Abstract
In eukaryotes, 14-3-3 dimers regulate hundreds of functionally diverse proteins (clients), typically in phosphorylation-dependent interactions. To uncover new clients, a 14-3-3 omega (At1g78300) from Arabidopsis was engineered with a “tandem affinity purification” (TAP) tag and expressed in transgenic plants. Purified complexes were analyzed by tandem mass spectrometry. Results indicate that 14-3-3 omega can dimerize with at least 10 of the 12 14-3-3 isoforms expressed in Arabidopsis. The identification here of 121 putative clients provides support for in vivo 14-3-3 interactions with a diverse array of proteins, including those involved in: (1) Ion transport, such as a K+ channel (GORK), a Cl− channel (CLCg), Ca2+ channels belonging to the glutamate receptor family (GLRs 1.2, 2.1, 2.9, 3.4, 3.7); (2) hormone signaling, such as ACC synthase (isoforms ACS-6, 7 and 8 involved in ethylene synthesis) and the brassinolide receptors BRI1 and BAK1; (3) transcription, such as 7 WRKY family transcription factors; (4) metabolism, such as phosphoenol pyruvate (PEP) carboxylase; and (5) lipid signaling, such as phospholipase D (β, and γ). More than 80% (101) of these putative clients represent previously unidentified 14-3-3 interactors. These results raise the number of putative 14-3-3 clients identified in plants to over 300.
Keywords: 14-3-3, tandem affinity purification, MudPIT, phosphorylation, 16O/18O, iTRAQ, interactome, proteomics, kinase
Introduction
In eukaryotes, 14-3-3 proteins regulate diverse cellular functions through hundreds of different protein-protein interactions (clients). In the yeast Saccharomyces cerevisiae, a double knockout of its 14-3-3 genes (BMH1 and BMH2) is lethal, indicating that some 14-3-3 interactions are essential. 14-3-3s are encoded by multi-gene families in animals (e.g. 7 isoforms in mammals) and plants. In Arabidopsis, mRNA expression has been detected for 12 of the 15 14-3-3 genes (GF14/phi, GF14/chi, GF14/omega, GF14/psi, GF14/upsilon, GF14/lamda, GF14/nu, GF14/kappa, GF14/mu, GF14/epsilon, GF14/omicron, GF14/iota) [1]. 11 of these 12 isoforms have been detected in a proteomic analysis [2]. Unique structural features associated with each isoform are expected to provide differences in subcellular localization, client specific interactions, and differences in regulation by phosphorylation or cation binding [3]. Since 14-3-3s can heterodimerize, the 12 expressed Arabidopsis isoforms can potentially form 78 different dimers, each with a unique combination of potential regulatory features.
Most 14-3-3 client interactions are thought to be promoted by phosphorylation of a target binding site on the client. The target binding sites include Mode-1 (K/R xx Sp/Tp x P) and Mode-2 (K/R xxx Sp/Tp x P) (where x represents any amino acid and the Sp/Tp is phosphorylated) ([4], [5]). A third motif, Mode-3 (YTpV), was found in H+-ATPase [6]. Other non-consensus sites have also been discovered, some of which do not require phosphorylation [7].
The two major challenges to understanding the biological functions of 14-3-3s are (i) the need to first identify the plethora of potential clients, and (ii) on an individual basis, determine how the client’s structural or enzymatic functions are altered. Many 14-3-3 interactions have been shown to regulate the client protein directly. For example, in plants, one of the best studied examples is the 14-3-3 activation of the plasma membrane H+-ATPase [6]. However, 14-3-3s can also regulate cellular functions by bringing together two different clients [8]. This mode of action is possible since 14-3-3s dimerize, and each 14-3-3 subunit has its own binding cleft. As the list of 14-3-3 clients grows, so does the number of unique combinations of clients potentially brought into the same complex as a result of a 14-3-3 scaffold.
Hundreds of potential 14-3-3 clients have now been identified in fungal, plant, and animals systems. Several proteome wide survey approaches have been used, including classic yeast two-hybrid searches, in vitro binding of cell extracts to a 14-3-3 affinity column, and copurification of in vivo complexes formed with epitope tagged 14-3-3s. For example, in animal systems the purification of in vivo complexes formed with epitope tagged 14-3-3 has contributed to the identification of more than 300 potential 14-3-3 clients ([9]–[11]). While each experimental strategy has its pros and cons, one advantage of purifying epitope tagged 14-3-3 complexes is that the identification of a potential client is accompanied by evidence supporting an in vivo interaction. One of the disadvantages of the yeast two-hybrid approach is that some 14-3-3 complexes will never be recovered; for example, in cases where the yeast is missing a kinase activity required for phosphorylation of a particular plant client. While S. cerevisiae has around 119 kinases, it does not have representatives for the more than 600 receptor kinases present in plants [12].
To date, most of the potential 14-3-3 clients from plants have been obtained by yeast two-hybrid and in vitro binding studies [13]. To complement these approaches, we performed a mass spectrometry (MS)-based proteomic analysis of tandem affinity purification (TAP)-tag affinity purified 14-3-3 complexes. For this purpose, we engineered a stable transgenic Arabidopsis plant expressing a tandem affinity tagged 14-3-3 omega. Our TAP-tag contained a standard protein A motif, but was modified to replace the calmodulin binding site with a 6 x His motif to avoid the co-purification of complexes associated with calmodulin binding proteins. After purifying TAP-tagged 14-3-3 complexes, in parallel with a control TAP-tagged green fluorescence protein (GFP), the co-purifying proteins were subjected to in-solution protease digestion followed by matrix assisted laser desorption ionization time of flight (MALDI)-based multiple dimensional protein identification technology (MudPIT) analysis. We identified 131 proteins that specifically co-purified with the TAP-tagged 14-3-3, including 10 14-3-3 isoforms and 121 putative clients. Of these, 101 represent new potential 14-3-3 clients that have not previously been identified. For example, our results support a role of 14-3-3 in regulating new clients involved in the transport of Ca2+, K+ and Cl−, enzymes involved in ethylene biosynthesis, brassinolide signal transduction, and WRKY transcription factors to name a few. This analysis brings the total number of potential 14-3-3 clients in plants to more than 300. This makes 14-3-3s one of the most connected nodes of interaction on the emerging protein-protein interaction map of the plant proteome.
Material and methods
Transgenic Plant Lines and Growth Conditions
Arabidopsis thaliana cv Columbia plants were transformed with 35s::GFP-TAP2 (ps346, ss687 or ss820) or 35s::14-3-3 omega-YFP-TAP2 (ps472, plant line TL3169) through an Agrobacterium (GV3101) -mediated floral dip transformation method [14]. Transgenic plants were selected on plates consisting of 1/2x MS salts (Sigma), 0.5g/L MES, pH 5.7, and 25ug/mL hygromycin B (Invitrogen) or 50ug/mL kanamycin (Sigma), respectively. Seedlings were then transferred after 10–14 days to 200mL liquid media consisting of 1/2x MS salts, 0.5g/L MES, pH 5.7, and 2% sucrose and grown for 6–7 weeks under low light conditions with continuous shaking at room temperature.
Constructs and cloning
Recombinant TEV protease
GST-TSPN-S2 (ps308) encodes a mutant version of tobacco etch virus (TEV) protease with reduced auto-cleavage activity [15]. It was constructed as a sandwich fusion between glutathione-s-transferase (GST) at the N-terminus and a Strep Tag (S2) at the C-terminus in a modified pGEX-4T vector (Pharmacia), which harbors an ampicillin resistance marker (see Supplemental Figure 1 for plasmid sequence).
Plant constructs
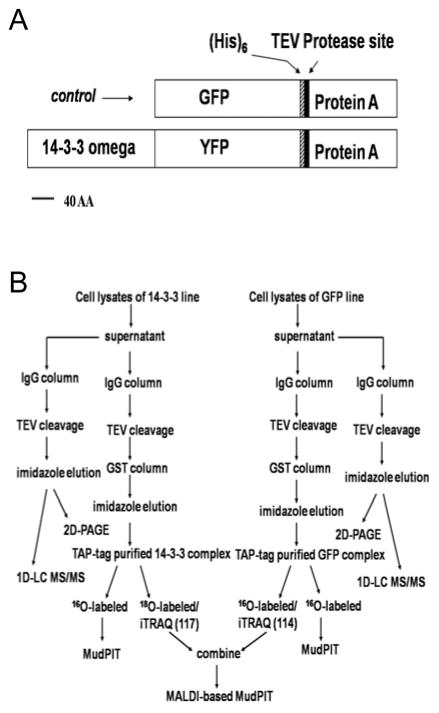
35s::GFP-TAP2 (ps346) encodes an N-terminal GFP fused to Protein A, a TEV protease cleavage site and a 6xHis, under the control of the cauliflower mosaic virus (CaMV) 35s promoter (Figure 1A). The parent plant transformation vector was pGreenII, providing hygromycin resistance in plants and kanamycin resistance in bacteria [16] (see Supplemental Figure 2 for plasmid sequence)
Figure 1. Flow chart of MS-based proteomic analysis of TAP-tag affinity purified 14-3-3 protein complex.
A) Diagram showing domain organization of GFP-TAP2 (control) and 14-3-3 omega-YFP-TAP2 (bait) fusion proteins. B) Soluble (supernatant) proteins were separated from membranes and organelles by ultracentrifugation and processed as shown. Purified samples were subjected to either MALDI-based or ESI-based MudPIT analysis. Quantitative analysis was conducted using 16O/18O and iTRAQ labeling methods followed by MudPIT analyses.
35s::14-3-3 omega-YFP-TAP2 (ps472) encodes an N-terminal 14-3-3 omega (At1g78300) fused to a YFP, the same affinity tag used in 35s::GFP-TAP2, and expressed under the control of a 35s promoter (Figure 1A). The parent plant transformation vector was pBIN, providing kanamycin resistances in plants and bacteria, [17] (see Supplemental Figure 3 for plasmid sequence).
Purification of GST-tagged TEV protease
Overnight cultures of E. coli harboring GST-TSPN-S2 (ps308) in 2XYT media + 200μg/mL ampicillin were diluted 10-fold, grown for 1 hr at 30°C, and expression of GST-TSPN-S2 induced by addition of 0.5mM isopropyl β-D-1-thiogalactopyranoside (IPTG). Cells were grown 3 more hours at 30°C. Cells were harvested, and the cell pellet resuspended in 20mL GST-BB (binding buffer; 20mM Tris-HCl, pH 8.5, 10mM EDTA, 100mM NaCl) + 1mM phenylmethylsulphonyl fluoride (PMSF) and 1mg/mL lysozyme. After incubation on ice for 10–30min, the mixture was frozen at −70°C until used. The sample was thawed quickly, and triton x100 added to 0.4% (v/v). Cells were broken by sonication for 6 min at 4°C. Cell debris was pelleted at 10,000×g for 10 min. The supernatant was mixed with 400μL of a 50% slurry of glutathione agarose (Sigma) pre-washed with 3 × 5mL GST-BB and incubated for 1 hour at 4°C. The glutathione agarose beads were pelleted at 200–300×g for 30s, washed with 3 × 10mL GST-BB, and transferred to a bio-spin column (Bio-Rad). TSPN fusion protein was eluted with 2 × 500μL GST-EB (elution buffer: 100mM Tris-HCl, pH 8.5, 500mM NaCl, 0.1mM EDTA, 10mM glutathione) and concentrated in a centricon (YM-30, Millipore). Glycerol was added to a final concentration of 50%. Aliquots were frozen and stored at −70°C. The concentration of TSPN was determined via the Bradford method [18], and greater than 90% purity verified by SDS-PAGE (10% polyacrylamide gel) and staining with Coomassie Brilliant Blue [18].
TAP-tag affinity purifications
Liquid grown plants were harvested, quick frozen in liquid nitrogen, ground to a fine powder with dry ice in a coffee grinder, and either used directly or stored at −70°C. Frozen plant powder was mixed with an equal amount (w/v) of Homogenization Buffer (HB: 100mM Tris pH 7.5, 10mM EDTA, 10% glycerol, 150mM NaCl, and Complete Protease Inhibitor Cocktail Tablet added fresh as per manufacturer’s recommendations (Roche)). The homogenate was filtered through four layers of cheesecloth pre-soaked in ice-cold HB into a pre-chilled flask on ice. The filtrate was then centrifuged at 6,000×g for 15 minutes at 4°C to pellet intact organelles and cell wall debris. The supernatant containing membranes and soluble proteins was then centrifuged at 112,000×g for 1 hr at 4°C in a swinging bucket rotor to pellet membranes. Membrane pellets were resuspended in Resuspension Buffer (RB: 100mM Tris pH 7.5, 10% glycerol, 1mM PMSF) homogenized in a dounce homogenizer and stored at −70°C. To 37.5mL of the soluble supernatant fraction, 500μL of 50% slurry of IgG sepharose 6 fast flow (GE Healthcare) pre-washed with 3 × 5 mL IgG-W (10mL Tris, pH 7.5, 150mM NaCl) was added and the mixture rocked at 4°C for 2hrs. Sepharose beads were then pelleted at 200×g for 10–20s, the supernatant was removed (unbound fraction), and beads were washed with 3 × 5mL IgG-W.
