Abstract
Background and Aims
Chromium and cysteine supplementation have been shown to improve glucose metabolism in animal studies. This study examined the hypothesis that chromium dinicocysteinate (CDNC), a complex of chromium and L-cysteine, is beneficial in lowering oxidative stress, vascular inflammation and glycemia in type 2 diabetic patients.
Methods
Type 2 diabetic patients enrolled in this study were given placebo for one month for stabilization and then randomized into one of three groups: placebo (P), chromium picolinate (CP) or CDNC, after which they received daily oral supplementation for 3 months. Of the 100 patients enrolled in the study, 74 patients completed it. There were 25 patients in the P supplemented group, 25 in the CP supplemented and 24 in the CDNC supplemented group who completed the study. Blood markers of glycemia, vascular inflammation, HOMA insulin resistance and oxidative stress were determined at randomization and after 3 months of supplementation with P, CP or CDNC.
Results
There was a significant decrease at 3 months in insulin resistance (p=0.02) and in the levels of protein oxidation (p=0.02) and TNF-α (p=0.01) in the CDNC supplemented cohort compared to baseline. However, there was no statistically significant change in these markers in the CP supplemented group compared to baseline. Insulin levels significantly decreased(p=0.01) for subjects receiving CDNC but not CP. There was no significant impact of supplementation on HbA1c or glucose levels in either of the groups.
Conclusions
CDNC supplementation lowers insulin resistance by reducing blood levels of TNF-α, insulin, and oxidative stress in type 2 diabetic patients. Therefore, CDNC supplementation has potential as an adjunct therapy for individuals with type 2 diabetes.
Keywords: Chromium, L-cysteine, oxidative stress, insulin resistance, diabetes
1 Introduction
Diabetes now affects 366 million people worldwide and is responsible for one death every seven seconds, or about 4.6 million deaths each year. According to the American Diabetes Association, 23.5 million or 10.7% of the US population aged 20 years and older have diabetes. Intensive blood glucose control dramatically reduces the devastating complications that result from poorly controlled diabetes. However, for many, achievement of tight glucose control is difficult with current regimens. Chromium supplementation in the form of commercially available chromium dinicotinate (CDN) or chromium picolinate (CP) is widely used by the diabetic patient population.
Trivalent chromium is an essential nutrient and has been shown to lower oxidative stress and improve glucose and lipid metabolism (1-5). Subclinical chromium deficiency may contribute to insulin resistance and cardiovascular disease, particularly in aging and diabetic populations (6). It has been proposed that chromium supplementation increases the amount of a chromium-containing oligopeptide present in the insulin-sensitive cells that bind to the insulin receptor, markedly increasing the activity of insulin-stimulated tyrosine kinase and phosphorylation of insulin receptor substrate-1 and glucose transporter GLUT4 (7).
Diabetes is associated with elevated levels of oxidative stress, which potentially impairs cellular glucose metabolism via a variety of mechanisms, including redox imbalance and insulin resistance (17-21).Recent studies report lower blood levels of L-cysteine and altered cysteine homeostasis in diabetic patients (8, 9). Along with a host of proteins, L-cysteine is a precursor of glutathione, which is considered essential for the reduction of cellular oxidative stress (10). Dietary supplementation with N-acetylcysteine (NAC) or whey protein and α-lactoalbumin (cysteine rich proteins) lowered the oxidative stress and insulin resistance induced by sucrose or fructose in rats and streptozotocin-treated diabetic mice (11-14). Oral supplementation with L-cysteine lowered oxidative stress, vascular inflammation and glycemia markers in ZDF rats, an animal model of type 2 diabetes (15). Recent studies report that L-cysteine supplementation lowered oxidative stress markers in type 2 diabetic patients and normal subjects (8, 16).
Previous animal studies reported the results of a head to head comparison between three chromium complexes in order to determine if any one of these complexes demonstrated a superior ability to modulate risk factors linked with diabetes (25). One of these, CDNC, a complex of trivalent chromium with L-cysteine and niacin, proved to be the most efficacious in decreasing fasting glucose levels, glycated hemoglobin levels, insulin levels and vascular inflammation in Zucker diabetic fatty rats as assessed by CRP, MCP-1, ICAM-1 and oxidative stress levels. We therefore undertook this pilot study to determine whether CDNC is superior to chromium picolinate at lowering oxidative stress and insulin resistance in type 2 diabetic patients. This study examined the effect of daily supplementation for 3 months on oxidative stress, insulin resistance, markers of vascular inflammation and glycemia in type 2 adult diabetic patients.
