Abstract
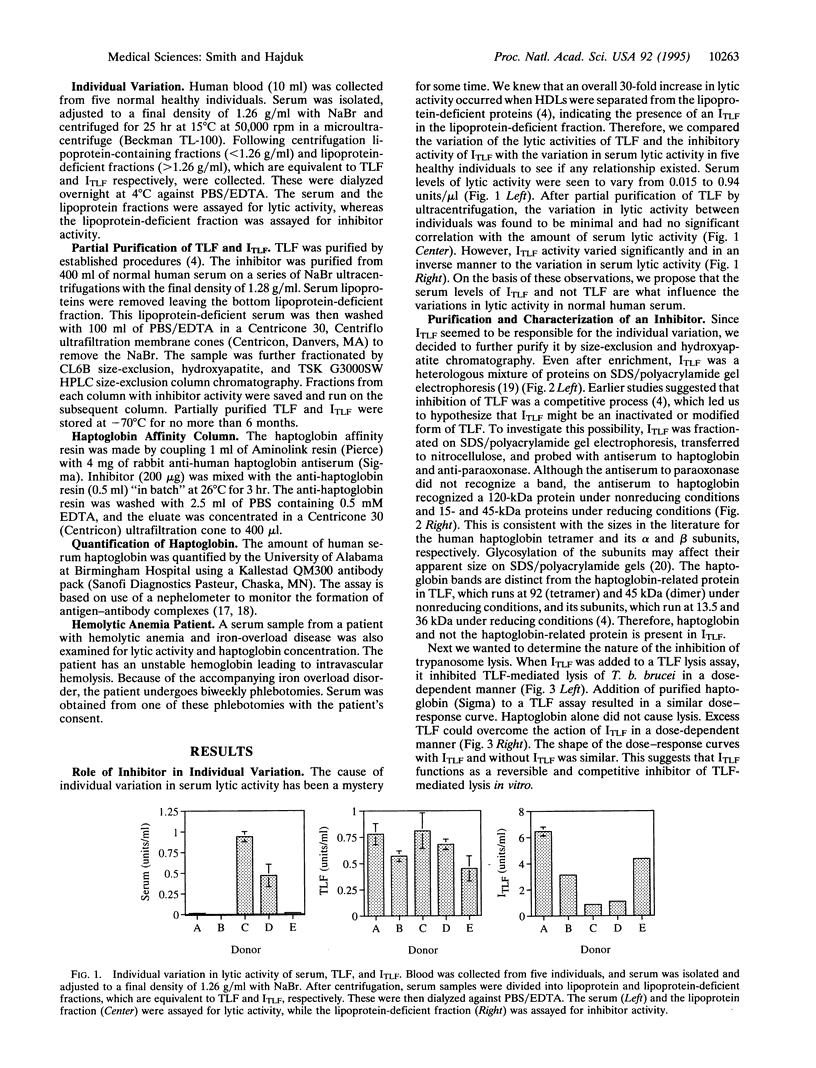
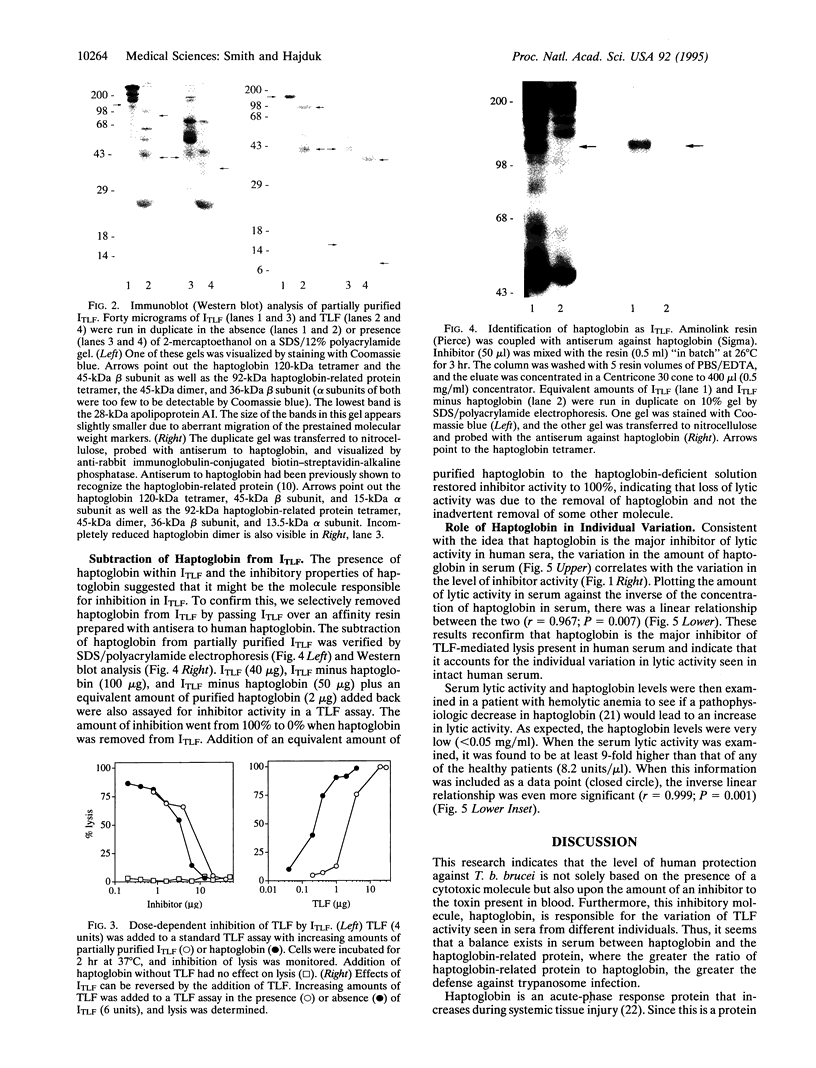
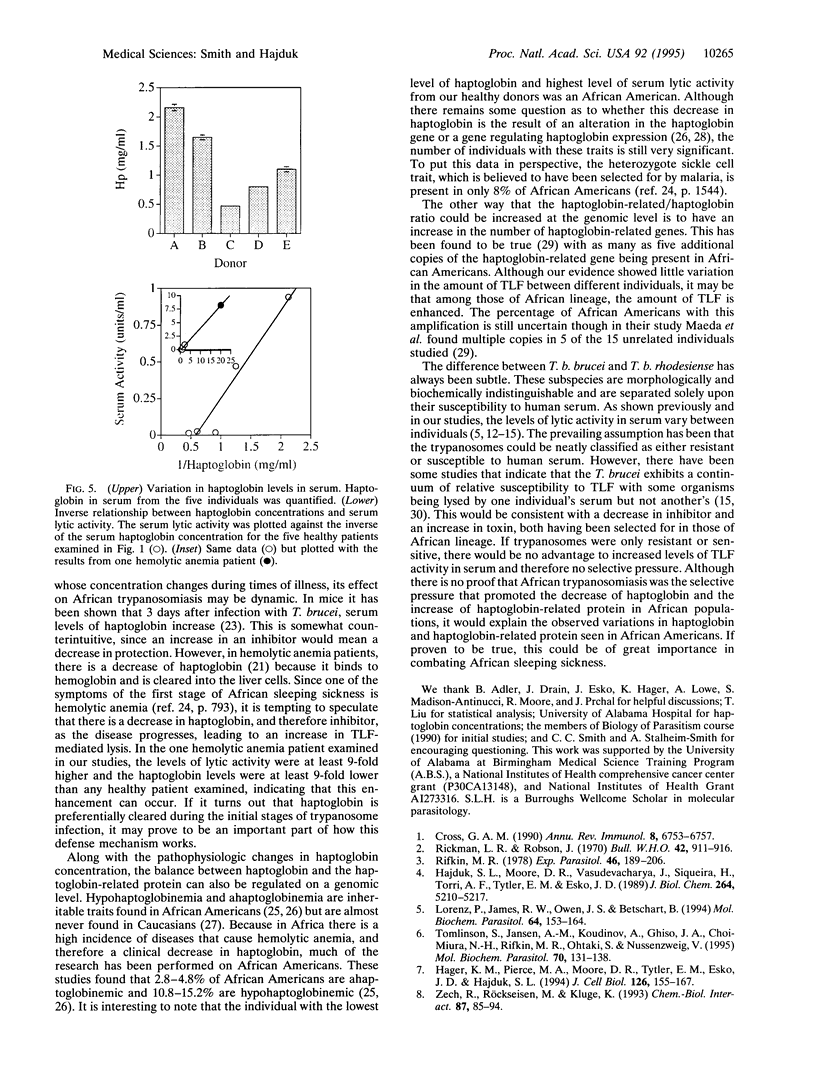
Trypanosomes are protozoan parasites of medical and veterinary importance. Trypanosoma brucei rhodesiense and Trypanosoma brucei gambiense infect humans, causing African sleeping sickness. However, Trypanosoma brucei brucei can only infect animals, causing the disease Nagana in cattle. Man is protected from this subspecies of trypanosomes by a toxic subtype of high density lipoproteins (HDLs) called the trypanosome lytic factor (TLF). The toxic molecule in TLF is believed to be the haptoglobin-related protein that when bound to hemoglobin kills the trypanosome via oxidative damage initiated by its peroxidase activity. The amount of lytic activity in serum varies widely between different individuals with up to a 60-fold difference in activity. In addition, an increase in the total amount of lytic activity occurs during the purification of TLF, suggesting that an inhibitor of TLF (ITLF) exists in human serum. We now show that the individual variation in trypanosome lytic activity in serum correlates to variations in the amount of ITLF. Immunoblots of ITLF probed with antiserum against haptoglobin recognize a 120-kDa protein, indicating that haptoglobin is present in partially purified ITLF. Haptoglobin involvement is further shown in that it inhibits TLF in a manner similar to ITLF. Using an anti-haptoglobin column to remove haptoglobin from ITLF, we show that the loss of haptoglobin coincides with the loss of inhibitor activity. Addition of purified haptoglobin restores inhibitor activity. This indicates that haptoglobin is the molecule responsible for inhibition and therefore causing the individual variation in serum lytic activity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., BLUMBERG B. S., AP REES Haptoglobin types in British, Spanish Basque and Nigerian African populations. Nature. 1958 Mar 22;181(4612):824–825. doi: 10.1038/181824a0. [DOI] [PubMed] [Google Scholar]
