Abstract
Objective
Although several studies have shown poorer survival among individuals with 25-hydroxy (OH) vitamin D deficiency, data on patients receiving dialysis are limited. Using data from the Comprehensive Dialysis Study (CDS), we tested the hypothesis that patients new to dialysis with low serum concentrations of 25-OH vitamin D would experience higher mortality and hospitalizations.
Design
The CDS is a prospective cohort study. We recruited participants from 56 dialysis units located throughout the United States.
Subjects and Intervention
We obtained data on demographics, comorbidites, and laboratory values from the CDS Patient Questionnaire as well as the Medical Evidence Form (CMS form 2728). Participants provided baseline serum samples for 25-OH vitamin D measurements.
Main Outcome Measure
We ascertained time to death and first hospitalization as well as number of first-year hospitalizations via the U.S. Renal Data System standard analysis files. We used Cox proportional hazards to determine the association between 25-OH vitamin D tertiles and survival and hospitalization. For number of hospitalizations in the first year, we used negative binomial regression.
Results
The analytic cohort was composed of 256 patients with Patient Questionnaire data and 25-OH vitamin D concentrations. The mean age of participants was 62 (±14.0) years, and mean follow-up was 3.8 years. Patients with 25-OH vitamin D concentrations in the lowest tertile (<10.6 ng/mL) at the start of dialysis experienced higher mortality (adjusted hazard ratio 1.75, 95% confidence interval [CI] 1.03–2.97) as well as hospitalization (adjusted hazard ratio 1.76, 95% CI 1.24–2.49). Patients in the lower 2 tertiles (<15.5 ng/mL) experienced a higher rate of hospitalizations in the first year (incidence rate ratio 1.70 [95% CI 1.06–2.72] for middle tertile, 1.66 [95% CI 1.10–2.51] for lowest tertile).
Conclusion
We found a sizeable increase in mortality and hospitalization for patients on dialysis with severe 25-OH vitamin D deficiency.
25-Hydroxy vitamin d (25-OH vitamin D) deficiency is common among patients receiving maintenance dialysis.1–4 However, the clinical significance of this finding is unclear because many patients on dialysis receive supplementation with 1,25- dihydroxy (1,25-(OH)2) vitamin D derivatives for treatment of secondary hyperparathyroidism.
25-OH vitamin D has an autocrine role in various tissues, including the skin, intestine, lymph nodes, vascular smooth muscle, and endothelium.5–7 Experts have suggested that local “extrarenal” 1-α-hydroxylase requires sufficient circulating 25-OH vitamin D to facilitate cell differentiation and maintain antiproliferative activity. Thus, important activities of 25-OH vitamin D may be independent of 1,25-(OH)2 vitamin D status or therapy, with deficiency potentially relevant for patients on dialysis.4,8
Limited data are available on health outcomes among patients on dialysis with 25-OH vitamin D deficiency. One study2 from the United States has linked vitamin D deficiency to higher odds of 90-day mortality; others from Europe have linked vitamin D deficiency to sudden cardiac death9 and cardiovascular mortality.10 Increased arterial stiffness11 and vascular calcifications12 have also been reported in small studies.
We have previously reported that approximately 90% of patients enrolled in the Comprehensive Dialysis Study (CDS), a cohort of patients new to hemodialysis or peritoneal dialysis in the United States, had 25-OH vitamin D deficiency, which is defined as a serum concentration less than 20 ng/mL.1 In this study, we test the hypothesis that low serum concentrations of 25-OH vitamin D are independently associated with higher mortality and likelihood of hospitalization in this diverse cohort of hemodialysis and peritoneal dialysis patients.
Methods
Study Design and Participants
The primary aim of the CDS was to determine the relations among nutritional status, inflammatory response, physical activity, rehabilitation, and health-related quality of life in patients new to dialysis. The study was performed through the U.S. Renal Data System Center (USRDS) and the Special Studies Centers in Nutrition and Rehabilitation/Quality of Life at 296 dialysis units throughout the United States.
The study design and methods have been previously described in detail.13 In brief, we invited (in writing) adult patients starting dialysis to enroll in the CDS between September 2005 and June 2007 at dialysis facilities selected to participate. The sampling frame consisted of 4,410 facilities, with probability proportional to estimated size sampling used to select 335 facilities, of which 297 units participated. The study was approved by institutional review boards for the USRDS Coordinating Center (University of Minnesota), the USRDS Rehabilitation/ Quality of Life Special Studies Center (Emory University), and the USRDS Nutrition Special Studies Center (University of California–San Francisco).