2 RESEARCH DESIGN AND METHODS
Patient Enrollment
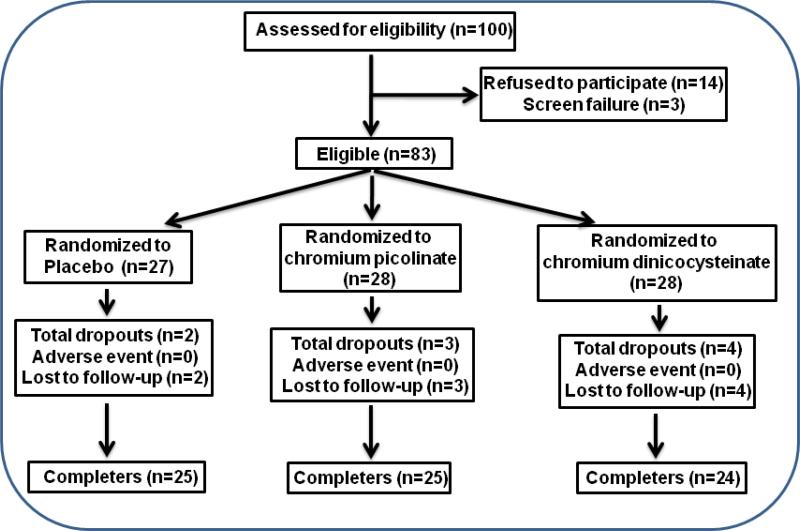
Informed written consent was obtained from all patients according to the protocol approved by the Louisiana State University Health Sciences Center Institutional Review Board (IRB). All patients included in this study were adults with type 2 diabetes. One hundred diabetic subjects were enrolled in this study. Fasting blood was collected at the screening visit and all subjects were given placebo supplementation for one month. After the one month placebo run-in period, subjects were randomized into three groups. Patients in one group continued taking placebo and those in the other two groups were given either chromium dinicocysteinate (orally, 400 μg Cr3+/day) or chromium picolinate (orally, 400 μg Cr3+/day) supplementation for up to 3 months while receiving the usual standard care for diabetes. Medication usage was tracked throughout the duration of the study. Both subjects and investigators were blinded to the supplement (placebo or either formulation of Cr3+) each subject received. Figure 1 gives flow chart for number of patients that were enrolled, screen failure, randomized and those completed the study in each supplementation group. Of the 100 subjects screened, 74 subjects completed the study. Among those who discontinued, 3 were screen failures, 14 refused to enter the study and 9 were lost to follow-up.
Figure 1.
Enrollment, screen failure, randomization and completion flow chart for all type 2 diabetic patients.
Inclusion/Exclusion Criteria
Adult type 2 diabetic patients ages 25-65 years were included in the study. Table 1 outlines the specific inclusion and exclusion criteria for patient enrollment in this study. Patients were excluded if they had any history of cardiovascular disease, sickle cell disease, treatment with insulin, or metabolic disorders, including: uncontrolled hypertension, hypothyroidism or hyperthyroidism. Patients were excluded at baseline or at any visit if they showed signs of significant hepatic dysfunction, defined as any underlying chronic liver disease or liver function tests greater than 1.5 times the upper limit of normal or renal dysfunction, defined as a serum creatinine greater than 1.5mg/dL. Women with a positive pregnancy test or those nursing infants were also excluded. Women who were sexually active were not included unless they agreed to use an effective form of contraceptive. All participants agreed not to take any supplemental vitamins or herbal products and those who started taking any supplements during the study period were withdrawn from the trial.
Table 1.
Inclusion and exclusion criteria for the random trial.
| Inclusion Criteria |
|---|
| Adults ages 30-55 years. |
| Participants must understand the risks and benefits of the protocol and sign an informed consent form provided |
| Willingness to participate in 4 additional clinic visits (Baseline, 1, 2 and 3 months) |
| Willingness to complete standard health history questionnaire before and during the study |
| Women with a negative pregnancy test |
| Exclusion Criteria |
| History of cardiovascular disease, sickle cell disease, insulin intake, and metabolic disorders including: hypertension, hypothyroid and hyperthyroid |
| Any sign of hepatic and renal dysfunction as assessed from blood chemistry tests |
| Presently taking weight loss or other dietary supplements |
| Women with a positive pregnancy test or nursing females |
Chromium or Placebo Supplementation
Diabetic patients who agreed to participate in this study were asked to come to the clinic after overnight fasting. Initially all of the study subjects were given a placebo for a one month run-in period prior to randomization. The outline of each clinic visit and clinical trial design is provided in Table 2. The blood levels determined at the randomization visit after the run-in period were considered baseline values. During the run-in period and before randomization, any subject indentified to have an exclusion criterion was excluded from the study. The remaining subjects were randomized to receive supplementation with a placebo, chromium dinicocysteinate (400 μg Cr3+/day), or chromium picolinate (400 μg Cr3+/day) for up to 3 months while receiving the usual standard care for diabetes. Both subjects and investigators were blinded to the medication (placebo or either formulation of chromium) each subject received. Pregnancy tests and renal and liver function tests were done at −1 (screening visit), 0 (baseline or randomization visit), and at the 1, 2 and 3 month supplementation visits to monitor for any sign of toxicity. All data presented here are from randomization (baseline) and after 3 months of supplementation. The placebo-run in period was designed to stabilize patients and prevent any effect due solely to inclusion in the study.
Table 2.
Trial design and time line of the clinical study.
| Screening | Trial Period | |||
|---|---|---|---|---|
| Visit 1 | Visit 2 | Visit 3 | Visit 4 | Visit 5 |
| •Subject Enrollment •Supplementation with placebo •Pre-randomization placebo-run-in period |
•Randomization •Supplementation •Data collection* |
Data collection | Data collection | Data collection |
| Placebo-run-in 1 month | 0 (baseline) | 1 month | 2 months | 3 months |
Overnight fasting (12 hours) blood samples, anthropomorphic data, record of daily insulin use and blood glucose levels were collected at each visit.