Fusion proteins were then eluted from the sepharose beads by cleavage with 2μM (50 μg/500 μL) GST purified GST-TSPN-S2 in cleavage buffer (IgG-W+ 0.1mM β-Mercaptoethanol) for 2 hrs at 16°C. Sepharose beads were pelleted, and supernatant removed and stored at −70°C (1st elution). To the sepharose beads another 2μM TSPN was added in cleavage buffer and the reaction was allowed to proceed overnight at 16°C before collecting a second elution. The 2hr and 16hr cleavage reactions were then pooled, mixed with 25 μL packed glutathione agarose beads (pre-washed with IgG-W (Sigma)) and incubated for 10 min at 4°C. The slurry was transferred to a spin column (Bio-Rad) and the flow through collected via centrifugation at 200×g at 4°C for 10s. This GST binding step was performed in order to help remove contaminating proteins carried over from the GST-TSPN-ST protease elution step.
After a GST clean-up, NaCl was added to the flow through to bring the concentration to 300mM NaCl and mixed with 100μL of a 50% slurry of Ni-NTA superflow (Qiagen) pre-washed with 3 × 2mL 6H-W1 (20mM Tris-HCl, pH 7.5, 300mM NaCl). Binding was allowed to proceed at 4°C for 1 hr. The mixture was then transferred to a bio-spin column (Bio-Rad), washed with 3 × 1 mL 6H-W1, followed by 2 × 75μL washes with 6H-W1 + 0.1% NP-40 to aid in the removal of nonspecific interactions. The column was then washed 2 × 75uL with 6H-W1 to remove traces of NP-40, and purified protein complexes were eluted from the column with 2 × 100μL 6H-EB: 20mM Tris-HCl, pH 7.5, 50mM NaCl, and 300mM imidazole.
Western blot detection of 14-3-3 omega-YFP-TAP2 bait and GFP-TAP2 proteins
Western blotting was performed essentially as described [19]. Briefly, protein samples were mixed with 3× loading and incubated for 10min at 37 °C. Samples were electrophoresed through an 10% polyacrylamide gel (29:1, acrylamide:bisacrylamide, Sigma) and transferred to nitrocellulose using a Bio-Rad transfer apparatus. Blots were incubated in blocking buffer (10 mM Tris, pH 7.6, 137 mM NaCl, 0.5% (v/v) Tween 20 (TBS-T), with 5% (w/v) nonfatdry milk) for at least 1 h at RT with shaking followed by a 1 hr incubation at RT with primary anti-GFP antibody (Clonetech). After washing, the blots were washed 4 × 10 min in TBS-T and incubated for 1 h at RT with secondary antibody diluted 1:10,000 in blocking buffer. The secondary antibody used for detection was a donkey anti-rabbit IgG conjugated with horseradish peroxidase (GE Healthcare). Following secondary antibody incubation, the blots were washed 4 times for 10 min in TBS-T, and detection was made using ECL (GE Healthcare).
Two-dimensional gel electrophoresis and imaging
2D gel electrophoresis was performed as previously described [20]. Purified protein samples were precipitated with 4 volumes of cold (−20°C) acetone. After incubation at −20° overnight, the precipitates were washed twice with −20°C acetone/water (4:1) and resulting pellets were dried for 15 min using a Speed Vac. The pellets were dissolved in 200 μL DeStreak Rehydration Solution (GE Healthcare) and spun at 16,000 rpm at 22° for 10 min. Bio-Lyte 3–10 Ampholyte (Bio-Rad) was added to each supernatant to a final concentration of 0.2% (v/v). 185 μL of each extract (81 μg for 14-3-3 and 97.1 μg for GFP) was loaded onto a 3–10L (linear) 11 cm IPG strip (Bio-Rad, Hercules, CA) by overnight passive rehydration. Isoelectric focusing was carried out on a Bio-Rad Protean IEF cell using a program as follows: 250 V, linear ramp for 20 minutes; 8000 V, linear ramp for 2 hours 30 minutes; and 8000 V for a total of 20,000 Vhr. Strips were stored at −80°C overnight, then thawed the next day and incubated twice for 10 minutes each in 8M urea, 2% SDS, 0.05 M Tris-HCl, pH 8.8, 20% glycerol. The first incubation contained 2% dithiothreitol (DTT) and the second contained 2.5% iodoacetamide. The strips were then layered on 8–16% acrylamide Criterion Tris-HCl gels and embedded in place with 0.5% agarose, along with electrophoresis until the dye front reached the bottom of the gel. Gels were washed with two changes of water (10 minutes each) and stained overnight with Sypro Ruby stain (Molecular Probes, Inc). Stained gels were imaged on a Bio-Rad VersaDoc imager. Images of gels were compared using Bio-Rad PDQuest version 7.3 software and spot sets were created.
In-gel and in-solution protease digestion of proteins
In-gel digestion was performed and modified as previously described [21]. The 2D-gel spots were excised individually by a ProteomeWorks Spot Cutter (Bio-Rad). Gel samples were washed twice with 25 mM ammonium bicarbonate and 100% acetonitrile (ACN), and reduced by 10 mM DTT and alkylated by 100 mM iodoacetamide. Samples were subjected to in-gel digestion by incubation with 75 ng trypsin (Promega) dissolved in 25 mM ammonium bicarbonate at 37°C for 6 hr on the Investigator ProPrep Digestion and Mass Spec Preparation Station (Genomics Solutions, Ann Arbor, MI). Peptide samples were spotted onto MALDI plates using Zip-Tip μC18 tips (Millipore). 0.5 μL and 0.5 μL matrix mix (5 mg/ml α-cyano-4-hydroxycinnamic acid (α-CHCA) with 10 mM ammonium phosphate) were then spotted onto the plate.
For in-solution digestion, TAP-affinity purified 14-3-3 and GFP protein complexes were digested by trypsin (Promega) in small aliquots. For each protein sample aliquot (5ug in 20μl), 30 μl of 100% ACN was added and incubated for 20 min at room temperature, followed by the addition of 40 μl 10 mM DTT in 25 mM ammonium bicarbonate and incubation for 10 min at 60°C. After cooling to room temperature (15 min), 20 μl of 55 mM iodoacetamide in 25 mM ammonium bicarbonate was added and incubation continued for 35 min at room temperature. Proteolysis was initiated by the addition of 35 μl (0.2 μg) trypsin ((Promega), dissolved in 25 mM ammonium bicarbonate) per aliquot reaction and incubated overnight at 37°C.
Peptide desalting by reverse phase μC18 Zip-Tip columns
Peptide desalting was performed as previously described [22]. The digested peptides were dried in a vacuum. Desalting of the pellets was performed by use of reverse phase Zip-Tip μC18 columns (Millipore). The C18 column was equilibrated by 100% ACN and washed by 0.1% (v/v) TFA (in water) three times. Peptide pellet was re-suspended in 0.1% (v/v) TFA and bound onto the column. The C18 column was washed by 0.1% (v/v) TFA three times and peptides were eluted by a solution containing 50% (v/v) ACN, 0.1% (v/v) TFA. The eluate was dried in a speed-vac.
Mass spectrometry analysis and protein identification
2D Gel mass spectrometric data was collected using an ABI 4700 Proteomics Analyzer MALDI TOF/TOF mass spectrometer (Applied Biosystems, CA), using their 4000 Series Explorer software v. 3.0 – 3.6. MS acquisition/processing settings were: Reflector Positive Mode (1-keV accelerating voltage), 700–4000 Da acquisition mass range, baseline subtraction enabled at peak width 50, S/N threshold 3, Cluster Area S/N Optimization enabled at S/N 3, internal calibration to within 20 ppm using trypsin autolysis peaks 842.51 and 2211.105. The eight most intense ions were selected for MSMS analysis. MSMS acquisition/processing settings were: 70 Da to precursor ion mass range acquisition, precursor window resolution of −1 to +4 Da, Collision Induced Dissociation (CID) on, baseline subtraction enabled at peak width 50, S/N threshold 5. Raw data was extracted for protein identification by GPS Explorer Software v. 3.0 – 3.6 (Applied Biosystems) and analyzed by Mascot v 1.9.05 (Matrix Science) [23] using NCBI nr database (NCBI 2005.03.22) containing 2,367,365 sequences. Analyses were performed as combination MS + MS/MS. Search settings included MS and MSMS minimum S/N filter 10, peak density filter 50 peaks per 200 Da, maximum number of peaks 65. Additional settings were: cleavage enzyme trypsin, variable modifications of oxidation of Methionine and Carbamidomethylation of Cysteines, max 2 missed cleavages, precursor mass tolerance 20ppm, fragment ion mass tolerance 0.2 Da.
For MALDI-based MudPIT analysis, tryptic digested peptides were fractionated by two dimensional liquid-chromatography (2D-LC). The first dimension was separated by a strong cation-exchange (SCX) column (5 μL, 300 Å, 25 mm length, tapered bore 4.0 mm inlet, 1.0 mm outlet) (Michrom Bioresources) using HPLC. The SCX-bound peptides were eluted with four different salt concentrations of ammonium acetate (Table 1) and separated by the second dimension, a reverse phase C18 column (5 μL, 100 Å, 0.1 × 150 nm) (Michrom). For reverse phase LC gradient formation, solvent A, 0.3% (v/v) formic acid (FA); solution was mixed with solvent C, 90% (v/v) ACN, 0.3% (v/v) FA to make 80% (v/v) ACN as final in 1 hr at a nanoflow rate of 0.7 μL/min. The eluted peptides were subjected to spotting onto ABI MALDI plates (400/plate) by a ProBot spotting robot (Dionex LC Packings) in alpha-cyano-4-hydroxycinnamic acid (Sigma) at a rate of 1 spot every 6 seconds followed by a MALDI-TOF-TOF MS/MS analysis using the ABI 4700 spectrometer. Matrix was spiked with Insulin B chain and Angiotensin 1–7 clip standards for MALDI calibration. Peptides were spotted onto MALDI plates, with a density of 400 spots per plate. Mass range of MS acquisition was set between 700 and 4000 Da. Laser intensity was set at 4500. For MS processing, baseline was subtracted at 50 with signal to noise ratio of 3. Smoothing was disabled. Signal to noise ratio threshold for cluster area optimization was set at 10. Internal calibration using masses 3494.651 and 899.466 was performed. For MS/MS acquisition, collision induced dissociation (CID) was used with laser intensity at 5500. For MS/MS processing, baseline was subtracted at 1000 with signal to noise ratio threshold at 10. Smoothing was disabled. Signal to noise ratio threshold for cluster area optimization was set at 15. Top eight abundant peaks per spot were selected for MS/MS analysis. MS/MS data obtained was subjected to MASCOT algorithm analysis against SwissProt database (20050303) containing 176,469 sequences and NCBI database (20050322) containing 2,367,365 sequences.
Table 1.
14-3-3 and control GFP samples analyzed by MS
| Sample - Prep # | Quantative analysis | Separation method | LC elution | MS method |
|---|---|---|---|---|
| 1 GFP-prep1 | n | 2D-PAGE | MALDI Tof-Tof | |
| 2 14-3-3-prep1 | n | 2D-PAGE | MALDI Tof-Tof | |
| 3 GFP-prep2 | n | 1D-LC | ACN | ESI Ion-Trap |
| 4 14-3-3-prep2 | n | 1D-LC | ACN | ESI Ion-Trap |
| 5 14-3-3-prep3 | n | n | n | |
| 6 GFP-prep3 (O18) | n | n | n | |
| 7 5 + 6 | O16/O18 | 1D-LC | ACN | ESI Ion-Trap |
| 8 GFP-prep4 | n | 2D-LC | A | MALDI Tof-Tof |
| 9 GFP-prep5 | n | 2D-LC | B | MALDI Tof-Tof |
| 10 14-3-3-prep4 | n | 2D-LC | A | MALDI Tof-Tof |
| 11 14-3-3-prep5 | n | 2D-LC | B | MALDI Tof-Tof |
| 12 14-3-3-prep5(O18) | n | 2D-LC | B | MALDI Tof-Tof |
| 13 9 + 12 | O16/O18 | 2D-LC | B | MALDI Tof-Tof |
| 14 GFP-prep6 | n | n | n | |
| 15 14-3-3-prep6 | n | n | n | |
| 16 14 + 15 | iTRAQ | 2D-LC | C | MALDI Tof-Tof |
| 17 14-3-3-prep7 | n | TiO2, 1D-LC | C | ESI Ion-Trap |
a) Prep #: biological sample replicates
b) ACN: acetonitrile
c) ammonium acetate steps (mM): 20, 100, 500, 1000
d) ammonium acetate steps (mM): 20, 75, 250, 1000
e) ammonium acetate steps (mM): 0, 10, 25, 50, 75, 100, 150, 200, 250, 500
f) Any prep not particularly labeled O18 were O16-labeled
g) n: not applicable
In some cases, a relative comparison of peptide ion frequencies was evaluated in control and 14-3-3 samples using a non-isobaric tag (iTRAQ) strategy. For iTRAQ samples, the mass tolerance for fragmented ion was set at 0.2 Da. Missed cleavage site was set at 1. Met oxidation and Carbamidomethyl Cys were selected for potential protein modification. Protein identification results were inspected by use of GPS TM software version 3.5 (Applied Biosystems). MS/MS spectra were manually inspected to ensure the quality of the identification. At least two peptides per protein were documented for protein identification. For iTRAQ analyses, the mass tolerance for precursor ion was set at 150 ppm and for fragmented ion was set at 0.2 Da. Missed cleavage site was set at 1. Parameters set for potential protein modifications included those to account for iTRAQ labeling and Met oxidation.
