Abstract
Background
Patients with somatization disorder (SD) have altered neural activity in the brain regions of the default mode network (DMN). However, the regional alteration of the DMN in SD remains unknown. The present study was designed to investigate the regional alterations of the DMN in patients with SD at rest.
Methods
Twenty-five first-episode, medication-naive patients with SD and 28 age-, sex-, education- matched healthy controls underwent a resting-state functional magnetic resonance imaging (fMRI) scan. The fractional amplitude of low-frequency fluctuations (fALFF) was applied to analyze the data.
Results
Patients with SD showed a dissociation pattern of resting-state fALFF in the DMN, with increased fALFF in the bilateral superior medial prefrontal cortex (MPFC, BA8, 9) and decreased fALFF in the left precuneus (PCu, BA7). Furthermore, significantly positive correlation was observed between the z values of the voxels within the bilateral superior MPFC and somatization subscale scores of the Symptom Check List (SCL-90) in patients with SD.
Conclusions
Our findings indicate that there is a dissociation pattern of the anterior and posterior DMN in first-episode, treatment-naive patients with SD. The results provide new insight for the importance of the DMN in the pathophysiology of SD.
Introduction
Somatization disorder (SD) is an illness with lots of physical complaints in a variety of systems that occurs at least 2 years resulting in significant impairment or treatment seeking. The somatic symptoms typically refer to gastrointestinal, cardio-respiratory, uro-genital, other internal systems, or musculoskeletal problems. The disorder usually begins before age 30 and occurs more often in women than in men (about 1∶5, men to women). The prevalence of SD in the general population may be around 4–7% [1]–[4]. Patients with SD suffer significantly psychosocial disability leading to a high rate of health care utilization, a decline of the life quality, absenteeism from work and reduction in productivity [5], [6]. Health care burden of SD is comparable to that of some severe mental illnesses like schizophrenia and depression [7]. Although the pathophysiology of SD remains unclear, the progress in neuroimaging technology makes it possible to study the neurobiology of SD [8].
Somatization accompanied by anxiety has been associated with decreased metabolism in some brain regions, including ventrolateral prefrontal cortex, ventral anterior cingulate gyrus (ACC) and anterior insular gyrus [9]. In a structural magnetic resonance imaging (MRI) study, patients with SD had significantly smaller mean volumes in amygdala compared to healthy controls [10]. Moreover, Fayed et al. found that enhanced activity in posterior cingulate cortex (PCC) in SD patients relative to controls [11]. Lemche et al. reported that when SD patients were in different emotional states (happy or sad), abnormal activity existed in the anterior ventral precuneus (PCu, BA7), posterior cingulate gyrus and anteromedial thalamus [12]. These findings provide evidence of neuroanatomical and neurophysiologic alterations in patients with SD. Recently, the default mode network (DMN) attracts more attention in the study of psychiatric disorders. The DMN, consisting of medial prefrontal cortex (MPFC), ACC, PCC/PCu and dorsomedial thalamus, exhibits high levels of activity at rest and becomes suspended or deactivated when specific goal-directed behavior is needed [13]–[17]. Furthermore, a recent systematic and critical review suggests that the DMN may act as an important role in the pathophysiology of SD [18]. We endorsed these studies for that they provide useful clues with altered activity of the DMN in SD. However, there is still lack of evidence about the changes of regional activity in SD.
The fractional amplitude of low-frequency fluctuations (fALFF), which is measured with resting-state functional magnetic resonance imaging (fMRI), is one of such methods to detect regional signal changes of spontaneous activity. The fALFF approach, an improved index of amplitude of low-frequency fluctuations (ALFF), is concerned with the percentile of the power spectrum in low-frequency (e.g.0.01–0.08 Hz) oscillations (LFOs) versus that of the entire detectable spectrum (e.g.0–0.25 Hz). In contrast to ALFF, fALFF can provide a higher specific measure of LFOs and effectively reflects intrinsic neural activity and physiological states within specific regions. Therefore, non-specific signal components could be effectively suppressed by this technology, and the sensitivity and specificity in examining regional spontaneous brain activity could be significantly improved [19], [20]. So far, this method has been successfully used to investigate the brain function in healthy subjects [21] and clinical populations [22]–[28].
The aim of the current study was to examine regional neural activity of the brain in SD patients by using the fALFF approach. According to the aforementioned studies [9]–[12], [18], which provide useful clues with altered activity of the DMN in SD. Therefore, we hypothesized that (1) the differences of fALFF exist in the DMN between the patients and the controls; (2) the differences of fALFF were expected to be related to some clinical variables, such as the somatization subscale of the Symptom Check List 90 (SCL-90) [29], the Hamilton depression scale (HAMD) [30] and the Hamilton anxiety scale (HAMA) [31] in the patient group.