Adverse Effects (AE)
The nurse kept track of all the symptoms reported by the patient at each visit. In some instances, these symptoms were similar to pre-existing conditions that were recorded in the patient charts. Other times, some symptoms were clearly unrelated to treatment (sports injuries, tooth extractions, decreased sex drive). To be in compliance with safety monitoring, all these symptoms regardless of relevance to the study were recorded. All of these were compiled into a single spreadsheet and summary is provided in Table 7. Overall, there do not appear to be any differences from one group to another group.
Table 7.
Summary of Occurrence of Adverse Events (AEs).
| Number of AEs | Severity | Subjects Reporting AEs | Treatment Related | |||
|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | ||||
| Placebo | 45 | 35 | 9 | 1 | 19 | None |
| Chromium Picolinate | 44 | 40 | 4 | 0 | 17 | None |
| Chromium Dinicocysteinate | 28 | 24 | 2 | 2 | 14 | None |
Blood Collection
Blood was drawn from patients after an overnight fast (8 hours). Following the blood collection at each visit, serum tubes for chemistry profile, EDTA tubes for HbA1C and complete blood counts were promptly delivered to the LSUHSC clinical laboratories. Additional tubes of EDTA-blood were brought to research laboratory. Clear plasma was separated via centrifugation at 3000 rpm (1500 × g) for 15 minutes. All plasma samples were stored at -80°C for analyses of the biochemical parameters.
TNF-α, IL-6, IL-8, ICAM-1, insulin, oxidative stress (protein oxidation) and insulin resistance assays
TNF-α, IL-6, IL-8, ICAM-1 and insulin levels in the plasma were determined by the sandwich ELISA method using commercially available kits from Fisher Thermo Scientific Co. (Rockford, IL). All appropriate controls and standards as specified by the manufacturer's kit were used. In the cytokine assay, control samples were analyzed each time to check the variation from plate to plate on different days of analysis. The protein oxidation was assessed by determining protein carbonyl level in the plasma using an ELISA kit from ENZO life sciences International Inc. (Plymouth Meeting, PA). Insulin resistance was calculated from blood glucose and insulin levels using HOMA insulin resistance method as described previously (21).
All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise mentioned. Chromium dinicocysteinate was furnished by InterHealth Nutraceuticals, Inc. (Benicia, CA). Chromium picolinate was obtained from Nutrition-21 (Purchase, NY). Data were analyzed statistically using the Wilcoxon signed rank test to determine significant changes after 3-month supplementation for each treatment group and the Kruskal-Wallis test to compare the three treatment groups on changes. SAS version 9.2 (Cary, NC) was used for statistical computing. A p value of less than 0.05 for a statistical test was considered significant.
3 Results
Table 3 gives the ages, weights, BMI, glucose, HbA1c and number of subjects in the placebo, chromium picolinate and chromium dinciocysteinate supplemented groups. Table IV also shows the glucose, glycated hemoglobin and insulin levels of patients at baseline and after 3 months of supplementation. The ages, weights, BMI, glycemia levels of patients were similar in all three groups (Table 3). There were no differences in the glucose or HbA1c levels after supplementation versus baseline in any of the groups (Table 4). However, there was a significant reduction in the insulin level after supplementation with CDNC versus before supplementation (Table 4). There was no significant difference in insulin levels after supplementation in the placebo or chromium picolinate groups.
Table 3.
Demographic and baseline characteristics of the trial subjects.
| Placebo (n=25) | Chromium Picolinate (n=25) | Chromium Dinicocysteinate (n=24) | |
|---|---|---|---|
| Age (years) | 48.64±1.99 | 51.12±2.03 | 48.79±1.82 |
| Gender: male/female (%) | 2/23 (8.70%) | 7/18 (38.89%) | 8/16 (50%) |
| Weight (kg) | 100.47±5.52 | 103.94±6.39 | 104.31±6.93 |
| BMI | 38.00±1.71 | 35.44±2.06 | 36.85±2.20 |
| Fasting Glucose (mg/dL) | 142.4±12.32 | 128.88±8.78 | 131.95±9.61 |
| HbA1C (%) | 7.84±0.34 | 7.54±0.32 | 7.65±0.35 |
Where applicable, values are expressed as Mean ± SEM. No significant difference was observed among the groups.
Table 4.
Effect of chromium supplementation (daily for 3 months at 400 μg Cr/day) on various blood biomarkers in type 2 diabetic patients. Values are Mean ± SEM. P < 0.05 is considered statistically significant.
