Abstract
Objectives
Evidence-based traumatic brain injury guidelines support cerebral perfusion pressure thresholds for adults at a class 2 level, but evidence is lacking in younger patients. The purpose of this study is to identify the impact of age-specific cerebral perfusion pressure thresholds on short-term survival among patients with severe traumatic brain injury.
Design
Institutional review board-approved, prospective, observational cohort study.
Patients
Data on all patients with a postresuscitation Glasgow Coma Score less than 9 were added in the Brain Trauma Foundation prospective New York State TBI-trac database.
Measurements and Main Results
We calculated the survival rates and relative risks of mortality for patients with severe traumatic brain injury based on predefined age-specific cerebral perfusion pressure thresholds. A higher threshold and a lower threshold were defined for each age group: 60 and 50 mm Hg for 12 years old or older, 50 and 35 mm Hg for 6–11 years, and 40 and 30 mm Hg for 0–5 years. Patients were stratified into age groups of 0–11, 12–17, and 18 years old or older. Three exclusive groups of CPP-L (events below low cerebral perfusion pressure threshold), CPP-B (events between high and low cerebral perfusion pressure thresholds), and CPP-H (events above high cerebral perfusion pressure threshold) were defined. As an internal events of hypotension and elevated intracranial pressure. Survival was significantly higher in 0–11 and 18 years old or older age groups for patients with CPP-H events compared with those with CPP-L events. There was a significant decrease in survival with prolonged exposure to CPP-B events for the 0–11 and 18 years old and older age groups when compared with the patients with CPP-H events (p = 0.0001 and p = 0.042, respectively). There was also a significant decrease in survival with prolonged exposure to CPP-L events in all age groups compared with the patients with CPP-H events (p < 0.0001 for 0- to 11-yr olds, p = 0.0240 for 12- to 17-yr olds, and p < 0.0001 for 18-yr old and older age groups). The 12- to 17-year olds had a significantly higher likelihood of survival compared with adults with prolonged exposure to CPP-L events (< 50 mm Hg). CPP-L events were significantly related to systemic hypotension for the 12- to 17-year-old group (p = 0.004) and the 18-year-old and older group (p < 0.0001). CPP-B events were significantly related to systemic hypotension in the 0- to 11-year-old group (p = 0.014). CPP-B and CPP-L events were significantly related to elevated intracranial pressure in all age groups.
Conclusions
Our data provide new evidence that cerebral perfusion pressure targets should be age specific. Furthermore, cerebral perfusion pressure goals above 50 or 60 mm Hg in adults, above 50 mm Hg in 6- to 17-year olds, and above 40 mm Hg in 0- to 5-year olds seem to be appropriate targets for treatment-based studies. Systemic hypotension had an inconsistent relationship to events of low cerebral perfusion pressure, whereas elevated intracranial pressure was significantly related to all low cerebral perfusion pressure events across all age groups. This may impart a clinically important difference in care, highlighting the necessity of controlling intracranial pressure at all times, while targeting SBP in specific instances.
Keywords: cerebral perfusion pressure, head trauma, severe traumatic brain injury, traumatic brain injury management
Traumatic brain injury (TBI) is the leading cause of death and disability in children 1–19 years old (1–5). The economic impact of TBI exceeds $2.5 billion annually in direct costs alone. Acute management of severe TBI currently focuses on monitoring intracranial pressure (ICP) and cerebral perfusion pressure (CPP) in neurocritical ICUs. No evidence has demonstrated that any specific pharmacologic therapies decrease morbidity or mortality in children with these injuries (6).
It is well established that secondary brain injury occurs in the setting of increased ICP and decreased cerebral perfusion (7). Therefore, management of patients with severe TBI has focused on decreasing ICP and optimizing CPP (8, 9). Current evidence-based TBI guidelines support ICP and CPP thresholds for adults at a class 2 level; a sustained CPP between 50 and 70 mm Hg or an ICP maintained below 20 mm Hg have been associated with favorable outcomes (10, 11). Although multiple studies have shown better outcomes and lower mortality with CPP greater than 60, 70, or 80 mm Hg, other studies have shown no benefit to maintaining higher CPP (12–18).
The 2012 Brain Trauma Foundation recommendations for the pediatric population were based on level III evidence, endorse maintaining CPP above 40 mm Hg in patients of all ages, and suggest that an age-related continuum between 40 and 65 mm Hg may exist (19, 20). However, no age-specific pediatric CPP thresholds have been determined. Wide variations of normal blood pressure (BP) and CPP exist across childhood due to changes in cerebrovascular autoregulation and normal development. Translating static adult ICP and CPP thresholds into treatment guidelines for children of widely varying age may be inconsistent physiologically. Multiple studies have shown that CPP below 40 mm Hg in all ages is associated with significantly poorer outcome and higher mortality (21, 22). Additionally, CPP above 50 mm Hg has been associated with increased survival in patients older than 10 in one study and in noninfant patients in another (23, 24). One other recent study has shown a correlation between poor outcome with initial CPP below 40 mm Hg and a good outcome with initial CPP above 60 mm Hg but less than 70 mm Hg (25).
Recent studies provide further evidence for an age-related CPP continuum, but they are based on small sample sizes. One study found a significant difference in mean CPP over the first 6 hours of monitoring between patients with good and poor outcomes in children aged 2–16 (26). Investigators also reported an age-related difference in mean CPP over this time period, suggesting that CPP targets should vary with age. Study investigators were unable, however, to make recommendations for target CPP values. An additional study found that multiple episodes of CPP below 45 mm Hg in children 2 years and younger were associated with unfavorable outcome but only contained 22 patients (27). Finally, a recent study used a derived measure of time and depth below CPP thresholds, applying the lowest age-acceptable mean arterial blood pressure (MAP) as a threshold (48 mm Hg for 2- to 6-yr olds, 54 mm Hg in 7- to 10-yr olds, and 58 mm Hg in 11- to 15-yr olds), to examine the relationship with outcome. They determined that the combination of longer time and further depth below thresholds correlated with poor outcome but were unable to determine if the thresholds used were meaningful themselves (28).
