Abstract
The synthesis of six cyclic depsipeptoids inspired by the natural depsipeptide sansalvamide A is described. An efficient and fast synthetic strategy was developed using a combination of consecutive isocyanide-based multicomponent reactions (Ugi and Passerini reactions). This methodology can be used to access a variety of cyclic oligodepsipeptoids.
Keywords: depsipeptoids, multicomponent reactions, Passerini reaction, sansalvamide A, Ugi reaction
Introduction
Peptoids are an interesting class of non-natural compounds that have recently received much attention due to their wide range of biological activities, which makes them attractive candidates for drug discovery [1–7]. This family of oligomers comprising poly-N-substituted glycines mimics the primary natural structure of peptides and exhibits greater proteolytic stability and increased cellular permeabilities in comparison to peptides [5–7]. A powerful synthetic tool for the preparation of a peptoid backbone is the Ugi four-component reaction (U-4CR) [8–14]. It has been demonstrated that the combination of multicomponent reactions with the use of microwave irradiation is able to efficiently produce complex molecules with a reduced number of steps and short reaction times [15–18].
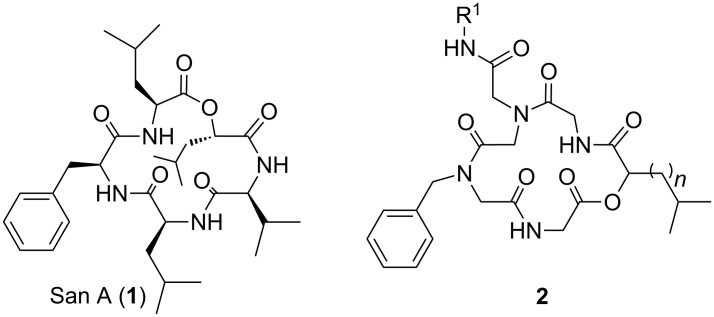
Depsipeptides are polymeric natural compounds, analogues of peptides, being formed by amino acids and hydroxy acids linked together by amide and ester bonds. These natural products show promising biological activities, especially regarding their therapeutic potential in cancer treatment [19]. An example of a cyclic depsipeptide is sansalvamide A (San A, Figure 1) [20–29], which was isolated from a marine fungus (Fusarium spp.) [20] and exhibits antitumor activity against multiple cancer cell lines. It is cytotoxic against colon (HCT-116) [20,23,25–26], pancreatic (S2-013 and AsPC-1) [22,28–29], prostate (PC-3), breast (MDA-MB231) and melanoma cancers (WM-115) [24]. It has been reported that substitution of an ester group by an amide in the structure of a peptide provides an efficient way to evaluate the role of protein hydrogen bonding [30–34]. Recent works have discovered that some analogues of San A inhibit Hsp 90, a key protein that enables many proteins involved in tumor progression [35–39].
Figure 1.
Sansalvamide A (1) and its depsipeptoid analogues (2).
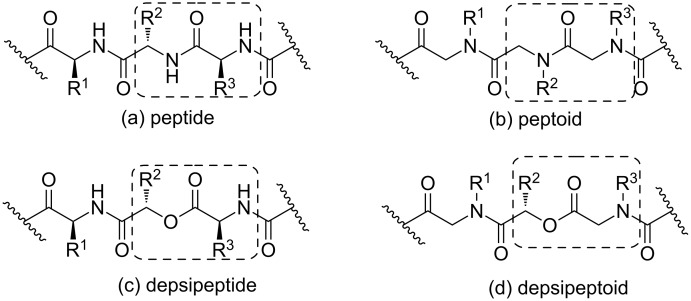
The Passerini three-component reaction (P-3CR) allows an easy access to depsipeptides using a convergent approach. It has become a powerful tool in combinatorial synthesis [40–43] and can be used strategically for the synthesis of depsipeptoids. By analogy to peptides and peptoids, a depsipeptoid would be a peptoid bearing an ester group instead of an amide group. Differences between peptide, peptoid, depsipeptide and depsipeptoid structures are outlined in Figure 2.