To further evaluate the confidence of protein identifications, a decoy database [24] with a randomized version of NCBI nr (20050322) containing 2,367,365 sequences and SwissProt (20050303) containing 176,469 sequences was generated. A combined and concentrated database containing both regular and random sequences was generated. The decoy was searched with the same parameters used for a regular database search. A false-positive identification rate (FDR) was calculated as ((FP/(FP + TP))*2) where FP represents false positive and TP represents true positive. The FDR for all searches was calculated using a peptide homology match cut-off threshold of better than E-value 0.1 [25], and is listed in Supplemental Table 7. The FDR rates were normally less than 0.01, with the worst case exception at 0.06. In all cases, true positives were confirmed to be the best hits in both SwissProt and NCBI databases.
Prediction of protein mass and peptides containing consensus 14-3-3 binding motifs
Gene ontology annotations were characterized by searching ATG number against the Arabidopsis information resource (TAIR) gene ontology (GO) database (http://www.arabidopsis.org/tools/bulk/go/index.jsp). The molecular mass of each protein was predicted using Compute pI/Mw algorithm (http://ca.expasy.org/tools/pi_tool.html). A bioinformatic analysis was performed to predict 14-3-3 binding peptides of these interacting proteins by use of MotifScan (http://scansite.mit.edu/motifscan.seq.ptml) [26] algorithm followed by a manual evaluation.
Quantitative proteomics analysis using 16O/18O and iTRAQ labeling
MS-based quantitative proteomic analysis using 16O/18O stable isotope labeling catalyzed by trypsin digestion was performed as described previously [27]. For 16O/18O labeling, trypsin dissolved in H216O and H218O (Sigma) was used. TAP affinity purified 14-3-3 fraction was subjected to in-solution trypsin digestion in H216O at 37°C overnight, whereas GFP fraction was subjected to in-solution digestion in H218O. After the digestion, two samples were combined into a single tube. Peptides were desalted by reverse phase C18 Zip-Tip columns (Millipore) for MS analysis. The 16O and 18O-labeled peptide has theoretical mass difference of 2 and 4 Da. The signal level of 16O peak was compared to the background level. Since in TOF-TOF analysis signal to noise ratio threshold for cluster area optimization was set at 10, the background level was calculated by dividing the signal level by 10. The 18O/16O ratio was calculated as such: (18O peak signal- background)/(16O peak signal- background). The 18O/16O ratio was calculated as such: (18O peak signal- background)/background.
iTRAQ labeling was performed as per manufacturer’s recommended protocol (Applied Biosystems) starting with the acetone precipitation of the TAP affinity purified proteins. The only deviation included two 5μg additions (instead of one 10μg addition) of trypsin (supplied in kit, Applied Biosystems). After the first addition of 5μg trypsin, the samples were incubated at 40°C for 3 hours, followed by a second aliquot of 5μg trypsin and incubation overnight at 37°C to ensure complete trypsin digestion.
To estimate protein concentration after the acetone precipitation via the Bradford method [18], an aliquot of each sample (after solublization in dissolution and denaturization buffer (provided in kit)) was dialyzed using mini-dialysis tubes (Pierce) against 1L 10 mM Tris pH 6.8 at room temperature for 1 hr to eliminate detergents and salts.
iTRAQ-labeled GFP and 14-3-3 samples were combined into a single sample and subjected to MALDI-based 2D-LC MS/MS analysis. In this case, the SCX-bound peptides were eluted by ten different concentration of ammonium acetate salt (0, 10, 25, 50, 75, 100, 150, 200, 250, 500 mM). The salt eluted peptides were spotted onto individual spot on 10 separate MALDI plates with time interval of six seconds at a nanoflow rate of 0.7 μL/min. Four hundred spots were spotted onto each MALDI plate.
An MS/MS analysis of top-three abundant peptides in each spot per plate was performed to build up exclusion list. The list includes masses of abundant peptides i.e. 14-3-3, GFP, GST, IgG, and trypsin (Supplemental Table 1). A complete MS/MS analyses on top eight abundant peaks per spot was performed further to retrieve sequence information of less abundant peptides by including the exclusion list. The iTRAQ (117/114) ratio was calculated by correction factor input for the GPS TM software version 3.5 (Applied Biosystems). using 114 as a reference. Although TOF-TOF was set for a theoretical mass selection window of −0.001 Da to + 2D for fragmentation analyses, the instrumentation used here was observed to allow significant amounts of contaminants between −1 to +3 Da. Thus, spectra used for iTRAQ quantitation were manually inspected to ensure that the signals corresponding to the peptide being identified and quantified in a fragmentation analysis represented more than 50% of the total signal.
Results
TAP-tag affinity purification of 14-3-3 protein complexes in vivo
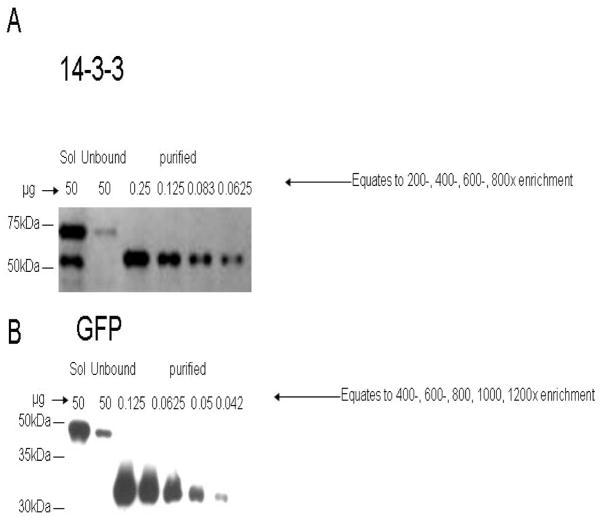
A tandem affinity purification strategy (TAP-tag purification) was used to purify 14-3-3 omega-YFP-TAP2 (called 14-3-3 samples or 14-3-3-TAP2Y) and GFP-TAP2 (called GFP samples) (Figure 1A) from the soluble extracts of liquid grown transgenic plants. The first affinity purification step was binding to an IgG sepharose column, followed by a TEV protease cleavage elution (Figure 1B). The second affinity purification was over a Ni-NTA column. A western blot analysis consistently showed at least a 200-fold enrichment of purified 14-3-3-TAP2Y (Figure 2A) and a 800-fold enrichment of purified GFP, as shown using an anti-GFP antibody (Figure 2B) to detect the fusion proteins. In experiments used for MudPIT analyses, this enrichment was improved several fold for both samples by including an extra GST removal step to better remove contaminants that had been added during our TEV protease elution from the IgG column. Regardless, in all purifications the enrichment for the 14-3-3 samples was around 4-fold less compared to the GFP control, consistent with the expectation that the 14-3-3 samples contained a large number of 14-3-3 interacting proteins. The TAP purified samples from multiple 14-3-3-TAP2Y and GFP-TAP2 preparations were analyzed by both 2D-gel separations and MudPIT, providing evidence for more than 131 14-3-3 specific interacting proteins (Tables 2 and 3).
Figure 2. Western blots showing an example of enrichment for GFP and 14-3-3 baits in a tandem affinity purification strategy.
Proteins were separated by SDS-PAGE and Western Blots probed with an anti-GFP antibody. For each bait, 50ug of protein were analyzed to show the signal strength of the GFP in the soluble extract “loaded” onto the 1st IgG purification column, and in the “unbound” flow through from that column. Aliquots of the purified fractions were then diluted and analyzed for relative enrichment compared to the starting soluble extract. A) 14-3-3 purification. B) GFP control purification. Data represents one replicate of several Western blots used to assess the fold enrichment of independent purifications. 14-3-3 complexes were typically enriched around 200-fold (GFP control, 800-fold) after Protein A and Ni2+ purification.
Table 2.
14-3-3 Isoforms in the 14-3-3 Complex
| Protein name | AGI number | MW (kD) | Protein score | Number of replicates |
|---|---|---|---|---|
| 14-3-3ω(GR14omega) | At1g78300 | 29.1 | 473 | 5 |
| 14-3-3χ(GR14chi) | At4g09000 | 29.9 | 198 | 3 |
| 14-3-3ε(GR14epsilon) | At1g22300 | 28.9 | 240 | 3 |
| 14-3-3κ(GF14kappa) | At5g65430 | 28 | 220 | 3 |
| 14-3-3λ(GF14lambda) | At5g10450 | 27.7 | 129 | 3 |
| 14-3-3o(GF14omicron) | At1g34760 | 27.5 | 133 | 3 |
| 14-3-3φ(GF14phi) | At1g35160 | 30.2 | 585 | 3 |
| 14-3-3μ(GF14mu) | At2g42590 | 28.9 | 210 | 2 |
| 14-3-3ψ(GF14psi) | At5g38480 | 28.6 | 204 | 2 |
| 14-3-3υ(GF14upsilon) | At5g16050 | 30.2 | 598 | 1 |
a) AGI number: The accession number of Arabidopsis genes
b) Protein score: the score the MASCOT software assigned to each identified protein after database searching
c) MW: predicted molecular mass of the 14-3-3 protein
d) Number of replicate: how often a protein identified among different replicates
Table 3.