Patients received information about the study at their dialysis facility or through the mail. Those willing to participate completed and returned a signed consent form or provided verbal consent by telephone. A written consent was required for laboratory draw. A patient was excluded if he or she had a speech, hearing, or cognitive impairment; if they had prior or pending transplantation; or if they had plans to move out of the area. Patients who did not have phones or did not speak English or Spanish were not eligible to participate. On average, patients enrolled in CDS had been on dialysis for 4.5 months (interquartile range 3.7–4.9 months) at the time of the Patient Questionnaire.
As part of the nutrition substudy of CDS, 266 patients recruited from 56 dialysis units provided serum samples in addition to participating in a standardized telephone Patient Questionnaire administered by DataBanque. On average, the samples were obtained at 6 months (interquartile range 5–7 months) from the start of dialysis. One patient had a 25-OH vitamin D concentration greater than the assay limit and was excluded from further analysis. Nine patients were missing vitamin D laboratory data; the remaining 256 patients comprise the analytic cohort.
Clinical Covariates
The CDS Patient Questionnaire interview included information on patient demographics, employment status, tobacco use, health-related quality of life, and physical activity. To complement the Patient Questionnaire, we obtained data from the Medical Evidence Form (CMS form 2728), including height, weight, comorbidities, and selected laboratory values (serum hemoglobin, creatinine, and albumin) noted before the initiation of dialysis. We used the USRDS Medicare claims file to obtain data on use of 1,25-(OH)2 vitamin D derivatives (paricalcitol or doxercalciferol) up to 6 months after the measurement of 25-OH vitamin D concentrations in CDS. Patients who had at least 1 claim for vitamin D derivatives within this time period were categorized as receiving treatment.
25-OH Vitamin D Measurement
Baseline serum samples were collected from participants in the nutrition substudy at the time of their routine laboratory studies. Specimens were received as serum and were shipped on ice via overnight delivery the day they were drawn after centrifugation at the dialysis unit. Samples were aliquoted and stored over liquid nitrogen until assay. We measured concentrations of 25-OH vitamin D via the Direct Enzyme Immunoassay (Immunodiagnostic Systems, Inc.). The assay has a mean intra-assay coefficient of variation of 5.5% and a mean interassay coefficient of variation of 6.6%. The range of the assay is 2.4 to 144 ng/mL. We made all measurements in duplicate and used the mean in analyses.
Outcome Measures
We obtained data on mortality and hospitalization from the USRDS Standard Analysis Files, with follow-up obtained to December 2009. We calculated time to death and time to first hospitalization as well as number of hospitalizations in the first year from the start date of dialysis; patients receiving a transplant were censored from the analysis at the time of transplant.
Statistical Analysis
Continuous data are presented using means (±standard deviation) and compared using analysis of variance (AN-OVA). Categorical data are presented as proportions and compared using the χ2 test. Using Cox proportional hazards regression, we determined the associations among 25-OH vitamin D tertiles and survival in 4 models: (1) unadjusted; (2) adjusted for age; (3) adjusted for age, sex, and race/ethnicity; and (4) adjusted for age, sex, race/ethnicity, Quetélet’s (body mass) index, diabetes mellitus, and treatment modality (hemodialysis vs. peritoneal dialysis) and accounting for correlation within centers. We adopted a similar approach to determine the association between 25-OH vitamin D concentration and time to first hospitalization. We report the P value for trend for the multivariable adjusted models. Because neither 1,25-(OH)2 vitamin D (calcitriol) nor activated vitamin D derivatives (e.g., paricalcitol, doxercalciferol) significantly changes absorption, storage, or utilization of 25-OH vitamin D, we did not include treatment with 1,25-(OH)2 vitamin D or activated vitamin D derivatives in the multivariable adjusted value. However, we tested whether treatment with intravenous 1,25-(OH)2 vitamin D or activated vitamin D derivatives modified the association between 25-OH vitamin D concentrations and survival. We also performed companion analyses to test the association between mortality and hospitalization per 10-ng/mL change in vitamin D concentrations. We tested the proportional hazard assumption using Schoenfield residuals. For the number of hospitalizations in the first year, we calculated event rates incorporating repeat hospitalizations using negative binomial regression. Because we relied on USRDS Medicare claims data to ascertain hospitalization, we performed a sensitivity analysis excluding patients that were not Medicare eligible by the end of follow-up. We considered an association as significant if the 2-tailed P value was less than .05. We conducted all analyses using SAS v9.2 and v9.3 (SAS Institute Inc., Cary, NC).