| Placebo (n=25) | Chromium Picolinate (n=25) | Chromium Dinicocysteinate (n=24) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3 Months | P-value | Baseline | 3 Months | P-value | Baseline | 3 Months | P-value | |
| Glucose (mg/dL) | 142.4±12.3 | 135.9±13.3 | NS | 128.9±8.8 | 137.2±12.0 | NS | 131.9±9.6 | 129.2±12.6 | NS |
| HbA1C (%) | 7.8±0.3 | 7.8±0.4 | NS | 7.5±0.3 | 7.4±0.3 | NS | 7.7±0.4 | 7.5±0.4 | NS |
| Insulin (μU/mL) | 30.6±5.9 | 32.4±5.2 | NS | 32.7±5.6 | 25.3±3.2 | NS | 34.7±5.6 | 24.3±3.9 | 0.01 |
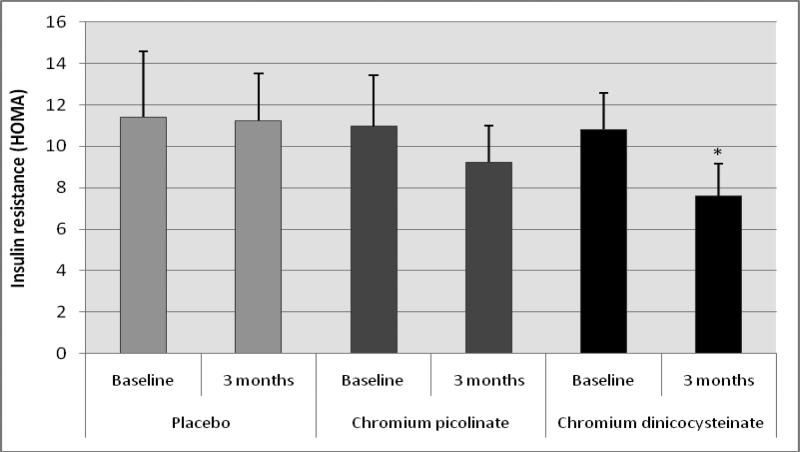
Figure 2 illustrates the effect of placebo, chromium picolinate or CDNC supplementation on insulin resistance in type 2 diabetic patients. There was a significant decrease in insulin resistance after supplementation with CDNC compared with baseline levels. However, there was no significant difference in insulin resistance after placebo or CP supplementation. This suggests that CDNC supplementation can lower insulin resistance levels as determined by HOMA-IR in type 2 diabetic patients. When subjects were divided in to male and female groups; similar significant reduction in HOMA-IR was observed after CDNC supplementation in female group (p=0.035) but not in male group (p=0.19), which is likely due to number of male (n= 8) participants were lower than female (n=16) participants. The differences were not significant when data was compared between CDNC versus CP or placebo-supplementation groups irrespective of when data was analyzed with all, male or female participants.
Figure 2.
Effect of chromium dinicocysteinate, chromium picolinate and placebo supplementation (daily for 3 months, 400 μg Cr/day) on insulin resistance levels in type 2 diabetic patients. Values are Mean ± SE. *p<0.02 compared with baseline values.
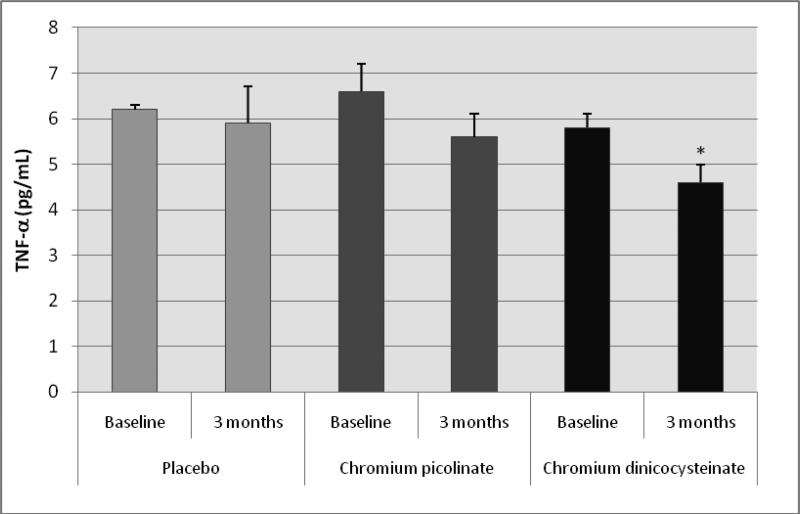
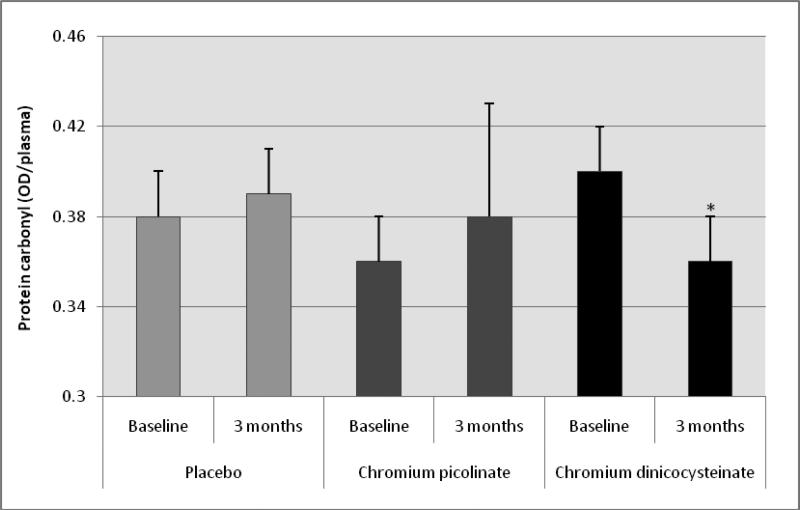
Table 5 shows no difference in levels of IL-6, IL-8 or ICAM-1 in any of the groups before versus after supplementation with placebo, CP or CDNC in type 2 diabetic patients. However, there was a significant decrease in levels of TNF-α (figure 3) and oxidative stress as determined by protein carbonyl (figure 4) in diabetic patients supplemented with CDNC for 3 months compared to baseline. Levels of oxidative stress or TNF-α did not change after placebo or CP supplementation in diabetic patients. This suggests that CDNC supplementation can lower oxidative stress and TNF-α levels in type 2 diabetic patients. When participants were divided in to male and female groups; similar significant reduction in TNF-α was observed after CDNC supplementation in female group (p=0.035) and in male group (p=0.01), for protein carbonyl differences were significant in female group (p=0.005) but not for male group. The differences in TNF-α or protein carbonyl were not significant when data was compared between CDNC versus CP or placebo-supplementation groups irrespective of when data was analyzed with all, male or female participants.