14-3-3 clients identified in the tandem affinity purified 14-3-3 samples
| 14-3-3 Client Protein Identified (Number of Replicates) | ATG number | Confidence % | Score |
|---|---|---|---|
| CARBOHYDRATE METABOLISM | |||
| PEP carboxylase (2) | At1g53310 | 100 | 43 |
| Glucose-6-phosphate dehydrogenase 3 (1) | At1g24280 | 99.9 | 29 |
| Glucose-6-phosphate dehydrogenase 1 (1) | At5g35790 | 99.6 | 27 |
| Acetyl-coenzyme A carboxyl transferase (2) | AtCg00500 | 100 | 43 |
| Fumarase 1 (2) | At2g47510 | 99.9 | 30 |
| Fumarase 2 (1) | At5g50950 | 99.9 | 30 |
| Granule-bound starch synthase I (1) | At1g32900 | 99.8 | 27 |
| Alpha-glucan water dikinase (1) | At1g10760 | 99.9 | 32 |
| Neutral invertase (1) | At1g35580 | 100 | 30 |
| Phosphomannomutase (2) | At2g45790 | 100 | 44 |
|
| |||
| NITROGEN METABOLISM | |||
| Ferredoxin-dependent Glu synthase 1 (3) | At5g04140 | 100 | 38 |
| Nitrate reductase (2) | At1g77760 | 99.7 | 25 |
|
| |||
| TRANSPORT | |||
| Phospholipid-transporting ATPase ALA1 (3) | At5g04930 | 99.1 | 23 |
| Potassium channel GORK (4) | At5g37500 | 99.9 | 32 |
| Cd/Zn translocating ATPase HMA2 (2) | At2g19110 | 100 | 63 |
| Chloride Channel CLC-g (3) | At5g33280 | 96.7 | 23 |
| ADP, ATP carrier protein 2 (1) | At5g13490 | 100 | 52 |
| YCF1.2 Photosystem II component (2) | AtCg01130 | 100 | 54 |
| Plasma membrane H+ ATPase AHA6 (1) | At2g07560 | 97.1 | 18 |
|
| |||
| CYTOSKELETON | |||
| Villin 2 (1) | At2g41740 | 99.8 | 27 |
| Tubulin alpha-6 chain (1) | At4g14960 | 100 | 61 |
| Tubulin beta-8 chain (2) | At5g23860 | 100 | 70 |
| Gamma-tubulin complex component 4 (2) | At3g53760 | 100 | 66 |
| Kinesin-4 (2) | At5g27000 | 100 | 43 |
| Actin 7 (1) | At5g09810 | 100 | 72 |
| ZYP1b, transverse filament-like | At1g22275 | 100 | 46 |
|
| |||
| TRANSCRIPTION | |||
| CURLY LEAF (1) | At2g23380 | 99 | 41 |
| Potential polycomb group protein EZA1 (4) | At4g02020 | 100 | 34 |
| WRKY transcription factor 6 (1) | At1g62300 | 100 | 40 |
| WRKY transcription factor 16 (3) | At5g45050 | 100 | 56 |
| WRKY transcription factor 18 (1) | At4g31800 | 99.9 | 30 |
| WRKY transcription factor 19 (3) | At4g12020 | 100 | 42 |
| WRKY transcription factor 27 (2) | At5g52830 | 99.9 | 28 |
| WRKY transcription factor 32 (4) | At4g30935 | 99.8 | 30 |
| WRKY transcription factor 40 (1) | At1g80840 | 100 | 39 |
| LUMINIDEPZENDENS (3) | At4g02560 | 100 | 37 |
| MADS box protein AGL5 (2) | At2g42830 | 100 | 33 |
| Phytochrome-interacting factor 4 (1) | At2g43010 | 99.9 | 31 |
| DNA-directed RNA pol 3 (3) | At2g24120 | 99.9 | 29 |
| DNA-directed RNA pol beta chain (2) | AtCg00190 | 99.8 | 27 |
| Vernalization-insensitive protein 3 (1) | At5g57380 | 100 | 36 |
| Pirin 1 (1) | At3g59220 | 100 | 35 |
| BAP28 like 18s rRNA maturation factor (4) | At3g06530 | 100 | 61 |
|
| |||
| TRANSLATION | |||
| Mitochondria ribosomal protein S4 (2) | AtMg00290 | 100 | 33 |
| Mitochondria ribosomal protein S3 (2) | AtMg00090 | 100 | 40 |
| 40S ribospmal protein S4 (2) | At5g07090 | 100 | 36 |
| 40S ribosomal protein S18 (2) | At1g34030 | 100 | 38 |
| 60S ribosomal protein L2 (1) | At2g18020 | 99.9 | 30 |
| 60S ribosomal protein L26B (1) | At5g67510 | 99.9 | 30 |
| EIF-4A-1 (1) | At3g13920 | 99.9 | 31 |
| EIF-2 beta (2) | At5g20920 | 99.4 | 21 |
|
| |||
| SIGNALING | |||
| ETO1-like protein 1 (3) | At4g02680 | 100 | 48 |
| ETO1-like protein 2 (3) | At5g58550 | 100 | 37 |
| ACC synthase 6 (1) | At4g11280 | 99.9 | 29 |
| ACC synthase 7 (2) | At4g26200 | 100 | 47 |
| ACC synthase 8 (2) | At4g37770 | 100 | 37 |
| Phospholipase D beta 1 (2) | At2g42010 | 100 | 37 |
| Phospholipase D gamma 1 (2) | At4g11850 | 99.9 | 31 |
| Guanine nucleotide-bind protein (AGB1)(1) | At4g34460 | 99.7 | 26 |
| Inositol-3-phosphate synthase isozyme 1 (1) | At4g39800 | 100 | 40 |
| Inositol-3-phosphate synthase isozyme 2 (2) | At2g22240 | 99.9 | 32 |
|
| |||
| KINASE | |||
| MAP kinase 7 (1) | At2g18170 | 99.6 | 24 |
| Calcium-dependent protein kinase 1 (2) | At5g04870 | 97.7 | 19 |
| Serine/threonine-protein kinase PBS1 (2) | At5g13160 | 100 | 41 |
| Casein kinase II, alpha chain 2 (2) | At3g50000 | 99.8 | 26 |
| Protein kinase AFC1 (1) | At3g53570 | 99.9 | 31 |
| Pantothenate kinase 2 (1) | At4g32180 | 100 | 42 |
|
| |||
| PHOPHOTASE | |||
| S/T-protein phosphatase BSU1 (2) | At1g03445 | 100 | 36 |
| S/T-protein phosphatase BSL1 (2) | At4g03080 | 99.9 | 31 |
| Kinase associated protein phosphatase (3) | At5g19280 | 100 | 34 |
|
| |||
| RECEPTORS | |||
| Glutamate receptor 1.2 (3) | At5g48400 | 99.6 | 32 |
| Glutamate receptor 2.9 (2) | At2g29100 | 100 | 43 |
| Glutamate receptor 3.4 (1) | At1g05200 | 100 | 42 |
| Glutamate receptor 3.7 (2) | At2g32400 | 99.9 | 35 |
| Glutamate receptor 2.1 (2) | At5g27100 | 100 | 33 |
| Receptor protein kinase CLAVATA1 (2) | At1g75820 | 100 | 34 |
| BRI1 receptor kinase (BAK1) (2) | At4g33430 | 99.9 | 28 |
| BRI1-like 2 (1) | At2g01950 | 99.9 | 34 |
| BRI1 (1) | At4g39400 | 99.7 | 28 |
| TOO MANY MOUTH (1) | At1g80080 | 99.8 | 29 |
|
| |||
| CHAPERONE | |||
| Hsp-60 (3) | At1g55490 | 99.7 | 25 |
| Hsp-101 (3) | At1g74310 | 99.9 | 30 |
| HSP70-2 (1) | At5g02490 | 99.9 | 28 |
| Hsp 81-2 (1) | At5g56030 | 100 | 42 |
| T-complex protein 1, epsioln subunit (3) | At1g24510 | 99.2 | 23 |
| J domain protein (1) | At5g49060 | 100 | 39 |
| OTHER | |||
| Putative nucleoporin interacting protein (2) | At2g41620 | 100 | 40 |
| LOB domain protein 15 (1) | At2g40470 | 99.9 | 32 |
| Lon protease homolog 2 (4) | At5g26860 | 99.9 | 46 |
| Aspartate carbamoyltransferase (3) | At3g20330 | 100 | 66 |
| PROLIFERA (3) | At4g02060 | 100 | 41 |
| RPP13-like disease resistant protein 1 (3) | At3g14470 | 100 | 40 |
| RPP13-like disease resistant protein 2 (2) | At3g46710 | 100 | 54 |
| Peroxidase 31 (2) | At3g28200 | 100 | 33 |
| Peroxidase 50 (2) | At4g37520 | 99.9 | 36 |
| Peroxidase 51 (1) | At4g37530 | 100 | 34 |
| Peroxidase 52 (3) | At5g05340 | 100 | 38 |
| Disease resistance protein (pCol) (2) | At4g14610 | 100 | 57 |
| Catalase 2 (2) | At4g35090 | 100 | 49 |
| Glycine dehydrogenase (2) | At4g33010 | 100 | 44 |
| Vesicle-fusing ATPase (2) | At4g04910 | 100 | 41 |
| Dynamin 2B (2) | At1g59610 | 100 | 37 |
| DNA topoisomerase I (2) | At5g55300 | 100 | 36 |
| Cytochrome P450 71A23 (2) | At3g48340 | 100 | 33 |
| Glucan endo-1, 3-beta-glucosidase (2) | At3g57260 | 99.8 | 27 |
| D-3-phosphoglycerate dehydrogenase (1) | At1g17745 | 100 | 51 |
| Branched-chain amino acid aminotransferase 1 (1) | At1g10060 | 100 | 47 |
| Cytochrome P450 71A13 (1) | At2g30770 | 100 | 44 |
| Cytochrome P450 86A2 (1) | At4g00360 | 99.9 | 28 |
| Signal recognition particle 54kD (1) | At5g03940 | 100 | 40 |
| Signal recognition particle 54kD (1) | At5g49500 | 97.9 | 19 |
| Putative thioredoxin-like 7 (1) | At2g33270 | 100 | 39 |
| GDP-mannose 4, 6 dehydrogenase 1 (1) | At5g66280 | 100 | 38 |
| P-2-dehydro-3-deoxyheptonate aldolase 1 (1) | At4g39980 | 100 | 37 |
| P-2-dehydro-3-deoxyheptonate aldolase 2 (1) | At4g33510 | 99.9 | 33 |
| Cell cycle protein FtsH homolog 1 (1) | At1g50250 | 100 | 37 |
| PP1/PP2A phosphatase pleiotropic regulator (1) | At3g16650 | 100 | 35 |
| Ubiquitin ligase SINAT2 (1) | At3g58040 | 99.9 | 32 |
| FRIGIDA (1) | At4g00650 | 99.8 | 30 |
| Cysteine synthase (1) | At4g14880 | 99.9 | 30 |
| Cysteine synthase (1) | At3g59760 | 100 | 49 |
| Katanin like protein (1) | At5g23430 | 99.6 | 26 |
| Cell division protein 48 homolog E (1) | At5g03340 | 99.8 | 26 |
| GIGANTEA protein (1) | At1g22770 | 99.7 | 26 |
| Serpin (1) | At1g62170 | 99.9 | 21 |
| Lyase (1) | At3g25810 | 99.9 | 68 |
| Putative unconventional myosin (1) | At2g31900 | 99.9 | 98 |
| Glutamyl-tRNA synthase (2) | At5g64050 | 97.5 | 19 |
| YCF1.2 Photosystem II component (2) | AtCg01130 | 100 | 54 |
| DNA repair protein UVH3 (2) | At3g28030 | 100 | 44 |
a) Number of replicates: how often a protein identified among different replicates
b) ATG number: The accession number of Arabidopsis genes
c) Confidence %: The confidence interval of the identified protein after database searching
d) Confidence % shown are for protein identification based on MASCOT
e) Score: the score the MASCOT software assigned to each identified protein after database searching
f) Only AHA6 has mode III motif g) Total peptide number identified is listed in Supplemental Table 5
At least 10 of the 14-3-3 isoforms interact with 14-3-3-omega-TAP2Y
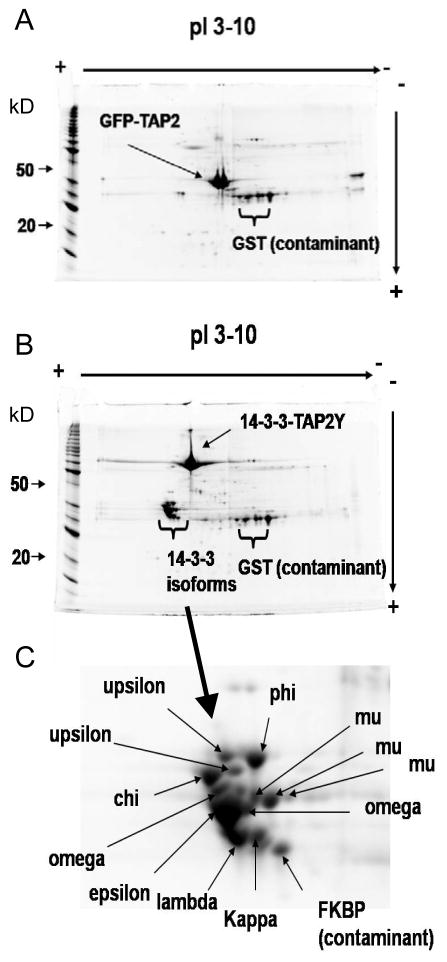
To identify the major interacting partners for 14-3-3-omega-TAP2Y, the control GFP and 14-3-3 purified samples were separated in a parallel 2D gel electrophoresis analysis and quantified by Sypro Ruby stain (Figure 3A and 3B). Protein spots from both gels were excised, digested, and analyzed by MALDI TOF-TOF MS/MS. The major spots common to GFP and 14-3-3 samples were dominated by expected contaminants, such as TEV protease, which was added as a GST-fusion protein during the elution of proteins bound to the IgG sepharose column. However, approximately fifty 14-3-3 sample specific spots were detected with varying intensity. All of the strongest staining spots were from eight different 14-3-3 isoforms (including a spot predicted to be the endogenous omega isoform) (Figure 3C), as identified by the detection of isoform specific peptides. In our MudPIT analysis described below, we also identified two more 14-3-3 isoforms (psi and omicron) (Table 2, Supplemental Table 2) indicating that our TAP-tagged 14-3-3 omega dimerized with at least 10 of the 12 expressed endogenous 14-3-3 isoforms. Unfortunately, none of the weakly stained spots contained enough protein to generate reliable MS/MS identification results. Nevertheless, this 2D-gel analysis provided evidence that the complex mixture of copurifying 14-3-3 clients was not dominated by a few highly abundant proteins, but rather represented a highly diverse mixture of many different proteins, each at a relatively low level of abundance compared to the 14-3-3 proteins.
Figure 3. Two dimensional gel electrophoresis fractionation of 14-3-3 protein complexes reveals 14-3-3 isoforms.
A) TAP-affinity purified GFP control. B) TAP-affinity purified 14-3-3 protein complexes. C) A region of B showing the 14-3-3 isoforms that copurified with the bait 14-3-3 omega-YFP-TAP2. Protein gels were stained by Sypro Ruby stain.
121 putative 14-3-3 clients were identified by MudPIT analyses
Since the 2D-gel based strategy was not sensitive enough to identify any clients in our complex mixture of 14-3-3 interactions, we switched our approach to an in-solution trypsin digestion followed by a 2D-LC MALDI-based TOF-TOF analysis. Tryptic digested peptides were bound to a SCX column and eluted by different salt concentrations (Table 1). The salt-eluted peptides were separated by a C18 reverse phase column, and peptide fractions were spotted onto MALDI plates for TOF-TOF MS analysis.
As a control for non-specific interactions we identified proteins that co-purified with our control TAP-tagged GFP in two independent purifications (Table 1). Thirty proteins were identified (Supplemental Table 3) using a Mascot score of 24 as a reasonable cut-off for a confident database match [28]. 75% of these proteins overlapped in both replicates. All of these proteins were considered non-specific contaminating interactions and were therefore excluded from our list below of potential 14-3-3 specific interactions.