Materials and Methods
Subjects
Twenty-six right-handed patients with SD, originally recruited from the Mental Health Center, the First Affiliated Hospital of Guangxi Medical University, China, participated in the whole study. The patients were recruited consecutively and diagnosed according to the Structured Clinical Interview of the DSM-IV (SCID) [32]. All patients were at their first episode and medication-naive. Exclusion criteria included age younger than 18 years or older than 60 years, history of loss of consciousness, mental retardation, cardiovascular disease, bipolar disorder, neurological illness, and alcohol or drug abuse. However, comorbidity with major depressive disorder (MDD) is allowable.
Thirty right-handed healthy controls were recruited from the community. None of them had a history of serious medical or neuropsychiatric illness, craniocerebral operations, a family history of major psychiatric or neurological illness in their first-degree relatives, and all were matched with the patients in terms of age, sex ratio and years of education.
All subjects were assessed with the HAMD, HAMA, somatization subscale of the SCL-90 at the scan day. The somatization subscale of SCL-90, consisting of 12 physical symptoms items (e.g. headaches, dizziness, pains in lower back, nausea, etc.), was used to assess the somatic symptom severity. The 17-item HAMD and 14-item HAMA, respectively, were used to measure the depressive symptom and the anxious symptom. The three scales were used widely in psychiatric research. The reliability and validity of the three scales are all above 0.8.
The study was approved by the Ethics Committee of the First Affiliated Hospital, Guangxi Medical University. All participants were given information about the procedures and signed an informed consent.
Imaging acquisition
Imaging was acquired by a 3.0 T Siemens scanner (Siemens, Erlangen, Germany) located in the First Affiliated Hospital, Guangxi Medical University, China. To minimize head movement and reduce scanner noise, foam padding and earplugs were used. During the scanning, subjects were instructed to lie still with their eyes closed and remain awake. Functional images were obtained with an echo-planar imaging (EPI) sequence. The following parameters were used for functional imaging as follows: repetition time/echo time (TR/TE) = 2000/30 ms, slices = 30, thickness = 4 mm, gap = 0.4 mm, field of view (FOV) = 24 cm, flip angle = 90°, data matrix = 64×64. For each subject, the fMRI scanning lasted for 500 s and 250 volumes were obtained.
Data preprocessing
Image preprocessing was conducted using statistical parametric mapping software (SPM8, http://www.fil.ion.ucl.ac.uk/spm). The first 10 volumes of each subject were discarded due to the signal reaching equilibrium and the participants adapting to the scanning noise. The remaining 240 volumes were corrected for acquisition delay between slices and for head motion. The subjects should have no more than 2 mm maximum displacement in x, y, or z and 2° of angular motion during the whole fMRI scan. The resulting images were spatially normalized to the Montreal Neurological Institute (MNI) EPI template in SPM8, and each voxel was resampled to 3×3×3 mm3. After this, the processed images were spatially smoothed with an 8 mm full width at half maximum (FWHM) Gaussian kernel.
fALFF data analysis
After preprocessing in SPM, linear trend was removed and fALFF analysis was performed using the REST software [33]. Briefly, the time course of each voxel was first converted to the frequency domain without band-pass filtering by using a Fast Fourier Transform (FFT) and the power spectrum was obtained. Since the power of a given frequency was proportional to the square of the amplitude of its frequency component, the square root was calculated at each frequency of the power spectrum and the averaged square root was obtained across 0.01–0.08 Hz at each voxel. The sum of amplitude across 0.01–0.08 Hz was divided by that across the entire frequency range. For a standardization purpose, the fALFF of each voxel was divided by the global mean fALFF value within a brain mask.
