Abstract
Objective
Children who are less fit reportedly have lower performance on tests of cognitive control and differences in brain function. This study examined the effect of an exercise intervention on brain function during two cognitive control tasks in overweight children.
Design and Methods
Participants included 43 unfit, overweight (BMI ≥ 85th percentile) children 8- to 11-years old (91% Black), who were randomly divided into either an aerobic exercise (n = 24) or attention control group (n = 19). Each group was offered a separate instructor-led after-school program every school day for 8 months. Before and after the program, all children performed two cognitive control tasks during functional magnetic resonance imaging (fMRI): antisaccade and flanker.
Results
Compared to the control group, the exercise group decreased activation in several regions supporting antisaccade performance, including precentral gyrus and posterior parietal cortex, and increased activation in several regions supporting flanker performance, including anterior cingulate and superior frontal gyrus.
Conclusions
Exercise may differentially impact these two task conditions, or the paradigms in which cognitive control tasks were presented may be sensitive to distinct types of brain activation that show different effects of exercise. In sum, exercise appears to alter efficiency or flexible modulation of neural circuitry supporting cognitive control in overweight children.
Introduction
One third of school-age children in the US are overweight or obese (1). The increase in obesity has been concomitant with decreased fitness (2), with potential implications for cognition and brain function. Lower-fit and overweight children show worse cognitive performance, including lower academic achievement and cognitive control (CC), than their fitter or leaner peers in several studies (3,4), although others have shown no relationship (5). In adults, it may be the case that fitness training benefits CC more than other types of cognition (6).
CC reportedly varies between children of different fitness levels, an issue which may be optimally evaluated by functional magnetic resonance imaging (fMRI) studies. Existing neuroimaging investigations of fitness often use flanker tasks, which require response selection within the context of response-congruent or -incongruent information (7). In one cross-sectional study, activation was investigated during an incongruent vs. congruent condition of a flanker task in higher- and lower-fit children who were matched on performance (8). Higher-fit children showed lower activation in pre-frontal cortex (PFC), anterior cingulate cortex (ACC), and superior parietal lobule (SPL), consistent with greater efficiency. Another study using the flanker task compared higher- and lower-fit children on performance and activation across time (9). Lower-fit children showed a decline in incongruent accuracy across task blocks. Higher-fit children maintained accuracy across task blocks and had higher PFC, ACC, and parietal activation early on in the incongruent condition with lower activation later (though activation did not decrease to below that of the lower-fit group). This pattern suggests improved adaptation of neural recruitment among the high-fit.
To determine whether exercise plays a causal role in altering brain function, randomized controlled trials are necessary. Randomized controlled trials using fMRI, however, are rare. In one of the few extant studies, older adults randomly assigned to an exercise group showed improved task performance and altered brain function compared to controls (10). The exercise group increased activation on a flanker incongruent vs. congruent condition in attentional regions (middle frontal gyrus [MFG], superior frontal gyrus [SFG], and SPL), but reduced activation in a region associated with conflict monitoring (ACC). Given the involvement of PFC and other regions that develop later in children, CC is potentially more responsive to intervention at a younger age (11). A recent study of 8- to 9-year-old children demonstrated that those assigned to physical activity for 9 months showed decreased brain activation in right anterior PFC compared to those in a waitlist control group on an incongruent flanker condition compared to fixation (12). An earlier study by our group randomized 222 overweight children 7- to 11-years old to a 3-month exercise intervention, which showed cognitive and fitness benefits (13). Twenty children provided fMRI data, with results showing that children assigned to exercise decreased posterior parietal cortex and increased PFC activation during an antisaccade task (a CC task) compared to controls.
In the current study it was hypothesized that exercise training would improve performance and alter brain activation in overweight children during CC task performance (antisaccade and flanker tasks). This study built upon our previous neuroimaging work by: (1) enhancing the sample size (N = 20 to N = 43), (2) extending the exercise intervention (3-8 months), and (3) including a robust attention-control group rather than a no-intervention control condition. If exercise training affects the brain during childhood, when circuitry and performance are rapidly changing, developmental trajectories potentially could be altered with positive long-term consequences.
Methods
Participants
Participants were recruited from public schools around Augusta, GA and were eligible if they were 8- to 11-years old, overweight (BMI ≥ 85th percentile (1)), and inactive (no regular physical activity program ≥ 1 h week–1). Participants in the current imaging study (powered to detect differences between groups on change in brain activation) are a subsample of participants from a larger exercise intervention study. Exclusions included any medical condition that would limit physical activity or affect study results (including neurological or psychiatric disorders). Children and parents completed written informed assent and consent in accordance with the Human Assurance Committee of Georgia Regents University. Each child's parent or guardian reported the child's age, sex, race, and health status. Parents also reported their own educational attainment, which was used as an index of socioeconomic status (1 = grade 7 or less; 2 = grades 8-9; 3 = grades 10-11; 4 = high school graduate; 5 = partial college; 6 = college graduate; 7 = post-graduate). The schools from which study participants were drawn report 80% of their students were eligible for free or reduced cost school meals (SD = 14%), with a range of 51-98%. The study took place at the Georgia Prevention Center at Georgia Regents University.
FMRI data were collected for 54 children at baseline and 43 children at post-test. Of the 11 participants lost after baseline, 4 refused to participate before randomization, 5 dropped out after randomization (1 from the exercise group, 4 from the control group), 1 refused to participate in post-test MRI (from the control group), and 1 was ruled out based on a neurological anomaly observed in the MRI scan (from the control group). Twenty-five children in the exercise group provided fMRI data at baseline and 24 at post-test. Twenty-five children in the control group provided fMRI data at baseline and 19 at post-test. The 6 children who participated in at least a portion of the study but refused to provide post-test MRI data did not significantly differ from the participants who provided post-test data in any of the baseline characteristics (variables listed in Table 1), or in baseline activation in any of the clusters that were significant in the group analysis (listed in Table 3). Participants were included in analyses only if they had both baseline and post-test MRI data, resulting in a total of 43 participants (exercise group n = 24, control group n = 19). Characteristics of the sample are provided in Table 1.
TABLE 1.
Baseline characteristics of participants
| Exercise | Control | |
|---|---|---|
| n | 24 | 19 |
| Age (years) | 9.7 (0.8) | 9.9 (0.9) |
| Female | 71% | 58% |
| Black | 92% | 90% |
| Left-handed | 4% | 16% |
| Parental education scale | 5.0 (1.1) | 4.7 (1.2) |
| Cognitive assessment system; | 94.0 (6.8) | 93.2 (12.0) |
| Full Scale standard score | ||
| BMI z-score | 1.91 (0.42) | 1.93 (0.57) |
| Overweight | 17% | 42% |
| Obese | 83% | 58% |
| Body fat | 36.9% (6.6) | 35.3% (7.7) |
| VO2 peak (ml/kg/min) | 27.5 (4.6) | 28.7 (4.9) |
| Needs improvement | 4% | 0% |
| Needs improvement; health risk | 96% | 100% |
Mean (SD), or percent.