Figure 2.
Generic structures of (a) peptide, (b) peptoid, (c) depsipeptide and (d) depsipeptoid.
Results and Discussion
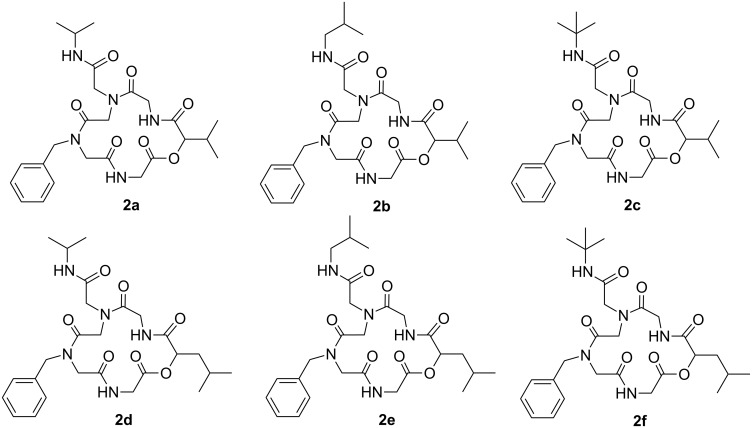
In continuing our research on the synthesis of peptoids with potential pharmacological activity [12,17–18,44–45] and using a fast and efficient microwave-assisted synthesis of peptoids [15,17–18], we decided to carry out the synthesis of depsipeptoid analogues of San A based on a strategy developed in our groups for the synthesis of cyclic RGD pentapeptoids [44]. This strategy was adapted by a combination of microwave-assisted Ugi and Passerini reactions. It is also important to highlight that the synthesis of cyclic depsipeptoids had not been explored yet. In this paper, we describe the synthesis of six pentadepsipeptoid analogues of San A (Figure 3).
Figure 3.
Structures of six pentadepsipeptoid analogues of San A.
The synthetic route for the synthesis of cyclic pentadepsipeptoids via consecutive Ugi reactions allows only three side chains connected to three nitrogen atoms. The pentapeptide of San A has in its structure five side chains attached to the α carbon atoms: one isopropyl, one benzyl and three isobutyl groups. To generate the pentapeptoid analogues of San A, the side chain groups isobutyl, isopropyl and benzyl linked to the α carbon atom present in the peptide were moved to the nitrogen atoms. It was decided to keep at least one benzyl group in the structure of the peptoids and vary the isopropyl and isobutyl groups, thus maintaining a greater similarity with the structure of the San A depsipeptide.
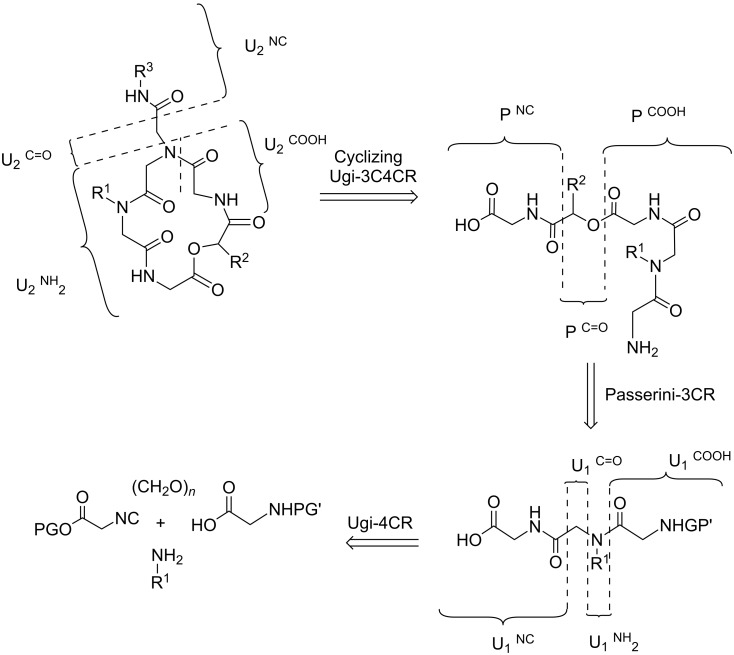
The retrosynthetic analysis of the depsipeptoids (Scheme 1) shows that the proposed compounds can be achieved using a strategy based on: (a) formation of a peptoid via Ugi reaction; (b) ester hydrolysis; (c) formation of an acyclic depsipeptoid scaffold through a Passerini reaction; (d) deprotection of the amine/acid groups and (e) a macrocyclization step via an intramolecular Ugi reaction.