Statistical analysis
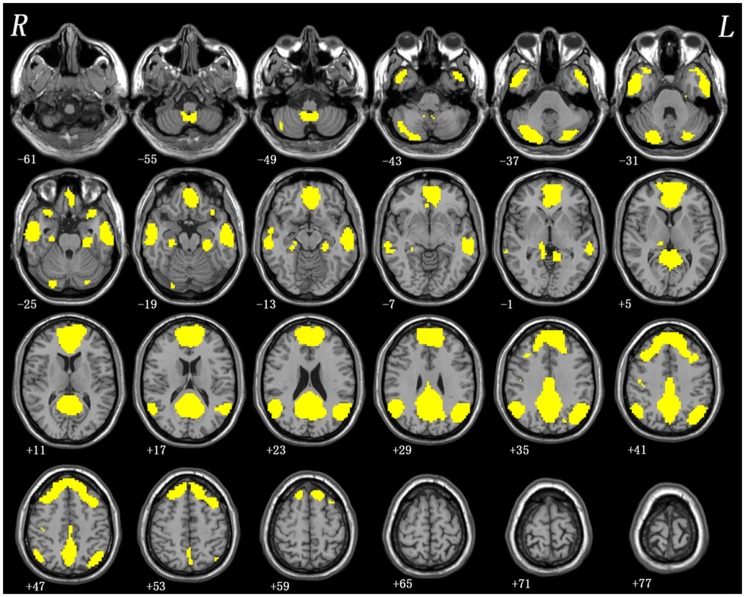
After assessing normal distributions, the fALFF analyses were performed with the independent two-sample t-tests via voxel-wise cross-subject statistics in the regions within the DMN mask, which was produced in our previous studies [34], [35] (see Figure 1). The resulting statistical map was corrected for multiple comparisons to a significant level of p<0.05 after AlphaSim correction (combined with the individual voxel p<0.01 and cluster size >52 voxels). This correction was confined within the DMN mask and determined by Monte Carlo simulations using the AFNI AlphaSim program (http://afni.nih.gov/afni/docpdf/AlphaSim.pdf). After testing the normality, voxel-based correlation analyses were implemented (p<0.05) to identify the relationship of the fALFF values in regions with significant group differences and the severity of anxiety, depression or somatic symptoms. The results were constrained within the DMN mask. The statistical results were corrected using AlphaSim program in the Resting-State fMRI Data Analysis Toolkit (REST) software [33] at a significant level of p<0.05 (combined height threshold p<0.01 and a minimum cluster size >52). The demographic and clinical data were compared by using two-sample t-test and Chi-square test (p<0.05).
Figure 1. The DMN mask produced in our previous studies.
DMN = default mode network.
Results
Demographics and clinical characteristics of the subjects
The data of three subjects (one patient and two healthy controls) were discarded from further analysis because of excessive head movement. The demographic and clinical data are presented in Table 1. The two groups were matched in age, sex ratio, and years of education. Compared with healthy control, patients with SD showed higher levels of depression, anxiety and somatic symptoms (p<0.001).
Table 1. Characteristics of the patients and healthy controls.
| Variables (mean ±standard deviation) | Patients | Controls | p value |
| Gender (male/female) | 4/21 | 6/22 | 0.732a |
| Age, years | 41.00±10.76 | 38.71±9.59 | 0.417b |
| Education, years | 7.72±4.39 | 7.82±2.59 | 0.920b |
| Illness duration, months | 59.12±62.22 | ||
| HAMD | 18.84±7.31 | 2.60±1.83 | <0.001b |
| HAMA | 22.96±10.95 | 0.53±0.99 | <0.001b |
| somatization subscale of SCL-90 | 28.48±10.37 | 14.32±3.44 | <0.001b |
HAMD: Hamilton depression scale; HAMA: Hamilton anxiety scale; SCL-90: Symptom Check List-90.
The p value for gender distribution in the two groups was obtained by chi-square test.
The p values were obtained by two sample t-tests.
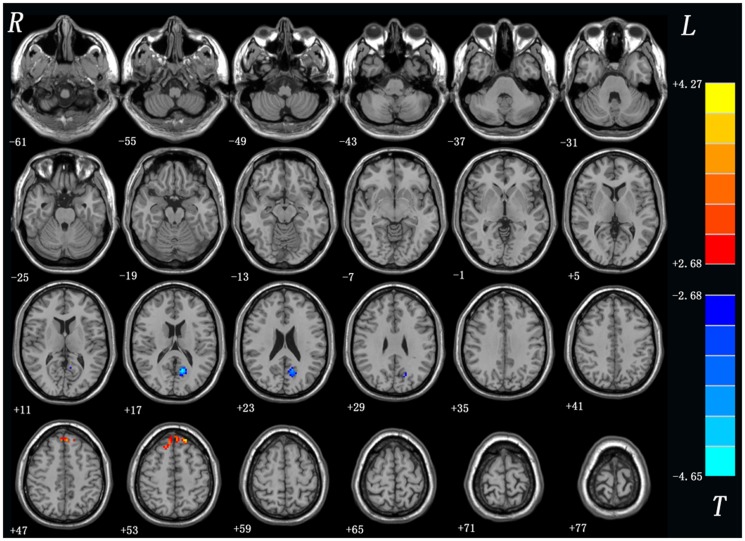
Differences in fALFF values between patients with SD and controls
As shown in Table 2 and Figure 2, the SD group exhibited significantly increased fALFF in the bilateral superior MPFC (BA8, 9) and decreased fALFF in left PCu (BA7) relative to the controls. No other difference was observed in the patients.
