Abstract
Background
Genome-wide association studies (GWAS) have identified numerous loci influencing cross-sectional lung function, but less is known about genes influencing longitudinal change in lung function.
Methods
We performed GWAS of the rate of change in forced expiratory volume in the first second (FEV1) in 14 longitudinal, population-based cohort studies comprising 27,249 adults of European ancestry using linear mixed effects model and combined cohort-specific results using fixed effect meta-analysis to identify novel genetic loci associated with longitudinal change in lung function. Gene expression analyses were subsequently performed for identified genetic loci. As a secondary aim, we estimated the mean rate of decline in FEV1 by smoking pattern, irrespective of genotypes, across these 14 studies using meta-analysis.
Results
The overall meta-analysis produced suggestive evidence for association at the novel IL16/STARD5/TMC3 locus on chromosome 15 (P = 5.71 × 10-7). In addition, meta-analysis using the five cohorts with ≥3 FEV1 measurements per participant identified the novel ME3 locus on chromosome 11 (P = 2.18 × 10-8) at genome-wide significance. Neither locus was associated with FEV1 decline in two additional cohort studies. We confirmed gene expression of IL16, STARD5, and ME3 in multiple lung tissues. Publicly available microarray data confirmed differential expression of all three genes in lung samples from COPD patients compared with controls. Irrespective of genotypes, the combined estimate for FEV1 decline was 26.9, 29.2 and 35.7 mL/year in never, former, and persistent smokers, respectively.
Conclusions
In this large-scale GWAS, we identified two novel genetic loci in association with the rate of change in FEV1 that harbor candidate genes with biologically plausible functional links to lung function.
Introduction
Forced expiratory volume in the first second (FEV1) is a reliable spirometric parameter that reflects the physiological state of the lungs and airways. Reduced FEV1 relative to forced vital capacity (FVC), is a defining feature of chronic obstructive pulmonary disease (COPD), a leading cause of death globally.[1] FEV1 is also a predictor of morbidity and mortality in the general population.[2], [3] Lung function reaches its peak in early adulthood, followed by a plateau, and then subsequently declines. As first reported by Fletcher and Peto,[4] decline in lung function is accelerated in smokers, leading to increased risks of COPD and premature death. While cigarette smoking is a key risk factor for accelerated loss of lung function, genetic variation is hypothesized to also play an important role.[5], [6] Family and twin studies of the longitudinal change in lung function report heritability estimates between 10 and 39%.[7], [8]
Recent large-scale genome-wide association studies (GWAS) identified 26 novel loci for cross-sectional lung function,[9]–[11] demonstrating the power of GWAS with large sample size to identify common genetic variants with modest effect sizes. However, cross-sectional measurements in adults reflect the combination of maximal attained lung growth and subsequent decline. GWAS that specifically study the longitudinal change in lung function are needed to distinguish the genetic contributions to age-related decline. To date, only one population-based GWAS meta-analysis of longitudinal change in lung function has been reported.[12] Separate analyses were conducted in 1,441 asthmatic and 2,667 non-asthmatic participants; association was found at one novel locus in each analysis, though only the locus in non-asthmatics replicated.
In this study, we conducted primary GWAS of the rate of change in FEV1 in each of 14 population-based cohort studies from the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) and SpiroMeta consortia, comprising 27,249 adult participants of European ancestry and 62,130 FEV1 measurements. We then performed meta-analysis of the cohort-specific results, followed up our most statistically significant associations in the AGES-Reykjavík cohort study and the Lung Health Study (LHS) for corroborative evidence, and explored the biological basis for identified associations using cell-specific gene expression studies, and expression quantitative trait loci (eQTL) look-up.
Methods
Study populations
All 14 cohort studies are members of the CHARGE or SpiroMeta Consortium (Table 1). The respective local Institutional Review Boards approved all study protocols, and written informed consent for genetic studies was obtained from all participants. Spirometry tests were performed at baseline and at least one follow-up time point by trained technicians and in accordance with the American Thoracic Society or European Respiratory Society recommendations (Methods S1 in File S1 for further details).[13] FEV1 measurements meeting acceptability criteria were included in the current study.
Table 1. Baseline characteristics of cohort studies included in the meta-analysis*.
| Cohort: | ARIC | B58C | BHS | CARDIA | CHS | FHS | Health ABC |
| No. of participants | 8,242 | 827 | 1,009 | 1,492 | 3,159 | 3,230 | 1,586 |
| No. of FEV1 measurements | 15,582 | 1,653 | 3,073 | 6,140 | 7,140 | 11,275 | 4,426 |
| No. of FEV1 per person | 2 | 2 | 7 | 5 | 3 | 5 | 4 |
| Follow-up duration, yr | 5.6 | 10 | 29 | 20.1 | 7.9 | 14.7 | 9.5 |
| Males, % | 46.5 | 48.6 | 41.6 | 46.9 | 39 | 47 | 52.7 |
| Baseline age, yr | 54.6 (5.7) | 35.0 (0.2) | 37.5 (12.8) | 27.5 (2.3) | 72.3 (5.4) | 50.9 (10.3) | 73.8 (2.8) |
| Baseline height, cm | 168.7 (9.4) | 170.1 (9.5) | 168.1 (8.9) | 171.2 (9.3) | 164.6 (9.4) | 168.4 (9.3) | 166.8 (9.3) |
| Current smokers, % | 20.2 | 27.1 | 20.9 | 24.8 | 10.8 | 24.6 | 6.4 |
| Former smokers, % | 32.6 | 41.5 | 16.5 | 17.3 | 35.7 | 39.8 | 49.9 |
| Baseline pack-years† | 25.9 (21.7) | 7.5 (11.4) | 8.2 (17.8) | 6.0 (6.5) | 33.2 (27.0) | 25.4 (21.3) | 36.8 (32.2) |
| Baseline FEV1, mL | 2972 (758) | 3631 (744) | 3230 (927) | 3818 (781) | 2123 (652) | 2989 (806) | 2308 (649) |
| Baseline FEV1/FVC, % | 74.1 (7.1) | 80.6 (5.8) | 78.2 (9.2) | 81.6 (6.5) | 70.5 (10.5) | 75.7 (8.0) | 74.7 (7.8) |
| Cohort: | KORA | LBC1921 | LBC1936 | PIVUS | RS | SAPALDIA | SHIP |
| No. of participants | 890 | 512 | 1,002 | 818 | 1,321 | 1,401 | 1,760 |
| No. of FEV1 measurements | 1,597 | 706 | 1,790 | 1,469 | 2,016 | 2,692 | 2,571 |
| No. of FEV1 per person | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| Follow-up duration, yr | 3.2 | 8.9 | 4.8 | 5.8 | 8.3 | 10.9 | 7.9 |
| Males, % | 47.2 | 41.4 | 50.8 | 49.9 | 45.1 | 48 | 49.4 |
| Baseline age, yr | 53.8 (4.5) | 79.1 (0.6) | 69.6 (0.8) | 70.2 (0.2) | 74.4 (5.6) | 41.1 (11.2) | 52.4 (13.6) |
| Baseline height, cm | 169.3 (9.3) | 163.2 (9.4) | 166.5 (8.9) | 169.0 (9.3) | 167.3 (9.1) | 169.4 (9.1) | 169.5 (9.7) |
| Current smokers, % | 20.5 | 7.0 | 12.9 | 10.2 | 11.1 | 26.9 | 32.8 |
| Former smokers, % | 40.9 | 50.4 | 42.6 | 39.6 | 56.7 | 25.8 | 23.8 |
| Baseline pack-years† | 11.2 (17.1) | 15.3 (22.3) | 16.9 (25.8) | 14.3 (15.8) | 25.7 (21.3) | 17.4 (18.0) | 11.3 (11.9) |
| Baseline FEV1, mL | 3280 (792) | 1887 (625) | 2371 (687) | 2452 (682) | 2215 (652) | 3516 (861) | 3238 (876) |
| Baseline FEV1/FVC, % | 77.5 (6.2) | 79.0 (11.8) | 78.3 (10.2) | 76.0 (10.0) | 74.8 (7.9) | 78.5 (8.2) | 83.1 (6.6) |
Definition of abbreviations: ARIC = Atherosclerosis Risk in Communities; B58C = British 1958 Birth Cohort; BHS = Busselton Health Study; CARDIA = Coronary Artery Risk Development in Young Adults; CHS = Cardiovascular Health Study = FHS, Framingham Heart Study; Health ABC = Health, Aging, and Body Composition; KORA = Cooperative Health Research in the Region of Augsburg; LBC1921 = Lothian Birth Cohort 1921; LBC1936 = Lothian Birth Cohort 1936; PIVUS = Prospective Investigation of the Vasculature in Uppsala Seniors; RS = Rotterdam Study; SAPALDIA = Swiss Study on Air Pollution and Lung Diseases in Adults; SD = standard deviation; SHIP = Study of Health in Pomerania.
