Abstract
Use of complementary approaches is common among breast cancer survivors. Potential interactions between aromatase inhibitors (AI) and high phytoestrogen foods, such as flaxseed (FS) are not often described. We conducted a pilot 2×2 factorial, randomized intervention study between tumor biopsy and resection, in 24 postmenopausal women with estrogen receptor positive (ER+) breast cancer, to assess the effects of flaxseed and anastrozole, and possible interactions between them, on serum steroid hormone and tumor-related characteristics associated with long-term survival (Roswell Park Cancer Institute, 2007–2010). The effect of each treatment vs placebo on outcomes was determined by linear regression adjusting for pre-treatment measure, stage, and grade. Although not statistically significant, mean ERβ expression was approximately 40% lower from pre- to post-intervention in the FS+AI group only. We observed a statistically significant negative association (β±SE −0.3±0.1; p=0.03) for androstenedione in the FS+AI group vs placebo and for DHEA with AI treatment (β±SE −1.6±0.6; p=0.009). Enterolactone excretion was much lower in the FS+AI group compared to the FS group. Our results do not support strong effects of flaxseed on AI activity for selected breast tumor characteristics or serum steroid hormone levels, but suggest AI therapy might reduce the production of circulating mammalian lignans from flaxseed.
Keywords: Breast cancer, clinical trial, intervention, flaxseed, aromatase inhibitors, hormones, complementary medicine
Introduction
The burden of breast cancer is significant, despite widespread screening and improved adjuvant treatments that have resulted in earlier diagnosis and longer survival (1). Consequently, breast cancer survivors are interested in modalities that might improve their recurrence free survival. For estrogen-receptor positive tumors, anti-estrogen therapy has been considered standard of care (2, 3). The goal of endocrine treatment is to prevent access of the tumor to estrogen, either through blocking the interaction of the estrogen receptor with co-regulatory proteins in tumor cells (Tamoxifen), or through a reduction or elimination of endogenous estrogen production (aromatase inhibitors) (4). Aromatase inhibitors block the enzyme aromatase that catalyzes the conversion of androgens into estrogens, the primary source of endogenous estrogens in postmenopausal women. The primary site of action for aromatase is in peripheral adipose tissue, and aromatase is particularly high in the breast. Anastrozole is a widely used non-steroidal aromatase inhibitor that competes with the endogenous ligands androstenedione and testosterone for the active site of aromatase by promoting metabolism to intermediates that bind irreversibly to the active site (5–7).
In addition to pharmacologic agents, complementary and alternative medicine approaches are widely used by cancer survivors in an attempt to prevent disease recurrence, to reduce the frequency and severity of side effects, and to provide potential health benefits. Complementary and alternative medicine use is particularly high among breast cancer survivors, and use is directly related to disease stage and severity (8–10). Vitamin and mineral supplementation are the most frequent, but use of other supplements is common (10). As patients tend to not discuss use of dietary supplements with their physicians (11–13), consideration of complementary and alternative medicine use in cancer treatment is important as dietary supplements may have substantial independent physiologic effects, and diet-drug interactions may occur.
Flaxseed is a naturally occurring food, often consumed as a dietary supplement, which contains high amounts of lignans. Lignans are considered phytoestrogens, naturally occurring diphenolic compounds structurally similar to endogenous estrogens and Tamoxifen (14). Lignans are found in a variety of plant foods, including whole grains, seeds, coffee, tea, vegetables and legumes, although the lignan content in flaxseed is over 100 times that found in other foods (15). The major circulating lignan, enterolactone, is considered a weak estrogen compared to estradiol. In human and experimental studies, flaxseed ingestion has been shown to affect both endocrine and growth factor pathways by modifying steroid hormone metabolism (16–18), modifying IGF and EGFR (19, 20, 20), and inhibiting aromatase and 17β-hydroxysteroid dehydrogenase (21, 22). Lignans inhibit cell proliferation in both estrogen receptor positive and negative cell lines (20, 23), reduce tumor growth and metastasis in a number of animal models (24–27), and work synergistically with Tamoxifen to reduce tumor growth (23). In human studies, ingestion of 25 g/day flaxseed for 4 weeks down-regulated Ki-67 by 34%, increased apoptosis by 30%, and reduced HER2neu by 71% in human breast cancer (28). Similar effects have been reported in two clinical studies of flaxseed and prostate cancer (29, 30). These qualities suggest a potential benefit of flaxseed in the adjuvant setting. However, the majority of human studies investigating the biologic effects of flaxseed have involved healthy women. There is a paucity of clinical data regarding the efficacy and safety of use of flaxseed among women after breast cancer, and many breast cancer survivors are prescribed hormone treatments such as Tamoxifen or aromatase inhibitors for up to 10 years after surgery, chemotherapy, and/or radiation.
Use of concomitant hormonal treatments is contraindicated during treatment with anastrozole, as these may reduce the efficacy of the drug (http://www.arimidex.net). However, no restriction is specified for foods containing high amounts of phytoestrogens such as soy or flaxseed. Although the phytoestrogens supplied by foods tend to be weak compared to estradiol, circulating levels resulting from usual dietary consumption can be much greater than that of endogenous estrogens. For example, as flaxseed provides over 100 times the amount of lignans obtainable in an average diet, concentrations of circulating lignans can reach pharmacologic levels through supplementation (31). Because the phytoestrogens in flaxseed can influence many of the same biologic pathways affected by anti-hormonal agents, the potential for physiologic effects exists and diet-drug interactions are possible (32). Potential synergistic or antagonistic effects between flaxseed and antiestrogens are of particular interest given the increasing use of aromatase inhibitors to treat postmenopausal women with hormone responsive disease. As an interaction has already been reported for flaxseed and Tamoxifen, similar interactions may be possible with anastrozole. Complementary and alternative medicine use is high among breast cancer patients, and is directly related to severity of disease (33). Furthermore, the potential benefit or negative impact of interactions with complementary and alternative medicine use is highest in this group of women.
Given the role of AIs in adjuvant treatment of breast cancer and the prevalent use of supplements such as flaxseed, we conducted a pilot 2×2 factorial, randomized intervention study between tumor biopsy and resection, in postmenopausal women diagnosed with estrogen receptor positive (ER+) breast cancer, to assess the effects of flaxseed and the aromatase inhibitor, anastrozole, on a number of steroid hormone and tumor-related characteristics associated with long-term survival, and to investigate the potential interaction between flaxseed and anastrozole on these biomarkers.