Table 2. Regions showing fALFF differences between groups.
| Brain regions | Voxels | MNI coordinates (mm) | t value | ||
| x | y | z | |||
| SD>controls | |||||
| bilateral superior medial frontal gyrus | 67 | −18 | 42 | 54 | 4.27 |
| SD<controls | |||||
| left precuneus | 75 | −12 | −63 | 18 | −4.64 |
x, y, z, coordinates of primary peak locations in the MNI space; t statistical value of peak voxel showing fALFF differences between the patients with SD and the controls. SD: somatization disorder.
Figure 2. fALFF differences between the patients with SD and the controls.
Red and blue denote higher and lower fALFF respectively and the color bars represent the t value of the group analysis. fALFF = fractional amplitudes of low-frequency fluctuations; SD = somatization disorder.
Correlations between the z values of the brain regions and HAMD, HAMA, somatization subscale of SCL-90 in patients
Significantly positive correlation was observed between the z values of the voxels within the bilateral superior MPFC and the somatization subscale scores of SCL-90 at 0.05 significance level (combined height threshold p<0 .01 and a minimum cluster size = 52) (see Figure 3) in the patient group. No significant correlation was found between the z values of other brain regions and the scores of HAMD and HAMA in patients.
Figure 3. Voxel-wise correlation between the MPFC and the somatization subscale scores of SCL-90 in the patients.
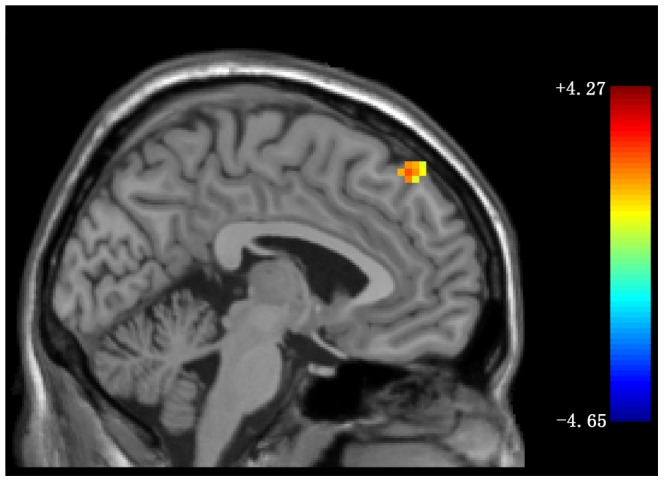
Sagittal view of the voxel-wise correlation analysis between z values of the voxels within the the bilateral superior MPFC (Montreal Neurological Institute coordinates: x = −4, y = 42, z = 52) and the somatization subscale scores of SCL-90 in the patient group at 0.05 significance level (combined height threshold p<0.01 and a minimum cluster size = 52). MPFC = medial prefrontal cortex; somatization subscale of SCL-90 = somatization subscale of the Symptom Check List.
Discussion
Using the fALFF method, the present study demonstrated that fALFF abnormalities of the DMN in medication-naive, first-episode patients with SD. Compared to healthy controls, the patients showed higher fALFF values in the bilateral superior MPFC, and lower fALFF values in the left PCu. Our findings provided evidence of aberrant regional activities in the DMN in patients with SD.