*Data are presented as mean (SD) unless otherwise indicated; total no. participants = 27,249, total no. FEV1 measurements = 62,130.
Pack-years are calculated among current and former smokers at study baseline.
Studies performed genotyping following standard quality control measures; imputation was conducted based on the HapMap CEU reference panel to generate genotype dosages for ∼ 2.5 million autosomal single nucleotide polymorphisms (SNPs) (Table S1 in File S1).
Statistical analysis
For the analysis of repeated measurement data such as longitudinal change in lung function, mixed effects models offer more flexibility and statistical power than alternative approaches; the model allows for the use of unbalanced data and does not exclude individuals with incomplete records. Each cohort study performed the GWAS using a linear mixed effects model. The model included a random intercept and a random slope, and fixed effects for time (a continuous variable quantifying the time distance between each FEV1 measurement and baseline), SNP and its interaction with time (SNP-by-time), baseline age, gender, standing height, smoking pattern during follow-up and its interaction with time (smoking-by-time), baseline smoking pack-years, study site, and principal components for genetic ancestry (as needed). Cohort-specific results for the SNP-by-time interaction term, which estimates the effect of genotype on the rate of change in FEV1, were shared, and two meta-analyses, one using all 14 studies and the other using the five studies with ≥3 FEV1 measurements per participant, were performed using METAL software with inverse variance weighting to combine effect estimates after applying genomic control correction.[14]
We sought corroborative evidence for SNPs with P < 1 × 10-5 in the AGES-Reykjavík cohort study (n = 1,494), and in LHS (n = 4,048), a clinical cohort study of smokers with mild COPD, in which a longitudinal GWAS was recently reported.[15]
Gene expression analyses
Expression profiles of genes at the novel loci were evaluated in human lung tissues and primary cell samples using RT-PCR (Table S7 in File S1). Using publicly available data from the Lung Genomics Research Consortium (LGRC), expression profiles of these genes were compared in lung specimens of 219 COPD patients and 137 controls, and sentinel (most associated) SNPs at the novel loci were also searched against an eQTL database of lymphoblastoid cell lines.[16]
This manuscript follows the PRISMA statement and a checklist is available online (Checklist S1).
Results
Population characteristics
The majority of the 14 cohort studies had FEV1 at two times, but five studies (BHS, CARDIA, CHS, FHS, Health ABC) had ≥3 FEV1 measurements per participant. The maximum length of follow-up ranged from 4 to 29 years. Studies with older participants generally had fewer current smokers and more former smokers, and had lower mean baseline FEV1.
Smoking patterns and rate of decline in FEV1
All 14 studies implemented a preliminary mixed model adjusted for all specified variables except the SNP terms and reported the estimated rate of change in FEV1 by smoking pattern (Table 2). The rate of decline in FEV1 in never smokers ranged from 10.0 to 39.7 mL/year, and was generally steeper in studies with older participants, as expected.[4] Across all 14 studies, the meta-analyzed rate of change in FEV1 was a decline of 26.9±0.3 mL/year in never smokers, and was 8.8±0.7, 2.6±0.6, and 2.3±0.5 mL/year steeper in persistent, intermittent, and former smokers, respectively (Table 2). We repeated the meta-analyses in the five cohort studies with ≥3 FEV1 measurements per participant, and found similar, although less statistically significant results.
Table 2. Model estimates for the rate of change in FEV1 in never smokers and effects of other smoking patterns (compared with never smokers) on the rate of change in FEV1 (mL/year)*.
| Study | Annual FEV1 change in never smokers | Additional Effect† of smoking patterns on annual FEV1 change | ||||||
| (referent group) | Persistent smokers | Intermittent smokers | Former smokers | |||||
| β | SE | β | SE | β | SE | β | SE | |
| ARIC | −14.0 | 1.3 | −12.4 | 1.7 | −5.5 | 2.1 | −5.3 | 1.4 |
| B58C | −29.6 | 1.5 | −9.4 | 2.8 | −2.2 | 3.4 | −3.0 | 3.0 |
| BHS | −23.0 | 1.0 | −20.0 | 3.0 | −8.0 | 2.0 | −9.0 | 2.0 |
| CARDIA | −26.4 | 0.5 | −6.7 | 1.3 | −0.2 | 1.0 | 1.0 | 1.2 |
| CHS | −35.0 | 1.1 | −2.2 | 3.3 | −4.6 | 2.2 | −2.4 | 1.7 |
| FHS | −26.0 | 0.6 | −8.1 | 1.3 | −2.9 | 1.0 | −1.1 | 0.8 |
| Health ABC | −39.7 | 1.3 | −12.9 | 6.1 | −6.8 | 4.4 | −2.6 | 1.7 |
| KORA | −22.1 | 3.7 | 2.2 | 7.2 | −10.4 | 9.3 | 2.8 | 5.2 |
| LBC1921 | −10.0 | 3.6 | −11.6 | 15.7 | 2.8 | 14.4 | −18.8 | 4.9 |
| LBC1936 | −32.3 | 3.6 | −19.0 | 9.9 | 40.1 | 16.8 | 4.3 | 5.3 |
| PIVUS | −21.1 | 2.5 | −15.9 | 8.2 | −21.7 | 13.4 | −3.9 | 3.9 |
| RS | −27.5 | 3.7 | −1.8 | 9.0 | 9.3 | 8.6 | −4.6 | 4.5 |
| SAPALDIA | −29.7 | 1.2 | −7.4 | 2.3 | −2.0 | 2.6 | −2.8 | 2.1 |
| SHIP | −31.8 | 2.8 | −0.4 | 10.9 | −0.1 | 3.9 | −15.0 | 7.3 |
| 14-cohort meta-analyzed estimate | −26.9 | 0.3 | −8.8 | 0.7 | −2.6 | 0.6 | −2.3 | 0.5 |
Definition of abbreviations: ARIC = Atherosclerosis Risk in Communities; B58C = British 1958 Birth Cohort; BHS = Busselton Health Study; CARDIA = Coronary Artery Risk Development in Young Adults; CHS = Cardiovascular Health Study; FHS = Framingham Heart Study; Health ABC = Health, Aging, and Body Composition; KORA = Cooperative Health Research in the Region of Augsburg; LBC1921 = Lothian Birth Cohort 1921; LBC1936 = Lothian Birth Cohort 1936; PIVUS = Prospective Investigation of the Vasculature in Uppsala Seniors; RS = Rotterdam Study; SAPALDIA = Swiss Study on Air Pollution and Lung Diseases in Adults; SE = standard error; SHIP = Study of Health in Pomerania.
