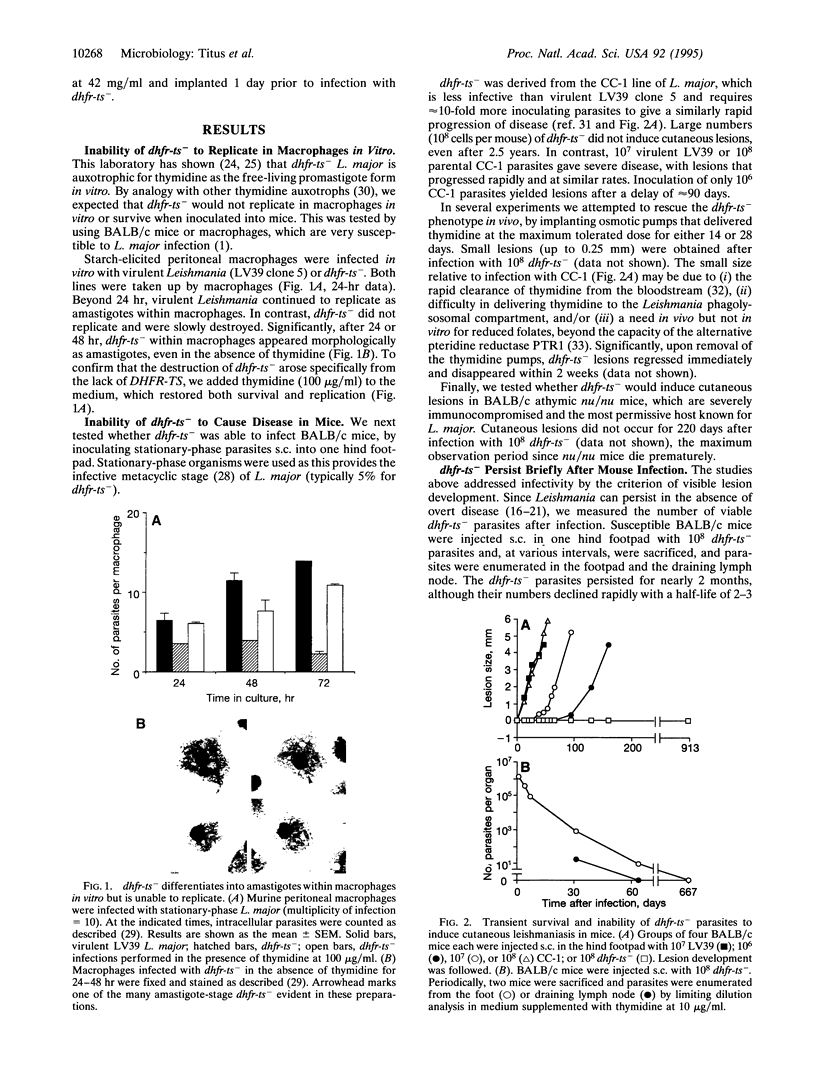
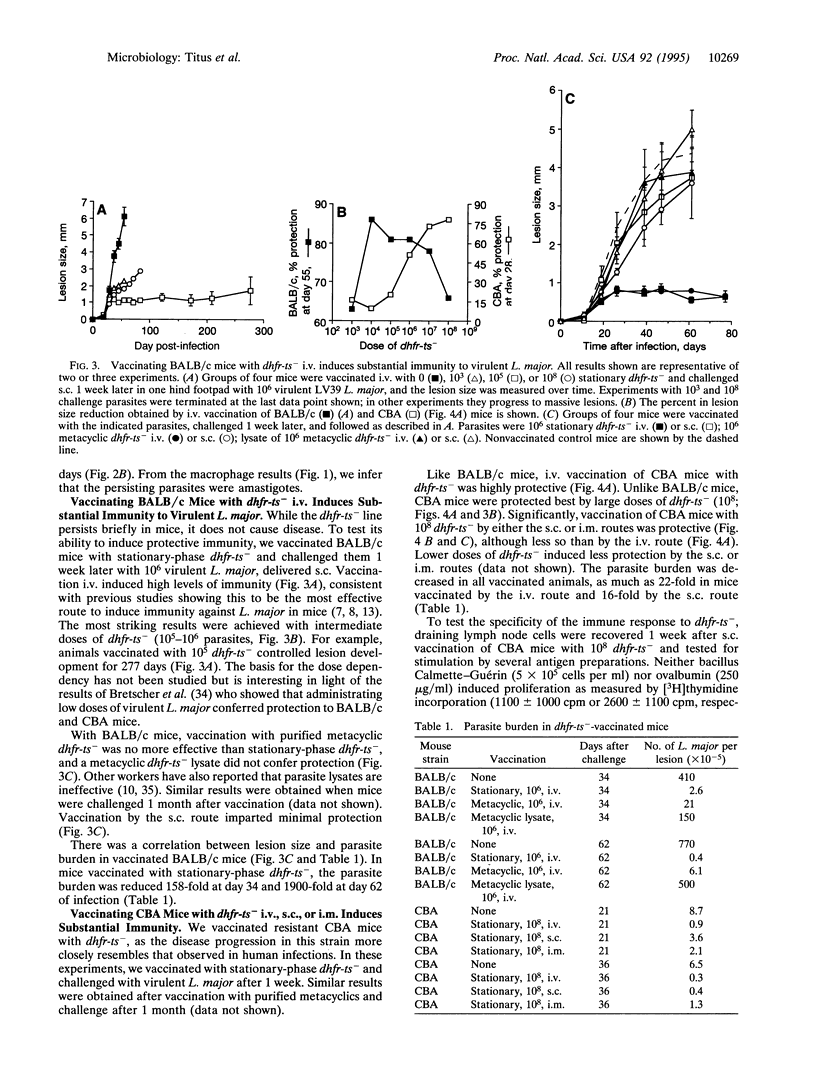
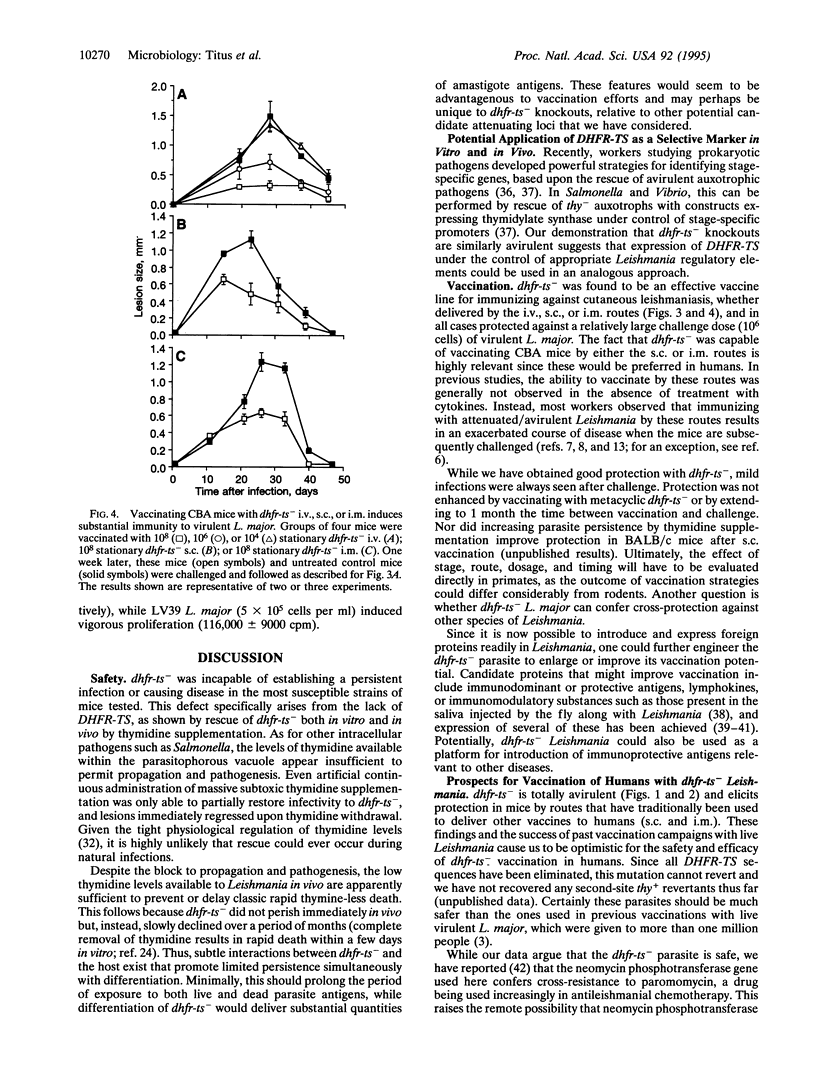
Abstract
Vaccination with live Leishmania major has been shown to yield effective immunization in humans; however, this has been discontinued because of problems associated with virulence of the available vaccine lines. To circumvent this, we tested the ability of a dhfr-ts- null mutant of L. major, obtained by gene targeting, to infect and then to vaccinate mice against challenge with virulent L. major. Survival and replication of dhfr-ts- in macrophages in vitro were dependent upon thymidine, with parasites differentiating into amastigotes prior to destruction. dhfr-ts- parasites persisted in BALB/c mice for up to 2 months, declining with a half-life of 2-3 days. Nonetheless, dhfr-ts- was incapable of causing disease in both susceptible and immunodeficient (nu/nu) BALB/c mice. Animal infectivity could be partially restored by thymidine supplementation. When inoculated by the i.v., s.c., or i.m. routes into mice, dhfr-ts- could elicit substantial resistance to a subsequent challenge with virulent L. major. Thus, Leishmania bearing auxotrophic gene knockouts can be safe and induce protective immunity. Potentially, dhfr-ts- could be used as a platform for delivery of immunogens relevant to other diseases.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebischer T., Moody S. F., Handman E. Persistence of virulent Leishmania major in murine cutaneous leishmaniasis: a possible hazard for the host. Infect Immun. 1993 Jan;61(1):220–226. doi: 10.1128/iai.61.1.220-226.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello A. R., Nare B., Freedman D., Hardy L., Beverley S. M. PTR1: a reductase mediating salvage of oxidized pteridines and methotrexate resistance in the protozoan parasite Leishmania major. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11442–11446. doi: 10.1073/pnas.91.24.11442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher P. A., Wei G., Menon J. N., Bielefeldt-Ohmann H. Establishment of stable, cell-mediated immunity that makes "susceptible" mice resistant to Leishmania major. Science. 1992 Jul 24;257(5069):539–542. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- Connell N. D., Medina-Acosta E., McMaster W. R., Bloom B. R., Russell D. G. Effective immunization against cutaneous leishmaniasis with recombinant bacille Calmette-Guérin expressing the Leishmania surface proteinase gp63. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11473–11477. doi: 10.1073/pnas.90.24.11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz A. K., Titus R., Beverley S. M. Plasticity in chromosome number and testing of essential genes in Leishmania by targeting. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1599–1603. doi: 10.1073/pnas.90.4.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz A., Beverley S. M. Gene replacement in parasitic protozoa. Nature. 1990 Nov 8;348(6297):171–173. doi: 10.1038/348171a0. [DOI] [PubMed] [Google Scholar]
- Cruz A., Coburn C. M., Beverley S. M. Double targeted gene replacement for creating null mutants. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7170–7174. doi: 10.1073/pnas.88.16.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczynski R. M. Immunization of susceptible BALB/c mice against Leishmania braziliensis. I. Resistance induced using as immunogen adherent or nonadherent cells from infected mice. Cell Immunol. 1985 Aug;94(1):1–10. doi: 10.1016/0008-8749(85)90080-2. [DOI] [PubMed] [Google Scholar]
- Greenblatt C. L. Cutaneous leishmaniasis: The prospects for a killed vaccine. Parasitol Today. 1988 Feb;4(2):53–54. doi: 10.1016/0169-4758(88)90067-1. [DOI] [PubMed] [Google Scholar]
- Gueiros-Filho F. J., Beverley S. M. On the introduction of genetically modified Leishmania outside the laboratory. Exp Parasitol. 1994 Jun;78(4):425–428. doi: 10.1006/expr.1994.1048. [DOI] [PubMed] [Google Scholar]
- Handman E., Hocking R. E., Mitchell G. F., Spithill T. W. Isolation and characterization of infective and non-infective clones of Leishmania tropica. Mol Biochem Parasitol. 1983 Feb;7(2):111–126. doi: 10.1016/0166-6851(83)90039-7. [DOI] [PubMed] [Google Scholar]
- Hill J. O., North R. J., Collins F. M. Advantages of measuring changes in the number of viable parasites in murine models of experimental cutaneous leishmaniasis. Infect Immun. 1983 Mar;39(3):1087–1094. doi: 10.1128/iai.39.3.1087-1094.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. G. Immunological regulation and control of experimental leishmaniasis. Int Rev Exp Pathol. 1986;28:79–116. [PubMed] [Google Scholar]