Results
The median serum 25-OH vitamin D concentration was 12.9 (interquartile range: 9.4–17.1) ng/mL. The mean age was 62 (±14) years, 116 patients (45%) were female, and 233 patients (91%) were on hemodialysis. The mean follow-up was 3.8 (±0.5) years. Compared with patients with 25-OH vitamin D concentrations in the highest tertile, patients with concentrations in the lower 2 tertiles were more likely to be younger and female, more likely to receive hemodialysis via a catheter, had higher body mass index, and had lower serum albumin concentrations (Table 1).
Table 1.
Baseline Characteristics by 25-OH Vitamin D Tertiles in the Analytic Cohort (n = 256)
| Lowest Tertile Mean ± SD or Percent (n) |
Middle Tertile Mean ± SD or Percent (n) |
Highest Tertile Mean ± SD or Percent (n) |
|
|---|---|---|---|
| Patient characteristics | |||
| Age (y) | 60.4 ± 13.6 | 60.4 ± 14.4 | 65.5 ± 13.4* |
| Female | 54.1 (46) | 54.7 (47) | 27.1 (23)* |
| Race, non-white | 34.1 (29) | 31.4 (27) | 23.5 (20) |
| College education or more | 51.9 (41) | 52.2 (36) | 44.3 (31) |
| Body mass index (kg/m2) | 31.3 ± 8.4 | 31.1 ± 8.6 | 27.7 ± 5.5* |
| Peritoneal dialysis | 14.1 (12) | 9.3 (8) | 3.5 (3) |
| Hemodialysis access via catheter† | 59.7 (40) | 56.5 (35) | 35.8 (24)* |
| Comorbidities | |||
| Diabetes | 63.5 (54) | 57.0 (49) | 56.0 (47) |
| Atherosclerotic heart disease | 41.2 (35) | 37.2 (32) | 33.3 (28) |
| Congestive heart failure | 41.2 (35) | 32.6 (28) | 26.2 (22) |
| Baseline serum laboratory values | |||
| Albumin (g/dL) | 3.4 ± 0.5 | 3.4 ± 0.4 | 3.6 ± 0.4* |
| Hemoglobin (g/L) | 10.1 ± 1.9 | 10.3 ± 1.5 | 10.4 ± 1.6 |
| Creatinine (mg/dL) | 6.3 ± 2.9 | 7.1 ± 4.4 | 7.4 ± 3.5 |
25-OH vitamin D, 25-hydroxy vitamin D; SD, standard deviation.
Tertiles represent serum concentrations of 25-OH vitamin D ≤10.6 ng/mL for the lowest and ≤15.5 ng/mL for the middle tertile. The maximum serum concentration in the highest tertile was 41.5 ng/mL.
Significant difference across the tertiles.
Percentages were calculated based on patients on hemodialysis only.
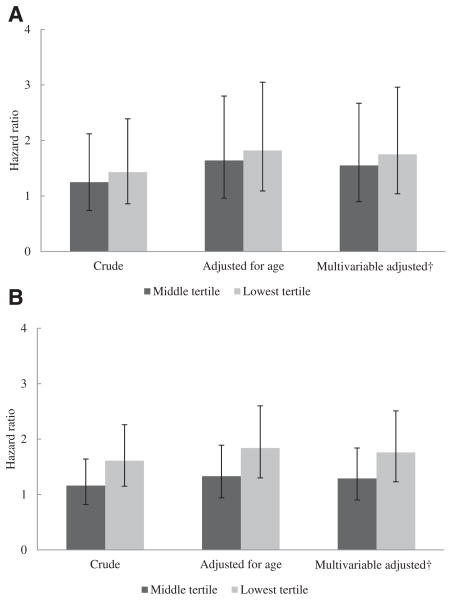
Lower vitamin D concentrations were associated with higher mortality and a higher likelihood of hospitalization (Fig. 1, A and B). In the multivariable adjusted model, other predictors of mortality included age (hazard ratio [HR] for each 10-year increase in age 1.49 [95% confidence interval {CI} 1.26–1.77]) and race/ethnicity (HR for non-white [vs. white] 0.47 [95% CI 0.25–0.86]) (Table 2). One hundred and seventy-seven (69%) patients received treatment with intravenous 1,25-(OH)2 vitamin D derivatives within 6 months after measurement of serum 25-OH vitamin D concentrations. Treatment with 1,25-(OH)2 vitamin D or activated vitamin D derivatives did not change the association between 25-OH vitamin D concentrations and mortality (P =.37) in the multivariable adjusted model.