*Data shown are the effect estimates (β and SE) of the time and smoking-by-time interaction terms in the preliminary mixed effects model fully adjusted for all specified variables except the SNP terms. Time represents the rate of change in FEV1 in never smokers and the smoking-by-time interaction term represents the effects of the other three smoking patterns on the rate of change in FEV1, compared with never smokers. Smoking categories are defined as persistent (smoke throughout follow-up), intermittent (stop and/or start smoking during follow-up) and former (smoke only prior to start of follow-up).
Effect estimates in smoking categories are added to estimates in never smokers to compute the actual rate of change in each group (for example, in ARIC, the point estimate of the rate of change in FEV1 in persistent smokers was −14.0 − 12.4 = −26.4 mL/year).
Discovery meta-analyses
Study-specific genomic inflation factors (λgc) were calculated for the SNP-by-time interaction term and used for study-level genomic control prior to the meta-analyses. Study-specific λgc values ranged from 0.96 to 1.11 (Table S1 in File S1) and the meta-analysis λgc was 1.01 for both the 14-study and five-study meta-analyses. Figures S1 and S2 in File S1 present the Manhattan and quantile-quantile (QQ) plots.
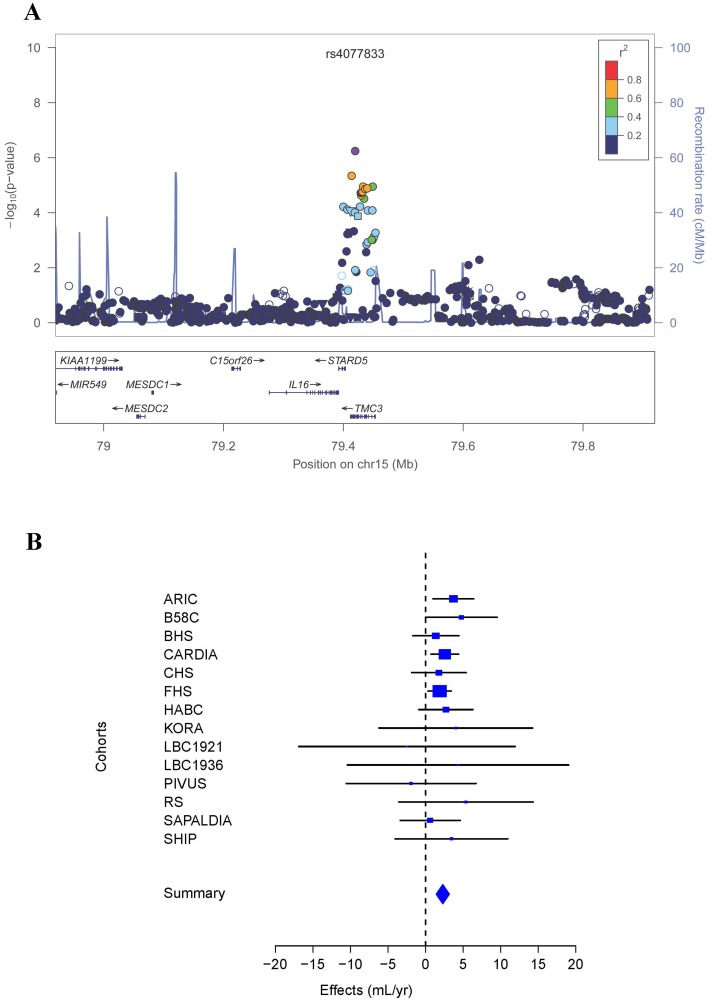
In the meta-analysis including all 14 cohort studies, 15 SNPs at nine independent loci were associated with the rate of change in FEV1 at P < 1 × 10−5, and none reached the genome-wide significance threshold of P < 5 × 10−8. The association results for the sentinel SNPs at these nine loci are presented in Table 3, and more detailed results for all 15 SNPs are included in Table S2 in File S1. The most statistically significant association, and the only one that reached P < 1 × 10−6, was for rs4077833, an intronic SNP located in the novel IL16/STARD5/TMC3 gene region on chromosome 15 (P = 5.71 × 10−7; Figures 1A and 1B). The C allele of rs4077833, with a frequency of 10%, was associated with an attenuation of the rate of decline in FEV1 by 2.3 mL/year in comparison to the G allele.
Table 3. Association of the most statistically significant SNPs with the rate of change in FEV1 (mL/year) in the meta-analysis of 14 cohort studies (n = 27,249)*.
| SNP | Chr | Position | Closest Gene(s) | Coded Allele | Frequency | β | SE | P Value |
| rs12137475 | 1 | 44059735 | ST3GAL3 | T | 0.11 | −3.5 | 0.8 | 3.90 × 10−6 |
| rs766488 | 1 | 61583103 | NFIA | A | 0.31 | 1.4 | 0.3 | 6.60 × 10−6 |
| rs17698444 | 1 | 215483178 | ESRRG/GPATCH2 | C | 0.89 | −2.2 | 0.5 | 2.62 × 10−6 |
| rs12692550 | 2 | 159958017 | BAZ2B | T | 0.17 | −1.7 | 0.4 | 5.16 × 10−6 |
| rs2260722 | 13 | 113236292 | TMCO3 | A | 0.72 | −1.5 | 0.3 | 1.83 × 10−6 |
| rs4077833 | 15 | 79419738 | IL16/STARD5/TMC3 | C | 0.10 | 2.3 | 0.5 | 5.71 × 10−7 |
| rs8027498 | 15 | 89595638 | SV2B | A | 0.25 | 1.4 | 0.3 | 9.41 × 10−6 |
| rs8051319 | 16 | 15794449 | MYH11 | T | 0.60 | 1.7 | 0.3 | 5.12 × 10−6 |
| rs740557 | 17 | 62451139 | CACNG4 | C | 0.85 | −2.3 | 0.5 | 3.59 × 10−6 |
Definition of abbreviations: Chr = chromosome; SE = standard error; SNP = single-nucleotide polymorphism.
*Data reported are the meta-analysis results of the SNP-by-time interaction term from the GWAS mixed effects model. A positive β-coefficient indicates an attenuation of FEV1 decline and a negative β-coefficient an acceleration of FEV1 decline.