To examine the relationship between minimum CPP thresholds and mortality rates in age-grouped pediatric TBI populations, we used data from the Brain Trauma Foundation's prospective database of patients with severe TBI, the New York State TBI-trac. The purpose of this study is to identify the impact of age-specific CPP thresholds on short-term survival among patients with severe TBI.
Methods
TBI-Trac Database
As part of a quality improvement initiative established in 2000 for patients with severe TBI, the Brain Trauma Foundation designed and implemented a program, funded by the New York State Department of Health, Division of Healthcare Financing and Acute and Primary Care reimbursement. The program uses an online Internet database, TBI-trac, to collect data on patients with severe TBI. This database is used by trauma centers for the purpose of tracking compliance with established guidelines and also as a prospective database to test hypotheses that could have evidence-based impact on improving TBI guidelines. Age-specific thresholds for CPP, ICP, and SBP, based on evidence presented in BTF guidelines from 2000, were built into the database (29). This database contains clinical information from prehospital sources, emergency departments, the first 10 days in the ICU, and 2-week mortality data from 22 of the 46 designated trauma centers in New York State. Of these 22 sites, 20 are level I trauma centers and two carry a level II trauma center designation. All trauma centers were voluntary participants in the prospective quality assurance program and database and treat severe TBI patients. This report is based on patients treated in these trauma centers between June 6, 2000, and August 21, 2008.
Study Population
The TBI-trac database contains all patients with isolated severe TBI or multitrauma TBI who meet the following criteria: arrival at the participating level I or II trauma center within 24 hours of injury with a Glasgow Coma Score (GCS) less than 9, including a GCS motor score less than 6 for at least 6 hours after injury and after appropriate resuscitation efforts. Patients with severe TBI who died in the emergency department or were admitted with the diagnosis of brain death were not eligible for enrollment in the database study. Nonpharmacologically paralyzed patients, on day 1 or 2 following trauma, with GCS of 3 or 4 and with fixed and dilated pupils were recorded but excluded from data analysis, due to their overall poor prognosis. Patients were stratified into age groups of 0–5, 6–11, 12–17, and 18 years old or older.
CPP Threshold Derivation
CPP, a global measure of blood flow to the brain, is derived by subtracting ICP from MAP. CPP thresholds prospectively defined in the database were extrapolated from population-based, age-adjusted BP values (29). A higher threshold and a lower threshold were defined for each age group: 60 and 50 mm Hg for 12 years old or older, 50 and 35 mm Hg for 6–11 years old, and 40 and 30 mm Hg for 0–5 years old. Trauma centers participating in the TBI-trac database recorded the total number of hours patients were below these thresholds on each ICU day; how frequently a variable was recorded in each center's record to derive this total was determined by individual ICU policies. In this study, total hours below these thresholds during ICU days were calculated. Then, CPP-L events were defined as CPP dropping below the lower threshold for each age group at any time during ICU stay. CPP-B events were defined as events below the high threshold but not below the low threshold (i.e., events of CPP between the high and low threshold) for any time during the ICU stay. Otherwise, monitored time without events below either threshold were defined as CPP-H events (i.e., above the high threshold). All three groups were exclusive. If a patient had any unrecorded hours over the period identified, we assumed they were in CPP-H event group.
Mortality
Survival or mortality was recorded as the study endpoint at 14 days post injury.
Hypotension
The Brain Trauma Foundation's TBI-trac database tracked total time per day patients spent below the SBP thresholds established based on the recommendations in previous guidelines: SBP less than 90 mm Hg in patients 12 years old and older, SBP less than 80 mm Hg in patients 6–11 years old, SBP less than 75 mm Hg for patients 1–5 years old, and SBP less than 65 mm Hg for patients less than 1 year old. As an internal control, we evaluated the association between events of CPP at different thresholds and events of hypotension.
Elevated ICP
The Brain Trauma Foundation's TBI-trac database tracked total time per day patients spent with elevated ICP: ICP greater than 25 mm Hg at any time during ICU stay in patients 1 year old and older and ICP greater than 20 mm Hg in patients 0–1 year old. As an internal control, we evaluated the association between events of CPP at different thresholds and events of elevated ICP.
Statistical Analysis
Patient characteristics were described as standard summary statistics—mean and SD, median and range for continuous variables and percentage for categorical variables. The chi-square or Fisher exact test, wherever applicable, was used to evaluate the association between two categorical variables. In addition to p value, the relative risk and 95% CIs were reported as risk of events (e.g., mortality, hypotension, and elevated ICP) relative to CPP thresholds. The CPP-H group was used for reference. The Kaplan-Meier method was used to calculate survival probabilities and survival function, and the log-rank test was used to compare survival curves between total hours of exposure to CPP-B, CPP-L, and CPP-H. All p values were two sided and unadjusted for multiple comparisons. Readers concerned with multiple comparisons (e.g., pairwise comparisons among CPP-B, CPP-L, and CPP-H) may wish to evaluate statistical significance at a modified α level of 0.05/3 comparisons = 0.017 based on Bonferroni method. All analyses were performed in SAS Version 9.2 (SAS Institute, Cary, NC).
Study Oversight
The research protocol was approved by the institutional review boards of each of the participating centers. The database was de-identified, thereby ensuring confidentiality for the data-sets at each institution. All patient data and outcomes were recorded from the patient medical records and entered directly into TBI-trac by the trained trauma nurse coordinator at each of the participating centers and kept electronically behind a secure, password and firewall-protected server.
Results
Demographics
A total of 2,641 patients were entered into the database during the study period, of which 2,074 met all inclusion and exclusion criteria (Fig. 1). The characteristics of the study population are detailed in Table 1. The 0- to 5-year-old group included 55 patients; the 6- to 11-year-old group included 65 patients; the 12- to 17-year-old group included 197 patients; and the 18-year-old and older group included 1,757 patients. The median admission GCS score was 4 in patients 0–5 years old and 5 in patients 6 years old and older. BP, ICP, and CPP monitoring was initiated as dictated by protocol of the admitting hospital, resulting in 14,893 total hours of patient monitoring.