Methods
We utilized a 2×2 factorial randomized intervention design between tumor biopsy and resection, in postmenopausal women diagnosed with estrogen receptor positive breast cancer and receiving surgery at Roswell Park Cancer Institute (RPCI). Because of the availability of biopsy and resection tumor samples, the pre-surgical setting provides a unique opportunity to rapidly obtain information on intervention related effects on growth factor and signaling pathways related to tumor characteristics in a short time period without the interference of other treatments.
Patients
The protocol for the study was reviewed and approved by the RPCI Institutional Review Board and all participants provided signed informed consent. The study was registered with clinicaltrials.gov (NCT00612560). Postmenopausal women with newly diagnosed ER+ breast cancer were recruited during their pre-surgical consultation from the RPCI Breast Clinic between 12/17/2007 and 12/31/2010.
Patients were eligible for inclusion if they were newly diagnosed with incident, operable, ER+ invasive breast cancer, clinical stage II or lower, between the ages of 18 and 80 years, postmenopausal, ECOG performance status ≤1, usual consumption of soy < 1/wk, willing to avoid soy, herbal supplements, aspirin, and ibuprofen during intervention, undergoing surgical therapy. Menopause was defined as no menstrual cycle in the past 12 months, hysterectomy with bilateral oophorectomy, or hysterectomy with intact ovaries if age > 55 years. Women were ineligible for the following reasons: inability to read or write English; history of previous invasive breast cancer; insulin dependent diabetes; history of coagulopathy, thrombocytopenia, or bleeding disorder; current (past 60 days) regular use of reproductive hormone therapy, Tamoxifen, aromatase inhibitors, or other estrogen inhibitors, flaxseed, or antibiotics; allergy to flaxseed, nuts, or other seeds; renal dysfunction (creatinine > 1.5 mg/dl); history of Crohns’ disease, ulcerative colitis, irritable bowel syndrome, celiac sprue, or other malabsorption syndrome, diverticulitis, or other bowel diagnosis; and current, regular (> 1/wk) use of prescription platelet-inhibiting agents. A total of 2677 women were screened for eligibility. The major reasons for ineligibility were a non-cancer diagnosis (benign breast disease, n=1169), premenopausal (n=315), ductal carcinoma in situ (n=253), negative estrogen receptor status (n=187), and previous history of invasive cancer (n=129). Of the screened women, 117 were eligible, 9 who were approached for participation were lost to follow-up, and 57 declined participation. Of the 51 consented women, 3 withdrew, 28 were randomized and started treatment, and the remainder became ineligible (started antibiotics, surgery date changed, other reasons). For analysis, we included women with sufficient data which resulted in a final sample size of 24.
Intervention
After consent, an appointment was made for a baseline study visit 13–16 days prior to the patient’s scheduled surgery date. At the baseline visit, each patient was assigned via a randomized permuted block method to 1 of 4 groups: 25 g/d ground flaxseed + 1/d placebo pill; 1 mg/d anastrozole; 25 g/d ground flaxseed + 1 mg/d anastrozole; or 1/d placebo pill control. Patients and study personnel were blinded to pill status, but it was not possible to blind patients to flaxseed treatment. Patients were instructed to consume the treatments at the same time each day, and were allowed to mix the flaxseed with food.
Whole flaxseed was provided as a single lot from a single source (Pizzey’s Milling, P.O. Box 132/Main Street South, Angusville, Manitoba, Canada). Flaxseed was ground and packaged into 25 g foil packets for distribution to patients. Each patient was provided with sufficient numbers of packets for the 13–16 days of the intervention. Anastrozole and placebo pills were provided by AstraZeneca Pharmaceuticals LP (Wilmington, DE).
Biologic sample collection
The effects of the intervention on tumor growth and signaling pathways were assessed in formalin fixed paraffin embedded biopsy and resection samples. The number of days between biopsy and resection ranged from 1–50 (mean [SD] 18.8 [11.6]) and did not vary by treatment group (mean±SEFS 19.1 [12.2]; AI 20.0 [9.2]; FS+AI 18.0 [11.5]; and placebo 18.6 [17.8]; F=0.03, p=0.99). Tumor tissue was collected from biopsies and definitive surgical specimens were prepared as part of standard clinical care using standardized pathology protocols. Resection samples were formalin fixed and paraffin embedded and stored in the Pathology Core Facility until slides were cut for immunohistochemistry. Women diagnosed at non-RPCI facilities provided medical release permission for the biopsy samples. Tumor sectioning and staining was performed in the RPCI Pathology Core Facility with pathologist’s supervision (CM) and stained slides were scored by a board-certified surgical pathologist (DH).
For assessment of effects on steroid and growth hormone metabolism, fasting whole blood samples were obtained by trained phlebotomists using a standardized phlebotomy protocol during the baseline enrollment visit and on the morning of surgical resection. To monitor compliance with the flaxseed arms of the study, overnight urine collections were obtained at baseline and the evening immediately prior to surgery. Blood and urine were processed within an hour of collection and frozen at −80ºC in the Division of Cancer Prevention Biorepository until assay.
Outcomes
Pre- to post-intervention changes in breast tumor markers of proliferation (Ki-67), apoptosis (caspase), and the hormone receptor ERβ were assessed with immunohistochemistry. The antibodies used for immunohistochemistry in this study are shown in the Appendix. Immunohistochemistry was performed in the RPCI Pathology Shared Core Facility, with all pathology personnel blinded to treatment assignment. Briefly, four-micron thick unstained sections of each clinical sample were placed onto electrostatically charged glass slides and baked overnight. Optimal primary antibody incubation and concentrations were determined via serial dilutions for each immunohistochemical assay with an identically fixed and embedded appropriate control tissue. All staining was performed utilizing the AutoStainer™ series of automated stainers (Dako-Cytomation, California). Antigen detection was executed via a peroxidase-conjugated secondary antibody / 3,3V-diaminobenzidine chromogen step.