Figure 1. Association of the chromosome 15 locus with the rate of change in FEV1 in the meta-analysis of 14 cohort studies.
A) Regional association plot, where the X-axis is Megabase (Mb) position and Y-axes are the negative log of the P value on the left and recombination rate on the right. The sentinel SNP is colored in purple and linkage disequilibrium to the sentinel SNP is depicted by degree of color according to the legend. B) Forest plot for rs4077833, where the size of the square for each study represents its contributing weight to the meta-analysis.
For estimation of longitudinal trajectory in lung function, having more than two measurements over time provides greater precision.[4] We performed a further meta-analysis with the five cohort studies (BHS, CHS, CARDIA, FHS, Health ABC) having ≥3 FEV1 measurements per participant, with a combined sample size of 10,476 participants and 32,054 FEV1 measurements (Methods S1 in File S1 for further details). A novel region on chromosome 11 had a genome-wide significant association (P < 5 × 10−8) with the rate of change in FEV1 (Table 4). The most statistically significant finding at this locus was for rs507211, an intronic SNP located in ME3 (Figures 2A and 2B). Six other SNPs, which are in linkage disequilibrium (LD) with rs507211 and are located in ME3, were identified at P < 1 × 10−6 (Table S3 in File S1). The rs507211 A allele, with a frequency of 25%, was associated with an attenuation of the rate of decline in FEV1 by 2.09 mL/year in comparison to the G allele (P = 2.18 × 10−8). Besides the ME3 locus, 17 SNPs from four other chromosomal regions had P values between 5 × 10−8 and 1 × 10−5 for associations with the rate of change in FEV1 (Tables 4 and Table S3 in File S1).
Table 4. Association of the most statistically significant SNPs with the rate of change in FEV1 (mL/year) in the meta-analysis of the five cohort studies with ≥3 FEV1 measurements per participant (n = 10,476).
| SNP | Chr | Position | Closest Gene(s) | Coded Allele | Frequency | β* | SE | P Value |
| rs10209501 | 2 | 28536881 | FOSL2/PLB1 | A | 0.33 | 1.6 | 0.4 | 7.09 × 10−6 |
| rs12692550 | 2 | 159958017 | BAZ2B | T | 0.18 | −2.0 | 0.4 | 2.02 × 10−6 |
| rs1729588 | 3 | 110790025 | FLJ25363/MIR4445 | A | 0.30 | 1.6 | 0.4 | 8.38 × 10−6 |
| rs10764053 | 10 | 19863644 | C10orf112 | T | 0.47 | 1.5 | 0.3 | 4.15 × 10−6 |
| rs507211 | 11 | 86054387 | ME3 | A | 0.25 | 2.1 | 0.4 | 2.18 × 10−8 |
Definition of abbreviations: Chr = chromosome; SE = standard error; SNP = single-nucleotide polymorphism.
*Data reported are the meta-analysis results of the SNP-by-time interaction term from the GWAS mixed effects model. A positive β-coefficient indicates an attenuation of FEV1 decline and a negative β-coefficient an acceleration of FEV1 decline.
Figure 2. Association of the chromosome 11 locus with the rate of change in FEV1 in the meta-analysis of the five cohort studies with ≥3 FEV1 measurements per participant.
A) Regional association plot, where the X-axis is Megabase (Mb) position, and the Y-axes are the negative log of the P value on the left and recombination rate on the right. The sentinel SNP is colored in purple and linkage disequilibrium to the sentinel SNP is depicted by degree of color according to the legend. B) Forest plot for rs507211, where the size of the square for each study represents its contributing weight to the meta-analysis.
Additional analyses
Corroborative evidence was sought for the sentinel SNP at each of the 14 loci associated at P < 1 × 10−5 (from both the 14-study and five-study meta-analyses) in 1,494 adults from the AGES-Reykjavík population-based cohort study (Table S4 in File S1). A P value of 0.004, representing the Bonferroni correction for 14 tests at the α = 0.05 level, was selected a priori as the threshold for statistical significance. No SNPs achieved this threshold. The lowest P value was for rs740577 in CACNG4 (P = 0.08), which showed consistent effect direction and magnitude with the original meta-analysis.
These same 14 SNPs were further examined in LHS, a clinical cohort study of 4,048 smokers with mild COPD for evidence of consistent association between healthy and diseased individuals.[17] None of the 14 SNPs were associated with the rate of change in FEV1 in LHS at P < 0.004 (Table S4 in File S1).
Previous meta-analyses in the CHARGE and SpiroMeta consortia identified 26 novel loci associated with cross-sectional FEV1 and/or FEV1/FVC at genome-wide significance.[9]-[11] We examined the sentinel SNPs from these loci in the meta-analysis of the 14 cohort studies for association with the rate of change in FEV1 (Table S5 in File S1). Given the a priori association with cross-sectional lung function, a P value threshold of 0.05 was used. Sentinel SNPs in PID1, HHIP, GPR126, and CFDP1 showed association with the rate of change in FEV1 (0.005 ≤ P ≤ 0.048).
Gene expression analyses
Three genes (IL16, STARD5, and TMC3) at the novel chromosome 15 locus and ME3 at the novel chromosome 11 locus were selected for follow-up mRNA expression profiling in human lung tissue, and primary cultures of human bronchial epithelial and airway smooth muscle cells, together with control tissues (peripheral blood mononuclear cells and brain). Transcripts of STARD5 and ME3 were found in all lung-derived tissues, transcripts of IL16 were found in lung tissue and smooth muscle cells, but not in epithelial cells, and TMC3 was not expressed in any of the lung-derived tissues (Table S6 in File S1).
Using the public LGRC data repository, we found that the expression profiles of IL16, STARD5, and ME3 in human lung samples showed statistically significant differences (P < 0.05) between COPD patients and controls (Figure S3 in File S1). Lower levels of IL16 (P = 0.004) were observed in COPD patients compared with controls, whereas higher levels of STARD5 (P = 3.22 × 10-9) and ME3 (P = 0.044) were observed in COPD patients compared with controls. Data on TMC3 expression were not available.
We performed additional follow-up analysis of the sentinel SNPs at the two novel loci using an eQTL database of lymphoblastoid cell lines (Table S8 in File S1). Trans-eQTL associations were observed between rs4077833 at the IL16/STARD5/TMC3 locus and a nuclear receptor, NR1I2 (chromosome 3; P = 6.84 × 10-4) and between rs507211 at the ME3 locus and KIAA1109 (chromosome 4; P = 5.20 × 10-4), which is part of a gene cluster (KIAA1109-TENR-IL2-IL21) that encodes two interleukins (IL2 and IL21).[18]
Discussion
Although the genetic contribution to cross-sectional lung function phenotypes has been addressed by large-scale GWAS, much less information is available for longitudinal lung function phenotypes. To identify novel loci that specifically affect lung function change over time, we performed a large-scale GWAS of the rate of change in FEV1 in 27,249 participants from 14 population-based cohort studies. We identified a novel locus (IL16/STARD5/TMC3) on chromosome 15 with suggestive evidence for association with the rate of change in FEV1. Given the greater precision to estimate longitudinal trends with more measurements, a meta-analysis of the five cohort studies with ≥3 FEV1 measurements per participant was performed, and it identified a second novel locus (ME3) on chromosome 11 at genome-wide statistical significance. For both loci, the minor allele was protective, and the magnitude of the association with the rate of change in FEV1 was similar to that of being an intermittent or former smoker versus a never-smoker.