Figure 1.
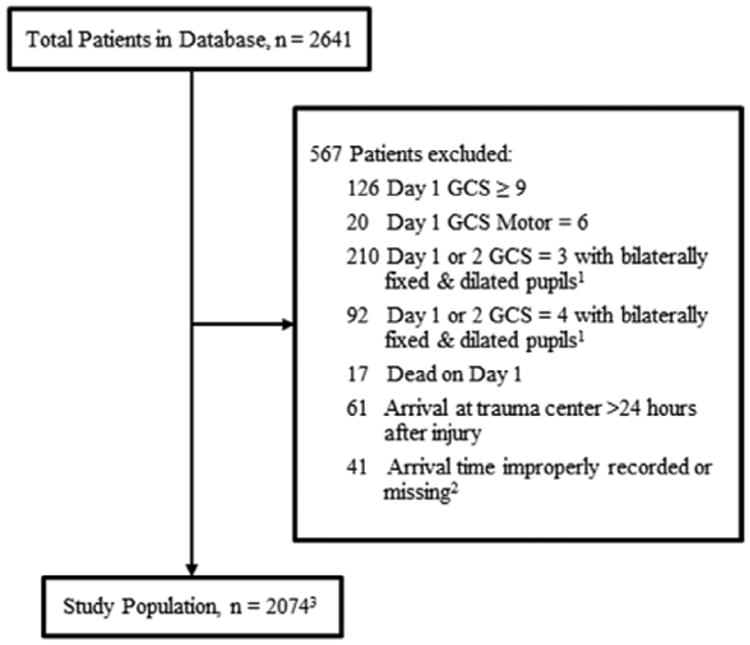
Database screening. 1Not pharmacologically paralyzed. 2Included in sensitivity analysis; data missing for arrival time at trauma center, time of trauma, or arrival time recorded as before trauma time. 3Of 2,074 patients, 14 patients do not have outcome data; included in sensitivity analysis. GCS = Glasgow Coma Score.
Table 1. Patient Demographics (n = 2,074).
| Characteristic | Age Group | |||
|---|---|---|---|---|
|
| ||||
| 0–5 Yr Old | 6–11 Yr Old | 12–17 Yr Old | ≥ 18 Yr Old | |
| n | 55 | 65 | 197 | 1,757 |
|
| ||||
| Age, yra | 2.1 (1.4) | 8.4 (1.7) | 15.3 (1.6) | 40.5 (19.0) |
|
| ||||
| Male, % | 66 | 63 | 70 | 77 |
|
| ||||
| Admission Glasgow Coma Scoreb | 4 (3–15) | 5 (3–14) | 5 (3–14) | 5 (3–15) |
|
| ||||
| Time to admission, hra | 2.36 (2.13) | 1.92 (1.75) | 1.84 (2.36) | 1.71 (2.51) |
|
| ||||
| Mechanism of injury, % | ||||
| Falls | 9 (16.7) | 6 (9.2) | 12 (6.1) | 391 (22.4) |
| Motor vehicle accident | 12 (22.2) | 25 (38.5) | 86 (43.7) | 607 (34.8) |
| Assault | 4 (7.4) | 0 (0) | 5 (2.5) | 127 (7.3) |
| Other | 29 (53.7) | 34 (52.3) | 94 (47.2) | 622 (35.6) |
|
| ||||
| Mortality, % | 16.4 | 15.4 | 13.7 | 19.1 |
Mean with sd in parentheses.
Median with range in parentheses.
The mechanism of injury included falls, motor vehicle accidents, assaults, and others. Of 2,074 patients (18.4%), 382 patients died within 2 weeks, and the mortality ranged from 13.7% to 19.1% across the four age groups.
Note that 41 of 2,641 patients (1.9%) were excluded from the study population due to errors in the reporting of their arrival or trauma time, which made determining their eligibility impossible. There were no significant differences in baseline characteristics between the included and excluded patients. In addition, of 2,074, there were missing outcome data for 14 patients that met criteria (0.7%) and were not included in the analysis. A sensitivity analysis confirmed that the exclusions of these 55 patients, all from the 18-year-old and older age group, did not influence the magnitude or direction of the reported effects for survival and CPP thresholds (p remained < 0.0001) or for risk of mortality by hours below CPP thresholds (p remained < 0.0001).
CPP and Survival
The relationship between any CPP events and 14-day survival is shown in Table 2. The 0- to 5-year-old and 6- to 11-year-old age groups combined after preliminary statistical analysis suggested inadequate power based on crude patient volume. When compared, the two groups were found to have similar survival at both thresholds (Supplemental Appendix A, Supplemental Digital Content 1, http://links.lww.com/PCC/A74).
Table 2. Relationship Between Mortality and Cerebral Perfusion Pressure Thresholds by Age Group.
| CPP Thresholds | Survivors (n = 1,681) | Nonsurvivors (n = 379) | Relative Risk (95% CI) | pa |
|---|---|---|---|---|
| 0–11 yr old | ||||
| n | 101 | 19 | ||
| CPP-H eventsb | 74 (91.4) | 7 (8.6) | Reference | |
| CPP-B eventsc | 24 (82.8) | 5 (17.2) | 1.99 (0.58, 5.80) | 0.29 |
| CPP-L eventsd | 3 (30.0) | 7 (70.0) | 8.10 (3.58, 18.31) | < 0.0001 |
|
| ||||
| 12–17 yr old | ||||
| n | 1 70 | 17 | ||
| CPP-H eventsb | 50 (87.7) | 7 (12.3) | Reference | |
| CPP-B eventsc | 78 (88.6) | 10 (11.4) | 0.93 (0.37, 2.29) | 0.87 |
| CPP-L eventsd | 42 (80.8) | 10 (19.2) | 1.57 (0.64, 2.81) | 0.32 |
|
| ||||
| ≥ 18 yr old | ||||
| n | 1,410 | 333 | ||
| CPP-H eventsb | 701 (84.0) | 134 (16.1) | Reference | |
| CPP-B eventsc | 562 (83.6) | 110 (16.4) | 1.02 (0.81, 1.28) | 0.87 |
| CPP-L eventsd | 147 (62.3) | 89 (37.7) | 2.35 (1.88, 2.95) | < 0.0001 |
CPP = cerebral perfusion pressure, CPP-H = high CPP threshold, CPP-B = total time between CPP threshold, CPP-L = total time below the low CPP threshold.