Serum steroid hormones (androstenedione, testosterone, and DHEA), urinary lignans, and IGF1, IGFBP3, and SHBG were all assayed in RPCI’s PK/PD Core Resource. IGF1, IGFBP3 and SHBG concentrations were measured with USCN ELISA Kits (E90050Hu; E90054Hu; E90396) (Houston, TX). Working standards were prepared according to the manufacturer’s instructions. Serum samples were diluted as per kit protocol. To each well, 100 μl of standard or sample was added. The plate was incubated for 2 hours at 37°C. One hundred μl of Detection Reagent A was added and the plate was incubated for 1 hour at 37°C. The plate was aspirated and washed three times. One hundred μl of Detection Reagent B was added and the plate was incubated for 30 minutes at 37°C. The plate was aspirated and washed five times. Ninety μl of Substrate solution was added and the plate was incubated for 15 – 25 minutes at 37°C. Fifty μl of Stop Solution was added and the plate was immediately read at 450 nm on a plate reader (Synergy HT; Bio-Tek Instruments). The concentration of each sample was determined based on the corresponding standard curve.
The dynamic ranges for IGF1, IGFBP3 and SHBG are 1.25 pg/ml – 40 pg/ml, 0.312 ng/ml – 20 ng/ml and 62.5 pg/ml – 4000 pg/ml respectively. For IGF1 and IGFBP3 the mean CV% for the calibrators was 6.7% and 6.11%, respectively. Percent CV is not available for SHBG since the curve was not run in duplicate. Average % difference for SHBG calibrators was 12.02%.
Androgens were detected by multiple reaction monitoring (MRM) using an AB SCIEX QTRAP® 5500 mass spectrometer with an electrospray ionization source in positive ion mode. Mass spectrometer conditions were ion spray voltage 5250 volts, turbo gas temperature 700 °C, nebulizer gas 55, turbo gas 65, curtain gas 16, CAD gas medium, and unit mass resolution for Q1 and Q3. Voltages for parent/fragment ion pair intensities were optimized using direct infusion and flow injection analysis.
Calibration and quality control (QC) samples were prepared in charcoal-stripped female human plasma and analyzed in each run. A 250 μl aliquot of sample was added to a glass screw-top tube followed by 750 μL of HPLC water, 100 μl of internal standard (IS) solution (d3-T/d3-DHT), and 4.0 ml of methyl-tert-butyl ether (MTBE). The samples were rotated for 15 minutes and centrifuged at 3,000 rpm and 4 °C for 15 minutes. The aqueous phase was frozen in a dry ice/acetone bath and the MTBE layer poured into a glass conical tube, and evaporated with nitrogen at 37 °C. The residue was reconstituted with 60.0 μl of 60% methanol, filtered, and 20 μl aliquot of filtrate injected.
HPLC analysis was performed using a Shimadzu Prominence UFLC System. Chromatographic separation was achieved using a Phenomenex Luna C18(2) column (3 μm, 2.0 mm × 150 mm, part number 00F-4251-B0) preceded by a Phenomenex SecurityGuard cartridge (C18). The analytical column was maintained at 60 °C and sample elution carried out at flow rate 175 μL/min with a biphasic gradient. Mobile phase A was 65% methanol with 0.4 ml of 1.0 M ammonium formate and 62.0 μl of concentrated formic acid per liter; mobile phase B was 100% methanol with 0.4 ml of 1.0 M ammonium formate and 62.0 μl of concentrated formic acid per liter.
Urine samples were analyzed for the lignans enterolactone and enterodiol using a Thermo Scientific Surveyor MS Pump Plus and Thermo Scientific TSQ Quantum Ultra. Column utilized was the Waters XBridge™ C18 3.5 μm, 2.1 × 50 mm (Part # 186003021 012337043188 31). The run time was 10 minutes and was performed in positive mode using a HESI probe. The mobile phase used was 60% of 0.1% ammonium acetate in water (pH 4.80 using 10% acetic acid) plus 40% methanol and the injection volume was 20 μl. All standard curves ranged from 0.5ng/ml to 100ng/ml and were prepared in 10% methanol. Bulk QCs 7.5 and 75ng/ml were also prepared in 10% methanol and stored for future analysis throughout the validation and patient studies. A 200μl aliquot of standards, quality control (QC) or patient samples were added to a 96 well plate. 240μl of a 1% Beta-Glucuronidase was added to hydrolyse the phytoestrogens to their aglycone forms and incubated overnight. Using a robotic liquid handler, a SPE Extraction Plate (Strata-X Phenomenex) was conditioned with 100% methanol followed by 30% methanol. The sample was added to the plate and was subsequently washed and eluted with 1:1 acetonitrile/methanol. The plate was dried under N2 and reconstituted with 40% methanol. The internal standard anthraflavin acid was used.
Statistical analyses
The characteristics of the participants in each study arm were described and compared using basic descriptive statistics. The comparability across treatment groups of participant characteristics and baseline (pre-treatment) measurements were assessed with ANOVA or chi-square tests as appropriate. Analysis of post-treatment measurements was conducted as intention-to-treat. Post-treatment summary statistics were reported by treatment arm. Effect of each treatment vs placebo was determined by a linear regression model of post-treatment measurement on treatment group, adjusted for pre-treatment value, age, and BMI. Residuals were analyzed using QQ plots but no transformations were made. Statistical significance was measured by marginal t-test vs placebo group. No correction for multiple testing was made.
Results
The demographic and clinical characteristics of study participants are summarized in Table 1. Although not statistically significant, participants in the AI and placebo groups were somewhat older than those in the FS or FS+AI groups (64.7±7.9, 64.2±8.5, 59.0±7.7, and 62.7±9.3, respectively). Most of the participants were overweight or obese with mean BMI ranging from 28.6–33.2 across treatment groups. The majority of participants had 12 or more years of education. Clinical breast cancer characteristics were comparable across groups as well. Mean tumor size ranged from 1.1 cm in the FS+AI and placebo groups to 1.3 cm in the FS and AI groups. The majority of tumors were stage I with grade fairly evenly distributed across treatments.
Table 1.