The sentinel SNP at the novel chromosome 15 locus is located in TMC3, although two neighboring genes, IL16 and STARD5 both harbor SNPs that are in modest LD with the sentinel SNP (Figure 1A). TMC3, a member of the transmembrane channel-like gene family, likely functions as an ion channel, transporter, or modifier,[19] and has been associated with deafness and skin cancer.[20], [21] IL16 is a pleiotropic immunomodulatory cytokine that acts as a chemoattractant for CD4+ cells and contributes to their recruitment and activation in response to inflammation.[22] Notably, asthma was the first disease where increased IL16 expression was observed.[23] Subsequent studies confirmed that in the non-diseased state IL16 is almost exclusively expressed by T lymphocytes in lymphatic tissue, whereas in asthmatic patients IL16 is also synthesized by airway epithelial cells to inhibit airway inflammation.[24]-[26] A promoter polymorphism (T-295C) in IL16 was associated with asthma in a Caucasian population in England,[27] although this finding was not confirmed in an Australian study.[28] STARD5 belongs to the steroidogenic acute regulatory lipid transfer domain protein superfamily, and is involved in the trafficking of cholesterol and other lipids between intracellular membranes.[29] Recent in vitro studies showed increased STARD5 expression and protein redistribution as a protective mechanism in response to induced endoplasmic reticulum (ER) stress and consequent over-accumulation of intracellular free cholesterol.[30] We confirmed the expression of STARD5 in all human lung tissues examined and of IL16 in human lung smooth muscle cells, but not epithelial cells, in line with previous observations. In contrast, no expression of TMC3 was detected in any of the tested human lung tissues. We also found significantly lower levels of IL16 in whole lung samples from COPD patients compared with controls, in contrast to its increased expression in asthma, and significantly higher levels of STARD5 in COPD patients compared with controls. Taken together, these results suggest IL16 as the most likely candidate accounting for the observed association, but further investigation is needed to elucidate underlying mechanisms.
The sentinel SNP at the novel chromosome 11 locus is located in ME3, whose protein product is a mitochondrial NADP(+)-dependent malic enzyme that catalyzes the oxidative decarboxylation of malate to pyruvate using NADP+ as a cofactor.[31] Mitochondrial malic enzymes play a role in the energy metabolism in tumors, and are considered potential therapeutic targets in cancer.[32], [33] We performed independent expression profiling of ME3 and confirmed its expression in all human lung tissues examined, and found significantly higher levels of ME3 in lung samples from COPD patients compared with controls. In addition, we looked up the sentinel SNP in ME3 in a recent GWAS of airway obstruction and found a P value of 0.049.[34] Taken together, these results support ME3 as a biologically plausible candidate in the regulation of lung function and pathogenesis of COPD.
The identification of trans-eQTL associations for the sentinel SNPs at both the IL16/STARD5/TMC3 and ME3 loci is interesting, and while the interpretation of trans-eQTL associations is ambiguous,[35] the regions these SNPs regulate merit further study.
Besides the GWAS meta-analyses, the assembly of 14 longitudinal cohort studies allowed us to meta-analyze the association of cumulative smoking patterns with the rate of change in FEV1 in the general population. The meta-analyzed estimate for the rate of decline in FEV1 in never smokers was 26.9 mL/year, and the annual decline was steeper in persistent, intermittent, and former smokers by 8.8, 2.6, and 2.3 mL/year, respectively. These findings provide a reference point for the effect of cigarette smoking on longitudinal lung function change in the general population.
There is phenotypic variation among the 14 cohort studies in aspects such as baseline age and cigarette smoking, and in factors that are of special importance to this longitudinal GWAS, such as the number of FEV1 measurements per participant and follow-up duration. Phenotypic heterogeneity represents a general challenge in genetic epidemiology, particularly in the investigation of longitudinal phenotypes. Thus, we performed a meta-analysis using the subset of cohort studies with ≥3 FEV1 measurements per participant, given that longitudinal trajectories are best estimated over longer time periods and with more measurements. There was little overlap between the top loci identified in the two meta-analyses at P < 1 × 10−5, suggesting that phenotypic heterogeneity affected the association results. Future meta-studies of lung function decline should aim to increase sample size while maintaining high phenotypic comparability among participating studies. In addition, the trajectory of lung function change, especially over a long period of time, is known to be nonlinear, which may require the use of nonlinear time effects in the statistical model. In this study, given that over half of the included cohort studies have FEV1 measurements at only two time points, our consideration was limited to a linear time effect. Further, the outcome studied, the rate of change in lung function, represents one of many ways to describe lung function change. Additional studies of other aspects of lung function change, such as reduced growth and premature decline, would be of interest.
We sought corroborative evidence in a single cohort study of 1,494 participants. This sample size is much smaller and arguably insufficient compared with replications applied to previous studies of cross-sectional lung function phenotypes. Thus, despite the lack of corroboration for the two novel loci identified in the meta-analyses, results from the complementary gene expression analyses provide compelling evidence for biologically plausible roles of the implicated genes in the longitudinal change in lung function.
None of the 14 sentinel SNPs were associated with the rate of change in FEV1 in the COPD patient-based LHS cohort. Similarly, a previous population-based GWAS of lung function decline noted a high degree of heterogeneity in findings when analyses were stratified by presence/absence of asthma.[12] The observed discrepancy of association results suggests that the genetic determination of lung function decline may be different in healthy individuals compared with COPD patients, may contribute differentially in a pre-diseased vs. post-diseased state in which medications may influence the rates of decline, or that LHS was underpowered for confirming our findings.
In this study, statistical models included a comprehensive list of confounders that are commonly adjusted for when modeling lung function phenotypes. Given the study's meta-analysis design and the objective to carry out the same statistical model in all cohort studies, additional covariates that were not available in all cohort studies could not be included. In addition, the adjustment of certain confounders, such as smoking, is challenging in a longitudinal study, and although we accounted for the two most important aspects of smoking, cumulative pattern and dosage, residual confounding due to smoking cannot be excluded.
In summary, we performed GWAS of the longitudinal change in lung function and subsequent meta-analyses, using harmonized data from more than 27,000 participants of European ancestry to identify genetic loci influencing the rate of change in FEV1. We identified the novel ME3 locus on chromosome 11 at genome-wide significance and found suggestive evidence for association at the novel IL16/STARD5/TMC3 locus on chromosome 15. Additional expression analyses confirmed the expression of ME3, IL16, and STARD5 in multiple lung tissues, and found differential expression profiles of these three genes in the lungs of COPD patients compared to non-COPD controls. These results support the involvement of these implicated genes in the longitudinal change in lung function in adults of European ancestry. Additional studies with larger sample size and in populations of other races/ethnicities are warranted.