Table 5.
Effect of chromium supplementation (daily for 3 months at 400 μg Cr/day) on various blood biomarkers in type 2 diabetic patients. Values are Mean ± SEM. No significant difference was observed among the treatment groups.
| Placebo (n=25) | Chromium Picolinate (n=25) | Chromium Dinicocysteinate (n=24) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3 Months | p-value | Baseline | 3 Months | p-value | Baseline | 3 Months | p-value | |
| IL-6 (pg/mL) | 7.2 ± 0.6 | 7.4 ± 0.9 | NS | 8.1 ± 0.7 | 8.9 ± 0.6 | NS | 6.9 ± 0.5 | 7.1 ± 0.6 | NS |
| IL-8 (Pg/mL) | 2.3 ± 0.3 | 2.8 ± 0.5 | NS | 2.9 ± 0.3 | 2.5 ± 0.4 | NS | 2.9 ± 0.7 | 3.3 ± 0.7 | NS |
| ICAM-1 (ng/mL) | 75.1 ± 10.1 | 80.8 ± 9.6 | NS | 107.8 ± 18.5 | 87.3 ± 13.4 | NS | 101.3 ± 14.6 | 91.8 ± 12.2 | NS |
Figure 3.
Effect of chromium dinicocysteinate, chromium picolinate and placebo supplementation (daily for 3 months, 400 μg Cr/day) on TNF-α levels in type 2 diabetic patients. Values are Mean ± SE. *p<0.01 compared with baseline values.
Figure 4.
Effect of chromium dinicocysteinate, chromium picolinate and placebo supplementation (daily for 3 months, 400 μg Cr/day) on protein carbonyl in type 2 diabetic patients. Values are Mean ± SE. *p<0.02 compared with baseline values.
There were no differences in the liver function tests (AST, AP, ALT) or the renal function tests (BUN/creatinine ratio, serum creatinine), nor in the complete blood counts of patients after supplementation compared to baseline in any of the groups (Table 6). This suggests that daily supplementation with either chromium picolinate or chromium dinicocysteinate was not toxic in type 2 diabetic patients. No differences were observed among the three cohorts with regards to medications used to control diabetes. Similarly, there was no adverse event reported related with supplementation in either of the groups (Table 7). No change in either body weight or BMI was detected using the DEXA-assessment of body composition in either group (data not shown here).
Table 6.
Effect of chromium supplementation (daily for 3 months at 400 μg Cr/day) on various blood biomarkers in type 2 diabetic patients. Values are Mean ± SEM. No significant difference was observed among the treatment groups.
| Placebo (n=25) | Chromium Picolinate (n=25) | Chromium Dinicocysteinate (n=24) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3 Months | P- value | Baseline | 3 Months | P-value | Baseline | 3 Months | P-value | |
| AST (U/L) | 19.2±2.7 | 19.4±1.8 | NS | 20.1±1.5 | 19.4±1.6 | 0.54 | 19.33±1.45 | 19.67±2.29 | NS |
| ALT (U/L) | 30.0±3.6 | 27.9±2.4 | NS | 27.3±1.9 | 28.9±1.9 | NS | 26.4±1.6 | 28.9±2.3 | NS |
| AP (U/L) | 78.0±3.8 | 75.9±3.3 | NS | 76.4±4.8 | 73.2±3.8 | NS | 80.5±5.3 | 80.7±6.9 | NS |
| Creatinine (mg/dL) | 0.86±0.20 | 0.85±0.05 | NS | 0.90±0.05 | 0.91±0.05 | NS | 0.85±0.04 | 0.82±0.04 | NS |
| BUN/Creatinine (ratio) | 17.6±1.2 | 18.22±1.45 | NS | 16.9±1.4 | 17.8±1.4 | NS | 16.1±1.0 | 15.6±1.2 | NS |
| RBC (M/μL) | 4.3±0.4 | 4.4±0.5 | NS | 4.4±0.1 | 4.3±0.1 | NS | 4.6±0.5 | 4.6±0.1 | NS |
| Hemoglobin (g/dL) | 12.4±0.3 | 12.4±0.3 | NS | 12.6±0.3 | 12.5±0.2 | NS | 13.2±0.2 | 12.9±0.3 | NS |
| Hematocrit (%) | 37.1±0.7 | 37.3±0.7 | NS | 37.6±0.7 | 37.2±0.7 | NS | 39.3±0.6 | 38.6±0.7 | NS |
4 Discussion
Uncontrolled hyperglycemia is known to increase reactive oxygen species (ROS) generation and oxidative stress in diabetic rats and diabetic patients (19, 20, 22, 23). Oxidative stress and TNF-α levels are elevated in the blood of many diabetics and are positively associated with an increase in vascular inflammation and insulin resistance (19, 20, 24). Insulin resistance and vascular inflammation are major risk factors that contribute to development of cardiovascular disease in diabetic patients.