Calculated from chi-square test.
CPP > 40 mm Hg for 0–5 yr old, > 50 mm Hg for 6–11 yr old, and > 60 mm Hg for > 12 yr old.
CPP 30–40 mm Hg for 0–5 yr old, 35–50 mm Hg for 6–11 yr old, and 50–60 mm Hg for > 12 yr old.
CPP < 30 mm Hg for 0–5 yr old, < 35 mm Hg for 6–11 yr old, and < 50 mm Hg for > 12 yr old.
In patients 0–11 years old, there was a statistically significant difference in survival rate between patients with CPP-H events and those with any CPP-L events (91.4% vs 30.0%, respectively, p < 0.0001). In patients 12–17 years old, there was not a significant difference in survival between patients with CPP-H events and those with any CPP-L events (87.7% vs 80.8%, respectively, p = 0.32). In patients 18 years old and older, survival rate was significantly higher for patients with CPP-H events compared with those with any CPP-L events (84.0% vs 62.3%, respectively, p < 0.0001). There was no significant difference in survival rate for patients with CPP-B events when compared with patients with CPP-H events at any age group.
Kaplan-Meier method plots for survival probabilities over cumulative hours of exposure to CPP-H, CPP-B, and CPP-L are shown in Figure 2. Compared with exposure to CPP-H, there was a significant difference in survival curves in patients 0–11 years old in both CPP-B and CPP-L event groups (p = 0.0001 and p < 0.0001, respectively), in patients 12–17 years old for the CPP-L event group (p = 0.024), and in patients 18 years old and older in both CPP-B and CPP-L event groups (p = 0.042 and p < 0.0001, respectively). There was no difference in survival curves for patients 12–17 years old in the CPP-B event group. Within the age groups, there was a significant difference in the survival curves between CPP-B and CPP-L in patients 12–17 years old and in patients 18 years old and older (p = 0.011 and p < 0.0001, respectively) but not in patients 0–11 years old (p = 0.42).
Figure 2.
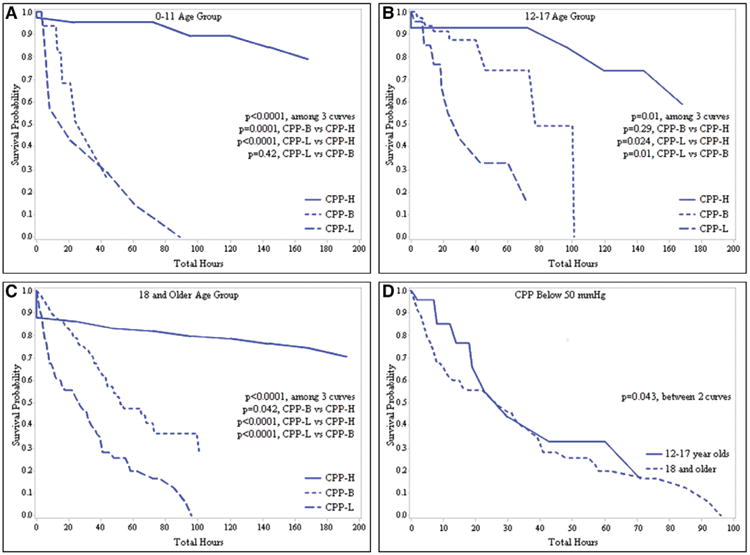
A–C, Kaplan-Meier survival plots for total monitored time above the high cerebral perfusion pressure (CPP) threshold (CPP-H, continuous line), total time between CPP thresholds (CPP-B, dotted line), and total time below the low CPP threshold (CPP-L, dashed line) at each age group. CPP-H = above 40 mm Hg for 0- to 5-yr olds, above 50 mm Hg for 6- to 11-yr olds, and above 60 mm Hg for 12-yr olds and older. CPP-B = between 30 and 40 mm Hg for 0- to 5-yr olds, between 35 and 50 mm Hg for 6- to 11-yr olds, and between 50 and 60 mm Hg for 12-yr olds and older. CPP-L = below 30 mm Hg for 0- to 5-yr olds, below 35 mm Hg for 6- to 11-yr olds, and below 50 mm Hg for 12-yr olds and older. D, Kaplan-Meier survival plot for time below 50 mm Hg for the 12- to 17-yr-old age group (continuous line) and the 18-yr-old and older age group (dashed line).
Additionally, we compared survival curves between patients 12–17 years old and patients 18 years old and older for cumulative exposure to CPP-L (i.e., total time CPP < 50 mm Hg). We found there was a significance difference, with the adolescent patients having comparatively better survival (p = 0.043).
CPP and Hypotension
The relationship between any CPP events and hypotension is shown in Table 3. Compared with the CPP-H group, hypotensive events were significantly more likely to occur for patients 0–11 years old in the CPP-B group (35% vs 14%, respectively, p = 0.01), for patients 12–17 years old in the CPP-L group (39% vs 14%, respectively, p = 0.004), and for patients 18 years old and older in the CPP-L group (44% vs 27%, respectively, p < 0.0001).