TABLE 3.
fMRI results: significant clusters in the whole-brain group by time interaction analyses
| Anatomical location | Center of mass (x,y,z in MNI coordinates) | Volume (voxels) | Direction | |
|---|---|---|---|---|
| Antisaccade | ||||
| 1. Bilateral ACC, extending into R medial frontal gyrus | –10.9, –44.4, -–1.9 | 81 | Ex | < C |
| 2. R precentral gyrus, extending into R IFG, postcentral gyrus, SPL, IPL, and insula | –57.5, 1.5, 22.5 | 91 | Ex | < C |
| 3. L precentral gyrus, extending into L postcentral gyrus | 59.8, 5.3, 27.3 | 51 | Ex | <C |
| 4. R postcentral gyrus, extending into R precentral gyrus, IFG, IPL, and insula | –47.8, 23.4, 46.3 | 62 | Ex | <C |
| 5. R SPL | –32.7, 50.8, 68.0 | 35 | Ex | <C |
| 6. L precentral gyrus, extending into bilateral medial frontal gyrus and paracentral lobule and L postcentral gyrus, SPL, precuneus, and IPL Incongruent vs. fixation | 20.4, 27.9, 69.5 | 418 | Ex | <C |
| 7. L superior temporal gyrus, extending into L insula | 57.5, 4.2, –3.4 | 55 | Ex | > C |
| 8. L medial frontal gyrus, extending into L SFG, MFG, and cingulate gyrus Incongruent vs. congruent | 21.6, –33.9, 30.6 | 153 | Ex | > C |
| 9. R ACC, extending into R SFG, MFG, cingulate gyrus, and medial frontal gyrus | – 23.5, – 29.5, 26.1 | 86 | Ex | >C |
| 10. L MFG, extending into L SFG, medial frontal gyrus, IFG, insula, cingulate gyrus, and ACC | 26, –19.7, 27.7 | 225 | Ex | >C |
R = right hemisphere and L = left hemisphere. MNI = Montreal Neurological Institute. ACC = anterior cingulate cortex. IFG = inferior frontal gyrus. SPL = superior parietal lobule. IPL = inferior parietal lobule. SFG = superior frontal gyrus. MFG = middle frontal gyrus.
Cognitive and health measures
The Cognitive Assessment System (CAS), a standardized individual assessment of children's cognitive processes, was administered (14). The Full Scale score of the CAS takes into account the four scales (Planning, Attention, Simultaneous and Successive processing), each of which include three subtests. A number of health measures also were collected at baseline and post-test. Body fat was measured with a dual-energy X-ray absorptiometry (DXA) scan using a Hologic Discovery W (Hologic, Bedford, MA). VO2 peak was measured with an aerobic fitness treadmill test (Modified Balke Protocol for Poorly Fit Children; (15)). The test used a Cardiac Science TM65 treadmill (Bothell, WA) with a Parvo Medics TrueOne 2400 Metabolic Measurement System (Sandy, UT). The proportion of each group in each Fitness Zone was also obtained according to current Fitnessgram criteria for children 10 and older.
Intervention
Participants were assigned randomly to one of two conditions: aerobic exercise or a sedentary attention control. Randomization (balanced by race, sex, and school to avoid imbalances on factors linked with differences in achievement) was performed by the study statistician and concealed until after baseline testing was completed, at which point the study coordinator informed the families. Both groups were offered an after school program every school day for ~8 months (average number of days offered = 138, SD = 9). All participants were transported by bus daily after school to the Georgia Prevention Center where they spent half an hour on supervised homework time and were provided with a snack. Lead instructors were rotated between the two groups every 2 weeks and research assistants were rotated between the two groups every week. Both groups could earn points that were redeemed for small prizes weekly for performing desired behaviors. The reward schedule was periodically calibrated to keep the rewards offered to the groups similar.
The groups differed in that they participated in either an aerobic exercise or an attention control program. The aerobic exercise group engaged in instructor-led aerobic activities (e.g., tag and jump rope) for 40 min per day. They wore heart rate monitors every day (S610i; Polar Electro, Oy, Finland) with which they could monitor their own performance and from which data were collected daily. Points in the exercise group were earned for an average daily heart rate above 150 beats per minute, with more points for higher average heart rates. Participants in the exercise group had an average heart rate of 161 beats per minute (SD = 9) at the intervention. The attention control group engaged in instructor-led sedentary activities (e.g., art and board games). Points in the control group were earned for participation and good behavior.
Data collection and analysis
MRI parameters
Images were acquired at Georgia Regents University on a GE Signa Excite HDx 3 Tesla MRI system (General Electric Medical Systems, Milwaukee, WI). For all MRI scans, head positions were stabilized with a vacuum pillow and/or foam padding. High-resolution T1-weighted structural brain images were acquired using a 3D FSPGR protocol (repetition time (TR) = 9.436 ms, echo time (TE) = 3.876 ms, flip angle = 20° , field of view (FOV) = 240 × 240 mm2, acquisition matrix = 512 × 512, 120 contiguous axial slices, slice thickness = 1.3 mm, total scan time = 3 min, 33 s). For functional scans, a single blocked antisaccade run (TR = 3,000 ms, TE = 35 ms, flip angle = 90° , FOV = 240 × 240 mm2, acquisition matrix = 128 × 128, 30 interleaved axial slices, slice thickness = 4 mm (skip 1 mm), 104 volumes) was always followed by two event-related flanker runs (each: TR = 2500 ms, TE = 35 ms, flip angle = 90° , FOV = 240 × 240 mm2, acquisition matrix = 128 × 128, 30 interleaved axial slices, slice thickness = 4 mm (skip 1 mm), 168 volumes per run). For each functional run, 4 volumes were acquired and discarded before stimulus presentation began to allow for scanner stabilization. Each functional run was collected obliquely, with the slices aligned to the superior margin of the participant's anterior commissure and the inferior margin of the posterior commissure.