- Abebe M., Bulto T., Endesha T., Nigatu W. Further studies on the Trypanosoma brucei group trypanosome, isolated from a patient infected in Anger-Didessa Valley, West Ethiopia, using the blood incubation infectivity test (BIIT). Short communication. Acta Trop. 1988 Jun;45(2):185–186. [PubMed] [Google Scholar]
- GIBLETT E. R., STEINBERG A. G. The inheritance of serum haptoglobin types in American Negroes: evidence for a third allele Hp-2m. Am J Hum Genet. 1960 Jun;12:160–169. [PMC free article] [PubMed] [Google Scholar]
- GITLIN D., EDELHOCH H. A study of the reaction between human serum albumin and its homologous equine antibody through the medium of light scattering. J Immunol. 1951 Jan;66(1):67–77. [PubMed] [Google Scholar]
- GOLDBERG R. J., CAMPBELL D. H. The light-scattering properties of an antigen-antibody reaction. J Immunol. 1951 Jan;66(1):79–86. [PubMed] [Google Scholar]
- Gillett M. P., Owen J. S. Trypanosoma brucei brucei: differences in the trypanocidal activity of human plasma and its relationship to the level of high density lipoproteins. Trans R Soc Trop Med Hyg. 1991 Sep-Oct;85(5):612–616. doi: 10.1016/0035-9203(91)90365-6. [DOI] [PubMed] [Google Scholar]
- Hager K. M., Pierce M. A., Moore D. R., Tytler E. M., Esko J. D., Hajduk S. L. Endocytosis of a cytotoxic human high density lipoprotein results in disruption of acidic intracellular vesicles and subsequent killing of African trypanosomes. J Cell Biol. 1994 Jul;126(1):155–167. doi: 10.1083/jcb.126.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajduk S. L., Moore D. R., Vasudevacharya J., Siqueira H., Torri A. F., Tytler E. M., Esko J. D. Lysis of Trypanosoma brucei by a toxic subspecies of human high density lipoprotein. J Biol Chem. 1989 Mar 25;264(9):5210–5217. [PubMed] [Google Scholar]