Demographic and clinical characteristics by treatment assignment of women with breast cancer participating in the FaBrC Study
| Flaxseed (n=6) |
Anastrazole (n=7) |
Flaxseed/Anastrazole (n=6) |
Placebo (n=5) |
|
|---|---|---|---|---|
| Mean (SD) | ||||
| Age, y | 59.0 (7.7) | 64.7 (7.9) | 62.7 (9.3) | 64.2 (8.5) |
| BMI, kg/m2 | 30.9 (8.9) | 28.6 (5.7) | 33.2 (11.2) | 29.5 (7.2) |
| Tumor size, cm | 1.3 (0.5) | 1.3 (1.3) | 1.1 (0.4) | 1.1 (0.8) |
| n (%) | ||||
| Education | ||||
| <12 y | 2 (33.3) | 3 (50.0) | 2 (40.0) | 1 (20.0) |
| ≥12 y | 4 (66.7) | 3 (50.0) | 3 (60.0) | 4 (80.0) |
| Clinical stage | ||||
| I | 4 (66.7) | 6 (85.7) | 5 (83.3) | 3 (60.0) |
| IIA | 1 (16.7) | 1 (14.3) | 1 (16.7) | 2 (40.0) |
| IIB | 1 (16.7) | |||
| Histologic grade | ||||
| I | 1 (16.7) | 1 (20.0) | ||
| II | 1 (16.7) | 3 (42.9) | 4 (66.7) | 1 (20.0) |
| III | 4 (66.7) | 4 (57.1) | 2 (33.3) | 3 (60.0) |
Differences in characteristics across treatment groups p>0.05 assessed with Wilcoxon Rank Sum test for ordinal responses and Pearson Chi square for categorical responses
Urinary lignan excretion was measured as a biomarker of compliance. As shown in Table 2, enterolactone and enterodiol excretion increased dramatically from pre- to post-intervention in women assigned to either FS or FS+AI treatments. Interestingly, despite equal doses of flaxseed in each group, enterolactone and enterodiol excretion was much lower in the FS+AI group compared to the FS group. No increases were observed in the AI or placebo groups.
Table 2.
Pre- and post-intervention urinary lignan excretion by treatment assignment
| Flaxseed (n=6) |
Anastrazole (n=7) |
Flaxseed/Anastrazole (n=6) |
Placebo (n=5) |
|
|---|---|---|---|---|
| Mean (SD) | ||||
| Enterolactone, ng/ml | ||||
| Pre-intervention | 42.7 (38.2) | 20.9 (12.5) | 27.1 (25.2) | 23.5 (20.6) |
| Post-intervention | 1262.0 (1604.6) | 27.5 (18.3) | 489.5 (539.6) | 13.6 (16.7) |
| Enterodiol, ng/ml | ||||
| Pre-intervention | 12.0 (8.1) | 10.4 (8.6) | 7.6 (5.0) | 23.7 (29.0) |
| Post-intervention | 111.3 (149.3) | 10.4 (8.6) | 162.5 (144.7) | 23.7 (29.0) |
Urinary lignans corrected for creatinine
Changes in Ki-67, caspase, and the Ki-67:caspase ratio and estrogen receptor β expression by treatment assignment are shown in Table 3. Compared to placebo, we observed no effect of FS, AI, or FS+AI on expression of growth related biomarkers or estrogen receptor β expression. Although differences in ERβ were not statistically significant across treatment groups, mean receptor expression was approximately 40% lower from pre- to post-intervention in the FS+AI group, whereas pre- to post-intervention expression was essentially unchanged in the other treatment groups.
Table 3.
Pre- and post-intervention tumor growth factor and estrogen receptor β expression by treatment assignment
| Flaxseed (n=6) |
Anastrazole (n=7) |
Flaxseed/Anastrazole (n=6) |
Placebo (n=5) |
|
|---|---|---|---|---|
| Mean (SD) | ||||
| Ki-67, % | ||||
| Pre-intervention | 13.2 (15.4) | 11.3 (6.3) | 7.2 (4.1) | 22.2 (27.6) |
| Post-intervention | 20.0 (23.2) | 8.5 (10.2) | 8.1 (15.7) | 20.2 (20.1) |
| (SE) | 5.5 (8.0) | −0.76 (8.6) | 0.51 (8.1) | |
| p-value | 0.69 | 0.93 | 0.95 | |
| Caspase, % | ||||
| Pre-intervention | 25.0 (20.6) | 26.3 (27.5) | 25.0 (20.5) | 28.0 (29.3) |
| Post-intervention | 22.0 (13.0) | 23.7 (24.7) | 15.9 (18.0) | 18.0 (16.4) |
| (SE) | 4.8 (8.0) | 10.9 (8.8) | −0.83 (7.4) | |
| p-value | 0.56 | 0.24 | 0.91 | |
| Ki-67:caspase ratio | ||||
| Pre-intervention | 0.6 (0.8) | 25.6 (49.6) | 0.9 (1.0) | 3.1 (6.1) |
| Post-intervention | 0.9 (0.7) | 0.5 (0.5) | 2.1 (4.0) | 3.4 (4.2) |
| (SE) | −2.3 (1.4) | −2.2 (1.8) | −2.7 (1.4) | |
| p-value | 0.13 | 0.25 | 0.08 | |
| ERβ, % | ||||
| Pre-intervention | 60.0 (7.1) | 45.0 (23.8) | 63.3 (15.1) | 62.0 (13.0) |
| Post-intervention | 56.0 (26.1) | 46.7 (32.1) | 40.0 (26.8) | 66.0 (32.9) |
| (SE) | −9.3 (17.1) | −1.4 (18.5) | −26.1 (16.5) | |
| p-value | 0.60 | 0.94 | 0.14 | |
Effect of each treatment (flax; aromatase inhibitor; flax+aromatase inhibitor) vs placebo was determined by linear regression of post-treatment measures on treatment group, adjusting for pre-treatment measure, stage, and grade
Pre- and post-intervention serum steroid hormone and growth hormone levels by treatment assignment are shown in Table 4. We observed a statistically significant negative association (β±SE −0.3±0.1; p=0.03) for androstenedione in the FS+AI group vs placebo, although there were no differences in mean expression from pre- to post-intervention. For DHEA, serum levels were significantly negatively associated with AI treatment (β±SE −1.6±0.6; p=0.009); although negative associations were observed with the other treatment groups, only AI was statistically significant. No treatment effects were observed for SHBG, IGF1, or IGFBP3.
Table 4.