- Kapler G. M., Coburn C. M., Beverley S. M. Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol Cell Biol. 1990 Mar;10(3):1084–1094. doi: 10.1128/mcb.10.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimsey P. B., Theodos C. M., Mitchen T. K., Turco S. J., Titus R. G. An avirulent lipophosphoglycan-deficient Leishmania major clone induces CD4+ T cells which protect susceptible BALB/c mice against infection with virulent L. major. Infect Immun. 1993 Dec;61(12):5205–5213. doi: 10.1128/iai.61.12.5205-5213.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBowitz J. H., Coburn C. M., McMahon-Pratt D., Beverley S. M. Development of a stable Leishmania expression vector and application to the study of parasite surface antigen genes. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9736–9740. doi: 10.1073/pnas.87.24.9736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Chang K. P. Extrachromosomal genetic complementation of surface metalloproteinase (gp63)-deficient Leishmania increases their binding to macrophages. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4991–4995. doi: 10.1073/pnas.89.11.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan M. J., Slauch J. M., Hanna P. C., Camilli A., Tobias J. W., Waldor M. K., Mekalanos J. J. Selection for bacterial genes that are specifically induced in host tissues: the hunt for virulence factors. Infect Agents Dis. 1993 Aug;2(4):263–268. [PubMed] [Google Scholar]
- Mahan M. J., Slauch J. M., Mekalanos J. J. Selection of bacterial virulence genes that are specifically induced in host tissues. Science. 1993 Jan 29;259(5095):686–688. doi: 10.1126/science.8430319. [DOI] [PubMed] [Google Scholar]
- Marchand M., Daoud S., Titus R. G., Louis J., Boon T. Variants with reduced virulence derived from Leishmania major after mutagen treatment. Parasite Immunol. 1987 Jan;9(1):81–92. doi: 10.1111/j.1365-3024.1987.tb00490.x. [DOI] [PubMed] [Google Scholar]