- Hawking F., Ramsden D. B., Whytock S. The trypanocidal action of human serum and of baboon plasma. Trans R Soc Trop Med Hyg. 1973;67(4):501–516. doi: 10.1016/0035-9203(73)90081-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lorenz P., James R. W., Owen J. S., Betschart B. Heterogeneity in the properties of the trypanolytic factor in normal human serum. Mol Biochem Parasitol. 1994 Mar;64(1):153–164. doi: 10.1016/0166-6851(94)90143-0. [DOI] [PubMed] [Google Scholar]
- Maeda N., McEvoy S. M., Harris H. F., Huisman T. H., Smithies O. Polymorphisms in the human haptoglobin gene cluster: chromosomes with multiple haptoglobin-related (Hpr) genes. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7395–7399. doi: 10.1073/pnas.83.19.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N. Nucleotide sequence of the haptoglobin and haptoglobin-related gene pair. The haptoglobin-related gene contains a retrovirus-like element. J Biol Chem. 1985 Jun 10;260(11):6698–6709. [PubMed] [Google Scholar]
- Malchy B., Rorstad O., Dixon G. H. The half-molecule of haptoglobin: studies on the product obtained by the selective cleavage of a haptoglobin disulfide. Can J Biochem. 1973 Mar;51(3):265–273. doi: 10.1139/o73-033. [DOI] [PubMed] [Google Scholar]
- NOSSLIN B. F., NYMAN M. Haptoglobin determination in diagnosis of haemolytic diseases. Lancet. 1958 May 10;1(7028):1000–1001. doi: 10.1016/s0140-6736(58)91804-x. [DOI] [PubMed] [Google Scholar]
- PARKER W. C., BEARN A. G. Control gene mutation as a possible explanation of certain haptoglobin phenotypes. Am J Hum Genet. 1963 Jun;15:159–181. [PMC free article] [PubMed] [Google Scholar]
- Pluschke G., Jenni L., van Alphen L., Lefkovits I. Complete two-dimensional gel electrophoresis pattern of de novo synthesized acute phase reactants. Clin Exp Immunol. 1986 Nov;66(2):331–339. [PMC free article] [PubMed] [Google Scholar]
- Rickman L. R., Robson J. The testing of proven Trypanosoma brucei and T. rhodesiense strains by the blood incubation infectivity test. Bull World Health Organ. 1970;42(6):911–916. [PMC free article] [PubMed] [Google Scholar]