This study demonstrates a significantly lower protein oxidation level in diabetic patients supplemented with CDNC but not in those supplemented with placebo or chromium picolinate. Oxidative stress is a known activator of NFkB, which undergoes nuclear translocation and serine phosphorylation at residue 276 of its p65 subunit. It then associates with surrounding chromatin components and binds with DNA, which promotes the transcription of pro-inflammatory cytokine TNF-a, which mediates the insulin resistance cascade. This study demonstrates that supplementation with CDNC significantly reduced circulating levels of TNF-α, insulin, and HOMA insulin resistance over a three month period. Conversely, supplementation with placebo or chromium picolinate did not show any effect on TNF-α, protein oxidation, HOMA insulin resistance or glycemia. Lack of effect of supplementation of CDNC or CP on changes in HbA1c may likely be due to the short duration of this clinical trial. Similarly, we did not observe a significant impact on blood glucose levels. It is unlikely that the effects of CDNC on circulating insulin, TNF-α, oxidative stress and insulin resistance reported herein are dependent on any chromium deficiency in the diabetic patients because these effects were seen only in CDNC supplemented group but not in CP supplemented group. It seems that the chromium dinicocysteinate adduct may preferentially activate the insulin receptor transduction system (25). Additional studies are required to address this issue and understand the underlying mechanisms involved.
Oxidative stress plays a key role in the regulatory pathway that progresses from hyperglycemia to monocyte and endothelial cell activation in the enhanced vascular inflammation of diabetes (19, 20, 24). The effect of CDNC on TNF-α inhibition may be mediated in part by reduction in the oxidative stress pathways due to an enhanced effect of chromium facilitated by L-cysteine (10, 25-28), as . L-cysteine (LC) supplementation inhibits NFkB activation and improves insulin signaling (15). When combined with chromium it produced better effects, which could explain the greater efficacy of CDNC over P or CP. This study indicates that CDNC supplementation is more effective in lowering circulating levels of TNF-α, protein oxidation and insulin resistance compared with P or CP supplementation in type 2 diabetic patients.
Normal glucose homeostasis is maintained by a delicate balance between insulin secretion by the pancreatic β-cells and insulin sensitivity of the peripheral tissues (muscle, liver, and adipose tissue). Visceral adiposity is considered a risk factor for insulin resistance metabolic syndrome (29) and type 2 diabetes in adults (30), as well as in first degree relatives of patients with type 2 diabetes with normal glucose levels (31). TNF-α is expressed in adipose tissues, with visceral fat responsible for more TNF-α production than subcutaneous fat. Obese mice lacking either TNF-α or its receptor show protection against developing insulin resistance. TNF-α inhibits tyrosine kinase phosphorylation of the insulin receptor, resulting in defects in insulin signaling and ultimately leading to insulin resistance and impaired glucose transport (32, 33). The mechanism involves Ser phosphorylation of IRS-2 mediated by TNF-α activation of MAP kinases (34). TNF-α may play a crucial role in the systemic insulin resistance of type 2 diabetes (35, 36). The association of endothelial dysfunction with insulin resistance in the absence of overt diabetes or metabolic syndrome provides evidence that atherosclerosis may actually begin earlier in the pathogenesis of insulin resistance, ultimately resulting in a progression of metabolic syndrome to prediabetes and then to type 2 diabetes (37, 38).
When people are insulin resistant, their muscle, fat, and liver cells do not respond properly to insulin. As a result, the body requires more insulin to maintain normoglycemia. Initially, the pancreas increases insulin production to counterbalance the insulin resistance and maintain normal glucose levels. However, in many individuals the pancreas eventually fails to keep up with the body's demand for insulin leading to hyperglycemia and setting the stage for the development of diabetes. Insulin resistance increases the chances of developing both type 2 diabetes and cardiovascular disease. Physical activity and weight loss can help people with insulin resistance or pre-diabetes delay or prevent developing type 2 diabetes. However, maintaining a healthy lifestyle is difficult for many individuals to sustain and many ultimately develop diabetes. In addition to the role that physical activity and weight loss, CDNC supplementation appears to be helpful in alleviating insulin resistance. As stated above, our ability to increase insulin sensitivity may help to manage disease progression by restoring glucose homeostasis at a lower level of insulin. Such an approach could spare the pancreas. This cascade may take time to be fully implemented biochemically, thereby explaining why we saw a trend towards lower glucose levels in the CDNC supplemented group that had not yet reached statistical significance. Further long-term studies are required to better determine if this trend reaches statistical significance.
In conclusion, CDNC supplementation has the potential to lower blood levels of oxidative stress, TNF-α and insulin resistance in type 2 diabetic patients. CDNC is a complex of L-cysteine and chromium dinicotinate. L-cysteine derivatives such as N-acetyl cysteine are known to scavenge oxygen radicals. Results of this study indicate that the presence of the cysteinate molecule in CDNC helps provide better protection against the oxidative stress and the activation of signal transduction pathways associated with the insulin resistance and vascular inflammation of type 2 diabetes. Whether or not CDNC supplementation can similarly prevent insulin resistance and vascular inflammation and thereby, delay or prevent the onset of diabetes in the pre-diabetic population is not known and will be interesting to investigate. If it works as well as this research hints that it may, then chromium dinicocysteinate supplementation could be used as an adjunct therapy for those with established diabetes. Furthermore, it might delay or prevent the development of diabetes in patients with insulin resistance or pre-diabetes.
Acknowledgements
The authors are grateful to research pharmacist Randy McNight and John Rowell, RN for the outstanding help in conducting this study. SKJ is supported by grants from NIDDK and the Office of Dietary Supplements of the National Institutes of Health RO1 DK072433, InterHealth Nutraceuticals, Inc. (Benicia, CA) and the Malcolm Feist Endowed Chair in Diabetes. The authors thank Ms Georgia Morgan for excellent editing of this manuscript.