To identify putative 14-3-3 specific interactions, we conducted 3 independent purifications, 2 of which were done in parallel with GFP controls described above (Table 1). For MS identifications of potential 14-3-3 interactors, we employed the following more rigorous set of criteria. First, we eliminated any protein that overlapped with a putative non-specific interactor in the GFP controls, even if that GFP hit had only been identified as single peptide with a Mascot score no better than 4, or a confidence as low as 90%. Second, all positive 14-3-3 hits required the identification of at least 2 peptides, with the final protein identification having a total Mascot score of at least 24, or an overall confidence level of 99%. Third, in 22 cases where the best individual peptide (among the multiple peptides supporting a specific protein identification) had a confidence below 90%, a manual inspection was done to validate the identification. In these cases, at least three immonium ions and three daughter ions (y or b ion) were required to match theoretical mass predictions, and represent the major peaks in the spectra. If more than 50% of the signal in the spectra appeared to be from a contaminating peptide, the spectrum was not used.
Using the above criteria, a total of 131 Arabidopsis proteins were identified as potential interactors with our TAP tagged 14-3-3 omega (Table 2 and 3, with details in Supplemental table 2 and 5). Nine of these proteins were identified as specific 14-3-3 isoforms other than omega. Twenty were putative client proteins that were expected based on the plant 14-3-3 interactions reported in the literature (Supplemental Table 4). These 2 subsets of interactions confirm that our tagged 14-3-3 formed heterodimers and that these complexes included well known 14-3-3 clients.
Corroborating 14-3-3 co-purification for 31 clients using quantitative MS
While 131 proteins were identified in the 14-3-3 purified fraction that were not found in the GFP fraction, 31 selected examples were further shown by quantitative MS strategies to be significantly enriched over background in a 14-3-3 purified sample. This was done by employing two different strategies. This enrichment evidence provides added confidence that a particular 14-3-3 interaction was real, and not just an artifact of not identifying a low abundance protein in one of the GFP control samples. While technical limitations prevented a quantitative enrichment of every putative 14-3-3 client, we never observed a case in which a putative client was also identified in the GFP samples. Thus, all the enrichment ratios are actually based on an increase over varrying levels of background noise from contaminating peptide(s).
The first approach was to label 14-3-3 sample peptides with 18O during a trypsin digestion. These labeled peptides were then mixed into a single sample with an equal amount of 16O labeled peptides from a GFP control purification. This mixed sample was then subjected to a 2D-LC MALDI-TOF-TOF MS analysis to provide a relative comparison of signal strength for different pairs of 16O/18O labeled peptides. This strategy identified 9 putative 14-3-3 clients with quantitative evidence for clear enrichment in the 14-3-3 sample (Supplemental Figure 4). These include, peroxidase 31, WRKY transcription factor 16, guard cell recycling outward K+ channel (GORK) channel, CURLY LEAF, cadmium/zinc-transporting ATPase 2 (HMA2), glutamate receptor 1.2, CLC-G channel, ALA 1, and invertase.
The second approach was to use a quantitative iTRAQ strategy. The 14-3-3 and GFP samples were labeled with either iTRAQ 117 or 114 mass reporters, respectively. The labeled samples were combined and subjected to a 2D-LC MALDI-TOF-TOF MS analysis (Table 1). As expected, peptides derived from known contaminants, such as added trypsin and GST-TEV protease, showed ratios of 1.1 and 1.4, respectively (Supplemental table 6), confirming that the two iTRAQ labeled samples were mixed equally. By comparing enrichment levels for shared and unique peptides associated with the GFP and 14-3-3 tags, we established normalization criteria for estimating minimum enrichment ratios (Supplement text 1). Using this approach, minimum enrichment ratios from 12 to 243 were found for 21 putative client proteins, including UVH3, PEP carboxylase, phosphoglucomutase, G-6-P dehydrogenase, Glutamyl-tRNA synthase, and phospholipase D alpha (Supplemental Figure 5).
Discussion
The TAP-tag purification strategy used here provides evidence for more than 131 different protein interactions with 14-3-3 omega, 10 of which were 14-3-3 isoforms (including itself) and 121 of which are putative 14-3-3 clients. Of these putative clients, 20 were previously reported in the literature. These include such proteins as nitrate reductase, neutral invertase, serpin, calcium-dependent protein kinase -1 (CDPK1/CPK1), and 1-aminocyclopropane-1-carboxylate (ACC) synthase. An additional 101 interactors have not been previously identified, raising the number of putative 14-3-3 clients identified in plants to more than 300 (see Supplemental Table 4). A current estimate for the number of 14-3-3 interactions in animal systems is also over 300 [8]. While plants and animals show some 14-3-3 clients in common (such as MAPKs and Glutamate Receptors, discussed below) the vast majority appear unique to either plants or animals. This suggests that 14-3-3 interactions have adapted to provide new regulatory interactions as eukaryotic organisms evolve.
Two lines of evidence suggest that many more interactors are yet to be identified. First, we only detected a 25% overlap with other studies, while still identifying 101 new interactions. Second, preliminary results based on single peptide identifications in this study provide an expectation for more than 100 additional 14-3-3 interactors (data not shown). Identifying all of the 14-3-3 interactions is a formidable challenge, especially since many of the interactions are phospho-dependent, transient, and include many low abundance proteins that are hard to detect.
Identifying 14-3-3 clients with in vivo interaction evidence
In plants, most of the putative 14-3-3 interactions have been identified by yeast two-hybrid strategies or the enrichment of 14-3-3 clients from plant extracts run through a 14-3-3 affinity chromatography column. In relatively few cases have interactions been verified with in vivo evidence. One advantage of the TAP-tag strategy used here is that each interactor identified comes with experimental support for an in vivo interaction [29].
While a TAP-tag strategy offers a productive strategy to identify potential clients, an important caveat is that the purification of 14-3-3 complexes may also include non-client proteins that are only indirectly associated with the complex through a secondary binding to a true client. While we have not conducted in vitro binding assays to verify a direct 14-3-3 interaction for any of the new potential clients identified here, most of the proteins do contain a mode 1 or mode 2 target binding site, consistent with typical 14-3-3 clients. Further confirmation may require in vitro binding assays in which the client has been phosphorylated by an appropriate protein kinase, and the binding shown to be competitive with a known 14-3-3 peptide substrate, such as a phospho Raf-1 peptide (e.g. [30]). Nevertheless, the copurification of a protein as part of a stable 14-3-3 complex still supports a working hypothesis that its interaction may have in vivo regulatory significance.
14-3-3 omega can heterodimerize with at least 9 other isoforms in vivo
While several studies have reported that 14-3-3 proteins can form heterodimers ([2], [11]), the extent to which this can occur in vivo between omega and the 11 other expressed Arabidopsis isoforms is not fully known. Evidence here indicates that isoform omega can hetero-dimerize in vivo with at least 9 of the other expressed isoforms. It is not clear if the failure to see an interaction with isoforms iota (GRF12, At1g34760) and nu (GRF7, At3g02520) is a function of unique properties of these isoforms, or a technical limitation of our experimental strategy.
The ability of our tagged 14-3-3 to interact with other isoforms demonstrates that a large C-terminal tag on 14-3-3 omega does not disrupt its hetero-dimerization potential. This is consistent with structure-function studies on 14-3-3s that indicate that the C-terminal end is primarily involved in client interactions (e.g. [31]) while the N-terminal end mediates dimerization (e.g. [32]). A C-terminal location for a TAP-tag was also used in an animal study that successfully identified 117 interactions [10]. While we cannot rule out that a C-terminal TAP-tag will modify some client binding interactions, the fact that heterodimers are formed with at least 9 other endogenous Arabidopsis 14-3-3 isoforms (which have an un-modified C-terminal domain) provides an expectation that most clients will still interact with the un-tagged half of the heterodimer, and therefore be detected by our experimental strategy.
Identification of 16 membrane proteins as 14-3-3 clients
Among the 121 putative clients identified here, 16 (13%) have evidence for membrane association. This increases by 4-fold the number of membrane proteins in plants with evidence for a 14-3-3 interaction. Based on our experimental design, membrane proteins were not expected to be highly represented since our protocol discarded membrane proteins that were pelleted during a high speed centrifugation. Nevertheless, partial proteolysis of membrane proteins is common in plant protein purifications. In our study, it appears that partial proteolysis produced a significant number of “shaved” protein fragments that retained their binding interaction with the TAP-tagged 14-3-3. An alternative explanation is that we simply purified a small number of membrane vesicles and identified abundant proteins associated with such vesicles. This appears unlikely for 3 reasons. First, our GFP control purifications showed no examples of an abundant integral membrane protein contaminant. Second, in the 14-3-3 samples, we saw no evidence for a general enrichment for some of the most abundant membrane associated or compartmentalized proteins, such as the chloroplast inner envelope proteins, BiP (an ER luminal protein), or RUBP-carboxylase. Finally, for each of the 16 integral membrane proteins identified, all of the peptides detected by mass spectrometry map to the cytosolic exposed face of the protein, according to topology models found at (http://aramemnon.botanik.uni-koeln.de/index.ep) [33]. This bias for cytosolic exposed peptides is most consistent with a protocol that selectively purified 14-3-3 bound protein fragments that were released by partial proteolysis, as opposed to a general non-specific recovery of abundant proteins associated with membrane vesicles.
Evidence that our protocol successfully purified known 14-3-3 membrane protein interactions was provided by the identification of a plasma membrane H+-ATPases (AHA6), one of the most well studied 14-3-3 target interactions in plants ([6], [34]). Three new groups of 14-3-3 membrane complexes (including K+, Ca2+, and Cl− channels) are highlighted in the next section.
Biological Implications
The 14-3-3 interactions detected here have implications to many aspects of plant biology, confirming the diversity of interactions identified by previous 14-3-3 target surveys ([35]–[39]). However, for each new putative client, the challenge will be to verify a direct 14-3-3 specific interaction and determine its functional consequence. The literature provides a precedent for 14-3-3 interactions resulting in: 1) enzyme activation and inactivation (e.g., H+-ATPase and nitrate reductase, respectively [34], 2) masking or unmasking of protein targeting information (e.g., Bzr1 transcription factor ([40], [41]), 3) bridging or blocking the formation of protein complexes [8]. From our list of 131 interactions, we offer a few examples below that highlight potential clients of special interest.
Channels for K+ and Cl−
The K+ ion channel GORK was identified here in three independent 14-3-3 copurifications and appeared with a greater than 109-fold enrichment over background in our 18O/16O 14-3-3/GFP comparison. GORK is a plasma membrane K+ channel, with one of its biological functions linked to the regulation of stomatal aperture and transpiration ([42], [43]). 14-3-3s have previously been proposed as a master regulator of ion homeostasis across the plasma membrane, with evidence for regulation of the plasma membrane proton pump as well as Kin and Kout channels [44]. More recently, 14-3-3 has been implicated in the regulation of two specific K+ channels, AtTPK1 (KCO1), a vacuolar membrane localized K+ channel [45] and KAT1, a PM inward rectifying K+ channel [46].
A putative chloride channel, CLCg, was also identified here in 2 independent co-purifications, with a greater than 47-fold enrichment over background in our 18O/16O 14-3-3/GFP comparison. Although the sub-cellular localization of CLCg is not yet known [47], the regulation of Cl− and K+ transport are expected to be linked in some situations requiring a net balance of positive and negative charges moving across the membrane. Our results raise the potential that 14-3-3 may be involved in a mechanism to coordinate the regulation of K+ and Cl− transport across different membranes.
Glutamate Receptors
Strong evidence was obtained here for 14-3-3 interactions with 5 different glutamate receptor isoforms (GLRs 1.2, 2.1, 2.9, 3.4 and 3.7) representing all 3 major subgroups. Isoform GLR1.2 was found in 2 independent purifications and displayed a greater than 519 fold enrichment over background in our 18O/16O 14-3-3/GFP comparison. In Arabidopsis, glutamate receptors are implicated in calcium signaling and are represented by 20 isoforms ([48]–[51]). All 5 GLRs identified here display a mode 1 or mode 2 14-3-3 binding site. Of the 20 Arabidopsis isoforms, all but 3 (i.e. 17) have potential mode 1 or 2 binding sites, suggesting that 14-3-3 interactions might be a common feature for most members of this family in plants. In animals, glutamate receptors have also been identified as 14-3-3 clients [11] providing a potential example of a common target of 14-3-3 regulation in both plants and animals.