- Melby P. C. Experimental leishmaniasis in humans: review. Rev Infect Dis. 1991 Sep-Oct;13(5):1009–1017. doi: 10.1093/clinids/13.5.1009. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Handman E. Heterologous protection in murine cutaneous leishmaniasis. Immunol Cell Biol. 1987 Oct;65(Pt 5):387–392. doi: 10.1038/icb.1987.44. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Handman E., Spithill T. W. Examination of variables in the vaccination of mice against cutaneous leishmaniasis using living avirulent cloned lines and killed promastigotes of Leishmania major. Int J Parasitol. 1985 Dec;15(6):677–684. doi: 10.1016/0020-7519(85)90015-3. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Handman E., Spithill T. W. Vaccination against cutaneous leishmaniasis in mice using nonpathogenic cloned promastigotes of Leishmania major and importance of route of injection. Aust J Exp Biol Med Sci. 1984 Apr;62(Pt 2):145–153. doi: 10.1038/icb.1984.14. [DOI] [PubMed] [Google Scholar]
- Modabber F. Experiences with vaccines against cutaneous leishmaniasis: of men and mice. Parasitology. 1989;98 (Suppl):S49–S60. doi: 10.1017/s0031182000072243. [DOI] [PubMed] [Google Scholar]
- Müller I. Role of T cell subsets during the recall of immunologic memory to Leishmania major. Eur J Immunol. 1992 Dec;22(12):3063–3069. doi: 10.1002/eji.1830221206. [DOI] [PubMed] [Google Scholar]
- Peters W., Bryceson A., Evans D. A., Neal R. A., Kaye P., Blackwell J., Killick-Kendrick R., Liew F. Y. Leishmania infecting man and wild animals in Saudi Arabia. 8. The influence of prior infection with Leishmania arabica on challenge with L. major in man. Trans R Soc Trop Med Hyg. 1990 Sep-Oct;84(5):681–689. doi: 10.1016/0035-9203(90)90145-5. [DOI] [PubMed] [Google Scholar]
- Rivier D., Shah R., Bovay P., Mauel J. Vaccine development against cutaneous leishmaniasis. Subcutaneous administration of radioattenuated parasites protects CBA mice against virulent Leishmania major challenge. Parasite Immunol. 1993 Feb;15(2):75–84. doi: 10.1111/j.1365-3024.1993.tb00587.x. [DOI] [PubMed] [Google Scholar]
- Sacks D. L., Perkins P. V. Identification of an infective stage of Leishmania promastigotes. Science. 1984 Mar 30;223(4643):1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- Scott P., Pearce E., Natovitz P., Sher A. Vaccination against cutaneous leishmaniasis in a murine model. I. Induction of protective immunity with a soluble extract of promastigotes. J Immunol. 1987 Jul 1;139(1):221–227. [PubMed] [Google Scholar]