Table 3. Relationship Between Hypotension and Cerebral Perfusion Pressure Thresholds by Age Group.
| CPP Thresholds | Hypotensiona | Relative Risk (95% CI) | pb | |
|---|---|---|---|---|
|
| ||||
| No (n = 1,481) | Yes (n = 593) | |||
| 0–11 yr old | ||||
| n | 96 | 24 | ||
| CPP-H eventsc | 70 (86.4) | 11 (13.6) | Reference | |
| CPP-B eventsd | 19 (65.5) | 10 (34.5) | 2.54 (1.21, 5.34) | 0.01 |
| CPP-L eventse | 7 (70.0) | 3 (30.0) | 2.21 (0.74, 6.60) | 0.18 |
|
| ||||
| 12–17 yr old | ||||
| n | 152 | 45 | ||
| CPP-H eventsc | 49 (86.0) | 8 (14.0) | Reference | |
| CPP-B eventsd | 71 (80.7) | 17 (19.3) | 1.38 (0.64, 2.98) | 0.41 |
| CPP-L eventse | 32 (61.5) | 20 (38.5) | 2.74 (1.32, 5.68) | 0.004 |
|
| ||||
| ≥ 18 yr old | ||||
| n | 1,233 | 524 | ||
| CPP-H eventsc | 621 (73.4) | 225 (26.6) | Reference | |
| CPP-B eventsd | 480 (71.2) | 194 (28.8) | 1.08 (0.92, 1.27) | 0.34 |
| CPP-L eventse | 132 (55.7) | 105 (44.3) | 1.67 (1.39, 2.00) | < 0.0001 |
CPP = cerebral perfusion pressure, CPP-H = high CPP threshold, CPP-B = total time between CPP threshold, CPP-L = total time below the low CPP threshold.
SBP < 65 mm Hg for 0–1 yr old, < 75 mm Hg for 1–5 yr old, < 80 mm Hg for 6–11 yr old, and < 90 mm Hg for > 12 yr old.
Calculated from chi-square test.
CPP > 40 mm Hg for 0–5 yr old, > 50 mm Hg for 6–11 yr old, and > 60 mm Hg for > 12 yr old.
CPP 30–40 mm Hg for 0–5 yr old, 35–50 mm Hg for 6–11 yr old, and 50–60 mm Hg for > 12 yr old.
CPP < 30 mm Hg for 0–5 yr old, < 35 mm Hg for 6–11 yr old, and < 50 mm Hg for > 12 yr old.
CPP and Elevated ICP
The relationship between any CPP events and elevated ICP is shown in Table 4. Events of elevated ICP were significantly more likely to occur for both patients with CPP-B and CPP-L events in all age groups (p < 0.0001 in all groups) compared with those in the CPP-H group. The percentage of patients with elevated ICP was highest in patients with CPP-L events and lowest in patients with CPP-H events.
Table 4. Relationship Between Elevated Intracranial Pressure and Cerebral Perfusion Pressure Thresholds by Age Group.
| CPP Thresholds | Elevated Intracranial Pressurea | Relative Risk (95% CI) | pb | |
|---|---|---|---|---|
|
| ||||
| No (n = 1,292) | Yes (n = 782) | |||
| 0–11 yr old | ||||
| n | 70 | 50 | ||
| CPP-H eventsc | 62 (76.5) | 19 (23.5) | Reference | |
| CPP-B eventsd | 7 (24.1) | 22 (75.9) | 3.23 (2.08, 5.04) | < 0.0001 |
| CPP-L eventse | 1 (10.0) | 9 (90.0) | 3.84 (2.46, 5.98) | < 0.0001 |
|
| ||||
| 12–17 yr old | ||||
| n | 91 | 106 | ||
| CPP-H eventsc | 53 (93.0) | 4 (7.0) | Reference | |
| CPP-B eventsd | 30 (34.1) | 58 (65.9) | 9.39 (3.61, 24.45) | < 0.0001 |
| CPP-L eventse | 8 (15.4) | 44 (84.6) | 12.06 (4.65, 31.24) | < 0.0001 |
|
| ||||
| ≥ 18 yr old | ||||
| n | 1,131 | 626 | ||
| CPP-H eventsc | 783 (92.6) | 63 (7.5) | Reference | |
| CPP-B eventsd | 283 (42.0) | 391 (58.0) | 7.79 (6.09, 9.96) | < 0.0001 |
| CPP-L eventse | 65 (27.4) | 172 (72.6) | 9.75 (7.59, 12.52) | < 0.0001 |
CPP = cerebral perfusion pressure, CPP-H = high CPP threshold, CPP-B = total time between CPP threshold, CPP-L = total time below the low CPP threshold.
Intracranial pressure > 20 mm Hg for 0–1 yr old and > 25 mm Hg for > 1 yr old.
Calculated from chi-square test.
CPP > 40 mm Hg for 0–5 yr old, > 50 mm Hg for 6–11 yr old, and > 60 mm Hg for > 12 yr old.
CPP 30–40 mm Hg for 0–5 yr old, 35–50 mm Hg for 6–11 yr old, and 50–60 mm Hg for > 12 yr old.
CPP < 30 mm Hg for 0–5 yr old, < 35 mm Hg for 6–11 yr old, and < 50 mm Hg for > 12 yr old.
Discussion
Implications for Current Therapy
Our data provide new evidence that CPP targets should be age specific. Although the direct comparison did not show a significant difference in survival for patients using 40 mm Hg for 0- to 5-year olds and 50 mm Hg for 6- to 17-year olds, there was a significant risk of death with prolonged exposure to time below these thresholds, as demonstrated by the significant difference in survival with prolonged exposure to a CPP below these thresholds. Based on these data, CPP goals above 50 or 60 mm Hg in adults, above 50 mm Hg in 6- to 17-year olds, and above 40 mm Hg in 0- to 5-year olds appear to be appropriate targets for treatment-based studies. Although the data were less conclusive for 12- to 17-year olds, there was still a clear association between total time with CPP below 50 mm Hg and survival probability, suggesting this is an appropriate target for treatment-based studies in this age group. There was no a clear relationship between CPP and systemic hypotension, whereas elevated ICP was related to all instances of low CPP. This likely represents the necessity for individualized care in severe TBI patients; although it is clear ICP should always be controlled, BP requirements are likely to be evaluated on a patient-by-patient basis.
It is less clear how to achieve adequate CPP without causing secondary harm. Liberal use of volume expansion and vasoconstricting agents to maintain CPP above 70 mm Hg carries a higher risk of acute respiratory distress syndrome in adults (18, 30). There is also evidence that strict CPP-guided therapy may not be the best surrogate of cerebral blood flow and oxygenation (31). Optimal CPPs appear to depend on the integrity of individual's cerebrovascular autoregulation systems and measured pressure reactivity (32–35). However, the technology used to assess pressure reactivity, that is, transcranial Doppler, currently is not suited to constant monitoring or widespread use (36).