Supporting Information
This is a single file that contains all supporting information for the paper. Briefly, File S1 contains the following items: Methods S1, which describes further details of the cohort studies and the statistical methodology; Table S1, Details of SNP genotyping, quality control (QC), imputation, and statistical analysis across the 14 cohort studies; Table S2, Regression results for single nucleotide polymorphisms associated with the rate of change in FEV1 (mL/year) at P < 1 × 10-5 in the meta-analysis of 14 cohort studies (N = 27,249); Table S3, Regression results for single nucleotide polymorphisms associated with the rate of change in FEV1 (mL/year) at P < 1 × 10-5 in the meta-analysis of the five cohort studies with three or more FEV1 measurements per participant (N = 10,476); Table S4, Association of the 14 sentinel SNPs from the meta-analyses in the AGES-Reykjavík study (AGES) and the Lung Health Study (LHS) for the rate of change in FEV1 (mL/year); Table S5, Association of previously reported loci in GWAS of cross-sectional lung function with the rate of change in FEV1 (mL/year) in the meta-analysis of 14 cohort studies (N = 27,249); Table S6, mRNA expression profiling of the implicated genes at the two novel loci in human lung and control tissues; Table S7, Primers for mRNA expression profiling; Table S8, Summary of eQTL look-up for the most significant SNPs at the novel chromosome 11 and 15 loci; Figure S1, Manhattan and QQ plots for the meta-analysis of the rate of change in FEV1 in 14 cohort studies; Figure S2, Manhattan and QQ plots for the meta-analysis of the rate of change in FEV1 in the five cohort studies with three or more FEV1 measurements per participant; Figure S3, mRNA expression profiling in human lung samples from 219 COPD patients and 137 controls for A) IL16, B) STARD5, and C) ME3, using publicly available microarray data from the Lung Genomics Research Consortium site (http://www.lung-genomics.org/). The y-axes reflect the probe intensities of each gene transcript in the binary logarithm form, with the red dots indicating the average probe intensities and the red bars indicating standard deviation. The P values were calculated using the two-sample t-test.
(DOCX)
PRISMA Checklist.
(DOCX)
Funding Statement
The AGES-Reykjavik Study is funded by NIH contract N01-AG-12100, the NIA Intramural Research Program, Hjartavernd (the Icelandic Heart Association), and the Althingi (the Icelandic Parliament). The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C), R01HL087641, R01HL59367 and R01HL086694; National Human Genome Research Institute contract U01HG004402; and National Institutes of Health contract HHSN268200625226C. The authors thank the staff and participants of the ARIC study for their important contributions. Infrastructure was partly supported by Grant Number UL1RR025005, a component of the National Institutes of Health and NIH Roadmap for Medical Research. Supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences ZO1 ES43012. The authors acknowledge use of phenotype and genotype data from the British 1958 Birth Cohort DNA collection, funded by the Medical Research Council grant G0000934 and the Wellcome Trust grant 068545/Z/02 (http://www.b58cgene.sgul.ac.uk/). Genotyping for the B58C-WTCCC subset was funded by the Wellcome Trust grant 076113/B/04/Z. The B58C-T1DGC genotyping utilized resources provided by the Type 1 Diabetes Genetics Consortium, a collaborative clinical study sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Allergy and Infectious Diseases (NIAID), National Human Genome Research Institute (NHGRI), National Institute of Child Health and Human Development (NICHD), and Juvenile Diabetes Research Foundation International (JDRF) and supported by U01 DK062418. B58C-T1DGC GWAS data were deposited by the Diabetes and Inflammation Laboratory, Cambridge Institute for Medical Research (CIMR), University of Cambridge, which is funded by Juvenile Diabetes Research Foundation International, the Wellcome Trust and the National Institute for Health Research Cambridge Biomedical Research Centre; the CIMR is in receipt of a Wellcome Trust Strategic Award (079895). The B58C-GABRIEL genotyping was supported by a contract from the European Commission Framework Programme 6 (018996) and grants from the French Ministry of Research. The 1994 Busselton follow-up Health Study was supported by Healthways, Western Australia. The Busselton Health Study is supported by The Great Wine Estates of the Margaret River region of Western Australia. The study gratefully acknowledges the assistance of the Western Australian DNA Bank (NHMRC Enabling Facility) with DNA samples and the support provided by The Ark at University of Western Australia for this study. The Coronary Artery Risk Development in Young Adults (CARDIA) study was funded by contracts N01-HC-95095, N01-HC-48047, N01-HC-48048, N01-HC-48049, N01-HC-48050, N01-HC-45134, N01-HC-05187, N01-HC-45205, and N01-HC-45204 from NHLBI to the CARDIA investigators. Genotyping of the CARDIA participants was supported by grants U01-HG-004729, U01-HG-004446, and U01-HG-004424 from the NHGRI. Statistical analyses were supported by grants U01-HG-004729 and R01-HL-084099 to MF. This Cardiovascular Health Study (CHS) research was supported by NHLBI contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and HHSN268200960009C; and NHLBI grants HL080295, HL087652, HL105756, HL103612, and HL085251 with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through AG023629 from the National Institute on Aging (NIA). A full list of CHS investigators and institutions can be found at http://chs-nhlbi.org. The provision of genotyping data was supported in part by the National Center for Advancing Translational Sciences, CTSI grant UL1TR000124, and the National Institute of Diabetes and Digestive and Kidney Disease Diabetes Research Center (DRC) grant DK063491 to the Southern California Diabetes Endocrinology Research Center. Framingham Heart Study (FHS) research was conducted in part using data and resources of the NHLBI and Boston University School of Medicine. The analyses reflect intellectual input and resource development from the FHS investigators participating in the SNP Health Association Resource (SHARe) project. This work was partially supported by NHLBI (contract no. N01-HC-25195) and its contract with Affymetrix for genotyping services (contract no. N02-HL-6-4278). A portion of this research utilized the Linux Cluster for Genetic Analysis (LinGA-II) funded by the Robert Dawson Evans Endowment of the Department of Medicine at Boston University School of Medicine and Boston Medical Center. JBW was supported by a Young Clinical Scientist Award from the Flight Attendant Medical Research Institute (FAMRI). The Health, Aging, and Body Composition Study was supported by NIA contracts N01AG62101, N01AG2103, and N01AG62106, NIA grant R01-AG028050, NINR grant R01-NR012459, and in part by the Intramural Research Program of the NIA, NIH. The genome-wide association study was funded by NIA grant 1R01AG032098–01A1 to Wake Forest Health Sciences, and genotyping services were provided by the Center for Inherited Disease Research, which is fully funded through a federal contract from the National Institutes of Health to The Johns Hopkins University, contract number HHSN268200782096C. This research was