MPFC and PCu are recognized as the core regions of the DMN. Anatomically, MPFC is consisted of cytoarchitectonically discrete areas that receive a wide range of sensory information from the body and the external environment via the orbital prefrontal cortex [36]–[38]. Moreover, MPFC has wide connections among affective-limbic areas (such as the amygdala, hippocampus, and hypothalamus), executive control and emotional processing areas (such as orbital frontal cortex, ACC) [39]. Such anatomical relationships support a role for these medial areas in the integration of the visceromotor aspects of emotional processing with information gathered from the internal and external environments. It is assumed that MPFC plays an important role in emotional processing, such as attention to emotion, identification, or regulation of emotion [40], [41]. Some researchers proposed that MPFC plays a role in the integration of emotional and cognitive processes by incorporating emotional biasing signals or markers into decision-making process [42]–[44]. The different spontaneous neural activity in MPFC may result in the dysfunction of this region, and lead to a loss of top-down regulation, which is regarded as the basis of the pathophysiology of emotional, cognitive and behavioral changes in SD. In somatoform pain disorder patients, MPFC has been found to be dysfunctional. A pain related to hypoactivity of MPFC was found in the somatoform pain disorder patients when under the same pain intensity and pain unpleasantness [45]. It suggests that dysfunction of MPFC may be correlated to somatic pain. Additionally, the correlation between MPFC and pain catastrophizing is supported by neuroimaging data in subjects with chronic pain (fibromyalgia) [46]. Consistent with its key role in both emotion and cognition, we found that increased fALFF existed in MPFC in SD patients, and the z values of the voxels within the MPFC was positively correlated to the somatization subscale scores of SCL-90. The significantly positive correlation suggests that fALFF in MPFC may be applied as an indicator for the somatic symptom severity. Our study thus extends the prior findings by providing new evidence that different regional activity of MPFC may be involved in the pathophysiology of SD.
PCu, which locates in the posteromedial cortex of the parietal lobe, has traditionally received little attention mainly because of its hidden location and rarely lesioned in strokes or accidents. However, its strategic location and wide-spread connections suggest that PCu is a major association area that may subserve a variety of fundamental cognitive and behavioral functions, including visuo-spatial imagery, episodic memory retrieval, self-processing operations and consciousness [47], [48]. It is noteworthy that PCu plays a key role in implementation of a wide range of higher-order cognitive functions. An fMRI study by Knauff et al. investigated the PCu was linked to the cognitive processes of mental imagery in deductive reasoning [49]. PCu has wide-spread associations with cortical and subcortical structures, indicating the complexity of its behavioral specializations. Gusnard et al. suggested that PCu and interconnected medial prefrontal cortices and PCC were engaged in continuous information gathering and representation of the self and external world [50]. Furthermore, PCu and prefrontal cortex are strongly interconnected, and these projections tend to concentrate at the level of BA 8, 9 [51]–[53]. Our findings showed that different neural activity was found in PCu and MPFC in SD patients. The interconnection between PCu and MPFC in SD patients is unclear. However, reduced PCu activation was observed to lead to a loss of MPFC deactivation in Parkinson's disease [54]. Hence, decreased fALFF in PCu in the current study suggests that PCu acts as a role in the pathophysiology of SD.
Previously, an anterior and posterior subsystem of the DMN was identified [55]–[57]. The anterior DMN have been detected hyperactivity in healthy individuals when under pain stimuli [58]. In an fMRI study, the chronic pain disorders showed higher power spectra in the anterior DMN [59]. Our study showed a dissociation pattern between the anterior and posterior parts of DMN, with hyperactivity in anterior regions of the DMN (bilateral superior MPFC) and hypoactivity in the posterior regions of the DMN (left PCu). The role of the DMN is known to participant in self-referential activity, episodic memory retrieval, motivation, cognition, and emotion modulation [13], [60]–[62]. The dissociation pattern of the DMN may result in somatic symptom complex and emotional, cognitive, behavioral changes.
In addition to a relatively small sample size, other limitations should be considered in explaining the results. First, high level of depression and anxiety was found in patients. It is well known that comorbidities existed among depression, anxiety and somatization with a high ratio [63]–[66]. Depression and anxiety may be inherent characteristics of SD, which can not be removed in the analysis. For the same reason, depression and anxiety might have biased the present findings. Second, due to the fact that SD is common in the females, a high proportion of females were enrolled in the present study, which might limit the generalizability of our results. Third, the present study mainly focused on the DMN. It is helpful for understanding the pathophysiological contribution of the DMN. For the same reason, some meaningful findings from other brain regions might be neglected. Fourth, the fALFF method applied in the present study has its own limitations. Although fALFF is regarded as representing regional signal dynamics, the exact physiological nature of fALFF is not entirely clear. Finally, the effects of instrument noise during resting fMRI scans could not be avoided. The noise would pass the LFOs (0.01–0.08 Hz) and influence our findings [67]. In future study, a more rigorous method should be explored to remove such noises.
Conclusions
Despite the limitations, our findings indicate that there is a dissociation pattern of the anterior and posterior DMN in first-episode, treatment-naive patients with SD. The results provide new insight for the importance of the DMN in the pathophysiology of SD.
Acknowledgments
We would like to thank Prof. Dr. Bernard. A. Goodman (Guangxi University, China) and Dr. Shaogang Liu (Guangxi University for Nationalities, China) for their assistance in completing this project. The authors also thank all individuals who served as the research participants.