Antisaccade task
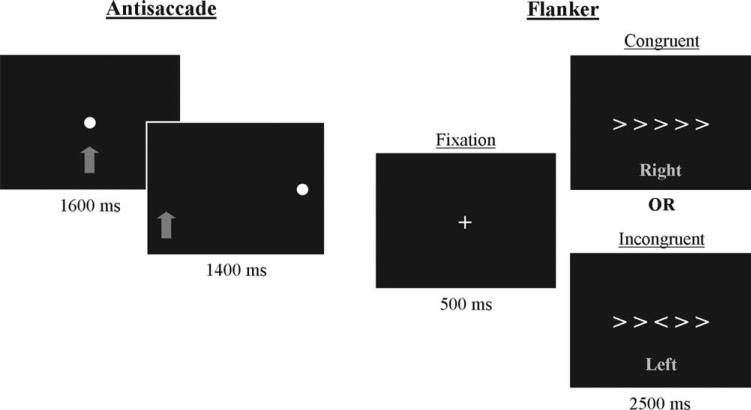
Antisaccade tasks require inhibition of a glance to a newly appearing cue and redirection of gaze to its mirror image (11,16). This task was presented in a blocked design alternating between fixation and antisaccade blocks. A blocked design was selected for the antisaccade task in order to replicate our previous study and to achieve greater detection power than might be available with an event-related design (17). During each of seven fixation blocks, participants were asked to look at a cue consisting of a blue filled circle that appeared at central fixation for 24 s. During each of six antisaccade blocks, eight trials were presented (3 s each for 24 s blocks and a total of 48 total trials across the run). An antisaccade trial began with a blue filled circle (the same cue as for a fixation trial) at central fixation for 1600 ms. Then fixation was extinguished and the cue was presented in the periphery (±10° of visual angle on the horizontal plane; half of trials in each visual field) for 1400 ms (see Figure 1). Participants were instructed to look at the cue when it was in the middle of the screen, but when it appeared at a peripheral location, to look to the mirror image (opposite side of the screen, the same distance from the center). Before entering the scanner, flash cards were used to explain the task and to have participants demonstrate their understanding.
FIGURE 1.
Antisaccade and flanker trials. In the antisaccade task, the participant was instructed to fixate on the cue when it was in the middle of the screen. When the cue appeared at a peripheral location, the participant was to look to the mirror image location (opposite side of the screen, the same distance from center). The arrow did not appear on the screen; in this figure it is used to indicate the correct eye position. In the flanker task, the participant was instructed to fixate on the cross. When the symbols appeared, the participant was to identify the direction of the central symbol and pressed a button with the corresponding hand. The text did not appear on the screen; in this figure, text indicates the correct response hand.
Stimuli were constructed using Presentation software (Neurobehavioral Systems, Albany, CA) and presented using a dual mirror system that both displayed visual stimuli on a rear projection screen and projected an image of the participant's eye to an infrared camera. The infrared camera was part of an MRI-compatible system (IView X MRI-LR, SensoMotoric Instruments, Berlin, Germany) that showed eye position in real time and recorded it for further analysis. Prior to the antisaccade task, eye position was calibrated.
Individual antisaccade trials were scored as a correct or an error response based on eye direction relative to target direction, with corrections to errors also quantified. Latencies for the correct, error and error correction responses (with error correction responses being those in which the initial glance was toward the cue but was then corrected to the mirror image location) were generated using Matlab (The Mathworks, Natick, MA) and procedures that have been used previously (18). Trials were eliminated from performance analysis if the latency was faster than 90 ms, if the eye movement was <10% of the distance to the target, if there was a blink before the saccade, if there was no response, or if the data were too noisy to be scored.
FMRI data were acquired during antisaccade performance from 43 participants who had data available at both baseline and post-test. One participant was excluded for excessive motion (>2 mm shift in x, y, or z directions), resulting in 42 complete datasets for this task (n = 24 exercise, n = 18 control). Of this group, eye movements were recorded for 93% of participants at baseline and 95% at post-test (failure to do so was because of technical difficulties, such as insufficient contrast between the pupil and iris or eyelashes rather than participant noncompliance).
Flanker task
Flanker tasks require response selection within the context of response-congruent and -incongruent information (7,10). This task was presented in an event-related design due in part to context effects that have been reported showing decreased activation in several brain regions (including ACC, PFC, and parietal cortex) as the number of preceding incongruent trials increased (19). As such, a blocked design (in which incongruent trials would happen consecutively) might not achieve maximum statistical power. In addition, an event-related design is similar to a previous flanker study in children (9). The flanker task contained fixation trials (60 trials per run, 120 total trials) and two types of trials: congruent and incongruent (40 trials of each type per run, 80 total trials of each type). During fixation trials, participants were asked to look at a cross that appeared at central fixation for 3 s. During flanker trials, a cross was presented at central fixation to start the trial. After 500 ms, the cross was extinguished, and an array of five symbols was presented for 2500 ms, in which each of the symbols pointed either right (>) or left (<). Symbols could be arrayed in either a congruent fashion, in which all of the symbols were oriented in the same direction, or in an incongruent fashion, in which the flanking symbols pointed in the opposite direction of the central symbol (see Figure 1). Participants were instructed to identify the orientation of the central symbol while ignoring the orientation of the flanking symbols, and they indicated their response by pressing a button with the corresponding hand. Before entering the scanner, flash cards were used to explain the task and to have participants demonstrate their understanding.
Stimuli were constructed using Presentation software (Neurobehavioral Systems, Albany, CA) and presented using a dual mirror system that both displayed visual stimuli on a rear projection screen and projected an image of the participant's eye to an infrared camera (see antisaccade task description). In the flanker task, the image of the participant's eye was observed to ensure that the participant was alert and attending to the stimuli. The response to the visual stimuli was a button press of either the right or left index finger, which was recorded using MRI-compatible button pads (Lumina system, Cedrus Corporation, San Pedro, CA).
Individual trials were scored as correct or error based on hand of button press relative to indicated target direction. Latencies for correct and error responses were also quantified. Measures were calculated separately for congruent and incongruent trials using SAS (SAS Institute, Cary, NC). Each individual's interference effect was also calculated similarly to previous studies of exercise: [(average incongruent correct latency – average congruent correct latency)/average congruent correct latency] × 100 (8). Trials were eliminated from performance analysis if the latency was faster than 100 ms, if there was a response during the fixation period, or if there was no response.
FMRI data were acquired during flanker task performance from 42 participants who had data available at both baseline and post-test (one fewer than the antisaccade task due to scanner malfunction on a flanker run). Two participants were excluded for excessive motion (>2 mm shift in x, y, or z directions), resulting in 40 complete data-sets for this task (n = 23 exercise, n = 17 control). Of the number analyzed, button responses were recorded for 98% of participants at both baseline and post-test (failure to do so was because of technical difficulties rather than participant noncompliance).
Performance data analysis
Analysis of task performance variables of interest was conducted in SPSS Version 20 (IBM, Armonk, NY). Between-group differences at baseline were investigated using independent samples t tests. Group by time repeated-measures ANOVAs were used to investigate whether the groups significantly differed in how they changed over time. Performance variables that were analyzed are listed in Table 2.
TABLE 2.