Ethylene Biosynthesis
Evidence for 14-3-3 interactions was found for a subset of proteins all linked to the biosynthesis of ethylene, including three isoforms of ACC synthase (ACS-6, 7, 8), an ETO-like protein, and S-adenosylmethionine synthase. Although not confirmed by a second peptide identification, preliminary evidence indicates a potential 14-3-3 interaction with ETHYLENE INSENSITIVE3 (EIN3). EIN3 is a transcription factor that plays important role in ethylene signaling [52]. The identification here of ACC synthase provides in vivo corroboration for an ACC synthase interaction previously identified by an in vitro binding reaction with barley 14-3-3 proteins [53] and rice 14-3-3 proteins [54]. ACC synthase is a key enzyme in ethylene biosynthesis, and is known to be regulated through binding of a protein called ETO1 [55]. Regulation of ACC synthase activity by CDPK phosphorylation has also been proposed, providing a potential link between stress-induced calcium signals and the biosynthesis of ethylene [56]. Some CDPKs have also been found to bind to a 14-3-3 [30]. This group of interactions raises a number of questions, such as whether CDPK phosphorylation promotes binding of 14-3-3 to ACS and ETO, and whether ETO and 14-3-3 have an antagonistic relationship with respect to regulating ACS. Regardless, these results suggest that multiple 14-3-3 interactions could have significant impacts on ethylene signaling.
Transcription
Based on previous studies, 14-3-3 proteins have been implicated in the regulation of transcription through interactions with transcription factors [57]. A total of 15 transcription factors were identified here as putative clients. The largest subgroup of transcription factors belonged to the WRKY family (isoforms, 6, 16, 18, 19, 27, 32, and 40), implicated in regulating many different sets of genes, some of which are linked to biotic and abiotic stress pathways [58]. For example, AtWRKY6 expression changes during leaf senescence and pathogen defense [59]. In addition, AtWRKY18 and AtWRKY40 are involved in disease resistance [60]. Of the seven WRKYs identified here, all but one of them have at least 1 consensus 14-3-3 binding motif. WRKY-16 has seven consensus binding sites.
Chaperones
Another major subgroup of 14-3-3 interactions belongs to different chaperones, such as the heat shock protein family (HSP60, HSp70, HSP81, and HSP101), a J Domain Protein, and T-complex protein-1. Since some chaperones are known to bind to many proteins, it is not clear if these interactions are non-specific or involve a specific 14-3-3 binding site. However, two of the heat shock proteins (HSP60 and HSP70) do have consensus 14-3-3 binding sites. Furthermore, a barley HSP60 ortholog was previously identified as a client protein by affinity chromatography [35]. Since this barley study eluted 14-3-3-interactors by competition with a client peptide, this experimental design supports an interpretation that the barley HSP60 ortholog was identified as a true 14-3-3 client rather than a non-specific interaction. If in vitro binding assays eventually confirm some of the chaperones identified here as true 14-3-3 clients, this would dramatically expand the 14-3-3 interactome to include indirect associations with a large fraction of the proteome that have chaperone interactions.
Carbohydrate Metabolism
Multiple clients have been found in several linked steps in carbohydrate metabolism [35]. The identification here of phosphoenol pyruvate (PEP) carboxylase provides a novel and significant addition to the emerging connections in this metabolic pathway. PEP carboxylase was found in 2 independent purifications and displayed a greater than 242-fold enrichment over background in our 18O/16O 14-3-3/GFP comparison. PEP- carboxylase, which catalyzes the assimilation of CO2 into a four-carbon compound oxaloacetate, functions in many aspects of metabolism, including (in a C3 plant such as Arabidopsis) replenishment of tricarboxylic acid cycle intermediates, pH control, carbon and nitrogen partitioning, and malate synthesis [61]. While phospho-regulation of PEP-carboxylase is well established, an additional role for 14-3-3 now appears to warrant consideration. Since PEP-carboxylase does not have a consensus 14-3-3 binding site, alternative non-consensus sites may be involved, such as RLFSVD or RLATPE, as predicted by (http://elm.eu.org/). Alternatively, binding may involve an already established phospho-regulatory site near the N-terminal end (consensus = (E/D)(R/K)xxSIDAQ(L/M)R) (where x represents any amino acid and the Sp/Tp is phosphorylated), which does show similarity to a mode 1 consensus 14-3-3 binding site.
Translation
A role for 14-3-3 in protein synthesis was previously proposed based on interactions with translation initiation factors, as shown by co-immunoprecipitation and in vitro translation assays in which 14-3-3 was depleted [62]. Here we provide additional evidence with the identification of several ribosomal proteins (r-proteins) (i.e. 40S r-protein S4 and S18; 60S r-protein L8 and L26B) as well as regulatory proteins, eIF4A and eIF2. A previous proteomics analysis of a purified polysomal mRNA fraction also produced evidence for an association with 14-3-3 phi [63].
Phospholipase D signaling
Phospholipase D activity has been implicated in regulating multiple pathways, including those that control cell growth and patterning, programmed cell death, vesicle trafficking, cytoskeletal organization, and abiotic stress ([64]–[69]). Here we identified 2 phospholipase D proteins (beta and gamma) as potential 14-3-3 clients, consistent with expectations from phospholipase/14-3-3 interactions documented in animal systems [70]. Interestingly, a 14-3-3 has also been shown to bind phosphatidic acid (PA) [71], which is a signaling molecule [72] generated from phospholipase D. In plants, PA was also shown to interact with phosphoenol pyruvate (PEP) carboxylase [71], which was identified here as a potential 14-3-3 client. This unexpected interconnection of 14-3-3, PA, and PEP-carboxylase, raises the question of whether other targets of PA may also be connected to 14-3-3 in some feedback regulatory network. Regardless, phospholipase D has been implicated in stress responses in plants [73] and provides a logical target for considering mechanistic models in which 14-3-3 helps regulate such responses, such as a “stay green” drought resistant phenotype seen in cotton [74].
Phospho-signaling
Since most 14-3-3 interactions are thought to be mediated by target site phosphorylation, a comprehensive understanding of the 14-3-3 interactome will require delineating the kinases and phosphatases that regulate 14-3-3 interaction dynamics. While kinases provide an upstream trigger that can potentiate many 14-3-3 binding interactions, they also appear to be well represented as downstream clients. Of the 121 putative clients identified here, 6 were members of various protein kinase families (Table 3).
A 14-3-3/Serine-Threonine Plant Receptor Kinase Signaling Module
Our investigation provides in vivo 14-3-3 interaction evidence for both subunits of the brassinosteroid receptor, BRI1 and BAK1, both of which are members of a large family of LRR-receptor kinases. Previous studies have identified a 14-3-3 interaction with BAK1 by use of yeast two-hybrid screen, and obtained support for an in vivo interaction through immuno-precipitation ([75], [76]). However a direct yeast two-hybrid (Y2H) test for a similar 14-3-3 interaction with the BRI1 subunit failed to show an interaction [41]. We offer two explanations for the failure of a Y2H strategy to detect a BRI1/14-3-3 interaction in difference to our success using a TAP-tag copurification strategy. The first is that the interaction does not occur in yeast because the putative target binding site on BRI1 was not being phosphorylated. Second, our in planta interaction may be detecting an indirect interaction that is mediated by the association of a proteolytic fragment of BRI1 with a non-client binding site associated with a BAK1/14-3-3 complex. Regardless, there is strong evidence that the brassinosteroid response pathway is regulated downstream of BRI1/BAK1 through a 14-3-3 interaction with the transcription factor BZR1, which when phosphorylated binds a 14-3-3, causing it to be retained in the cytoplasm ([40], [41])
Our study also identified a related BRI1-like receptor kinase, 2 additional leucine repeat receptor kinases, and clavata, which is involved in shoot and floral meristem development. Another example of a 14-3-3 interaction with a 5th LRR-receptor kinase (SERK1) has been found through a Y2H study, with confirmation of an in vivo interaction provided by a FRET strategy using CFP and YFP tags [75].
The growing number of 14-3-3/LRR-receptor kinase interactions suggests that 14-3-3 interactions may have co-evolved with a subset of the plant receptor kinase as a regulatory module. Unlike typical animal receptor kinases, which are predominately tyrosine kinases, the plant receptor kinases are Ser/Thr kinases, and are therefore candidates for phospho-regulation through autoregulation of mode 1 and mode 2 14-3-3 binding-sites.
A MAPK signaling module
Our investigation provides in vivo evidence for a 14-3-3 interaction with AtMAPK7. In vitro binding studies have documented a 14-3-3 interaction with an autophosphorylated MAPK from maize (ZmMPK6) [77]. In yeast, there is evidence for 14-3-3 regulation of two MAPK pathways, one that functions in pseudo-hyphal development (RAS/Kss1-MAPK) [78] and one in osmotolerance (Hog1/MPK1). These interactions have been proposed as part of an explanation for why yeast with reduced 14-3-3 expression cannot grow under several stress conditions. MAPK/14-3-3 interactions are also established in mammalian systems, such as MEK kinase interactions with 14-3-3 zeta and epsilon in animals [79]. For AtMAPK7, a peptide containing only a consensus 14-3-3 binding site (RFIKSLP) has also been found to be an excellent peptide substrate for phosphorylation by a calcium dependent protein kinase (Harper, unpublished). This raises an interesting hypothesis that calcium signals may influence some MAPK signaling pathways through CDPK mediated MAPK/14-3-3 interactions.
Chloroplast and Mitochondrial Functions
Several examples have been reported for 14-3-3 protein interactions within the mitochondria and chloroplast (e.g. [80]–[82]). For example, evidence indicates that a 14-3-3 can regulate the activity of the ATP synthase in both chloroplasts and mitochondria [81]. Here we identified 2 plastid and 2 mitochondrial encoded proteins as potential clients of 14-3-3 omega (e.g. an acetyl-coenzyme A carboxyl transferase in the chloroplast). While it is not clear how 14-3-3 omega is translocated into these organelles, our results support previous research indicating important functional roles for 14-3-3 within the mitochondria and chloroplast.
Conclusion
The size of the 14-3-3 interactome has now exceeded 300 putative clients each in yeast, mammals, and plants. Each organism’s client list reveals similar regulatory themes. For example, all three groups show evidence for an ancient 14-3-3/MAPK signaling module. However many clients appear to be phylogenetically unique. For example, several clients identified here belong to kinase families not found in animals or yeast, such as the LRR-Ser/Thr receptor kinases and CDPKs. This phylogentic diversity of clients supports a perspective that 14-3-3 interactions are continuing to evolve into new functional partnerships.
Since the 12 different 14-3-3s expressed in Arabidopsis can potentially dimerize into 78 different complexes, and each 14-3-3 dimer can bind two different clients, the potential number of different 14-3-3/client complexes that can be formed with more than 300 different clients could theoretically exceed a million. Adding to this potential complexity is the fact that some of the putative clients have multiple binding interactions of their own. For example, a 14-3-3 interaction with one of the chaperones may indirectly link 14-3-3 with a very large percentage of the proteome. Thus, 14-3-3s may represent one of the busiest and most dynamic interaction nodes in the emerging global map of eukaryotic protein-protein interactions.
Supplementary Material
Supplemental Table 1. Mass exclusion list for MS/MS analysis of iTRAQ-labeled peptides.
Supplemental Table 2. Representative peptides and analysis for specific 14-3-3 isoforms identified in this study.
Supplemental Table 3. Exclusion list of non-specific interactions found in control GFP samples.
Supplemental Table 4. Previously documented 14-3-3 interactors in plants.
Supplemental Table 5. Representative peptides and analysis for potential 14-3-3 clients identified in this study.
Supplemental Table 6. Mass list of iTRAQ-labeled GFP, trypsin and 14-3-3 omega for normalization of enrichment ratios based on equal amounts of bait proteins.
Supplemental Table 7. Calculated FDR of each MS sample.
Supplemental Figure 1. Plasmid sequence of GST-TSPN-S2 (ps308).
Supplemental Figure 2. Plasmid sequence of GFP-TAP2 (ps346).
Supplemental Figure 3. Plasmid sequence of 35s::14-3-3 omega-YFP-TAP2 (ps472).
Supplemental Figure 4. MS and MS/MS spectrum for 16O/18O labeled peptides enriched in the 14-3-3 fractions.
Supplemental Figure 5. iTRAQ quantitative ratio distribution of 14-3-3 binding proteins.
Supplemental Text 1. Description of quantitative mass spec analyses.
Acknowledgments
This work was supported by grants to JFH from NSF (MCB-0114769, DBI- 0420033 and DBI-0436450), the DOE (DE-FG03-94ER20152) and NIH 1RO1 GM070813-01 for the development of the affinity tagging system, and to IFC from National Science Council, Taiwan (NSC96-3111-B-002-001 and NSC97-2311-B-002-005-MY3). We appreciated the help from Technology Commons, College of Life Science, National Taiwan University. Mass Spectrometry and Bioinformatics were made possible by the INBRE Program of the National Center for Research Resources (NIH grant P20 RR-016464).