Pre- and post-intervention serum steroid hormone and growth hormone levels by treatment assignment
| Flaxseed | Anastrazole | Flaxseed/Anastrazole | Placebo | |
|---|---|---|---|---|
| Mean (SD) | ||||
| Androstenedione, ng/ml | ||||
| Pre-intervention | 0.4 (0.1) | 0.6 (0.4) | 0.4 (0.1) | 0.5 (0.2) |
| Post-intervention | 0.6 (0.2) | 0.5 (0.2) | 0.4 (0.2) | 0.8 (0.2) |
| (SE) | −0.1 (0.1) | −0.2 (0.1) | −0.3 (0.1) | |
| p-value | 0.22 | 0.05 | 0.03 | |
| Testosterone, ng/ml | ||||
| Pre-intervention | 0.2 (0.1) | 0.2 (0.1) | 0.2 (0.1) | 0.2 (0.1) |
| Post-intervention | 0.2 (0.1) | 0.2 (0.1) | 0.2 (0.1) | 0.2 (0.1) |
| (SE) | −0.02 (0.02) | −0.03 (0.02) | −0.03 (0.02) | |
| p-value | 0.31 | 0.24 | 0.21 | |
| DHEA, ng/ml | ||||
| Pre-intervention | 1.6 (0.6) | 2.3 (2.2) | 1.5 (0.5) | 1.6 (0.6) |
| Post-intervention | 2.2 (0.9) | 1.7 (1.2) | 1.8 (1.1) | 3.0 (1.1) |
| (SE) | −0.7 (0.5) | −1.6 (0.6) | −1.1 (0.6) | |
| p-value | 0.25 | 0.009 | 0.08 | |
| SHBG, mcg/ml | ||||
| Pre-intervention | 19.0 (4.8) | 15.2 (4.0) | 14.6 (4.5) | 17.1 (5.1) |
| Post-intervention | 19.1 (4.1) | 14.8 (3.4) | 13.8 (2.5) | 16.7 (4.5) |
| (SE) | 1.6 (1.4) | −0.3 (1.3) | −1.1 (1.4) | |
| p-value | 0.26 | 0.80 | 0.43 | |
| IGF1, pg/ml | ||||
| Pre-intervention | 902.8 (648.8) | 959.1 (408.6) | 887.7 (654.4) | 1008.3 (686.7) |
| Post-intervention | 895.5 (776.0) | 819.8 (334.3) | 938.1 (652.9) | 982.0 (728.7) |
| (SE) | 47.4 (141.7) | −118.4 (139.8) | 109.3 (146.7) | |
| p-value | 0.74 | 0.41 | 0.47 | |
| IGFBP3, ng/ml | ||||
| Pre-intervention | 2895.5 (748.5) | 2625.2 (743.4) | 3927.0 (1284.5) | 3222.8 (1047.3) |
| Post-intervention | 3166.9 (405.1) | 2552.2 (715.3) | 3602.0 (1506.8) | 2933.2 (1358.6) |
| (SE) | 264.4 (366.6) | −94.2 (366.7) | −57.1 (394.0) | |
| p-value | 0.48 | 0.80 | 0.89 | |
Effect of each treatment (flax; aromatase inhibitor; flax+aromatase inhibitor) vs placebo was determined by linear regression of post-treatment measures on treatment group, adjusting for pre-treatment measure, age, and BMI
Discussion
Complementary and alternative medicine approaches are widely used by cancer survivors, especially for breast cancer, in an attempt to reduce the likelihood of recurrence and to improve outcomes (8–10). Many complementary approaches consist of foods, herbs, or supplements that are considered safe by consumers, despite the fact that food-drug interactions have been described previously in the literature (e.g., grapefruit and cholesterol lowering drugs, etc). Flaxseed is a commonly consumed food that is high in phytoestrogens with the potential to interact with adjuvant anti-hormonal treatments such as aromatase inhibitors.
In this small clinical trial, we found little evidence of an interaction between flaxseed and a commonly prescribed aromatase inhibitor, anastrozole, in the modification of several breast tumor characteristics related to prognosis or in serum hormone levels. It is likely that interactions between FS and AI are subtle; larger differences would have been detectable even in a small sample such as ours. We did, however, observe expected reductions in DHEA in association with AI treatment, as well as a significant decrease associated with AI and an interaction between FS+AI for a reduction in androstenedione. Both FS and AI inhibit aromatase, so a reduction in androgen production is not surprising.
It is unclear why there was no effect of AI or FS alone on markers previously shown to be affected by these agents, such as ki67. AI treatment is targeted towards women with ER+ tumors. Compared to pre-treatment measures, on- or post-treatment changes in the ki67 in residual disease appears to reflect information on both tumor biology and response to therapy and appear to be more predictive of long-term outcome following neo-adjuvant endocrine therapy(34). All women in this trial had ER+ breast cancer, and the majority of tumors were stage I and higher grade. Additionally, ki67, on average, was low across the treatment groups. Ki67 has been reported to correlate with other biomarkers in breast cancer such as grade and ER expression, with ER-positive cancer typically exhibiting lower levels of proliferation (35). As such, the tumors of the women participating in this trial may have been too homogeneous to show a large effect from treatment. Conversely, the treatment time for each group was 13–16 days which may have been too short for a pronounced effect on tumor characteristics or hormone levels. However, testing a chemotherapeutic agent in breast cancer presents challenges, given the very good prognosis, long survival of most breast cancer patients, and lack of intermediate biomarkers of progression. The time period between biopsy and resection offers the best opportunity to examine agent effects on tumor characteristics, but it does have the limitation of being short in duration. Identification of viable intermediate biomarkers of progression is needed for studies such as ours.
Of particular interest, we observed a fairly substantial, although not statistically significant, effect of AI on urinary lignan excretion in the FS+AI group compared to the FS only group which, to our knowledge, has not been previously reported. The mechanism behind this reduction in urinary lignan excretion is unclear. Conversion of plant lignans such as secoisolariciresinol to the mammalian lignan enterolactone is dependent upon the mammalian gut bacteria (36, 37). Further metabolism of enterolactone may occur via modulation of other CYP enzymes that participate in hydroxylation reactions yielding unmeasured metabolites (38). Alternately, the gut microbiota produce p450 enzymes and participate in metabolism of natural products similarly to human enzymes (39, 40). AIs may be inhibiting microbial p450 metabolism with subsequent reductions in mammalian lignan production. This dampening effect on lignan production (see Figure 1) may explain, in part, the lack of a detectable interaction in that group and warrants further examination. Concomitant consumption of AI and FS may be reducing the chemopreventive effect of FS, but the addition of FS does not appear to affect AI action.
Figure 1.