further supported by RC1AG035835. The KORA study was funded by the Helmholtz Zentrum München - German Research Center for Environmental Health, German Federal Ministry of Education and Research, State of Bavaria, Munich Center of Health Sciences (MC Health), Ludwig-Maximilians-Universität, as part of LMUinnovativ, and Competence Network ASCONET, subnetwork COSYCONET (FKZ 01GI0882). The Lothian Birth Cohorts 1921 and 1936 were funded by the Lifelong Health and Wellbeing Initiative (BBSRC, EPSRC, ESRC and MRC). The authors thank the cohort participants and team members who contributed to these studies. Phenotype collection in the Lothian Birth Cohort 1921 was supported by the BBSRC, The Royal Society and The Chief Scientist Office of the Scottish Government. Phenotype collection in the Lothian Birth Cohort 1936 was supported by Research Into Ageing (continues as part of Age UK The Disconnected Mind project). Genotyping of the cohorts was funded by the UK Biotechnology and Biological Sciences Research Council (BBSRC). The work was undertaken by the University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross council Lifelong Health and Wellbeing Initiative (G0700704/84698). Funding from the BBSRC, Engineering and Physical Sciences Research Council (EPSRC), Economic and Social Research Council (ESRC), and MRC is gratefully acknowledged. The Lung Health Study (LHS) was supported by GENEVA (U01HG 004738), and by the Mary Beryl Patch Turnbull Scholar Program (KCB, in part). The PIVUS study was funded by the Swedish Foundation for Strategic Research (ICA08-0047), the Swedish Research Council (2012-1397), the Swedish Heart-Lung Foundation (20120197), the Swedish Society of Medicine, and Uppsala University. CML is a Wellcome Trust Research Career Development Fellow (086596/Z/08/Z). The computations were performed on resources provided by SNIC through Uppsala Multidisciplinary Center for Advanced Computational Science (UPPMAX) under Project p2013056. APM acknowledges funding from the Wellcome Trust under grants WT098017, WT064890 and WT090532, and APM is a Senior Research Fellow in Basic and Biomedical Science (grant number WT098017). The Rotterdam Studies were funded by the Netherlands Organization of Scientific Research NWO Investments, nr. 175.010.2005.011, 911-03-012, Research Institute for Diseases in the Elderly, 014-93-015; RIDE2, the Netherlands Genomics Initiative (NGI)/Netherlands Organization for Scientific Research (NWO) project nr. 050-060-810, the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII) Municipality of Rotterdam. SAPALDIA was supported by the Swiss National Science Foundation (grants no 33CS30-134276/1, 33CSCO-108796, 3247BO-104283, 3247BO-104288, 3247BO-104284, 3247-065896, 3100-059302, 3200-052720,3200-042532, 4026-028099, 3233-054996, PDFMP3-123171), the Federal Office for Forest, Environment, and Landscape, the Federal Office of Public Health, the Federal Office of Roads and Transport, the canton's government of Aargau, Basel-Stadt, Basel-Land, Geneva, Luzern, Ticino, Valais, Zurich, the Swiss Lung League, the canton's Lung League of Basel Stadt/Basel Landschaft, Geneva, Ticino, Valais and Zurich, Schweizerische Unfallversicherungsanstalt (SUVA), Freiwillige Akademische Gesellschaft, UBS Wealth Foundation, Talecris Biotherapeutics GmbH, Abbott Diagnostics. Genotyping in the GABRIEL framework was supported by grants European Commission 018996 and Wellcome Trust WT 084703MA. SHIP is part of the Community Medicine Research net of the University of Greifswald, Germany, which is funded by the Federal Ministry of Education and Research, the Ministry of Cultural Affairs as well as the Social Ministry of the Federal State of Mecklenburg-West Pomerania, and the network ‘Greifswald Approach to Individualized Medicine (GANI_MED)’ funded by the Federal Ministry of Education and Research, and the German Asthma and COPD Network (COSYCONET), under 01ZZ9603, 01ZZ0103, 01ZZ0403, 03IS2061A, and BMBP 01GI0883. Genome-wide data have been supported by the Federal Ministry of Education and Research and a joint grant from Siemens Healthcare, Erlangen, Germany and the Federal State of Mecklenburg- West Pomerania (03ZIK012). The University of Greifswald is a member of the ‘Center of Knowledge Interchange’ program of the Siemens AG and the Caché Campus program of the InterSystems GmbH. The research undertaken by MDT, LVW and MSA was partly funded by the National Institute for Health Research (NIHR). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. MDT holds a Medical Research Council Senior Clinical Fellowship (G0902313). The expression analysis undertaken by IPH and EH was funded by the Medical Research Council of UK (grant no. G1000861). The University of Greifswald is a member of the ‘Center of Knowledge Interchange’ program of the Siemens AG and the Caché Campus program of the InterSystems GmbH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, et al. (2007) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 176: 532–555. [DOI] [PubMed] [Google Scholar]
- 2. Schunemann HJ, Dorn J, Grant BJ, Winkelstein W Jr, Trevisan M (2000) Pulmonary function is a long-term predictor of mortality in the general population: 29-year follow-up of the Buffalo Health Study. Chest 118: 656–664. [DOI] [PubMed] [Google Scholar]
- 3. Young RP, Hopkins R, Eaton TE (2007) Forced expiratory volume in one second: not just a lung function test but a marker of premature death from all causes. Eur Respir J 30: 616–622. [DOI] [PubMed] [Google Scholar]
- 4. Fletcher C, Peto R (1977) The natural history of chronic airflow obstruction. Br Med J 1: 1645–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, et al. (2006) Global burden of COPD: systematic review and meta-analysis. Eur Respir J 28: 523–532. [DOI] [PubMed] [Google Scholar]
- 6. Eisner MD, Anthonisen N, Coultas D, Kuenzli N, Perez-Padilla R, et al. (2010) An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 182: 693–718. [DOI] [PubMed] [Google Scholar]
- 7. Gottlieb DJ, Wilk JB, Harmon M, Evans JC, Joost O, et al. (2001) Heritability of longitudinal change in lung function. The Framingham study. Am J Respir Crit Care Med 164: 1655–1659. [DOI] [PubMed] [Google Scholar]
- 8. Finkel D, Pedersen NL, Reynolds CA, Berg S, de Faire U, et al. (2003) Genetic and environmental influences on decline in biobehavioral markers of aging. Behav Genet 33: 107–123. [DOI] [PubMed] [Google Scholar]
- 9. Hancock DB, Eijgelsheim M, Wilk JB, Gharib SA, Loehr LR, et al. (2009) Meta-analyses of genome-wide association studies identify multiple loci associated with pulmonary function. Nat Genet 42: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Repapi E, Sayers I, Wain LV, Burton PR, Johnson T, et al. (2009) Genome-wide association study identifies five loci associated with lung function. Nat Genet 42: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Soler Artigas M, Loth DW, Wain LV, Gharib SA, Obeidat M, et al. (2011) Genome-wide association and large-scale follow up identifies 16 new loci influencing lung function. Nat Genet 43: 1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Imboden M, Bouzigon E, Curjuric I, Ramasamy A, Kumar A, et al. (2012) Genome-wide association study of lung function decline in adults with and without asthma. The Journal of allergy and clinical immunology 129: 1218–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. (2005) Standardisation of spirometry. Eur Respir J 26: 319–338. [DOI] [PubMed] [Google Scholar]
