-MS in metabolomics. Electrophoresis 30:276-291 [DOI] [PubMed] [Google Scholar]
- Takahashi N, Washio J, Mayanagi G. (2010). Metabolomics of supragingival plaque and oral bacteria. J Dent Res 89:1383-1388 [DOI] [PubMed] [Google Scholar]
- Tatevossian A. (1990). Fluoride in dental plaque and its effects. J Dent Res 69(Spec Iss):645-652 [DOI] [PubMed] [Google Scholar]
- ten Cate JM. (1999). Current concepts on the theories of the mechanism of action of fluoride. Acta Odontol Scand 57:325-329 [DOI] [PubMed] [Google Scholar]
- Timischl B, Dettmer K, Kaspar H, Thieme M, Oefner PJ. (2008). Development of a quantitative, validated capillary electrophoresis-time of flight-mass spectrometry method with integrated high- confidence analyte identification for metabolomics. Electrophoresis 29:2203-2214 [DOI] [PubMed] [Google Scholar]
- Trahan L. (1995). Xylitol: a review of its action on mutans streptococci and dental plaque—its clinical significance. Int Dent J 45(1 Suppl 1):77S-92S [PubMed] [Google Scholar]
- Trahan L, Bareil M, Gauthier L, Vadeboncoeur C. (1985). Transport and phosphorylation of xylitol by a fructose phosphotransferase system in Streptococcus mutans. Caries Res 19:53-63 [DOI] [PubMed] [Google Scholar]
- Trahan L, Néron S, Bareil M. (1991). Intracellular xylitol-phosphate hydrolysis and efflux of xylitol in Streptococcus sobrinus. Oral Microbiol Immunol 6:41-50 [DOI] [PubMed] [Google Scholar]
- Vogel GL, Zhang Z, Chow LC, Schumacher GE. (2002). Changes in lactate and other ions in plaque and saliva after a fluoride rinse and subsequent sucrose administration. Caries Res 36:44-52 [DOI] [PubMed] [Google Scholar]
- Vogel GL, Schumacher GE, Chow LC, Takagi S, Carey CM. (2008). Ca pre-rinse greatly increases plaque and plaque fluid F. J Dent Res 87: 466-469 [DOI] [PMC free article] [PubMed] [Google Scholar]