Performance results of participants included in the fMRI analyses
| Baseline | Post-test | Change (post-test - baseline) | |||||
|---|---|---|---|---|---|---|---|
| Task | Variable | Exercise | Control | Exercise | Control | Exercise | Control |
| Antisaccade | n | 22 | 17 | 23 | 17 | 21 | 16 |
| Percent correct | 56 (26) | 53 (20) | 67 (21) | 63 (24) | 10 (24)b | 10 (13)b | |
| Correct latency (ms) | 461 (80) | 425 (110) | 480 (91) | 438 (92) | 11 (90) | 11 (159) | |
| Error latency (ms) | 398 (87)a | 326 (73)a | 370 (106) | 359 (83) | –9(111) | 22 (80) | |
| Percent of errors corrected | 76 (20) | 65 (27) | 69 (33) | 69 (29) | –11 (38) | 5 (37) | |
| Error correction latency (ms) | 332 (88) | 292 (66) | 315 (100) | 345 (113) | –12 (114) | 43 (102) | |
| Flanker | n | 22 | 17 | 22 | 17 | 21 | 17 |
| Congruent percent correct | 86 (26) | 92 (23) | 97 (4) | 98 (2) | 10 (24)b | 6 (22)b | |
| Congruent correct latency (ms) | 1122 (224)a | 998 (106)a | 1024 (158) | 1010 (172) | –71 (171) | 12 (166) | |
| Incongruent percent correct | 73 (35) | 88 (21) | 85 (26) | 89 (25) | 9 (33) | 0 (35) | |
| Incongruent correct latency (ms) | 1,278 (292) | 1,191 (175) | 1,171 (220) | 1,143 (194) | –52 (223) | –48 (197) | |
| Interference effect | 14 (18) | 20 (15) | 14 (13) | 14(11) | 1 (17) | –6 (14) | |
Mean and SD. Change scores for variables of interest were calculated by subtracting each individual's baseline value from their post-test value.
Significant baseline difference between groups, P < 0.05.
Significant change over time, P< 0.05.
MRI data analysis
FMRI analyses were conducted as in previously published data from our laboratory (13) using Analysis of Functional Neuroimages (AFNI version 2011_12_21_1014; (20)). For each functional run, volumes were despiked, slice time corrected, and registered to a representative volume to correct for head movement. The representative volume was identified by the following criteria: the median volume of the longest window of time points with the lowest number of outlier voxels. Each run was then aligned to each individual's T1-weighted structural MR image, transformed into a standardized space based on a publicly available template created for 7- to 11-year olds in MNI space (21), and resampled to 4 × 4 × 4 mm3 voxels. A 4-mm full-width at half-maximum (FWHM) Gaussian filter was applied to each functional dataset and data were normalized to a mean of 100.
Following preprocessing, hemodynamic response function (HRF)-convolved stimulus timing was entered into a general linear model analysis, along with three nuisance motion regressors (rotation in each plane: x, y, and z) and nuisance regressors detrending for linear, quadratic, and cubic drift. At this point the two flanker runs were concatenated in time during the regression analysis resulting in one flanker dataset per person. The HRF for each of the flanker and the antisaccade functional datasets was represented by the convolution of the stimulus duration and a gamma variate. For the antisaccade task, the contrast of interest was the antisaccade blocks vs. fixation. For the flanker task, there were three contrasts of interest: congruent trials vs. fixation, incongruent trials vs. fixation, and incongruent vs. congruent trials.
A two-way group (exercise, control) by time (baseline, post-test) ANOVA was performed on the datasets to compare activation changes between the two groups and results at P < 0.025 are reported. To protect against false positives, a threshold/cluster method derived from Monte Carlo simulations (accounting for the smoothness of the data and with a connectivity radius of 4 mm) was applied to the F-map (22). Based on these simulations, a family-wise alpha of 0.05 was preserved with three-dimensional clusters having a minimum volume of 35 voxels for the antisaccade task and 37 voxels for the flanker task. The resulting clustered F-maps were used to identify significant group by time interactions. Because between-group differences in brain activation were evident at pre-test, supplementary analyses were conducted for each cluster that showed a significant group by time interaction. Post-test between group t tests were conducted. In addition, group by time ANOVAs were conducted for each cluster that showed a significant group by time interaction while controlling for pretest activation. This was done in order to investigate whether group by time differences remained significant regardless of between-group differences at pre-test. Both left- and right-handed children were included in analyses to maximize generalizability. Sensitivity analyses compared results excluding the left-handed children. Correlation analyses were conducted between attendance and change in brain activation for the exercise and control groups separately.
Correlations of brain activation with health, cognition, and task performance
We conducted exploratory correlation analyses of change over time in task performance, CAS Full Scale score, and health variables versus change over time in brain activation. First, change scores were calculated for each performance, cognitive, and health variable and for percent signal change in each reported fMRI cluster for each individual. Next, for every variable, correlations were conducted across groups to investigate whether there was a relationship between the change in the variable of interest and the change in activation in each cluster.
Results
Health and task performance results
The groups did not significantly differ at baseline on any of the characteristics listed in Table 1. No group by time interactions were found in adiposity, fitness, or cognition in this subset of participants in the trial (data not shown). In performance measures, the exercise and control groups differed at baseline on antisaccade error latency (the exercise group had slower latency, t(37) = –2.755, P = 0.009) and on flanker congruent correct latency (the exercise group had slower latency, t(37) = –2.109, P = 0.042). Controlling for either of these variables did not alter any of the results in this study. There were no significant group by time interactions in any of these variables (see Table 2).
The groups did significantly differ in the percentage of days attended at the program out of the number of days offered, t(41) = 2.25, P = 0.03. The control group attended 75% of days offered (standard deviation = 20%). The exercise group attended 58% of days offered (standard deviation = 29%). There were no significant correlations between percentage of days attended and change in activation over time in any of the fMRI clusters reported in either the control or exercise group.