Scheme 1.
Retrosynthetic analysis of the cyclic depsipeptoids.
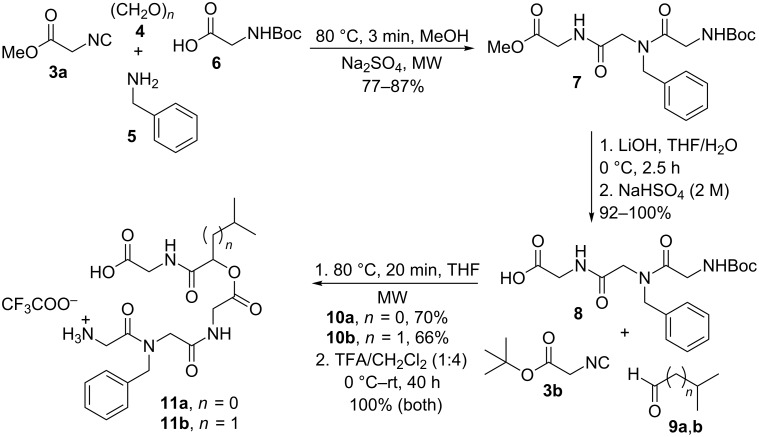
The general strategy for the synthesis of cyclic pentadepsipeptoids is depicted in Scheme 2. The synthesis of analogues 2 was initiated by an Ugi 4-component reaction (U-4CR) using methyl isocyanoacetate (3a), paraformaldehyde (4), benzylamine (5) and N-Boc-glycine (6) in MeOH (Scheme 2) in a microwave (MW) reactor (3 min at 80 °C) to provide the peptoid 7 in 77–87% yield. Peptoid 7 was subjected to hydrolysis in the presence of LiOH (THF/H2O, 0 °C, 2.5 h) followed by treatment with 2 M NaHSO4 providing the corresponding acid 8 in 92–100% yield. Acid 8 was then employed in a Passerini reaction with isobutyraldehyde (9a) or isovaleraldehyde (9b) and tert-butyl isocyanoacetate (3b), in a MW reactor (20 min at 80 °C) in THF, affording the acyclic depsipeptoids 10a and 10b in 70% and 66% yield, respectively. Removal of the Boc protecting group and ester hydrolysis were achieved after treatment of the acyclic depsipeptoids 10a,b with TFA in CH2Cl2, giving the corresponding amino acids 11a,b as TFA salt in quantitative yields.
Scheme 2.
Synthesis of acyclic depsipeptoids 11a,b.