References
- 1.Rosenquist M, Alsterfjord M, Larsson C, Sommarin M. Data mining the Arabidopsis genome reveals fifteen 14-3-3 genes. Expression is demonstrated for two out of five novel genes. Plant Physiol. 2001;127:142–149. doi: 10.1104/pp.127.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuller B, Stevens SM, Jr, Sehnke PC, Ferl RJ. Proteomic analysis of the 14-3-3 family in Arabidopsis. Proteomics. 2006;6:3050–3059. doi: 10.1002/pmic.200500729. [DOI] [PubMed] [Google Scholar]
- 3.Sehnke PC, Rosenquist M, Alsterfjord M, DeLille J, et al. Evolution and isoform specificity of plant 14-3-3 proteins. Plant Mol Biol. 2002;50:011–1018. doi: 10.1023/a:1021289127519. [DOI] [PubMed] [Google Scholar]
- 4.Muslin AJ, Tanner JW, Allen PM, Shaw AS. Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell. 1996;84:889–897. doi: 10.1016/s0092-8674(00)81067-3. [DOI] [PubMed] [Google Scholar]
- 5.Yaffe MB, Rittinger K, Volinia S, Caron PR, et al. The structural basis for 14-3-3: phosphopeptide binding specificity. Cell. 1997;91:961–971. doi: 10.1016/s0092-8674(00)80487-0. [DOI] [PubMed] [Google Scholar]
- 6.Maudoux O, Batoko H, Oecking C, Gevaert K, et al. A plant plasma membrane H+ –ATPase expressed in yeast is activated by phosphorylation at its penultimate residue and binding of 14-3-3 regulatory proteins in the absence of fusicoccin. J Biol Chem. 2000;275:17762–17770. doi: 10.1074/jbc.M909690199. [DOI] [PubMed] [Google Scholar]
- 7.Ottmann C, Yasmin L, Weyand M, Veesenmeyer JL, et al. Phosphorylation independent interaction between 14-3-3 and exoenzyme S: from structure to pathogenesis. Embo J. 2007;26:902–913. doi: 10.1038/sj.emboj.7601530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Heusden GP. 14-3-3 proteins: regulators of numerous eukaryotic proteins. IUBMB Life. 2005;57:623–629. doi: 10.1080/15216540500252666. [DOI] [PubMed] [Google Scholar]
- 9.Jin J, Smith FD, Stark C, Wells CD, et al. Proteomic, functional, and domainbased analysis of in vivo 14-3-3 binding proteins involved in cytoskeleton regulation and cellular organization. Curr Biol. 2004;14:1436–1450. doi: 10.1016/j.cub.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 10.Benzinger A, Muster N, Koch HB, Yates JR, 3rd, Hermeking H. Targeted proteomic analysis of 14-3-3 sigma, a p53 effector commonly silenced in cancer. Mol Cell Proteomics. 2005;4:785–795. doi: 10.1074/mcp.M500021-MCP200. [DOI] [PubMed] [Google Scholar]
- 11.Angrand PO, Segura I, Volkel P, Ghidelli S, et al. Transgenic mouse proteomics identifies new 14-3-3 associated proteins involved in cytoskeleton rearrangements and cell signaling. Mol Cell Proteomics. 2006;5:2211–2227. doi: 10.1074/mcp.M600147-MCP200. [DOI] [PubMed] [Google Scholar]
- 12.Wang D, Harper JF, Gribskov M. Systematic trans-genomic comparison of protein kinase between Arabidopsis and Saccharomyces cerevisiae. Plant Physiol. 2003;132:2152–2165. doi: 10.1104/pp.103.021485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morsy M, Gouthu S, Orchard S, Thorneycroft D, et al. Charting plant interactomes; possibilities and challenges. Trends Plant Sci. 2008;13:183–191. doi: 10.1016/j.tplants.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 15.Lucast LJ, Batey RT, Doudna JA. Large-Scale Purification of a Stable Form of Recombinant Tobacco Etch Virus Protease. Biotechniques. 2001;30:544–546. 548, 550. doi: 10.2144/01303st06. passim. [DOI] [PubMed] [Google Scholar]
- 16.Hellens RP, Allan AC, Friel EN, Bolitho K, et al. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods. 2005;1:13. doi: 10.1186/1746-4811-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984;12:8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradford MM. A rapid and sensitive method for the quantation of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 19.Curran AC, Hwang I, Corbin J, Martinez S, et al. Autoinhibition of a calmodulin-dependent calcium pump involves a structure in the stalk that connects the transmembrane domain to the ATPase catalytic domain. J Biol Chem. 2000;275:30301–30308. doi: 10.1074/jbc.M002047200. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto-Mizuma S, Wang GX, Liu LL, Schegg K, et al. Altered properties of volume-sensitive osmolyte and anion channels (VSOACs) and membrane protein expression in cardiac and smooth muscle myocytes from Clcn3−/− mice. J Physiol. 2004;557:439–456. doi: 10.1113/jphysiol.2003.059261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenfeld J, Capdevielle J, Guillemot JC, Ferrara P. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal Biochem. 1992;203:173–179. doi: 10.1016/0003-2697(92)90061-b. [DOI] [PubMed] [Google Scholar]
- 22.Williams AJ, Werner-Fraczek J, Chang IF, Bailey-Serres J. Regulated phosphorylation of 40S ribosomal protein S6 in root tips of maize. Plant Physiol. 2003;132:2086–2097. doi: 10.1104/pp.103.022749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence database using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Elias JE, Haas W, Faherty BK, Gygi SP. Comparative evaluation of mass spectrometry platforms used in large-scale proteomics investigations. Nat Methods. 2005;2:667–675. doi: 10.1038/nmeth785. [DOI] [PubMed] [Google Scholar]
- 25.Thompson MR, Thompson DK, Hettich RL. 2009. Systematic assessment of the benefits and caveats in mining microbial post-translational modifications from shotgun proteomic data: the response of Shewanella oneidensis to chromate exposure. J Proteome Res. 2008;7:648–658. doi: 10.1021/pr070531n. [DOI] [PubMed] [Google Scholar]
- 26.Obenauer JC, Cantley LC, Yaffe MB. Proteome-wide prediction of cell signaling interactions using short sequence motifs. Nucleic Acids Res. 2003;31:3635–3641. doi: 10.1093/nar/gkg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson CJ, Hegeman AD, Harms AC, Sussman MR. A quantitative analysis of Arabidopsis plasma membrane using trypsin-catalyzed (18)O labeling. Mol Cell Proteomics. 2006;5:1382–1395. doi: 10.1074/mcp.M500414-MCP200. [DOI] [PubMed] [Google Scholar]
- 28.Olsen JV, Ong SE, Mann M. Trypsin cleaves exclusively C-terminal to arginine and lysine residues. Mol Cell Proteomics. 2004;3:608–614. doi: 10.1074/mcp.T400003-MCP200. [DOI] [PubMed] [Google Scholar]
- 29.Chang IF. Mass spectrometry-based proteomic analysis of the epitope-tag affinity purified protein complexes in eukaryotes. Proteomics. 2006;6:6158–6166. doi: 10.1002/pmic.200600225. [DOI] [PubMed] [Google Scholar]
- 30.Moorhead G, Douglas P, Cotelle V, Harthill J, et al. Phosphorylation dependent interactions between enzymes of plant metabolism and 14-3-3 proteins. Plant J. 1999;18:1–12. doi: 10.1046/j.1365-313x.1999.00417.x. [DOI] [PubMed] [Google Scholar]
- 31.Athwal GS, Huber SC. Divalent cations and polyamines bind to loop 8 of 14-3-3 proteins, modulating their interaction with phosphorylated nitrate reductase. Plant J. 2002;29:119–129. doi: 10.1046/j.0960-7412.2001.01200.x. [DOI] [PubMed] [Google Scholar]
- 32.Abarca D, Madueno F, Martinez-Zapater JM, Salinas J. Dimerization of Arabidopsis 14-3-3 proteins: structural requirements within the N-terminal domain and effect of calcium. FEBS Lett. 1999;462:377–382. doi: 10.1016/s0014-5793(99)01560-4. [DOI] [PubMed] [Google Scholar]
- 33.Schwacke R, Schneider A, van der Graaff E, Fischer K, et al. ARAMEMNON, a novel database for Arabidopsis integral membrane proteins. Plant Physiol. 2003;131:16–26. doi: 10.1104/pp.011577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuglsang AT, Visconti S, Drumm K, Jahn T, et al. Binding of 14-3-3 protein to the plasma membrane H+-ATPase AHA2 involves the three C-terminal residues Tyr946–Thr-Val and requires phosphorylation of Thr947. J Biol Chem. 1999;274:36774–36780. doi: 10.1074/jbc.274.51.36774. [DOI] [PubMed] [Google Scholar]
- 35.Schoonheim PJ, Veiga H, da Pereira DC, Friso G, et al. A comprehensive analysis of the 14-3-3 interactome in barley leaves using a complementary proteomics and two-hybrid approach. Plant Physiol. 2007;143:670–683. doi: 10.1104/pp.106.090159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aducci P, Camoni L, Marra M, Visconti S. From cytosol to organelles: 14-3-3 proteins as multifunctional regulators of plant cell. IUBMB Life. 2002;53:49–55. doi: 10.1080/15216540210813. [DOI] [PubMed] [Google Scholar]
- 37.Roberts MR. 14-3-3 proteins find new partners in plant cell signaling. Trends Plant Sci. 2003;8:218–223. doi: 10.1016/S1360-1385(03)00056-6. [DOI] [PubMed] [Google Scholar]
- 38.Kanczewska J, Marco S, Vandermeeren C, Maudoux O, et al. Activation of the plant plasma membrane H+-ATPase by phosphorylation and binding of 14-3-3 proteins converts a dimer into a hexamer. Proc Natl Acad Sci USA. 2005;102:11675–11680. doi: 10.1073/pnas.0504498102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duby G, Boutry M. The plant plasma membrane proton pump ATPase: a highly regulated P-type ATPase with multiple physiological roles. Pflugers Arch. 2008 doi: 10.1007/s00424-008-0457-x. [DOI] [PubMed] [Google Scholar]
- 40.Ryu H, Kim K, Cho H, Park J, et al. Nucleocytoplasmic shuttling of BZR1 mediated by phosphorylation is essential in Arabidopsis brassinosteroid signaling. Plant Cell. 2007;19:2749–2762. doi: 10.1105/tpc.107.053728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gampala SS, Kim TW, He JX, Tang W, et al. An essential role for 14-3-3 proteins in brassionsteroid signal transduction in Arabidopsis. Dev Cell. 2007;13:177–189. doi: 10.1016/j.devcel.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ache P, Becker D, Ivashikina N, Dietrich P, et al. GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K+– selective, K+– sensing ion channel. FEBS Lett. 2000;486:93–98. doi: 10.1016/s0014-5793(00)02248-1. [DOI] [PubMed] [Google Scholar]
- 43.Hosy E, Vavasseur A, Mouline K, Dreyer I, et al. The Arabidopsis outward K+ channel GORK is involved in regulation of stomata movements and plant transpiration. Proc Natl Acad Sci USA. 2003;100:5549–5554. doi: 10.1073/pnas.0733970100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van den Wijngaard PW, Sinnige MP, Roobeek I, Reumer A, et al. Abscisic acid and 14-3-3 proteins control K+ channel activity in barley embryonic root. Plant J. 2005;41:43–55. doi: 10.1111/j.1365-313X.2004.02273.x. [DOI] [PubMed] [Google Scholar]
- 45.Latz A, Becker D, Hekman M, Muller T, et al. TPK1, a Ca(2+)-regulated Arabidopsis vacuole two-pore K(+) channel is activated by 14-3-3 proteins. Plant J. 2007;52:449–459. doi: 10.1111/j.1365-313X.2007.03255.x. [DOI] [PubMed] [Google Scholar]
- 46.Sottocornola B, Visconti S, Orsi S, Gazzarrini S, et al. The potassium channel KAT1 is activated by plant and animal 14-3-3 proteins. J Biol Chem. 2006;281:35735–35741. doi: 10.1074/jbc.M603361200. [DOI] [PubMed] [Google Scholar]
- 47.Marmagne A, Vinauger-Douard M, Monachello D, de Longevialle AF, et al. Two members of the Arabidopsis CLC (chloride channel) family, AtCLCe and AtCLCf, are associated with thylakoid and Golgi membranes, respectively. J Exp Bot. 2007;58:3385–3393. doi: 10.1093/jxb/erm187. [DOI] [PubMed] [Google Scholar]
- 48.Chiu JC, Brenner ED, DeSalle R, Nitabach MN, et al. Phylogenetic and expression analysis of the glutamate-receptor-like gene family in Arabidopsis thaliana. Mol Biol Evol. 2002;19:1066–1082. doi: 10.1093/oxfordjournals.molbev.a004165. [DOI] [PubMed] [Google Scholar]