Antisaccade imaging results
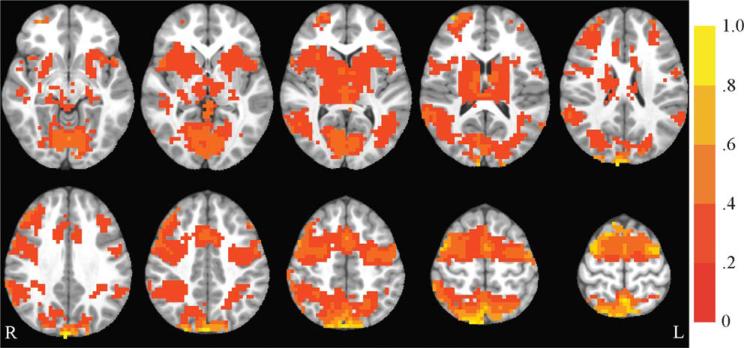
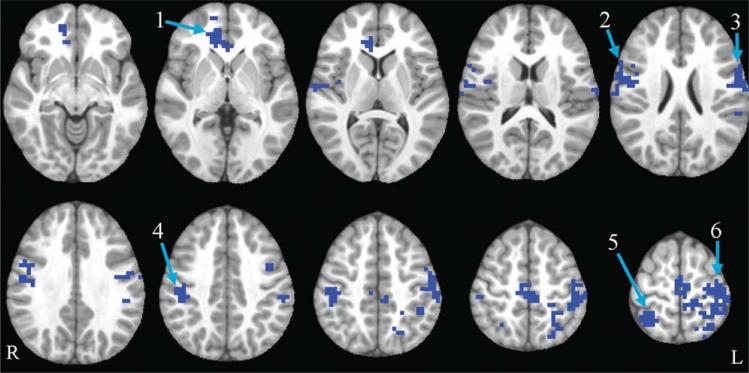
The antisaccade vs. fixation contrast was examined. Saccadic circuitry includes regions that are important for visual perception, visuo-spatial attention, inhibitory control, and generation of a saccade to a spatial location (23,24). Collapsing across all participants, activation in typical saccadic circuitry was observed at baseline (see Figure 2; see also (13)). Results from the whole-brain group (exercise, control) by time (baseline, post-test) ANOVA revealed that the following regions showed a significant group by time interaction: bilateral precentral gyrus, medial frontal gyrus, paracentral lobule, postcentral gyrus, superior parietal lobule (SPL), inferior parietal lobule (IPL), and anterior cingulate cortex (ACC); right inferior frontal gyrus (IFG) and insula; and left precuneus. In all of these regions, the exercise group showed a pattern of decreasing activation over time, while the control group showed the opposite pattern, increasing activation over time. All of these regions showed significant between-group differences at post-test, and all group by time interactions remained significant when controlling for pretest activation. These results remained significant when left-handed children were excluded for a sensitivity analysis. For further details, see Table 3 and Figure 3.
FIGURE 2.
Antisaccade activation at baseline. Axial slices (top left z = 28 through bottom right z = 64, spacing = 8 mm) displaying significant antisaccade-correlated activation across all participants at baseline. The background anatomical image is the pediatric template that was used during alignment and is shown using radiological convention. Scale indicates percent signal change.
FIGURE 3.
Antisaccade group by time interaction. Axial slices (top left z = –8 through bottom right z = 64, spacing = 8 mm) displaying significant group by time interactions in the antisaccade task. All clusters shown are blue, indicating that the exercise group decreased and the control group increased. The background anatomical image is the pediatric template that was used during alignment and is shown using radiological convention. Numbers correspond to labels in the first column of Table 3
Flanker imaging results
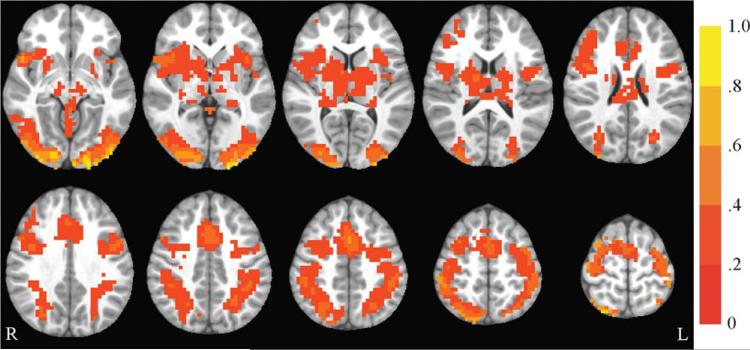
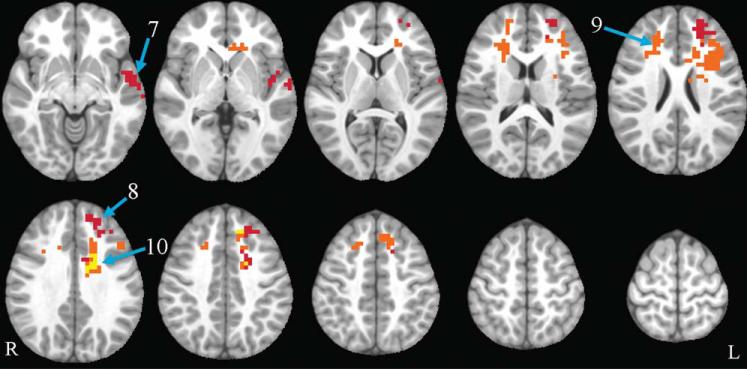
Three contrasts were examined: (1) congruent vs. fixation, (2) incongruent vs. fixation, and (3) incongruent vs. congruent. Flanker circuitry includes regions important for visual perception, visuo-spatial attention, conflict monitoring, and motor responses (25,26). Collapsing across all participants, activation in typical flanker circuitry was observed at baseline (see Figure 4 for an example of incongruent vs. fixation activation). Results for congruent vs. fixation from the whole-brain group (exercise, control) by time (baseline, post-test) ANOVA revealed no significant group by time interactions. The ANOVA for the incongruent vs. fixation contrast revealed a significant group by time interaction for the following regions: left medial frontal gyrus, superior frontal gyrus (SFG), middle frontal gyrus (MFG), superior temporal gyrus (STG), cingulate gyrus, and insula. In all regions in this contrast, the exercise group showed increased activation over time, while the control group showed decreased activation. The cluster including left STG and insula, but not the cluster including medial frontal gyrus, SFG, MFG, and cingulate gyrus, showed significant between-group differences at post-test and its group by time interaction remained significant when controlling for pretest activation. All results remained significant when left-handed were excluded for a sensitivity analysis.
FIGURE 4.
Incongruent vs. fixation activation at baseline. Axial slices (top left z = –8 through bottom right z = 64, spacing = 8 mm) displaying significant activation for the incongruent vs. fixation contrast across all participants at baseline. The background anatomical image is the pediatric template that was used during alignment and is shown using radiological convention. Scale indicates percent signal change.
The ANOVA for the incongruent vs. congruent contrast revealed a significant group by time interaction for the following regions: bilateral SFG, medial frontal gyrus, MFG, cingulate gyrus, and anterior cingulate cortex (ACC); and left IFG and insula. In all regions in this contrast, the exercise group showed increased activation over time, while the control group showed decreased activation. None of these regions, however, showed significant between-group differences at post-test and none of these group by time interactions remained significant when controlling for pretest activation. All results remained significant when left-handed children were excluded for a sensitivity analysis. For further details, see Table 3 and Figure 5.
FIGURE 5.