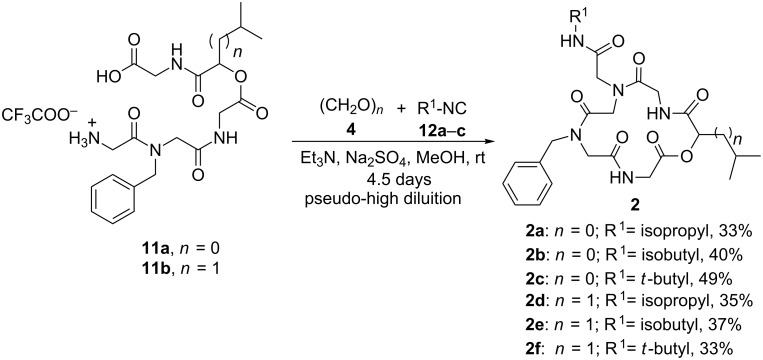
In the last step, the depsipeptoid amino acid salts 11a,b were subjected to an Ugi three-component four-center reaction (U-3C4CR) (Scheme 3). Compounds 11a,b were added under pseudo-high dilution conditions (addition rate: 0.6 mL/h; addition time: 83 h; concentration: 0.80 mmol/L) to a suspension of paraformaldehyde 4, triethylamine, sodium sulfate and isopropyl/isobutyl/tert-butyl isocyanide 12a–c in methanol to yield the target cyclic pentadepsipeptoids 2a–f after purification by column chromatography (yields ranged from 33–49% depending on the substrate). The structures of the final products obtained are shown in Figure 3.
Scheme 3.
Synthesis of macrocycles 2a-f.
Conclusion
In summary, the approach developed herein allows the synthesis of a wide range of cyclic depsipeptoids. Different structures can be obtained by changes in the amine component in the first Ugi reaction, in the carbonyl component in the Passerini reaction or in the isocyanide and carbonyl components in the macrocyclization step. The general route and procedure developed allows an easy access to complex molecules with a significantly reduced number of steps in short reaction times, and high yields in most of the steps. The strategic combination of two isocyanide-based multicomponent reactions and microwave irradiation makes this a very useful and attractive protocol. The obtained depsipeptoids will be tested for different biological activities.
Supporting Information
General procedures, NMR and mass spectra of all compounds.
Acknowledgments
The authors thank the Instituto de Química, Universidade de Brasília, FINEP-CT INFRA nº 970/01, CAPES, CNPq, and DAAD for financial support.
This article is part of the Thematic Series "Multicomponent reactions II" and is dedicated to Prof. Ronaldo A. Pilli on the occasion of his 60th anniversary.
References
- 1.Wessjohann L A, Andrade C K Z, Vercillo O E, Rivera D G. Targets Heterocycl Syst. 2006;10:24–53. [Google Scholar]
- 2.Wessjohann L A, Rhoden C R B, Rivera D G, Vercillo O E. Top Heterocycl Chem. 2010;23:199–226. doi: 10.1007/7081_2009_25. [DOI] [Google Scholar]
- 3.Messeguer J, Masip I, Montolio M, del Rio J A, Soriano E, Messeguer A. Tetrahedron. 2010;66:2444–2454. doi: 10.1016/j.tet.2010.01.090. [DOI] [Google Scholar]
- 4.Fowler S A, Blackwell H E. Org Biomol Chem. 2009;7:1508–1524. doi: 10.1039/b817980h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller S M, Simon R J, Ng S, Zuckermann R N, Kerr J M, Moos W H. Bioorg Med Chem Lett. 1994;4:2657–2662. doi: 10.1016/S0960-894X(01)80691-0. [DOI] [Google Scholar]
- 6.Wender P A, Mitchell D J, Pattabiraman K, Pelkey E T, Steinman L, Rothbard J B. Proc Natl Acad Sci U S A. 2000;97:13003–13008. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwon Y-U, Kodadek T. J Am Chem Soc. 2007;129:1508–1509. doi: 10.1021/ja0668623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wessjohann L A, Rivera D G, Vercillo O E. Chem Rev. 2009;109:796–814. doi: 10.1021/cr8003407. [DOI] [PubMed] [Google Scholar]