- Shankar A., Mitchen T. K., Hall L. R., Turco S. J., Titus R. G. Reversion to virulence in Leishmania major correlates with expression of surface lipophosphoglycan. Mol Biochem Parasitol. 1993 Oct;61(2):207–216. doi: 10.1016/0166-6851(93)90067-8. [DOI] [PubMed] [Google Scholar]
- Tattersall M. H., Brown B., Frei E., 3rd The reversal of methotrexate toxicity by thymidine with maintenance of antitumour effects. Nature. 1975 Jan 17;253(5488):198–200. doi: 10.1038/253198a0. [DOI] [PubMed] [Google Scholar]
- Titus R. G., Kelso A., Louis J. A. Intracellular destruction of Leishmania tropica by macrophages activated with macrophage activating factor/interferon. Clin Exp Immunol. 1984 Jan;55(1):157–165. [PMC free article] [PubMed] [Google Scholar]
- Titus R. G., Marchand M., Boon T., Louis J. A. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985 Sep;7(5):545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- Titus R. G., Müller I., Kimsey P., Cerny A., Behin R., Zinkernagel R. M., Louis J. A. Exacerbation of experimental murine cutaneous leishmaniasis with CD4+ Leishmania major-specific T cell lines or clones which secrete interferon-gamma and mediate parasite-specific delayed-type hypersensitivity. Eur J Immunol. 1991 Mar;21(3):559–567. doi: 10.1002/eji.1830210305. [DOI] [PubMed] [Google Scholar]
- Titus R. G., Ribeiro J. M. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science. 1988 Mar 11;239(4845):1306–1308. doi: 10.1126/science.3344436. [DOI] [PubMed] [Google Scholar]
- Tobin J. F., Reiner S. L., Hatam F., Zheng S., Leptak C. L., Wirth D. F., Locksley R. M. Transfected Leishmania expressing biologically active IFN-gamma. J Immunol. 1993 Jun 1;150(11):5059–5069. [PubMed] [Google Scholar]
- Yang D. M., Fairweather N., Button L. L., McMaster W. R., Kahl L. P., Liew F. Y. Oral Salmonella typhimurium (AroA-) vaccine expressing a major leishmanial surface protein (gp63) preferentially induces T helper 1 cells and protective immunity against leishmaniasis. J Immunol. 1990 Oct 1;145(7):2281–2285. [PubMed] [Google Scholar]
- de Rossell R. A., de Duran R. J., Rossell O., Rodríguez A. M. Is leishmaniasis ever cured? Trans R Soc Trop Med Hyg. 1992 May-Jun;86(3):251–253. doi: 10.1016/0035-9203(92)90297-p. [DOI] [PubMed] [Google Scholar]