Flanker group by time interactions. Axial slices (top left z = –8 through bottom right z = 64, spacing = 8 mm) displaying significant group by time interactions in the incongruent vs. fixation and incongruent vs. congruent contrast. All clusters shown are warm colors, indicating that the exercise group increased and the control group decreased. Red corresponds to incongruent vs. fixation, orange corresponds to incongruent vs. congruent, and yellow shows areas where the two contrasts overlap. The background anatomical image is the pediatric template that was used during alignment and is shown using radiological convention. Numbers correspond to labels in the first column of Table 3.
Correlations of brain activation with health, cognition, and task performance
There were no significant correlations between change in health variables (percent body fat or VO2 peak) and change in brain activation. There also were no significant correlations between change in Cognitive Assessment System Full Scale Standard Score and change in brain activation. The only significant correlation between antisaccade activation and task performance was that a decrease in activation in the right superior parietal lobule was correlated with faster error latencies (r(37) = 0.33, P = 0.04). For the incongruent vs. fixation or the incongruent versus congruent contrasts of the flanker task, there were no correlations between performance and activation.
Discussion
Overweight, unfit children randomly assigned to an 8-month exercise intervention showed significantly different changes in brain activation within the context of two separate CC tasks compared to children in the attention control condition, which apart from being sedentary, was very similar to the exercise intervention. Both groups showed improved task performance on antisaccade and flanker tasks, possibly due to common factors, such as maturation, education, practice, or benefits of the after school programs. No group by time interactions were detected in task performance, perhaps because the exercise and attention control conditions were more similar than in prior studies. Importantly, group differences in brain changes were seen even in the absence of performance differences. From baseline to post-test, the exercise group decreased activation during the anti-saccade task and increased activation during the incongruent aspect of the flanker task compared to the control group. While the groups differed in attendance, it is unlikely that the difference in attendance between the groups explained differences in brain function, as attendance did not correlate with change in brain activation. These results indicate that functional neuroimaging may be more sensitive than performance measures to exercise-induced changes, and that the groups might differ in task strategies used, given activation differences despite similar task performance.
Decreased activation during the antisaccade task for the exercise group was found in several regions known to support antisaccade performance, including inferior frontal gyrus (IFG) and anterior cingulate cortex (ACC). Specifically, IFG has been implicated in attention and inhibition (27), while ACC is likely important for error monitoring (28). Within the parietal lobe, exercise-related decreases were seen in superior and inferior regions, which are involved in visuo-spatial processing, attentional shifting and target selection (23,29). The right insula also was affected by exercise; its role in saccadic performance is less well-understood but it may be important for task-set maintenance and salience detection (30). Alterations also were found in two motor regions which support saccade preparation and generation (31,32): (1) bilateral precentral gyrus and (2) medial frontal gyrus and paracentral lobule.
The antisaccade results partly replicate our previous exercise intervention, in which children assigned to the exercise condition decreased activation on an antisaccade task in posterior parietal regions compared to those in a no-intervention control condition (13). The results from the earlier study showed increased prefrontal cortex (PFC) activation in an antisaccade task in children assigned to exercise, not seen here. It is possible that effects observed after 3 months of an exercise intervention may no longer exist after 8 months. Perhaps exercise initially increases PFC activation, which then decreases as antisaccade circuitry becomes more efficient.
There is precedent for decreased antisaccade activation in least one study in adults in which intensive daily antisaccade practice decreased activation on a blocked-design antisaccade task, possibly reflecting learning or improved efficiency (33). Decreased sustained (i.e., blocked-design) activation in a CC task with exercise also is consistent with a cross-sectional study that reported that children who were more fit had lower activation in several brain regions on the incongruent vs. congruent condition of a blocked-design flanker task. Although it was a different task (flanker rather than antisaccade), the previous study nevertheless showed that higher-fit children had lower activation than lower-fit children in several regions that were found in the current study with a blocked-design antisaccade task (including precentral gyrus, ACC, and superior parietal lobule; (8)). Decreased activation patterns may reflect more efficient CC. Another possible explanation is that the decrease in activation may reflect a shift from reactive control (more transient, occurring after an event) to proactive control (which involves actively maintaining goal-related information in working memory such that the system can respond to subsequent events) in the exercise group, as suggested by Voss et al. (34). This was not a distinction that we were able to make with our current data, however.
For the flanker task, activation increased in the exercise group compared to the control group. For both the incongruent vs. fixation and incongruent vs. congruent contrasts, the exercise group showed increases in regions supporting CC, including superior frontal, medial frontal, middle frontal, and cingulate gyri, although these interactions did not survive adjustment for pre-test activation. These regions are associated with working memory and spatial attention (35). Increased activation was observed in other regions supporting higher-order functions such as the insula, which has been associated with salience detection, as well as successful interference suppression in children (36). The interaction in the insula survived adjustment for pre-test activation in the incongruent vs. fixation contrast. In the incongruent vs. congruent contrast alone, the exercise group showed increased bilateral ACC activation, which is important for processing increased conflict in incongruent vs. congruent trials (37). Increased activation in the ACC did not survive adjustment for pre-test activation. For the incongruent vs. fixation contrast alone, the exercise group showed an increase in left superior temporal gyrus, which is involved in target detection (34) and which did survive adjustment for pre-test activation. In both contrasts, the differences in activation appear to be more extensive in the left hemisphere than in the right hemisphere. This is consistent with Bunge et al., who demonstrated that children who perform better than others recruit left prefrontal cortex more extensively and suggested that this could be related to use of a verbal strategy during performance in this task (36). That is, the exercise group may be utilizing their verbal abilities to perform this task (recoding nonverbal stimuli with verbal labels), resulting in greater recruitment in the left hemisphere.
The flanker results are consistent with previous exercise literature but frontal regions, especially, should be interpreted with caution due to the fact that they are influenced by pretest differences. As expected, we did not observe differences in the congruent vs. fixation contrast, lending support to previous suggestions that CC may be more susceptible to exercise-induced benefits than other aspects of cognition, such as more basic perceptual or motor tasks (6). Because both groups exhibited relatively low congruent error rate at baseline, the congruent vs. fixation contrast may be too simple to reveal an exercise effect.
Both the incongruent vs. fixation and incongruent vs. congruent contrasts demonstrated increased middle and superior frontal gyrus activation in the exercise group compared to the control group in our study. This is consistent with an exercise intervention in older adults in which participants increased activation on a flanker task in those regions (10). The incongruent vs. congruent contrast in our study showed increased ACC activation in the exercise group compared to the control group, which is the opposite of decreased ACC activation demonstrated in an exercise intervention in older adults. Divergent results between these two studies may be related to differing effects of exercise with age. Alternately, they may underlie between-study differences in interference measures, as interference decreased in the exercise group in the study of older adults but did not differentially change between groups in our study. Increased ACC activity, as reported in our results, however, also is reported by at least one cross-sectional study showing that higher-fit children have higher middle frontal gyrus and ACC activation on an event-related flanker task (9).