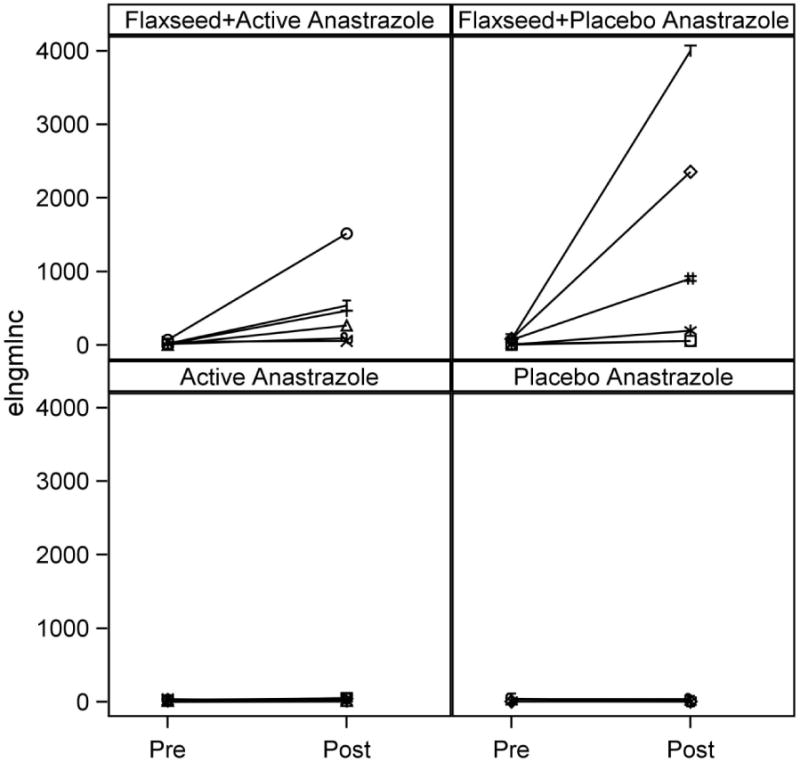
Pre- to postintervention urinary enterolactone excretion by treatment group
We also observed a 40% decrease in expression of ERβ from pre- to postintervention for women in the FS+AI treatment group only. DHEA activates estrogen receptors α and β (41). In our study, both AI and FS+AI treatments reduced DHEA production with comparable effects. Although there appeared to be no effect of DHEA lowering on ERβ expression in the AI group, the addition of FS may have been sufficient to produce a noticeable impact. Despite the lack of independent effects of AI and FS on ERβ, each has been reported to affect expression of this receptor. Flaxseed lignans have been shown to down-regulate ERβ expression in estrogen-dependent MCF7 breast cancer cells (42), but conflicting effects have been reported for anastrozole (43, 44) Given the small sample size, the combination of AI and FS may have been necessary to produce the observed decreased ERβ expression.
Assessment of the role of complementary approaches in adjuvant breast cancer treatment relies on adequate participation in clinical trials. Recruitment to clinical trials has always been challenging (45), and non-therapeutic trials offer less incentive for patients diagnosed with life-threatening diseases such as cancer. We had several challenges to recruitment in this study. Our target population was postmenopausal women with stage I or II ER+ breast cancer, as these are the patients who would be prescribed aromatase inhibitors. Prognosis for lower stage, ER+ disease is very good, providing less incentive for participation in a non-therapeutic trial. Another challenge to recruitment was study design. Although the “Window of Opportunity” setting provides the chance to assess differences in tumor biology from biopsy to surgery, the narrow time window for recruitment is a concern. Our recruitment success was comparable to a similar study of flaxseed in prostate cancer patients between biopsy and surgery (46, 47). Future studies would require a multi-center approach, or alternatively, the development of intermediate biomarkers of prognosis.
A large body of experimental data and limited human data support the potential for flaxseed to be used in breast cancer chemoprevention and treatment, but given the capacity for flaxseed lignans to inhibit aromatase, we hypothesized that food-drug interactions were possible and that the action of aromatase could be dampened. In this small pilot study, we did not observe an effect of flaxseed on AI activity with regards to selected breast tumor characteristics, growth hormone, or serum steroid hormone levels, although the sample size was likely too small to show small effects. On the contrary, there was a suggestion that AI therapy might reduce the production of circulating mammalian lignans from flaxseed. This dampening of lignan production by AIs would have implications for the use of flaxseed as a potential chemopreventive natural agent as the effective dose would be limited. Replication in larger studies of these intriguing findings is warranted.