- 14. Willer CJ, Li Y, Abecasis GR (2010) METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26: 2190–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hansel NN, Ruczinski I, Rafaels N, Sin DD, Daley D, et al. (2013) Genome-wide study identifies two loci associated with lung function decline in mild to moderate COPD. Hum Genet 132: 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dixon AL, Liang L, Moffatt MF, Chen W, Heath S, et al. (2007) A genome-wide association study of global gene expression. Nat Genet 39: 1202–1207. [DOI] [PubMed] [Google Scholar]
- 17. Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, et al. (1994) Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. JAMA 272: 1497–1505. [PubMed] [Google Scholar]
- 18. van Heel DA, Franke L, Hunt KA, Gwilliam R, Zhernakova A, et al. (2007) A genome-wide association study for celiac disease identifies risk variants in the region harboring IL2 and IL21. Nat Genet 39: 827–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kurima K, Yang Y, Sorber K, Griffith AJ (2003) Characterization of the transmembrane channel-like (TMC) gene family: functional clues from hearing loss and epidermodysplasia verruciformis. Genomics 82: 300–308. [DOI] [PubMed] [Google Scholar]
- 20. Ramoz N, Rueda LA, Bouadjar B, Montoya LS, Orth G, et al. (2002) Mutations in two adjacent novel genes are associated with epidermodysplasia verruciformis. Nat Genet 32: 579–581. [DOI] [PubMed] [Google Scholar]
- 21. Vreugde S, Erven A, Kros CJ, Marcotti W, Fuchs H, et al. (2002) Beethoven, a mouse model for dominant, progressive hearing loss Dfn236. Nat Genet 30: 257–258. [DOI] [PubMed] [Google Scholar]
- 22. Cruikshank WW, Kornfeld H (1998) Center DM (1998) Signaling and functional properties of interleukin-16. International reviews of immunology 16: 523–540. [DOI] [PubMed] [Google Scholar]
- 23. Bellini A, Yoshimura H, Vittori E, Marini M, Mattoli S (1993) Bronchial epithelial cells of patients with asthma release chemoattractant factors for T lymphocytes. The Journal of allergy and clinical immunology 92: 412–424. [DOI] [PubMed] [Google Scholar]
- 24. Cruikshank WW, Long A, Tarpy RE, Kornfeld H, Carroll MP, et al. (1995) Early identification of interleukin-16 (lymphocyte chemoattractant factor) and macrophage inflammatory protein 1 alpha (MIP1 alpha) in bronchoalveolar lavage fluid of antigen-challenged asthmatics. Am J Respir Cell Mol Biol 13: 738–747. [DOI] [PubMed] [Google Scholar]
- 25. Krug N, Cruikshank WW, Tschernig T, Erpenbeck VJ, Balke K, et al. (2000) Interleukin 16 and T-cell chemoattractant activity in bronchoalveolar lavage 24 hours after allergen challenge in asthma. Am J Respir Crit Care Med 162: 105–111. [DOI] [PubMed] [Google Scholar]
- 26. Laberge S, Ernst P, Ghaffar O, Cruikshank WW, Kornfeld H, et al. (1997) Increased expression of interleukin-16 in bronchial mucosa of subjects with atopic asthma. Am J Respir Cell Mol Biol 17: 193–202. [DOI] [PubMed] [Google Scholar]
- 27. Burkart KM, Barton SJ, Holloway JW, Yang IA, Cakebread JA, et al. (2006) Association of asthma with a functional promoter polymorphism in the IL16 gene. The Journal of allergy and clinical immunology 117: 86–91. [DOI] [PubMed] [Google Scholar]
- 28. Akesson LS, Duffy DL, Phelps SC, Thompson PJ, Kedda MA (2005) A polymorphism in the promoter region of the human interleukin-16 gene is not associated with asthma or atopy in an Australian population. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology 35: 327–331. [DOI] [PubMed] [Google Scholar]
- 29. Rodriguez-Agudo D, Ren S, Hylemon PB, Redford K, Natarajan R, et al. (2005) Human StarD5, a cytosolic StAR-related lipid binding protein. Journal of lipid research 46: 1615–1623. [DOI] [PubMed] [Google Scholar]
- 30. Rodriguez-Agudo D, Calderon-Dominguez M, Medina MA, Ren S, Gil G, et al. (2012) ER stress increases StarD5 expression by stabilizing its mRNA and leads to relocalization of its protein from the nucleus to the membranes. Journal of lipid research 53: 2708–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chang GG, Tong L (2003) Structure and function of malic enzymes, a new class of oxidative decarboxylases. Biochemistry 42: 12721–12733. [DOI] [PubMed] [Google Scholar]
- 32. Moreadith RW, Lehninger AL (1984) The pathways of glutamate and glutamine oxidation by tumor cell mitochondria. Role of mitochondrial NAD(P)+−dependent malic enzyme. J Biol Chem 259: 6215–6221. [PubMed] [Google Scholar]
- 33. Teller JK, Fahien LA, Davis JW (1992) Kinetics and regulation of hepatoma mitochondrial NAD(P) malic enzyme. J Biol Chem 267: 10423–10432. [PubMed] [Google Scholar]
- 34.Wilk JB, Shrine NR, Loehr LR, Zhao JH, Manichaikul A, et al.. (2012) Genome Wide Association Studies Identify CHRNA5/3 and HTR4 in the Development of Airflow Obstruction. Am J Respir Crit Care Med. [DOI] [PMC free article] [PubMed]
- 35. Montgomery SB, Dermitzakis ET (2011) From expression QTLs to personalized transcriptomics. Nat Rev Genet 12: 277–282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This is a single file that contains all supporting information for the paper. Briefly, File S1 contains the following items: Methods S1, which describes further details of the cohort studies and the statistical methodology; Table S1, Details of SNP genotyping, quality control (QC), imputation, and statistical analysis across the 14 cohort studies; Table S2, Regression results for single nucleotide polymorphisms associated with the rate of change in FEV1 (mL/year) at P < 1 × 10-5 in the meta-analysis of 14 cohort studies (N = 27,249); Table S3, Regression results for single nucleotide polymorphisms associated with the rate of change in FEV1 (mL/year) at P < 1 × 10-5 in the meta-analysis of the five cohort studies with three or more FEV1 measurements per participant (N = 10,476); Table S4, Association of the 14 sentinel SNPs from the meta-analyses in the AGES-Reykjavík study (AGES) and the Lung Health Study (LHS) for the rate of change in FEV1 (mL/year); Table S5, Association of previously reported loci in GWAS of cross-sectional lung function with the rate of change in FEV1 (mL/year) in the meta-analysis of 14 cohort studies (N = 27,249); Table S6, mRNA expression profiling of the implicated genes at the two novel loci in human lung and control tissues; Table S7, Primers for mRNA expression profiling; Table S8, Summary of eQTL look-up for the most significant SNPs at the novel chromosome 11 and 15 loci; Figure S1, Manhattan and QQ plots for the meta-analysis of the rate of change in FEV1 in 14 cohort studies; Figure S2, Manhattan and QQ plots for the meta-analysis of the rate of change in FEV1 in the five cohort studies with three or more FEV1 measurements per participant; Figure S3, mRNA expression profiling in human lung samples from 219 COPD patients and 137 controls for A) IL16, B) STARD5, and C) ME3, using publicly available microarray data from the Lung Genomics Research Consortium site (http://www.lung-genomics.org/). The y-axes reflect the probe intensities of each gene transcript in the binary logarithm form, with the red dots indicating the average probe intensities and the red bars indicating standard deviation. The P values were calculated using the two-sample t-test.
(DOCX)
PRISMA Checklist.
(DOCX)