Funding Statement
The study was supported by Grants from the National Natural Science Foundation of China (Grant nos. 81260210, and 30900483), the Natural Science Foundation of Guangxi (Grant no. 2013GXNSFAA019107), and the special funding by the Ministry of Health of the Peoples' Republic of China (Grant no. 201002003). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Katon W, Lin E, Von Korff M, Russo J, Lipscomb P, et al. (1991) Somatization: a spectrum of severity. Am J Psychiatry 148: 34–40. [DOI] [PubMed] [Google Scholar]
- 2. Rief W, Hessel A, Braehler E (2001) Somatization symptoms and hypochondriacal features in the general population. Psychosom Med 63: 595–602. [DOI] [PubMed] [Google Scholar]
- 3.Sadock BJ, Kaplan HI, Sadock VA (2007) Kaplan & Sadock's synopsis of psychiatry: behavioral sciences/clinical psychiatry. 10th edn. Philadelphia: Lippincott Williams & Wilkins. pp 634–640. [Google Scholar]
- 4. Lipowski ZJ (1988) Somatization: the concept and its clinical application. Am J Psychiatry 145: 1358–1368. [DOI] [PubMed] [Google Scholar]
- 5. Barsky AJ, Orav EJ, Bates DW (2005) Somatization increases medical utilization and costs independent of psychiatric and medical comorbidity. Arch Gen Psychiatry 62: 903–910. [DOI] [PubMed] [Google Scholar]
- 6. Koch H, van Bokhoven MA, ter Riet G, van der Weijden T, Dinant GJ, et al. (2007) Demographic characteristics and quality of life of patients with unexplained complaints: a descriptive study in general practice. Qual Life Res 16: 1483–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Konnopka A, Schaefert R, Heinrich S, Kaufmann C, Luppa M, et al. (2012) Economics of medically unexplained symptoms: a systematic review of the literature. Psychother Psychosom 81: 265–275. [DOI] [PubMed] [Google Scholar]
- 8. Dimsdale JE, Levenson J (2013) What's next for somatic symptom disorder? Am J Psychiatry 170: 1393–1395. [DOI] [PubMed] [Google Scholar]
- 9. Brody AL, Saxena S, Mandelkern MA, Fairbanks LA, Ho ML, et al. (2001) Brain metabolic changes associated with symptom factor improvement in major depressive disorder. Biol Psychiatry 50: 171–178. [DOI] [PubMed] [Google Scholar]
- 10. Atmaca M, Sirlier B, Yildirim H, Kayali A (2011) Hippocampus and amygdalar volumes in patients with somatization disorder. Prog Neuropsychopharmacol Biol Psychiatry 35: 1699–1703. [DOI] [PubMed] [Google Scholar]
- 11. Fayed N, Andres E, Rojas G, Moreno S, Serrano-Blanco A, et al. (2012) Brain dysfunction in fibromyalgia and somatization disorder using proton magnetic resonance spectroscopy: a controlled study. Acta Psychiatr Scand 126: 115–125. [DOI] [PubMed] [Google Scholar]
- 12. Lemche E, Giampietro VP, Brammer MJ, Surguladze SA, Williams SC, et al. (2013) Somatization severity associated with postero-medial complex structures. Sci Rep 3: 1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greicius MD, Krasnow B, Reiss AL, Menon V (2003) Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, et al. (2001) A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raichle ME, Gusnard DA (2005) Intrinsic brain activity sets the stage for expression of motivated behavior. J Comp Neurol 493: 167–176. [DOI] [PubMed] [Google Scholar]
- 16. Raichle ME, Snyder AZ (2007) A default mode of brain function: a brief history of an evolving idea. NeuroImage 37: 1083–1090. [DOI] [PubMed] [Google Scholar]
- 17. Fox MD, Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8: 700–711. [DOI] [PubMed] [Google Scholar]
- 18. Browning M, Fletcher P, Sharpe M (2011) Can neuroimaging help us to understand and classify somatoform disorders? A systematic and critical review. Psychosom Med 73: 173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, et al. (2008) An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Meth 172: 137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, et al. (2010) The oscillating brain: complex and reliable. NeuroImage 49: 1432–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kunisato Y, Okamoto Y, Okada G, Aoyama S, Nishiyama Y, et al. (2011) Personality traits and the amplitude of spontaneous low-frequency oscillations during resting state. Neurosci Lett 492: 109–113. [DOI] [PubMed] [Google Scholar]