- Rifkin M. R. Identification of the trypanocidal factor in normal human serum: high density lipoprotein. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3450–3454. doi: 10.1073/pnas.75.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin M. R. Trypanosoma brucei: some properties of the cytotoxic reaction induced by normal human serum. Exp Parasitol. 1978 Dec;46(2):189–206. doi: 10.1016/0014-4894(78)90131-5. [DOI] [PubMed] [Google Scholar]
- SUTTON H. E., KARP G. W., Jr VARIATIONS IN HETEROZYGOUS EXPRESSION AT THE HAPTOGLOBIN LOCUS. Am J Hum Genet. 1964 Dec;16:419–434. [PMC free article] [PubMed] [Google Scholar]
- Seed J. R., Sechelski J. B., Ortiz J. C., Chapman J. F. Relationship between human serum trypanocidal activity and host resistance to the African trypanosomes. J Parasitol. 1993 Apr;79(2):226–232. [PubMed] [Google Scholar]
- Smith A. B., Esko J. D., Hajduk S. L. Killing of trypanosomes by the human haptoglobin-related protein. Science. 1995 Apr 14;268(5208):284–286. doi: 10.1126/science.7716520. [DOI] [PubMed] [Google Scholar]
- Stadnyk A., Gauldie J. The acute phase protein response during parasitic infection. Immunol Today. 1991 Mar;12(3):A7–12. doi: 10.1016/s0167-5699(05)80004-0. [DOI] [PubMed] [Google Scholar]
- Tomlinson S., Jansen A. M., Koudinov A., Ghiso J. A., Choi-Miura N. H., Rifkin M. R., Ohtaki S., Nussenzweig V. High-density-lipoprotein-independent killing of Trypanosoma brucei by human serum. Mol Biochem Parasitol. 1995 Mar;70(1-2):131–138. doi: 10.1016/0166-6851(95)00019-w. [DOI] [PubMed] [Google Scholar]
- Tytler E. M., Moore D. R., Pierce M. A., Hager K. M., Esko J. D., Hajduk S. L. Reconstitution of the trypanolytic factor from components of a subspecies of human high-density lipoproteins. Mol Biochem Parasitol. 1995 Jan;69(1):9–17. doi: 10.1016/0166-6851(94)00172-j. [DOI] [PubMed] [Google Scholar]
- Zech R., Röckseisen M., Kluge K., Dewald K., Armstrong V. W., Chemnitius J. M. Lipoproteins and hydrolysis of organophosphorus compounds. Chem Biol Interact. 1993 Jun;87(1-3):85–94. doi: 10.1016/0009-2797(93)90028-w. [DOI] [PubMed] [Google Scholar]