- 9.Dömling A. Chem Rev. 2006;106:17–89. doi: 10.1021/cr0505728. [DOI] [PubMed] [Google Scholar]
- 10.Dömling A, Wang W, Wang K. Chem Rev. 2012;112:3083–3135. doi: 10.1021/cr100233r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dömling A, Beck B, Eichelberger U, Sakamuri S, Menon S, Chen Q-Z, Lu Y, Wessjohann L A. Angew Chem, Int Ed. 2006;45:7235–7239. doi: 10.1002/anie.200601259. Erratum: Angew. Chem., Int. Ed. 2007,46, 2347–2348. doi:10.1002/anie.200790053. [DOI] [PubMed] [Google Scholar]
- 12.Pando O, Stark S, Denkert A, Porzel A, Preusentanz R, Wessjohann L. J Am Chem Soc. 2011;133:7692–7695. doi: 10.1021/ja2022027. [DOI] [PubMed] [Google Scholar]
- 13.Rivera D G, León F, Concepción O, Morales F E, Wessjohann L A. Chem–Eur J. 2013;19:6417–6428. doi: 10.1002/chem.201201591. [DOI] [PubMed] [Google Scholar]
- 14.Neves Filho R A W, Stark S, Westermann B, Wessjohann L A. Beilstein J Org Chem. 2012;8:2085–2090. doi: 10.3762/bjoc.8.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barreto A F S, Vercillo O E, Birkett M A, Caulfied J C, Wessjohann L A, Andrade C K Z. Org Biomol Chem. 2011;9:5024–5027. doi: 10.1039/c1ob05471f. [DOI] [PubMed] [Google Scholar]
- 16.Barreto A F S, Vercillo O E, Andrade C K Z. J Braz Chem Soc. 2011;22:462–467. doi: 10.1590/S0103-50532011000300008. [DOI] [Google Scholar]
- 17.Barreto A F S. Reações Multicomponentes de Isocianetos Consecutivas Assistidas por Micro-ondas: Síntese de Ciclopeptóides e Ciclodepsipeptóides Análogos da Verticilida e Sansalvamida A. Brazil: Universidade de Brasília; 2013. [Google Scholar]
- 18.Barreto A F S, Vercillo O E, Wessjohann L A, Andrade C K Z. Blucher Chem Proc. 2011;1:298. http://blucherproceedings.com.br/articles/download/2095. [Google Scholar]
- 19.Hamel E, Covell D G. Curr Med Chem: Anti-Cancer Agents. 2002;2:19–53. doi: 10.2174/1568011023354263. [DOI] [PubMed] [Google Scholar]
- 20.Belofsky G N, Jensen P R, Fenical W T. Tetrahedron Lett. 1999;40:2913–2916. doi: 10.1016/S0040-4039(99)00393-7. [DOI] [Google Scholar]
- 21.Lee Y, Silverman R B. Org Lett. 2000;2:3743–3746. doi: 10.1021/ol0002830. [DOI] [PubMed] [Google Scholar]
- 22.Gu W, Liu S, Silverman R B. Org Lett. 2002;4:4171–4174. doi: 10.1021/ol0269392. [DOI] [PubMed] [Google Scholar]
- 23.Carroll C L, Johnston J V C, Kekec A, Brown J D, Parry E, Cajica J, Medina I, Cook K M, Corral R, Pan P-S, et al. Org Lett. 2005;7:3481–3484. doi: 10.1021/ol051161g. [DOI] [PubMed] [Google Scholar]
- 24.Liu S, Gu W, Lo D, Ding X-Z, Ujiki M, Adrian T E, Soff G A, Silverman R B. J Med Chem. 2005;48:3630–3638. doi: 10.1021/jm048952t. [DOI] [PubMed] [Google Scholar]
- 25.Otrubova K, Styers T J, Pan P-S, Rodriguez R, McGuire K L, McAlpine S R. Chem Commun. 2006:1033–1034. doi: 10.1039/b517434a. [DOI] [PubMed] [Google Scholar]
- 26.Styers T J, Kekec A, Rodriguez R, Brown J D, Cajica J, Pan P-S, Parry E, Carroll C L, Medina I, Corral R, et al. Bioorg Med Chem. 2006;14:5625–5631. doi: 10.1016/j.bmc.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez R, Pan P-S, Pan C-M, Ravula S, Lapera S, Singh E, Styers T J, Brown J D, Cajica J, Parry E, et al. J Org Chem. 2007;72:1980–2002. doi: 10.1021/jo061830j. [DOI] [PubMed] [Google Scholar]