A recent intervention study in 8- to 9-year-old children found different effects compared to our study, with a region-of-interest analysis showing that those assigned to an exercise program for 9 months compared to a waitlist control condition showed decreased activation in incongruent flanker trials compared to fixation (12). One reason why these studies demonstrate differing effects of exercise may be due to the differing samples of children included in the studies. The children in our study are older, predominantly Black, have lower VO2 and higher BMI, lower cognitive scores (when CAS Full Scale score is compared to IQ reported by Chaddock et al.) and have slower flanker reaction times. It also is possible that differences in task design between the two studies may contribute to differences in observed effects of exercise (as the flanker task in our study, unlike the study by Chaddock et al., did not occur within the context of no-go trials).
Interestingly, the antisaccade and flanker tasks demonstrated activation changes with exercise that occurred in opposing directions. This could be related to differing cognitive demands in the two tasks. The group by time brain activation results from the flanker and anti-saccade tasks did not spatially overlap, indicating that although they utilize some common cognitive processes, their circuitries (and/or the effects of exercise upon them) may be distinct. Another possible explanation for this difference is inherent in the paradigm designs. The antisaccade task was blocked and the flanker task was event-related. Because a blocked-design is more sensitive to sustained activation and an event-related design is more sensitive to transient activation, it is possible that sustained and transient neural activation are differentially affected by exercise. Sustained activation is maintained throughout performance of a task, whereas transient activation includes processing specifically involved in each trial of a task (38). There is evidence that these types of activation show different developmental patterns. For example, in a study using a letter-matching task, the right lateral inferior frontal gyrus exhibited greater sustained activity in children than adults, but greater transient activity in adults than in children (39).
Experimental support for differing effects of exercise on sustained and transient brain activation patterns comes from two previous cross-sectional studies of fitness conducted in children. Both were versions of a flanker task, with one a blocked-design and the other an event-related design. The blocked-design task showed that higher-fit children had lower activation than lower-fit children on the incongruent compared to congruent condition of a flanker task (8). The event-related task found a different pattern, with higher-fit children having higher activation than lower-fit children on the incongruent compared to congruent condition, at least early in the task (9). In the current study, there is only one modest correlation between change in activation and change in performance, and there were no group by time differences in task performance on either task. Therefore, it may be that changes in activation primarily reflect differences in task strategy between the groups, rather than differences in task performance abilities. Decreased sustained activation in the exercise group could be related to greater efficiency in task set maintenance or performance (33), while increased transient activation could be related to more flexible modulation of CC processes (9).
This study provided novel causal evidence for the effects of regular aerobic exercise on neural circuitry in overweight children and is one of the first to demonstrate that exercise alters brain function on two different cognitive tasks in the same sample. Through the use of a robust attention control group, we controlled for potentially beneficial common factors that derive purely from participating in an after school program, such as attention from adults, social interaction, and supervised homework time. Use of a randomized trial with an attention control condition allowed precise delineation of alterations in neural circuitry activation patterns due specifically to exercise. The sample was overweight, unfit and predominantly Black, an under-studied population at risk for adverse health and educational outcomes. An additional strength of this study as compared to previous fMRI investigations of exercise interventions by both our group and others is the much larger sample size, with a total of 23 additional children compared to our prior study (13) and 20 additional children compared to a recent 9-month intervention (12). Because the groups in the current study did not differ in the change in aerobic fitness or adiposity over the course of the intervention, and change in fitness or adiposity did not correlate with change in brain activation, it is not clear whether the differences observed in brain function were caused by increased fitness or decreased body fat in the exercise group as compared to the control group. The imaging study was a subsample of a larger exercise study powered for such health outcomes.
Future work is needed to determine whether transient and sustained neural activation patterns consistently show differential effects of exercise. It should also investigate whether exercise promotes the development of different task strategies (e.g., proactive versus reactive, or verbal strategies). Some of the differences in activation during the flanker task, while expected given previous literature, were related to group differences at pretest and thus would especially benefit from replication. Another topic of study should be whether children who are at different developmental stages or who are not overweight demonstrate changes in brain function similar to those reported here. Many promising hypotheses from cross-sectional studies have not been borne out by randomized trials, the most reliable evidence for causality (4). It is not clear whether high adiposity and low fitness are associated with different cognitive profiles, or whether an exercise intervention would provide greater cognitive benefits to fatter or more unfit children than their healthier peers. While the groups did not differentially change task performance over time, there may be vulnerable subgroups (e.g., children with developmental disabilities) that show greater performance improvements from exercise.
The current study provides strong new evidence that exercise causes alterations in neural circuitry supporting CC in overweight children. Specifically, children assigned to an aerobic exercise group demonstrated decreased activation in several brain regions on an antisaccade task compared to the control group, possibly reflecting increased efficiency. They also demonstrated increased activation in both the incongruent versus fixation and incongruent versus congruent contrasts of a flanker task compared to the control group, possibly reflecting greater flexible modulation of CC. The opposite patterns of exercise-induced change in brain activation for the two tasks may relate to the different cognitive demands of the two tasks. Another possibility is that opposite patterns of exercise-induced change are related to the task design, as the antisaccade was presented in a blocked design and the flanker task was presented in an event-related design. These designs are more sensitive to sustained versus transient activation, respectively, suggesting that exercise may differentially affect these two types of brain activation. Because there were no group by time differences in task performance and correlations between brain activation and task performance were minimal, it may be that differences in brain activation induced by exercise reflect differences in task strategy, rather than in task performance abilities. While the current study cannot specify which strategies may be altered, possibilities include a shift to verbal strategies supporting nonverbal task performance, or a shift from reactive to proactive control. Changes in these brain functions may have wide-ranging consequences, as CC is associated with other cognitive domains, such as school performance and social functioning (40). If exercise improves brain function supporting CC, children could see benefits in many aspects of daily life.