Less is known about cerebrovascular autoregulation in the pediatric TBI population, but impaired autoregulation is associated with poor outcome and survival (37–40). As in adults, assessment of cerebral autoregulation and pressure reactivity may allow for the determination of optimal CPP (37, 38). Although the effects of vasoconstricting agents and volume expansion in adult TBI patients have been examined, less is known about the effects of various techniques to improve BP and CPP in children. One recent study found a clinically important trend in the effects of norepinephrine compared with dopamine, phenylephrine, and epinephrine on both CPP and ICP values, which warrants further attention (41).
This study, with 317 patients under the age of 17, is the largest to analyze survival outcomes in severe pediatric TBI. Multi-institutional collaboration and prospective data collection provide further strength to our statistical analysis. By defining age-specific CPP thresholds before collecting the data, we removed a major potential bias from the data review.
Implications for Future Research
This study highlights the necessity of multicenter collaborations in analyzing data on relatively rare events, such as pediatric severe TBI. We suggest that multistate collaborations similar to the initiative taken by New York State will provide even more robust data and allow for the successful tracking and implementation of care guidelines. Further prospective analysis of long-term outcomes in this patient population should be performed based on these thresholds, with particular attention paid to the adolescent age group.
Although recent studies have examined outcomes of pediatric patients using ICP- or CPP-targeted approaches, they have been small and have been statistically inconclusive (42, 43). In addition to larger randomized blinded studies examining the effectiveness of ICP versus CPP-guided therapy, further research is needed to elucidate the connection between cerebral blood flow variables and brain tissue oxygenation. It is also clear, as has been previously described, that there is a 2D nature to these insults, and both the duration and depth of insult to CPP have an important impact on outcome (28).
Limitations
Our outcome assessments are limited by the fact that we only tracked short-term mortality rather than long-term measures. Other limitations include rare instances of incomplete data recording and data collected only on dichotomous variables. Automated data collection software can provide the ability to continuously record variables but was not available in this study.
The study was done, in part, to test the adherence of trauma centers to treatment guidelines. However, we did not track the specific treatments and interventions performed on each patient in response to specific clinical scenarios. In addition, the effects of assault and multisystem trauma on survival were not examined. Although it is widely accepted that morbidity and mortality rates associated with nonaccidental trauma (NAT) in children are higher than those associated with accidental trauma, it is likely that this is due to the severity of the injuries rather than the mechanism themselves (44–46). As our study was not aimed at differentiating outcome based on mechanism of injury, children with NAT were not analyzed separately from the rest of the study population.
Our pediatric age groups were defined based on approximate ranges of adolescence versus preadolescence, but pubertal status of these patients was not recorded. Our 0- to 11-year-old group was initially stratified into two separate groups (0–5 and 6–11) based on expected differences in physiology. These groups were combined in the final analyses due to similar results between the two groups with respect to survival at both thresholds.
Nonpharmacologically paralyzed patients, on day 1 or 2 following trauma, with GCS of 3 or 4 and with fixed and dilated pupils were excluded from data analysis due to their overall poor prognosis (47–50). Although there is some evidence that these patients will also benefit from aggressive treatment, multiple studies have shown mortality rates approaching 100% in these patients (51, 52). No analysis was done with these patients, as their very high mortality rate would have likely led to a skewed result.
Conclusion
Management of CPP in severely brain-injured patients plays an important role in short-term survival. CPP targets should be age specific. Our data show decreased survival in patients 18 years old and older who experience prolonged CPP below 50 or 60 mm Hg, in patients 6–17 years old with prolonged CPP below 50 mm Hg, and in patients 0–5 years old with prolonged CPP below 40 mm Hg.
Furthermore in our study, systemic hypotension had an inconsistent relationship to events of low CPP, whereas elevated ICP was significantly related to all low CPP events across all age groups. This may impart a clinically important difference in care, highlighting the necessity of controlling ICP at all times, while targeting SBP in specific instances.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (http://journals.lww.com/pccmjournal).
Supported, in part, by the New York State Department of Health to the Brain Trauma Foundation.
Presented, in part, at the American Association of Neurological Surgeons Annual Meeting, Denver, CO, April 13, 2011.