- 22. Kunisato Y, Okamoto Y, Okada G, Aoyama S, Demoto Y, et al. (2011) Modulation of default-mode network activity by acute tryptophan depletion is associated with mood change: a resting state functional magnetic resonance imaging study. Neurosci Res 69: 129–134. [DOI] [PubMed] [Google Scholar]
- 23. Hoptman MJ, Zuo XN, Butler PD, Javitt DC, D'Angelo D, et al. (2010) Amplitude of low-frequency oscillations in schizophrenia: a resting state fMRI study. Schizophr Res 117: 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lai CH, Wu YT (2012) Patterns of fractional amplitude of low-frequency oscillations in occipito-striato-thalamic regions of first-episode drug-naive panic disorder. J Affect Disord 142: 180–185. [DOI] [PubMed] [Google Scholar]
- 25. Liu CH, Ma X, Wu X, Fan TT, Zhang Y, et al. (2013) Resting-state brain activity in major depressive disorder patients and their siblings. J Affect Disord 149: 299–306. [DOI] [PubMed] [Google Scholar]
- 26. Liu F, Guo W, Liu L, Long Z, Ma C, et al. (2013) Abnormal amplitude low-frequency oscillations in medication-naive, first-episode patients with major depressive disorder: a resting-state fMRI study. J Affect Disord 146: 401–406. [DOI] [PubMed] [Google Scholar]
- 27. Guo W, Liu F, Zhang J, Zhang Z, Yu L, et al. (2013) Dissociation of regional activity in the default mode network in first-episode, drug-naive major depressive disorder at rest. J Affect Disord 151: 1097–1101. [DOI] [PubMed] [Google Scholar]
- 28. Guo W, Liu F, Liu J, Yu L, Zhang Z, et al. (2013) Is there a cerebellar compensatory effort in first-episode, treatment-naive major depressive disorder at rest? Prog Neuropsychopharmacol Biol Psychiatry 46: 13–18. [DOI] [PubMed] [Google Scholar]
- 29. Derogatis LR, Rickels K, Rock AF (1976) The SCL-90 and the MMPI: a step in the validation of a new self-report scale. Br J Psychiatry 128: 280–289. [DOI] [PubMed] [Google Scholar]
- 30. Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hamilton M (1959) The assessment of anxiety states by rating. Br J Med Psychol 32: 50–55. [DOI] [PubMed] [Google Scholar]
- 32.First MB, Spitzer RL, Gibbon M, Williams JBW (1997) Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), New York: Biometric Research Department. 671 p. [Google Scholar]
- 33. Song XW, Dong ZY, Long XY, Li SF, Zuo XN, et al. (2011) REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PloS one 6: e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guo W, Su Q, Yao D, Jiang J, Zhang J, et al. (2014) Decreased regional activity of default-mode network in unaffected siblings of schizophrenia patients at rest. Eur Neuropsychopharmacol 24: 545–552. [DOI] [PubMed] [Google Scholar]
- 35. Guo W, Yao D, Jiang J, Su Q, Zhang Z, et al. (2014) Abnormal default-mode network homogeneity in first-episode, drug-naive schizophrenia at rest. Prog Neuropsychopharmacol Biol Psychiatry 49: 16–20. [DOI] [PubMed] [Google Scholar]
- 36. Carmichael ST, Price JL (1995) Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol 363: 642–664. [DOI] [PubMed] [Google Scholar]
- 37. Barbas H (1993) Organization of cortical afferent input to orbitofrontal areas in the rhesus monkey. Neuroscience 56: 841–864. [DOI] [PubMed] [Google Scholar]
- 38. Rolls ET, Baylis LL (1994) Gustatory, olfactory, and visual convergence within the primate orbitofrontal cortex. J Neurosci 14: 5437–5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alalade E, Denny K, Potter G, Steffens D, Wang L (2011) Altered cerebellar-cerebral functional connectivity in geriatric depression. PloS one 6: e20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Phillips ML, Drevets WC, Rauch SL, Lane R (2003) Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry 54: 504–514. [DOI] [PubMed] [Google Scholar]
- 41. Teasdale JD, Howard RJ, Cox SG, Ha Y, Brammer MJ, et al. (1999) Functional MRI study of the cognitive generation of affect. Am J Psychiatry 156: 209–215. [DOI] [PubMed] [Google Scholar]
- 42. Damasio A (2008) Descartes' error: Emotion, reason and the human brain. Random House [Google Scholar]
- 43. Bechara A, Damasio AR, Damasio H, Anderson SW (1994) Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50: 7–15. [DOI] [PubMed] [Google Scholar]
- 44. Bechara A, Damasio H, Tranel D, Damasio AR (1997) Deciding advantageously before knowing the advantageous strategy. Science 275: 1293–1295. [DOI] [PubMed] [Google Scholar]