Acknowledgments
Funding agencies: This research was supported by the National Institutes of Health (R01 HL87923) and the National Science Foundation Graduate Research Fellowship Program.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomkinson GR, Olds TS. Secular changes in pediatric aerobic fitness test performance: the global picture. Med Sport Sci. 2007;50:46–66. doi: 10.1159/000101075. [DOI] [PubMed] [Google Scholar]
- 3.Taras H, Potts-Datema W. Obesity and student performance at school. J School Health. 2005;75:291–295. doi: 10.1111/j.1746-1561.2005.00040.x. [DOI] [PubMed] [Google Scholar]
- 4.Davis CL, Cooper S. Fitness, fatness, cognition, behavior, and academic achievement among overweight children: do cross-sectional associations correspond to exercise trial outcomes? Prev Med. 2011;52:S65–S69. doi: 10.1016/j.ypmed.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LeBlanc MM, Martin CK, Han H, et al. Adiposity and physical activity are not related to academic achievement in school-aged children. J Dev Behav Pediatr. 2012;33:486–494. doi: 10.1097/DBP.0b013e31825b849e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colcombe SJ, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 7.Eriksen BA, Eriksen CW. Effects of noise letters upon identification of a target letter in a non-search task. Percept Psychophys. 1974;16:143–149. [Google Scholar]
- 8.Voss MW, Chaddock L, Kim JS, et al. Aerobic fitness is associated with greater efficiency of the network underlying cognitive control in preadolescent children. Neuroscience. 2011;199:166–176. doi: 10.1016/j.neuroscience.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaddock L, Erickson KI, Prakash RS, et al. A functional MRI investigation of the association between childhood aerobic fitness and neurocognitive control. Biol Psychol. 2012;89:260–268. doi: 10.1016/j.biopsycho.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Colcombe SJ, Kramer AF, Erickson KI, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci USA. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luna B, Velanova K, Geier CF. Development of eye-movement control. Brain Cogn. 2008;68:293–308. doi: 10.1016/j.bandc.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaddock-Heyman L, Erickson KI, Voss MW, et al. The effects of physical activity on functional MRI activation associated with cognitive control in children: a randomized controlled intervention. Front Hum Neurosci. 2013;7:72. doi: 10.3389/fnhum.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis CL, Tomporowski PD, McDowell JE, et al. Exercise improves executive function and achievement and alters brain activation in overweight children: a randomized, controlled trial. Health Psychol. 2011;30:91–98. doi: 10.1037/a0021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naglieri JA, Das JP. Cognitive Assessment System: Interpretive Handbook. Riverside Publishing; Itasca, IL: 1997. [Google Scholar]
- 15.American College of Sports Medicine . ACSM's Guidelines for Exercise Testing and Prescription. 6th edn. Lippincott Williams & Wilkins; Baltimore, MD: 2000. [Google Scholar]
- 16.Hallett PE. Primary and secondary saccades to goals defined by instructions. Vis Res. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- 17.Amaro E, Barker GJ. Study design in fMRI: basic principles. Brain Cogn. 2006;60:220–232. doi: 10.1016/j.bandc.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Dyckman KA, Camchong J, Clementz BA, et al. An effect of context on saccade-related behavior and brain activity. NeuroImage. 2007;36:774–784. doi: 10.1016/j.neuroimage.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Durston S, Davidson MC, Thomas KM, et al. Parametric manipulation of conflict and response competition using rapid mixed-trial event-related fMRI. NeuroImage. 2003;20:2135–2141. doi: 10.1016/j.neuroimage.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 21.Fonov V, Evans AC, Botteron K, et al. Unbiased average age-appropriate atlases for pediatric studies. NeuroImage. 2011;54:313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward BD. Simultaneous Inference for fMRI Data. Biophysics Research Institute, Medical College of Wisconsin; Milwaukee, WI: 1997. [Google Scholar]
- 23.Ettinger U, Ffytche DH, Kumari V, et al. Decomposing the neural correlates of antisaccade eye movements using event-related fMRI. Cereb Cortex. 2008;18:1148–1159. doi: 10.1093/cercor/bhm147. [DOI] [PubMed] [Google Scholar]
- 24.McDowell JE, Dyckman KA, Austin BP, et al. Neurophysiology and neuroanatomy of reflexive and volitional saccades: evidence from studies of humans. Brain Cogn. 2008;68:255–270. doi: 10.1016/j.bandc.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casey BJ, Thomas KM, Welsh TF, et al. Dissociation of response conflict, attentional selection, and expectancy with functional magnetic resonance imaging. Proc Natl Acad Sci USA. 2000;97:8728–8733. doi: 10.1073/pnas.97.15.8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hazeltine E, Poldrack R, Gabrieli JD. Neural activation during response competition. J Cogn Neurosci. 2000;12:118–129. doi: 10.1162/089892900563984. [DOI] [PubMed] [Google Scholar]
- 27.Hampshire A, Chamberlain SR, Monti MM, et al. The role of the right inferior frontal gyrus: inhibition and attentional control. NeuroImage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ford KA, Goltz HC, Brown MR, et al. Neural processes associated with antisaccade task performance investigated with event-related fMRI. J Neurophysiol. 2005;94:429–440. doi: 10.1152/jn.00471.2004. [DOI] [PubMed] [Google Scholar]
- 29.Krafft C, Schwarz N, Chi L, et al. The location and function of parietal cortex supporting of reflexive and volitional saccades, a meta-analysis of over a decade of functional MRI data. In: Costa A, Collalba E, editors. Horizons in Neuroscience Research. Nova Science Publishers; Hauppauge, NY: 2012. [Google Scholar]
- 30.Dosenbach NUF, Visscher KM, Palmer ED, et al. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Connolly JD, Goodale MA, Menon RS, et al. Human fMRI evidence for the neural correlates of preparatory set. Nat Neurosci. 2002;5:1345–1352. doi: 10.1038/nn969. [DOI] [PubMed] [Google Scholar]
- 32.Brown MRG, Vilis T, Everling S. Frontoparietal activation with preparation for antisaccades. J Neurophysiol. 2007;98:1751–1762. doi: 10.1152/jn.00460.2007. [DOI] [PubMed] [Google Scholar]
- 33.Lee J, Park C, Dyckman KA, et al. Practice-related changes in neural activation patterns investigated via wavelet-based clustering analysis. Hum Brain Mapp . doi: 10.1002/hbm.22066. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braver TS, Barch DM, Gray JR, et al. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- 35.Kirschen MP, Chen SHA, Schraedley-Desmond P, et al. Load- and practice-dependent increases in cerebro-cerebellar activation in verbal working memory: an fMRI study. NeuroImage. 2005;24:462–472. doi: 10.1016/j.neuroimage.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 36.Bunge SA, Dudukovic NM, Thomason ME, et al. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luks TL, Simpson GV, Dale CL, et al. Preparatory allocation of attention and adjustments in conflict processing. NeuroImage. 2007;35:949–958. doi: 10.1016/j.neuroimage.2006.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Visscher KM, Miezin FM, Kelly JE, et al. Mixed blocked/event-related designs separate transient and sustained activity in fMRI. NeuroImage. 2003;19:1694–1708. doi: 10.1016/s1053-8119(03)00178-2. [DOI] [PubMed] [Google Scholar]
- 39.Burgund ED, Lugar HM, Miezin FM, et al. The development of sustained and transient neural activity. NeuroImage. 2006;29:812–821. doi: 10.1016/j.neuroimage.2005.08.056. [DOI] [PubMed] [Google Scholar]
- 40.Best JR, Miller PH, Jones LL. Executive functions after age 5: changes and correlates. Dev Rev. 2009;29:180–200. doi: 10.1016/j.dr.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]