References
- 1.Vincent J-B. Recent advances in the nutritional biochemistry of trivalent chromium. Proc. Nutr. Soc. 2004;63:41–47. doi: 10.1079/PNS2003315. [DOI] [PubMed] [Google Scholar]
- 2.Cefalu W-T, Hu F-B. Role of chromium in human health and in diabetes. Diab. Care. 2004;27:2741–2751. doi: 10.2337/diacare.27.11.2741. [DOI] [PubMed] [Google Scholar]
- 3.Balk EM, Tatsioni A, Lichtenstein AH, Lau J, Pittas AG. Effect of chromium supplementation on glucose metabolism and lipids. Diabetes Care. 2007;30:2154–2163. doi: 10.2337/dc06-0996. [DOI] [PubMed] [Google Scholar]
- 4.Hummel M, Standl E, Schnell O. Chromium in metabolic and cardiovascular disease. Horm. Metabolic Disease. 2007;39:743–751. doi: 10.1055/s-2007-985847. [DOI] [PubMed] [Google Scholar]
- 5.Anderson R-A, Cheng N, Bryden N-A, Polansky M-M, Cheng N, Chi J, Feng J. Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes. 1997;46:1786–1791. doi: 10.2337/diab.46.11.1786. [DOI] [PubMed] [Google Scholar]
- 6.Rajpathak S, Rimm E-B, Li T, Morris J-S, Stampfer M-J, Willet W-C, Hu F-B. Lower toenail chromium in men with diabetes and cardiovascular disease compared with healthy men. Diabetes Care. 2004;27:2211–2216. doi: 10.2337/diacare.27.9.2211. [DOI] [PubMed] [Google Scholar]
- 7.Cefalu WT, Wang ZQ, Zhang XH, Baldor LC, Russel JC. Oral chromium picolinate improves carbohydrate and lipid metabolism and enhance skeletal muscle Glut-4 translocation in obese, hyperinsulinemic (JCR-LA corpulent) rats. J Nutr. 2002;132:1107–1114. doi: 10.1093/jn/132.6.1107. [DOI] [PubMed] [Google Scholar]
- 8.Sekhar RV, McKay SV, Patel SG, et al. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care. 2011;34:162–167. doi: 10.2337/dc10-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darmaun D, Smith SD, Sweeten S, Hartman BK, Welch C, Mauras N. Poorly controlled type 1 diabetes is associated with altered glutathione homeostasis in adolescents: apparent resistance to N-acetylcysteine supplementation. Pediatr Diabetes. 2008;9:577–582. doi: 10.1111/j.1399-5448.2008.00436.x. [DOI] [PubMed] [Google Scholar]
- 10.Dröge W. Oxidative stress and ageing: is ageing a cysteine deficiency syndrome? Philos Trans R Soc Lond B Biol Sci. 2005;360:2355–2372. doi: 10.1098/rstb.2005.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blouet C, Mariotti F, Mikogami T, Tome D, Huneau JF. Meal cysteine improves postprandial glucose control in rats fed a high-sucrose meal. J Nutr Biochem. 2007;18:519–524. doi: 10.1016/j.jnutbio.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Blouet CB, Mariotti F, Azzout-Marniche D, et al. Dietary cysteine alleviates sucrose-induced oxidative stress and insulin resistance. Free Radic Biol Med. 2007;42:1089–1097. doi: 10.1016/j.freeradbiomed.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Song D, Hutchings S, Pang CCY. Chronic N-acetylcysteine prevents fructose-induced insulin resistance and hypertension in rats. Eu J Pharmacol. 2005;508:205–210. doi: 10.1016/j.ejphar.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Hsu CC, Yen HF, Yin MC, Tsai CM, Hsieh CH. Five cysteine-containing compounds delay diabetic deterioration in Balb/cA mice. J Nutr. 2004;134:3245–3249. doi: 10.1093/jn/134.12.3245. [DOI] [PubMed] [Google Scholar]
- 15.Jain SK, Velusamy T, Croad JL, Rains JL, Bull R. L-cysteine supplementation lowers blood glucose, glycated hemoglobin, CRP, MCP-1, oxidative stress and inhibits NFkB activation in the livers of Zucker diabetic rats. Free Radic Biol Med. 2009;46:1633–1638. doi: 10.1016/j.freeradbiomed.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iyer SS, Accardi CJ, Ziegler TR, et al. Cysteine redox potential determines pro-inflammatory IL-1beta levels. PLoS One. 2009;4:e5017. doi: 10.1371/journal.pone.0005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh U, Devraj S, Jialal I. Vitamin E, oxidative stress and inflammation. Ann. Rev. Nutr. 2005;25:151–174. doi: 10.1146/annurev.nutr.24.012003.132446. [DOI] [PubMed] [Google Scholar]
- 18.Rains JL, Jain SK. Oxidative stress, insulin signaling and diabetes. Free Rad Biol Med. 2011 doi: 10.1016/j.freeradbiomed.2010.12.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pennathur S, Heinecke JW. Mechanisms for oxidative stress in diabetic cardiovascular disease. Antioxidant Redox Signal. 2007;9:955–969. doi: 10.1089/ars.2007.1595. [DOI] [PubMed] [Google Scholar]