For information regarding this article, bba2003@nyp.org
References
- 1.Division of Vital Statistics, National Center for Health Statistics. Deaths, Percent of Total Deaths, and Death Rates for the 15 Leading Causes of Death in 5-year Age Groups, by Race and Sex: United States, 1999-2006. Atlanta, GA: Centers for Disease Control and Prevention; 2009. [Google Scholar]
- 2.Heron M, Sutton PD, Xu J, et al. Annual summary of vital statistics: 2007. Pediatrics. 2010;125:4–15. doi: 10.1542/peds.2009-2416. [DOI] [PubMed] [Google Scholar]
- 3.Keenan HT, Bratton SL. Epidemiology and outcomes of pediatric traumatic brain injury. Dev Neurosci. 2006;28:256–263. doi: 10.1159/000094152. [DOI] [PubMed] [Google Scholar]
- 4.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: A brief overview. J Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Thurman DJ, Alverson C, Dunn KA, et al. Traumatic brain injury in the United States: A public health perspective. J Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Xiong Y, Mahmood A, Chopp M. Emerging treatments for traumatic brain injury. Expert Opin Emerg Drugs. 2009;14:67–84. doi: 10.1517/14728210902769601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearl GS. Traumatic neuropathology. Clin Lab Med. 1998;18:39–64. [PubMed] [Google Scholar]
- 8.Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7:728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 9.Scaife ER, Statler KD. Traumatic brain injury: Preferred methods and targets for resuscitation. Curr Opin Pediatr. 2010;22:339–345. doi: 10.1097/MOP.0b013e3283395f2b. [DOI] [PubMed] [Google Scholar]
- 10.Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care; AANS/CNS. Bratton SL, Chestnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury IX Cerebral perfusion thresholds. J Neurotrauma. 2007;24(Suppl 1):S59–S64. doi: 10.1089/neu.2007.9987. [DOI] [PubMed] [Google Scholar]
- 11.Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care; AANS/CNS. Bratton SL, Chestnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury VIII Intracranial pressure thresholds. J Neurotrauma. 2007;24(Suppl 1):S55–S58. doi: 10.1089/neu.2007.9988. [DOI] [PubMed] [Google Scholar]
- 12.Andrews PJ, Sleeman DH, Statham PF, et al. Predicting recovery in patients suffering from traumatic brain injury by using admission variables and physiological data: A comparison between decision tree analysis and logistic regression. J Neurosurg. 2002;97:326–336. doi: 10.3171/jns.2002.97.2.0326. [DOI] [PubMed] [Google Scholar]
- 13.Changaris DG, McGraw CP, Richardson JD, et al. Correlation of cerebral perfusion pressure and Glasgow Coma Scale to outcome. J Trauma. 1987;27:1007–1013. doi: 10.1097/00005373-198709000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Clifton GL, Miller ER, Choi SC, et al. Fluid thresholds and outcome from severe brain injury. Crit Care Med. 2002;30:739–745. doi: 10.1097/00003246-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 15.McGraw C. A cerebral perfusion pressure greater than 80 mm HG is more beneficial. In: Hoff J, Betz A, editors. ICP VII. Berlin: Springer-Verlag; 1989. pp. 839–841. [Google Scholar]
- 16.Rosner MJ, Daughton S. Cerebral perfusion pressure management in head injury. J Trauma. 1990;30:933–940. doi: 10.1097/00005373-199008000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Juul N, Morris GF, Marshall SB, et al. Intracranial hypertension and cerebral perfusion pressure: Influence on neurological deterioration and outcome in severe head injury. J Neurosurg. 2000;92:1–6. doi: 10.3171/jns.2000.92.1.0001. [DOI] [PubMed] [Google Scholar]
- 18.Robertson CS, Valadka AB, Hannay HJ, et al. Prevention of secondary ischemic insults after severe head injury. Crit Care Med. 1999;27:2086–2095. doi: 10.1097/00003246-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Kochanek PM, Carney NA, Adelson PD, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents-Second edition Chapter 5 Cerebral perfusion pressure thresholds. Pediatr Crit Care Med. 2012;13:S24–S29. doi: 10.1097/PCC.0b013e31823f435c. [DOI] [PubMed] [Google Scholar]
- 20.Kochanek PM, Carney NA, Adelson PD, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents-Second edition Chapter 4 Threshold for treatment of intracranial hypertension. Pediatr Crit Care Med. 2012;13:S18–S23. doi: 10.1097/PCC.0b013e31823f435c. [DOI] [PubMed] [Google Scholar]
- 21.Downard C, Hulka F, Mullins RJ, et al. Relationship of cerebral perfusion pressure and survival in pediatric brain-injured patients. J Trauma. 2000;49:654–658. doi: 10.1097/00005373-200010000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Elias-Jones AC, Punt JA, Turnbull AE, et al. Management and outcome of severe head injuries in the Trent region 1985-90. Arch Dis Child. 1992;67:1430–1435. doi: 10.1136/adc.67.12.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutchison JS, Frndova H, Lo TY, et al. Hypothermia Pediatric Head Injury Trial Investigators; Canadian Critical Care Trials Group. Impact of hypotension and low cerebral perfusion pressure on outcomes in children treated with hypothermia therapy following severe traumatic brain injury: A post hoc analysis of the Hypothermia Pediatric Head Injury Trial. Dev Neurosci. 2010;32:406–412. doi: 10.1159/000323260. [DOI] [PubMed] [Google Scholar]
- 24.Kaiser G, Pfenninger J. Effect of neurointensive care upon outcome following severe head injuries in childhood-a preliminary report. Neuropediatrics. 1984;15:68–75. doi: 10.1055/s-2008-1052344. [DOI] [PubMed] [Google Scholar]
- 25.Català-Temprano A, Teruel GC, Lasaosa FJC, et al. Intracranial pressure and cerebral perfusion pressure as risk factors in children with traumatic brain injuries. J Neurosurg Pediatr. 2007;106:463–466. doi: 10.3171/ped.2007.106.6.463. [DOI] [PubMed] [Google Scholar]
- 26.Chambers IR, Stobbart L, Jones PA, et al. Age-related differences in intracranial pressure and cerebral perfusion pressure in the first 6 hours of monitoring after children's head injury: Association with outcome. Childs Nerv Syst. 2005;21:195–199. doi: 10.1007/s00381-004-1060-x. [DOI] [PubMed] [Google Scholar]
- 27.Mehta A, Kochanek PM, Tyler-Kabara E, et al. Relationship of intracranial pressure and cerebral perfusion pressure with outcome in young children after severe traumatic brain injury. Dev Neurosci. 2010;32:413–419. doi: 10.1159/000316804. [DOI] [PubMed] [Google Scholar]