- 49.Sanders D, Pelloux J, Brownlee C, Harper JF. Calcium at the crossroads of signaling. Plant Cell. 2002;14 (Suppl):S401–417. doi: 10.1105/tpc.002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qi Z, Stephens NR, Spalding EP. Calcium entry medicated by GLR3. 3, an Arabidopsis glutamate receptor with a broad agonist profile. Plant Physiol. 2006;142:963–971. doi: 10.1104/pp.106.088989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang S, Kim HB, Lee H, Choi JY, et al. Overexpression in Arabidopsis of a plasma membrane-targeting glutamate receptor from small radish increases glutamate-mediated Ca2+ influx and delays fungal infection. Mol Cells. 2006;21:418–427. [PubMed] [Google Scholar]
- 52.Guo H, Ecker JR. The ethylene signaling pathway: new insights. Curr Opin Plant Biol. 2004;7:40–49. doi: 10.1016/j.pbi.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 53.Alexander RD, Morris PC. A proteomic analysis of 14-3-3 binding proteins from developing barley grains. Proteomics. 2006;6:1886–1896. doi: 10.1002/pmic.200500548. [DOI] [PubMed] [Google Scholar]
- 54.Yao Y, Du Y, Jiang L, Liu JY. Interaction between ACC synthase 1 and 14-3-3 proteins in rice: a new insight. Biochemistry (Mosc) 2007;72:1003–1007. doi: 10.1134/s000629790709012x. [DOI] [PubMed] [Google Scholar]
- 55.Wang KL, Yoshida H, Lurin C, Ecker JR. Regulation of ethylene gas biosynthesis by the Arabidopsis ETO1 protein. Nature. 2004;428:945–950. doi: 10.1038/nature02516. [DOI] [PubMed] [Google Scholar]
- 56.Hernandez Sebastia C, Hardin SC, Clouse SD, Kieber JJ, Huber SC. Identification of a novel motif for CDPK phosphorylation in vitro that suggest ACC synthase may be a CDPK substrate. Arch Biochem Biophys. 2004;428:81–91. doi: 10.1016/j.abb.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 57.Igarashi D, Ishida S, Fukazawa J, Takahashi Y. 14-3-3 proteins regulate intracellular localization of the bZIP transcriptional activator RSG. Plant Cell. 2001;13:2483–2497. doi: 10.1105/tpc.010188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eulgem T, Rushton PJ, Robatzek S, Somssich IE. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000;5:199–206. doi: 10.1016/s1360-1385(00)01600-9. [DOI] [PubMed] [Google Scholar]
- 59.Robatzek S, Somssich IE. Targets of AtWRKY16 regulation regulation during plant senescence and pathogen defense. Genes Dev. 2002;16:1139–1149. doi: 10.1101/gad.222702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu X, Chen C, Fan B, Chen Z. Physical and functional interactions between pathogen-induced Arabidopsis WRKY18, WRKY40, and WRKY60 transcription factors. Plant Cell. 2006;18:1310–1326. doi: 10.1105/tpc.105.037523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chollet R, Vidal J, O’Leary MH. PHOSPHOENOLPYRUVATE CARBOXYLASE: A Ubiquitous, Highly Regulated Enzyme in Plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:273–298. doi: 10.1146/annurev.arplant.47.1.273. [DOI] [PubMed] [Google Scholar]
- 62.Wilker EW, van Vugt MA, Artim SA, Huang PH, et al. 14-3-3σ controls mitotic translation to facilitate cytokinesis. Nature. 2007;446:329–332. doi: 10.1038/nature05584. [DOI] [PubMed] [Google Scholar]
- 63.Chang IF. Ph D dissertation. 2004. Genomic and proteomic characterization of Arabidopsis cytosolic ribosomes. [Google Scholar]
- 64.Sang Y, Zheng S, Li W, Huang B, Wang X. Regulation of plant water loss by manipulating the expression of phospholipase D{alpha} Plant J. 2001;28:135–144. doi: 10.1046/j.1365-313x.2001.01138.x. [DOI] [PubMed] [Google Scholar]
- 65.Lee S, Park J, Lee Y. Phosphatidic acid induces actin polymerization by activating protein kinases in soybean cells. Mol Cells. 2003;15:313–319. [PubMed] [Google Scholar]
- 66.Ohashi Y, Oka A, Rodrigues-Pousada R, Possenti M, et al. Modulation of phospholipid signaling by GLABRA2 in root-hair pattern formation. Science. 2003;300:1427–1430. doi: 10.1126/science.1083695. [DOI] [PubMed] [Google Scholar]
- 67.Potocky M, Elias M, Profotova B, Novotna Z, et al. Phosphatidic acid produced by phospholipase D is required for tobacco pollen tube growth. Planta. 2003;217:122–130. doi: 10.1007/s00425-002-0965-4. [DOI] [PubMed] [Google Scholar]
- 68.Li W, Li M, Zhang W, Welti R, Wang X. The plasma membrane-bound phospholipase D{delta} enhances freezing tolerance in Arabidopsis thaliana. Nat Biotechnol. 2004;22:427–433. doi: 10.1038/nbt949. [DOI] [PubMed] [Google Scholar]
- 69.Wang X. Regulatory functions of phospholipase D and phosphatidic acid in plant growth, development, and stress responses. Plant Physiol. 2005;139:566–573. doi: 10.1104/pp.105.068809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Andoh T, Kato T, Jr, Matsui Y, Toh-e A. Phosphoinositide-specific phospholipase C forms a complex with 14-3-3 proteins and is involved in expression of UV resistance in fission yeast. Mol Gen Genet. 1998;258:139–147. doi: 10.1007/s004380050716. [DOI] [PubMed] [Google Scholar]
- 71.Testerink C, Dekker HL, Lim ZY, Johns MK, et al. Isolation and identification of phosphatidic acid targets from plants. Plant J. 2004;39:527–536. doi: 10.1111/j.1365-313X.2004.02152.x. [DOI] [PubMed] [Google Scholar]
- 72.Testerink C, Munnik T. Phosphatidic acid: a multifunctional stress signaling lipid in plants. Trends Plant Sci. 2005;10:368–375. doi: 10.1016/j.tplants.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 73.Bargmann BO, Munnik T. The role of phospholipase D in plant stress responses. Curr Opin Plant Biol. 2006;9:515–522. doi: 10.1016/j.pbi.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 74.Yan J, He C, Wang J, Mao Z, et al. Overexpression of the Arabidopsis 14-3-3 protein GF14 lambda in cotton leads to a “stay green” phenotype and improves stress tolerance under moderate drought conditions. Plant Cell Physiol. 2004;45:1007–1014. doi: 10.1093/pcp/pch115. [DOI] [PubMed] [Google Scholar]
- 75.Rienties IM, Vink J, Borst JW, Russinova E, de Vries SC. The Arabidopsis SERK1 protein interacts with the AAA-ATPase AtCDC48, the 14-3-3 protein GF14lambda and the PP2C phosphatase KAPP. Planta. 2005;221:394–405. doi: 10.1007/s00425-004-1447-7. [DOI] [PubMed] [Google Scholar]
- 76.Karlova R, Boeren S, Russinova E, Aker J, et al. The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE1 protein complex includes BRASSINOSTEROID-INSENSITIVE1. Plant Cell. 2006;18:626–638. doi: 10.1105/tpc.105.039412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lalle M, Visconti S, Marra M, Camoni L, et al. ZmMPK6, a novel maize MAP kinase that interacts with 14-3-3 proteins. Plant Mol Biol. 2005;59:713–722. doi: 10.1007/s11103-005-0862-x. [DOI] [PubMed] [Google Scholar]
- 78.Roberts RL, Mosch HU, Fink GR. 14-3-3 proteins are essential for RAS/MAPK cascade signaling during pseudohyphal development in S. cerevisiae. Cell. 1997;89:1055–1065. doi: 10.1016/s0092-8674(00)80293-7. [DOI] [PubMed] [Google Scholar]
- 79.Fanger GR, Widmann C, Porter AC, Sather S, et al. 14-3-3 proteins interact with specific MEK kinases. J Biol Chem. 1998;273:3476–3483. doi: 10.1074/jbc.273.6.3476. [DOI] [PubMed] [Google Scholar]
- 80.Riedel J, Tischner R, Mack G. The chloroplastic glutamine synthase (GS-2) of tobacco is phosphorylated and associated with 14-3-3 proteins inside the chloroplast. Planta. 2001;213:396–401. doi: 10.1007/s004250000509. [DOI] [PubMed] [Google Scholar]
- 81.Bunney TD, van Walraven HS, de Boer AH. 14-3-3 protein is a regulator of the mitochondria and chloroplast ATP synthase. Proc Natl Acad Sci USA. 2001;98:4249–4254. doi: 10.1073/pnas.061437498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ito J, Heazlewood JL, Millar AH. Analysis of the soluble ATP-binding proteome of plant mitochondria identified new proteins and nucleotide triphosphate interactions within the matrix. J Proteome Res. 2006;5:3459–3469. doi: 10.1021/pr060403j. [DOI] [PubMed] [Google Scholar]
- 83.Cotelle V, Meek SE, Provan F, Milne FC, et al. 14-3-3s regulate global cleavage of their diverse binding partners in sugar-starved Arabidopsis cells. EMBO J. 2000;19:2869–2876. doi: 10.1093/emboj/19.12.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Camoni L, Harper JF, Palmgren MG. 14-3-3 proteins activate a plant calcium-dependent protein kinase (CDPK) FEBS Lett. 1998;430:381–384. doi: 10.1016/s0014-5793(98)00696-6. [DOI] [PubMed] [Google Scholar]
- 85.Klychnikov OI, Li KW, Lill H, de Boer AH. The V-ATPase from etiolated barley (Hordeun vulgare L) shoots is activated by blue light and interacts with 14-3-3 proteins. J Exp Bot. 2007;58:1013–1023. doi: 10.1093/jxb/erl261. [DOI] [PubMed] [Google Scholar]
- 86.Mayfield JD, Folta KM, Paul AL, Ferl RJ. The 14-3-3 proteins mu and upsilon influence transition to flowering and early phytochrome response. Plant Physiol. 2007;145:1692–1702. doi: 10.1104/pp.107.108654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kinoshita T, Emi T, Tominaga M, Sakamoto K, et al. Blue-light- and phosphorylation-dependent binding of a 14-3-3 protein to phototropins in stomata guard cells of broad bean. Plant Physiol. 2003;133:1453–1463. doi: 10.1104/pp.103.029629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ishida S, Fukazawa J, Yuasa T, Takahashi Y. Involvement of 14-3-3 signaling protein binding in the functional regulation of the transcriptional activator REPRESSION OF SHOOT GROWTH by gibberellins. Plant Cell. 2004;16:2641–2651. doi: 10.1105/tpc.104.024604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lima L, Seabra A, Melo P, Cullimore J, Carvalho H. Phosphorylation and subsequent interaction with 14-3-3 proteins regulate plastid glutamine synthetase in Medicargo truncatula. Planta. 2006;223:558–567. doi: 10.1007/s00425-005-0097-8. [DOI] [PubMed] [Google Scholar]
- 90.Harthill JE, Meek SE, Morrice N, Peggie MW, et al. Phosphorylation and 14-3-3 binding of Arabidopsis trehalose-phosphate synthase 5 in response to 2-deoxyglucose. Plant J. 2006;47:211–223. doi: 10.1111/j.1365-313X.2006.02780.x. [DOI] [PubMed] [Google Scholar]
- 91.Kulma A, Villadsen D, Campbell DG, Meek SE, et al. Phosphorylation and 14-3-3 binding of Arabidopsis 6-phosphofructose-2-kinase/fructose-2, 6-bisphosphatase. Plant J. 2004;37:654–667. doi: 10.1111/j.1365-313x.2003.01992.x. [DOI] [PubMed] [Google Scholar]
- 92.Bustos DM, Iglesias AA. Phosphorylated non-phosphorylated glyceraldehydes-3-phosphate dehydrogenase from heterotrophic cells of wheat interacts with 14-3-3 proteins. Plant Physiol. 2003;133:2081–2088. doi: 10.1104/pp.103.030981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Klahre U, Kost B. Tobacco RhoGTPase ACTIVATING PROTEIN1 spatially restricts signaling of RAC/Rop to the apex of pollen tubes. Plant Cell. 2006;18:3033–3046. doi: 10.1105/tpc.106.045336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Mass exclusion list for MS/MS analysis of iTRAQ-labeled peptides.
Supplemental Table 2. Representative peptides and analysis for specific 14-3-3 isoforms identified in this study.
Supplemental Table 3. Exclusion list of non-specific interactions found in control GFP samples.
Supplemental Table 4. Previously documented 14-3-3 interactors in plants.
Supplemental Table 5. Representative peptides and analysis for potential 14-3-3 clients identified in this study.
Supplemental Table 6. Mass list of iTRAQ-labeled GFP, trypsin and 14-3-3 omega for normalization of enrichment ratios based on equal amounts of bait proteins.
Supplemental Table 7. Calculated FDR of each MS sample.
Supplemental Figure 1. Plasmid sequence of GST-TSPN-S2 (ps308).
Supplemental Figure 2. Plasmid sequence of GFP-TAP2 (ps346).
Supplemental Figure 3. Plasmid sequence of 35s::14-3-3 omega-YFP-TAP2 (ps472).
Supplemental Figure 4. MS and MS/MS spectrum for 16O/18O labeled peptides enriched in the 14-3-3 fractions.
Supplemental Figure 5. iTRAQ quantitative ratio distribution of 14-3-3 binding proteins.
Supplemental Text 1. Description of quantitative mass spec analyses.