Acknowledgments
Supported by 1R21AT004024-01from NIH National Center for Complementary and Alternative Medicine; the Pathology Resource Network is a Roswell Park Cancer Center Support Grant shared resource, supported by NIH National Cancer Institute P30CA016056-32
Appendix: Antibodies used for Immunohistochemical staining
| Antibody | Company | Clone | Dilution |
|---|---|---|---|
| KI-67 | Dako | MIB-1 | 1:50 |
| Cleaved Caspase 3 | Cell Signaling | polyclonal | 1:100 |
| ER β | GeneTex | 14C8 | 1:50 |
Footnotes
References
- 1.Cianfrocca M, Goldstein LJ. Prognostic and predictive factors in early stage breast cancer. The Oncologist. 2004;9:606–616. doi: 10.1634/theoncologist.9-6-606. [DOI] [PubMed] [Google Scholar]
- 2.Howell A. New developments in the treatment of postmenopausal breast cancer. Trends Endocrinol Metab. 2005;16:420–428. doi: 10.1016/j.tem.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann M, Rody A. Long-term risk of breast cancer recurrence: the need for extended adjuvant therapy. J Cancer Res Clin Oncol. 2005;131:487–494. doi: 10.1007/s00432-005-0668-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saladores PH, Precht JC, Schroth W, Brauch H, Schwab M. Impact of metabolizing enzymes on drug response of endocrine therapy in breast cancer. Expert Rev Mol Diagn. 2013;13:349–365. doi: 10.1586/erm.13.26. [DOI] [PubMed] [Google Scholar]
- 5.Geisler J, King N, Dowsett M, Ottestad L, Lundgren S, et al. Influence of anastrozole (Arimidex), a selective, non-steroidal aromatase inhibitor, on in vivo aromatisation and plasma oestrogen levels in postmenopausal women with breast cancer. Br J Cancer. 1996;74:1286–1291. doi: 10.1038/bjc.1996.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geisler J, Haynes B, Anker G, Dowsett M, Lonning PE. Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J Clin Oncol. 2002;20:751–757. doi: 10.1200/JCO.2002.20.3.751. [DOI] [PubMed] [Google Scholar]
- 7.Johannessen DC, Engan T, Di SE, Zurlo MG, Paolini J, et al. Endocrine and clinical effects of exemestane (PNU 155971), a novel steroidal aromatase inhibitor, in postmenopausal breast cancer patients: a phase I study. Clin Cancer Res. 1997;3:1101–1108. [PubMed] [Google Scholar]
- 8.Rakovitch E, Pignol JP, Chartier C, Ezer M, Verma S, et al. Complementary and alternative medicine use is associated with an increased perception of breast cancer risk and death. Breast Cancer Res Treat. 2005;90:139–148. doi: 10.1007/s10549-004-3779-1. [DOI] [PubMed] [Google Scholar]
- 9.Helyer LK, Chin S, Chui BK, Fitzgerald B, Verma S, et al. The use of complementary and alternative medicines among patients with locally advanced breast cancer – a descriptive study. BMC Cancer. 2006;6:39. doi: 10.1186/1471-2407-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerber B, Scholz C, Reimer T, Briese V, Janni W. Complementary and alternative therapeutic approaches in patients with early breast cancer: a systematic review. Breast Cancer Res Treat. 2006;95:199–209. doi: 10.1007/s10549-005-9005-y. [DOI] [PubMed] [Google Scholar]
- 11.Navo MA, Phan J, Vaughan C, Palmer JL, Michaud L, et al. An assessment of the utilization of complementary and alternative medication in women with gynecologic or breast malignancies. J Clin Oncol. 2004;22:671–677. doi: 10.1200/JCO.2004.04.162. [DOI] [PubMed] [Google Scholar]
- 12.Richardson MA, Sanders T, Palmer JL, Greisinger A, Singletary SE. Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology. J Clin Oncol. 2000;18:2505–2514. doi: 10.1200/JCO.2000.18.13.2505. [DOI] [PubMed] [Google Scholar]
- 13.Shen J, Andersen R, Albert PS, Wenger N, Glaspy J, et al. Use of complementary/alternative therapies by women with advanced-stage breast cancer. BMC Complement Altern Med. 2002;2:8. doi: 10.1186/1472-6882-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang C, Makela T, Hase T, Adlercreutz H, Kurzer MS. Lignans and flavonoids inhibit aromatase enzyme in human preadipocytes. J Steroid Biochem Mol Biol. 1994;50:205–212. doi: 10.1016/0960-0760(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 15.Thompson LU, Robb P, Serraino M, Cheung F. Mammalian lignan production from various foods. Nutr Cancer. 1991;16:43–52. doi: 10.1080/01635589109514139. [DOI] [PubMed] [Google Scholar]
- 16.Haggans CJ, Hutchins AM, Olson BA, Thomas W, Martini MC, et al. Effect of flaxseed consumption on urinary estrogen metabolites in postmenopausal women. Nutr Cancer. 1999;33:188–195. doi: 10.1207/S15327914NC330211. [DOI] [PubMed] [Google Scholar]
- 17.Hutchins AM, Martini MC, Olson BA, Thomas W, Slavin JL. Flaxseed consumption influences endogenous hormone concentrations in postmenopausal women. Nutr Cancer. 2001;39:58–65. doi: 10.1207/S15327914nc391_8. [DOI] [PubMed] [Google Scholar]
- 18.Hutchins AM, Martini MC, Olson BA, Thomas W, Slavin JL. Flaxseed influences urinary lignan excretion in a dose-dependent manner in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2000;9:1113–1118. [PubMed] [Google Scholar]
- 19.Chen J, Stavro PM, Thompson LU. Dietary flaxseed inhibits human breast cancer growth and metastasis and downregulates expression of insulin-like growth factor and epidermal growth factor receptor. Nutr Cancer. 2002;43:187–192. doi: 10.1207/S15327914NC432_9. [DOI] [PubMed] [Google Scholar]
- 20.Dabrosin C, Chen J, Wang L, Thompson LU. Flaxseed inhibits metastasis and decreases extracellular vascular endothelial growth factor in human breast cancer xenografts. Cancer Lett. 2002;185:31–37. doi: 10.1016/s0304-3835(02)00239-2. [DOI] [PubMed] [Google Scholar]
- 21.Brooks JD, Thompson LU. Mammalian lignans and genistein decrease the activities of aromatase and 17beta-hydroxysteroid dehydrogenase in MCF-7 cells. J Steroid Biochem Mol Biol. 2005;94:461–467. doi: 10.1016/j.jsbmb.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Adlercreutz H, Bannwart C, Wahala K, Makela T, Brunow G. Inhibition of human aromatase by mammalian lignans and isoflavonoid phytoestrogens. J Steroid Biochem Mol Biol. 1993;44:147–153. doi: 10.1016/0960-0760(93)90022-o. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Hui E, Ip T, Thompson LU. Dietary flaxseed enhances the inhibitory effect of tamoxifen on the growth of estrogen-dependent human breast cancer (mcf-7) in nude mice. Clin Cancer Res. 2004;10:7703–7711. doi: 10.1158/1078-0432.CCR-04-1130. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Tan KP, Ward WE, Thompson LU. Exposure to flaxseed or its purified lignan during suckling inhibits chemically induced rat mammary tumorigenesis. Exp Biol Med (Maywood) 2003;228:951–958. doi: 10.1177/153537020322800811. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Thompson LU. Lignans and tamoxifen, alone or in combination, reduce human breast cancer cell adhesion, invasion and migration in vitro. Breast Cancer Res Treat. 2003;80:163–170. doi: 10.1023/A:1024513815374. [DOI] [PubMed] [Google Scholar]