- 20.Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxidant Redox Signal. 2005;7:1040–1052. doi: 10.1089/ars.2005.7.1040. [DOI] [PubMed] [Google Scholar]
- 21.Yaturu S, Daberry B, Rains J, Jain S-K. Resistin and adiponectin levels in subjects with coronary artery disease and type 2 diabetes. Cytokine. 2006;34:219–223. doi: 10.1016/j.cyto.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Jain S-K. Hyperglycemia can cause membrane lipid peroxidation and osmotic fragility in human red blood cells. J. Biol. Chem. 1989;264:21340–21345. [PubMed] [Google Scholar]
- 23.Jain S-K, McVie R, Duett J, Herbst J-J. Erythrocyte membrane lipid peroxidation and glycosylated hemoglobin in diabetes. Diabetes. 1989:1539–1543. doi: 10.2337/diab.38.12.1539. [DOI] [PubMed] [Google Scholar]
- 24.Otani H. Oxidative stress as pathogenesis of cardiovascular risk associated with metabolic syndrome. Antioxidant Redox Signal. 2011;15:1911–1926. doi: 10.1089/ars.2010.3739. [DOI] [PubMed] [Google Scholar]
- 25.Jain SK, Croad JL, Velusamy T, Rains JL, Bull R. Chromium dinicocysteinate supplementation can lower blood glucose, CRP, MCP-1, ICAM-1, creatinine, apparently mediated by elevated blood vitamin C and adiponectin and inhibition of NFkB, Akt, and Glut-2 in livers of zucker diabetic fatty rats. Molecular Nutrition and Food Research. 2010;54:1371–80. doi: 10.1002/mnfr.200900177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jain S-K, Kannan K. Chromium chloride inhibits oxidative stress and TNF-α secretion caused by exposure to high glucose in cultured monocytes. Biochem. Biophys. Res. Commun. 2001;289:687–691. doi: 10.1006/bbrc.2001.6026. [DOI] [PubMed] [Google Scholar]
- 27.Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;249:C849–68. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jain S-K, Rains J, Croad J. Effect of chromium niacinate and chromium picolinate supplementation on fasting blood glucose, HbA1, TNF-α, IL-6, CRP, cholesterol, triglycerides and lipid peroxidation levels in streptozotocine treated diabetic rats. Free Radical Biol. Med. 2007;43:1124–1131. doi: 10.1016/j.freeradbiomed.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merino-Ibarra E, Artieda M, Cenarro A, et al. Ultrasonography for the evaluation of visceral fat and the metabolic syndrome. Metabolism. 2005;54:1230–5. doi: 10.1016/j.metabol.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi T, Boyko EJ, McNeely MJ, Leonetti DL, Kahn SE, Fujimoto WY. Visceral adiposity, not abdominal subcutaneous fat area, is associated with an increase in future insulin resistance in Japanese Americans. Diabetes. 2008;57:1269–75. doi: 10.2337/db07-1378. [DOI] [PubMed] [Google Scholar]
- 31.Nyholm B, Nielsen MF, Kristensen K, et al. Evidence of increased visceral obesity and reduced physical fitness in healthy insulin-resistant first-degree relatives of type 2 diabetic patients. Eur J Endocrinol. 2004;150:207–14. doi: 10.1530/eje.0.1500207. [DOI] [PubMed] [Google Scholar]
- 32.Lorenzo M, Fernandez-Veledo S, Vila-Bedmar R, Garcia-Guerra L, De Alvaro C, Nieto-Vazquez I. Insulin resistance induced by tumor necrosis factor-alpha in myocytes and brown adipocytes. J Anim Sci. 2008;86:E94–104. doi: 10.2527/jas.2007-0462. [DOI] [PubMed] [Google Scholar]
- 33.Feinstein R, Kanety H, Papa MZ, Lunenfeld B, Karasik A. Tumor necrosis factor-alpha suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. J Biol Chem. 1993;268:26055–8. [PubMed] [Google Scholar]
- 34.Nieto-Vazquez I, Fernandez-Veledo S, Kramer DK, Vila-Bedmar R, Garcia-Guerra L, Lorenzo M. Insulin resistance associated to obesity: the link TNF-alpha. Arch Physiol Biochem. 2008;114:183–94. doi: 10.1080/13813450802181047. [DOI] [PubMed] [Google Scholar]
- 35.Greenfield JR, Campbell LV. Relationship between inflammation, insulin resistance and type 2 diabetes: ‘cause or effect’? Curr Diabetes Rev. 2006;2:195–211. doi: 10.2174/157339906776818532. [DOI] [PubMed] [Google Scholar]
- 36.Hotamisligil GS, Spiegelman BM. Tumor necrosis factor alpha: a key component of the obesity-diabetes link. Diabetes. 1994;43:1271–8. doi: 10.2337/diab.43.11.1271. [DOI] [PubMed] [Google Scholar]
- 37.Hartge MM, Unger T, Kintscher U. The endothelium and vascular inflammation in diabetes. Diab Vasc Dis Res. 2007;4:84–8. doi: 10.3132/dvdr.2007.025. [DOI] [PubMed] [Google Scholar]
- 38.Hsueh WA, Quinones MJ. Role of endothelial dysfunction in insulin resistance. Am J Cardiol. 2003;92:10J–7J. doi: 10.1016/s0002-9149(03)00611-8. [DOI] [PubMed] [Google Scholar]