- 28.Pan P-S, McGuire K L, McAlpine S R. Bioorg Med Chem Lett. 2007;17:5072–5077. doi: 10.1016/j.bmcl.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 29.Davis M R, Styers T J, Rodriguez R A, Pan P-S, Vasko R C, McAlpine S R. Org Lett. 2008;10:177–180. doi: 10.1021/ol702403r. [DOI] [PubMed] [Google Scholar]
- 30.Seebach D, Mahajan Y R, Senthilkumar R, Rueping M, Jaun B. Chem Commun. 2002:1598–1599. doi: 10.1039/b204187c. [DOI] [PubMed] [Google Scholar]
- 31.Aravinda S, Shamala N, Das C, Balaram P. Biopolymers. 2002;64:255–267. doi: 10.1002/bip.10192. [DOI] [PubMed] [Google Scholar]
- 32.Haque T S, Little J C, Gellman S H. J Am Chem Soc. 1996;118:6975–6985. doi: 10.1021/ja960429j. [DOI] [Google Scholar]
- 33.Yang X, Wang M, Fitzgerald M C. Bioorg Chem. 2004;32:438–449. doi: 10.1016/j.bioorg.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Powers E T, Deechongkit S, Kelly J W. Adv Protein Chem. 2005;72:39–78. doi: 10.1016/S0065-3233(05)72002-7. [DOI] [PubMed] [Google Scholar]
- 35.Vasko R C, Rodriguez R A, Cunningham C N, Ardi V C, Agard D A, McAlpine S R. ACS Med Chem Lett. 2010;1:4–8. doi: 10.1021/ml900003t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sellers R P, Alexander L D, Johnson V A, Lin C-C, Savage J, Corral R, Moss J, Slugocki T L, Singh E K, Davis M R, et al. Bioorg Med Chem. 2010;18:6822–6856. doi: 10.1016/j.bmc.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ardi V C, Alexander L D, Johnson V A, McAlpine S R. ACS Chem Biol. 2011;6:1357–1366. doi: 10.1021/cb200203m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kunicki J B, Petersen M N, Alexander L D, Ardi V C, McConnell J R, McAlpine S R. Bioorg Med Chem Lett. 2011;21:4716–4719. doi: 10.1016/j.bmcl.2011.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis M R, Singh E K, Wahyudi H, Alexander L D, Kunicki J B, Nazarova L A, Fairweather K A, Giltrap A M, Jolliffe K A, McAlpine S R. Tetrahedron. 2012;68:1029–1051. doi: 10.1016/j.tet.2011.11.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berłożecki S, Szymanski W, Ostaszewski R. Tetrahedron. 2008;64:9780–9783. doi: 10.1016/j.tet.2008.07.064. [DOI] [Google Scholar]
- 41.Gulevich A V, Shpilevaya I V, Nenajdenko V G. Eur J Org Chem. 2009:3801–3808. doi: 10.1002/ejoc.200900330. [DOI] [Google Scholar]
- 42.Leon F, Rivera D G, Wessjohann L A. J Org Chem. 2008;73:1762–1767. doi: 10.1021/jo7022125. [DOI] [PubMed] [Google Scholar]
- 43.Henze M, Kreye O, Brauch S, Nitsche C, Naumann K, Wessjohann L A, Westermann B. Synthesis. 2010:2997–3003. doi: 10.1055/s-0030-1258182. [DOI] [Google Scholar]
- 44.Vercillo O E, Andrade C K Z, Wessjohann L A. Org Lett. 2008;10:205–208. doi: 10.1021/ol702521g. [DOI] [PubMed] [Google Scholar]
- 45.Neves Filho R A W, Westermann B, Wessjohann L A. Beilstein J Org Chem. 2011;7:1504–1507. doi: 10.3762/bjoc.7.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
General procedures, NMR and mass spectra of all compounds.