- 26.Thompson LU, Rickard SE, Orcheson LJ, Seidl MM. Flaxseed and its lignan and oil components reduce mammary tumor growth at a late stage of carcinogenesis. Carcinogenesis. 1996;17:1373–1376. doi: 10.1093/carcin/17.6.1373. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Chen J, Thompson LU. The inhibitory effect of flaxseed on the growth and metastasis of estrogen receptor negative human breast cancer xenograftsis attributed to both its lignan and oil components. Int J Cancer. 2005;116:793–798. doi: 10.1002/ijc.21067. [DOI] [PubMed] [Google Scholar]
- 28.Thompson LU, Chen JM, Tong L, Strasser-Weippt K, Goss PE. Dietary flaxseed alters tumor biological markers in postmenopausal breast cancer. Clin Cancer Res. 2005;11:3828–3835. doi: 10.1158/1078-0432.CCR-04-2326. [DOI] [PubMed] [Google Scholar]
- 29.mark-Wahnefried W, Robertson CN, Walther PJ, Polascik TJ, Paulson DF, et al. Pilot study to explore effects of low-fat, flaxseed-supplemented diet on proliferation of benign prostatic epithelium and prostate-specific antigen. Urology. 2004;63:900–904. doi: 10.1016/j.urology.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 30.mark-Wahnefried W, Price DT, Polascik TJ, Robertson CN, Anderson EE. Pilot study of dietary fat restriction and flaxseed supplementation in men with prostate cancer before surgery: exploring the effects on hormonal levels, prostate-specific antigen, and histopathologic features. Urology. 2001;58:47–52. doi: 10.1016/s0090-4295(01)01014-7. [DOI] [PubMed] [Google Scholar]
- 31.Nesbitt PD, Lam Y, Thompson LU. Human metabolism of mammalian lignan precursors in raw and processed flaxseed. Am J Clin Nutr. 1999;69:549–555. doi: 10.1093/ajcn/69.3.549. [DOI] [PubMed] [Google Scholar]
- 32.Humfrey CD. Phytoestrogens and human health effects: weighing up the current evidence. Nat Toxins. 1998;6:51–59. doi: 10.1002/(sici)1522-7189(199804)6:2<51::aid-nt11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 33.Boucher BA, Cotterchio M, Curca IA, Kreiger N, Harris SA, et al. Intake of phytoestrogen foods and supplements among women recently diagnosed with breast cancer in Ontario, Canada. Nutr Cancer. 2012;64:695–703. doi: 10.1080/01635581.2012.687426. [DOI] [PubMed] [Google Scholar]
- 34.Dowsett M, Smith IE, Ebbs SR, Dixon JM, Skene A, et al. Short-term changes in Ki-67 during neoadjuvant treatment of primary breast cancer with anastrozole or Tamoxifen alone or combined correlate with recurrence-free survival. Clin Cancer Res. 2005;11:951s–958s. [PubMed] [Google Scholar]
- 35.Trihia H, Murray S, Price K, Gelber RD, Golouh R, et al. Ki-67 expression in breast carcinoma: its association with grading systems, clinical parameters, and other prognostic factors–a surrogate marker? Cancer. 2003;97:1321–1331. doi: 10.1002/cncr.11188. [DOI] [PubMed] [Google Scholar]
- 36.Clavel T, Henderson G, Alpert CA, Philippe C, Rigottier-Gois L, et al. Intestinal bacterial communities that produce active estrogen-like compounds enterodiol and enterolactone in humans. Appl Environ Microbiol. 2005;71:6077–6085. doi: 10.1128/AEM.71.10.6077-6085.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clavel T, Borrmann D, Braune A, Dore J, Blaut M. Occurrence and activity of human intestinal bacteria involved in the conversion of dietary lignans. Anaerobe. 2006;12:140–147. doi: 10.1016/j.anaerobe.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Niemeyer HB, Honig D, Lange-Bohmer A, Jacobs E, Kulling SE, et al. Oxidative metabolites of the mammalian lignans enterodiol and enterolactone in rat bile and urine. J Agric Food Chem. 2000;48:2910–2919. doi: 10.1021/jf0000530. [DOI] [PubMed] [Google Scholar]
- 39.Kelly SL, Kelly DE. Microbial cytochromes P450: biodiversity and biotechnology. Where do cytochromes P450 come from, what do they do and what can they do for us? Philos Trans R Soc Lond B Biol Sci. 2012;368:0476–2013. doi: 10.1098/rstb.2012.0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Podust LM, Sherman DH. Diversity of P450 enzymes in the biosynthesis of natural products. Nat Prod Rep. 2012;29:1251–1266. doi: 10.1039/c2np20020a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller KK, Al-Rayyan N, Ivanova MM, Mattingly KA, Ripp SL, et al. DHEA metabolites activate estrogen receptors alpha and beta. Steroids. 2013;78:15–25. doi: 10.1016/j.steroids.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richter DU, Abarzua S, Chrobak M, Scholz C, Kuhn C, et al. Effects of phytoestrogen extracts isolated from flax on estradiol production and ER/PR expression in MCF7 breast cancer cells. Anticancer Res. 2010;30:1695–1699. [PubMed] [Google Scholar]
- 43.Smollich M, Gotte M, Fischgrabe J, Radke I, Kiesel L, et al. Differential effects of aromatase inhibitors and antiestrogens on estrogen receptor expression in breast cancer cells. Anticancer Res. 2009;29:2167–2171. [PubMed] [Google Scholar]
- 44.Bershtein LM, Poroshina TE, Zimarina TS, Tsyrlina EV, Zhil’tsova EK, et al. Expression of estrogen receptors-alpha and -beta in primary breast neoplasms and tumors exposed to neoadjuvant hormonal therapy. Bull Exp Biol Med. 2004;138:494–496. doi: 10.1007/s10517-005-0079-7. [DOI] [PubMed] [Google Scholar]
- 45.Caldwell PH, Hamilton S, Tan A, Craig JC. Strategies for increasing recruitment to randomised controlled trials: systematic review. PLoS Med. 2010;7:e1000368. doi: 10.1371/journal.pmed.1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Demark-Wahnefried W, Polascik TJ, George SL, Switzer BR, Madden JF, et al. Flaxseed supplementation (not dietary fat restriction) reduces prostate cancer proliferation rates in men presurgery. Cancer Epidemiol Biomarkers Prev. 2008;17:3577–3587. doi: 10.1158/1055-9965.EPI-08-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Demark-Wahnefried W, George SL, Switzer BR, Snyder DC, Madden JF, et al. Overcoming challenges in designing and implementing a phase II randomized controlled trial using a presurgical model to test a dietary intervention in prostate cancer. Clin Trials. 2008;5:262–272. doi: 10.1177/1740774508091676. [DOI] [PMC free article] [PubMed] [Google Scholar]