- 45. Gundel H, Valet M, Sorg C, Huber D, Zimmer C, et al. (2008) Altered cerebral response to noxious heat stimulation in patients with somatoform pain disorder. Pain 137: 413–421. [DOI] [PubMed] [Google Scholar]
- 46. Gracely RH, Geisser ME, Giesecke T, Grant MA, Petzke F, et al. (2004) Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain 127: 835–843. [DOI] [PubMed] [Google Scholar]
- 47. Cavanna AE, Trimble MR (2006) The precuneus: a review of its functional anatomy and . behavioural correlates. Brain 129: 564–583. [DOI] [PubMed] [Google Scholar]
- 48. Ogiso T, Kobayashi K, Sugishita M (2000) The precuneus in motor imagery: a magnetoencephalographic study. Neuroreport 11: 1345–1349. [DOI] [PubMed] [Google Scholar]
- 49. Knauff M, Fangmeier T, Ruff CC, Johnson-Laird PN (2003) Reasoning, models, and images: behavioral measures and cortical activity. J Cogn Neurosci 15: 559–573. [DOI] [PubMed] [Google Scholar]
- 50. Gusnard DA, Raichle ME, Raichle ME (2001) Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2: 685–694. [DOI] [PubMed] [Google Scholar]
- 51. Petrides M, Pandya DN (1984) Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Comp Neurol 228: 105–116. [DOI] [PubMed] [Google Scholar]
- 52. Leichnetz GR (2001) Connections of the medial posterior parietal cortex (area 7 m) in the monkey. Anat Rec 263: 215–236. [DOI] [PubMed] [Google Scholar]
- 53. Cavada C, Goldman-Rakic PS (1989) Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol 287: 422–445. [DOI] [PubMed] [Google Scholar]
- 54. Argyelan M, Carbon M, Ghilardi MF, Feigin A, Mattis P, et al. (2008) Dopaminergic suppression of brain deactivation responses during sequence learning. J Neurosci 28: 10687–10695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, et al. (2008) Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex 18: 1856–1864. [DOI] [PubMed] [Google Scholar]
- 56. Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M (2007) Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci USA 104: 13170–13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li B, Liu L, Friston KJ, Shen H, Wang L, et al. (2013) A treatment-resistant default mode subnetwork in major depression. Biol Psychiatry 74: 48–54. [DOI] [PubMed] [Google Scholar]
- 58. Otti A, Guendel H, Laer L, Wohlschlaeger AM, Lane RD, et al. (2010) I know the pain you feel-how the human brain's default mode predicts our resonance to another's suffering. Neuroscience 169: 143–148. [DOI] [PubMed] [Google Scholar]
- 59. Otti A, Guendel H, Wohlschlager A, Zimmer C, Noll-Hussong M (2013) Frequency shifts in the anterior default mode network and the salience network in chronic pain disorder. BMC psychiatry 13: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, et al. (2005) The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102: 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fransson P (2006) How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia 44: 2836–2845. [DOI] [PubMed] [Google Scholar]
- 62. Amft M, Bzdok D, Laird AR, Fox PT, Schilbach L, et al. (2014) Definition and characterization of an extended social-affective default network. Brain Struct Funct doi: 10.1007/s00429-013-0698-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Simon GE, VonKorff M, Piccinelli M, Fullerton C, Ormel J (1999) An international study of the relation between somatic symptoms and depression. New Engl J Med 341: 1329–1335. [DOI] [PubMed] [Google Scholar]
- 64. Lowe B, Spitzer RL, Williams JB, Mussell M, Schellberg D, et al. (2008) Depression, anxiety and somatization in primary care: syndrome overlap and functional impairment. Gen Hosp Psychiatry 30: 191–199. [DOI] [PubMed] [Google Scholar]
- 65. Kroenke K, Spitzer RL, deGruy FV III, Hahn SR, Linzer M, et al. (1997) Multisomatoform disorder. An alternative to undifferentiated somatoform disorder for the somatizing patient in primary care. Arch Gen Psychiatry 54: 352–358. [DOI] [PubMed] [Google Scholar]
- 66. Brown FW, Golding JM, Smith GR Jr (1990) Psychiatric comorbidity in primary care somatization disorder. Psychosom Med 52: 445–451. [DOI] [PubMed] [Google Scholar]
- 67. Lowe MJ, Mock BJ, Sorenson JA (1998) Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage 7: 119–132. [DOI] [PubMed] [Google Scholar]