- 28.Chambers IR, Jones PA, Lo TY, et al. Critical thresholds of intracranial pressure and cerebral perfusion pressure related to age in paediatric head injury. J Neurol Neurosurg Psychiatry. 2006;77:234–240. doi: 10.1136/jnnp.2005.072215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kokoska ER, Smith GS, Pittman T, et al. Early hypotension worsens neurological outcome in pediatric patients with moderately severe head trauma. J Pediatr Surg. 1998;33:333–338. doi: 10.1016/s0022-3468(98)90457-2. [DOI] [PubMed] [Google Scholar]
- 30.Contant CF, Valadka AB, Gopinath SP, et al. Adult respiratory distress syndrome: A complication of induced hypertension after severe head injury. J Neurosurg. 2001;95:560–568. doi: 10.3171/jns.2001.95.4.0560. [DOI] [PubMed] [Google Scholar]
- 31.Cruz J. The first decade of continuous monitoring of jugular bulb oxy-hemoglobinsaturation: Management strategies and clinical outcome. Crit Care Med. 1998;26:344–351. doi: 10.1097/00003246-199802000-00039. [DOI] [PubMed] [Google Scholar]
- 32.Jaeger M, Dengl M, Meixensberger J, et al. Effects of cerebrovascular pressure reactivity-guided optimization of cerebral perfusion pressure on brain tissue oxygenation after traumatic brain injury. Crit Care Med. 2010;38:1343–1347. doi: 10.1097/CCM.0b013e3181d45530. [DOI] [PubMed] [Google Scholar]
- 33.Johnson U, Nilsson P, Ronne-Engström E, et al. Favorable outcome in traumatic brain injury patients with impaired cerebral pressure auto-regulation when treated at low cerebral perfusion pressure levels. Neurosurgery. 2011;68:714–721. doi: 10.1227/NEU.0b013e3182077313. [DOI] [PubMed] [Google Scholar]
- 34.Schramm P, Klein KU, Pape M, et al. Serial measurement of static and dynamic cerebrovascular autoregulation after brain injury. J Neurosurg Anesthesiol. 2011;23:41–44. doi: 10.1097/ANA.0b013e3181f35854. [DOI] [PubMed] [Google Scholar]
- 35.Steiner LA, Czosnyka M, Piechnik SK, et al. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med. 2002;30:733–738. doi: 10.1097/00003246-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Sorrentino E, Budohoski KP, Kasprowicz M, et al. Critical thresholds for transcranial Doppler indices of cerebral autoregulation in traumatic brain injury. Neurocrit Care. 2011;14:188–193. doi: 10.1007/s12028-010-9492-5. [DOI] [PubMed] [Google Scholar]
- 37.Brady KM, Shaffner DH, Lee JK, et al. Continuous monitoring of cerebrovascular pressure reactivity after traumatic brain injury in children. Pediatrics. 2009;124:e1205–e1212. doi: 10.1542/peds.2009-0550. [DOI] [PubMed] [Google Scholar]
- 38.Figaji AA, Zwane E, Fieggen AG, et al. Pressure autoregulation, intracranial pressure, and brain tissue oxygenation in children with severe traumatic brain injury. J Neurosurg Pediatr. 2009;4:420–428. doi: 10.3171/2009.6.PEDS096. [DOI] [PubMed] [Google Scholar]
- 39.Sharples PM, Matthews DS, Eyre JA. Cerebral blood flow and metabolism in children with severe head injuries. Part 2: Cerebrovascular resistance and its determinants. J Neurol Neurosurg Psychiatry. 1995;58:153–159. doi: 10.1136/jnnp.58.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaiwat O, Sharma D, Udomphorn Y, et al. Cerebral hemodynamic predictors of poor 6-month Glasgow Outcome Score in severe pediatric traumatic brain injury. J Neurotrauma. 2009;26:657–663. doi: 10.1089/neu.2008.0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Di Gennaro JL, Mack CD, Malakouti A, et al. Use and effect of vasopressors after pediatric traumatic brain injury. Dev Neurosci. 2010;32:420–430. doi: 10.1159/000322083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grinkevičiūtė DE, Kėvalas R, Matukevičius A, et al. Significance of intracranial pressure and cerebral perfusion pressure in severe pediatric brain injury. Medicina (Kaunas) 2008;44:119–125. [PubMed] [Google Scholar]
- 43.Prabhakaran P, Reddy AT, Oakes WJ, et al. A pilot trial comparing cerebral perfusion pressure-targeted therapy to intracranial pressure-targeted therapy in children with severe traumatic brain injury. J Neurosurg. 2004;100:454–459. doi: 10.3171/ped.2004.100.5.0454. [DOI] [PubMed] [Google Scholar]
- 44.Adamo MA, Drazin D, Smith C, et al. Comparison of accidental and nonaccidental traumatic brain injuries in infants and toddlers: Demographics, neurosurgical interventions, and outcomes. J Neurosurg Pediatr. 2009;4:414–419. doi: 10.3171/2009.6.PEDS0939. [DOI] [PubMed] [Google Scholar]
- 45.Rhine T, Wade SL, Makoroff KL, et al. Clinical predictors of outcome following inflicted traumatic brain injury in children. J Trauma Acute Care Surg. 2012;73:S248–S253. doi: 10.1097/TA.0b013e31826b0062. [DOI] [PubMed] [Google Scholar]
- 46.Shein SL, Bell MJ, Kochanek PM, et al. Risk factors for mortality in children with abusive head trauma. J Pediatr. 2012;161:716–722.e1. doi: 10.1016/j.jpeds.2012.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lannoo E, Van Rietvelde F, Colardyn F, et al. Early predictors of mortality and morbidity after severe closed head injury. J Neurotrauma. 2000;17:403–414. doi: 10.1089/neu.2000.17.403. [DOI] [PubMed] [Google Scholar]
- 48.Ono J, Yamaura A, Kubota M, et al. Outcome prediction in severe head injury: Analyses of clinical prognostic factors. J Clin Neurosci. 2001;8:120–123. doi: 10.1054/jocn.2000.0732. [DOI] [PubMed] [Google Scholar]
- 49.Tien HC, Cunha JR, Wu SN, et al. Do trauma patients with a Glasgow Coma Scale score of 3 and bilateral fixed and dilated pupils have any chance of survival? J Trauma. 2006;60:274–278. doi: 10.1097/01.ta.0000197177.13379.f4. [DOI] [PubMed] [Google Scholar]
- 50.White JR, Farukhi Z, Bull C, et al. Predictors of outcome in severely head-injured children. Crit Care Med. 2001;29:534–540. doi: 10.1097/00003246-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 51.Chamoun RB, Robertson CS, Gopinath SP. Outcome in patients with blunt head trauma and a Glasgow Coma Scale score of 3 at presentation. J Neurosurg. 2009;111:683–687. doi: 10.3171/2009.2.JNS08817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nijboer JMM, van der Nallt J, ten Duis H. Patients beyond salvation? Various categories of trauma patients with a minimal Glasgow Coma Score. Injury. 2010;41:52–57. doi: 10.1016/j.injury.2009.05.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