Figure 1.
A. Hazard ratios for time to death in the lower tertiles of 25-hydroxy vitamin D (25-OH vitamin D) concentrations. B. Hazard ratios for time to first hospitalization in the lower tertiles of 25-OH vitamin D concentrations. The reported hazard ratios are in comparison with the highest tertile (≥25-OH vitamin D concentrations >15.5 ng/mL). The error bars represent 95% confidence intervals; P value for trend for the multivariable adjusted model in panel A = .0375, in panel B = .0017. †Adjusted for age, gender, race, body mass index, diabetes, treatment modality (hemodialysis vs. peritoneal dialysis), and facility.
Table 2.
Correlates of Mortality and Time to Hospitalization
| Hazard Ratio | Mortality (95% CI) | Time to Hospitalization (95% CI) |
|---|---|---|
| Lowest tertile of 25-OH vitamin D* | 1.75 (1.03–2.97) | 1.76 (1.24–2.49) |
| Middle tertile of 25-OH vitamin D* | 1.55 (0.86–2.80) | 1.29 (0.88–1.91) |
| Age (per 10 y) | 1.49 (1.26–1.77) | 1.22 (1.09–1.37) |
| Female gender | 1.19 (0.78–1.83) | 1.21 (0.89–1.64) |
| Non-white race/ethnicity | 0.47 (0.25–0.86) | 0.87 (0.64–1.19) |
| Body mass index (per kg/m2) | 0.99 (0.96–1.02) | 0.99 (0.97–1.02) |
| Diabetes | 1.21 (0.78–1.86) | 1.26 (0.93–1.72) |
| Peritoneal dialysis | 0.92 (0.48–1.78) | 0.95 (0.60–1.50) |
CI, confidence interval; 25-OH vitamin D, 25-hydroxy vitamin D.
The presented results have been adjusted for correlation within facility.
Reference category was the highest tertile (i.e., 25-OH vitamin D concentrations >15.5 ng/mL).
On companion analyses, the unadjusted HRs per 10-ng/ mL change in vitamin D concentrations were 0.74 (95% CI 0.51–1.08) for mortality and 0.73 (95% CI 0.57–0.93) for hospitalization. On multivariable adjusted analyses, the HRs were 0.63 (95% CI 0.42–0.97) for mortality and 0.69 (95% CI 0.52–0.91) for hospitalization.
A total of 111 (56.6%) patients were hospitalized in the first year; 93 (36.3%) had repeat hospitalizations. For patients in the lowest tertile of 25-OH vitamin D concentrations, vitamin D deficiency was associated with higher rates of hospitalization in the first year on unadjusted and adjusted analyses (Table 3). In adjusted analyses, patients in the middle tertile of 25-OH vitamin D concentrations also experienced a significant increase in rate of hospitalizations in the first year.
Table 3.
IRR for Number of Hospitalizations in the First Year in the Lower 2 Tertiles of 25-OH Vitamin D
| Number of Hospitalizations in the First Year | IRR for Lowest Tertile (95% CI) | IRR for Middle Tertile (95% CI) |
|---|---|---|
| Crude | 1.42 (1.00–2.01) | 1.49 (0.97–2.26) |
| Adjusted for age | 1.78 (1.21–2.61) | 1.80 (1.18–2.74) |
| Adjusted for age, gender, race | 1.72 (1.13–2.63) | 1.74 (1.11–2.74) |
| Multivariable adjusted* | 1.66 (1.10–2.51) | 1.70 (1.06–2.72) |
CI, confidence interval; IRR, incidence rate ratio; 25-OH vitamin D, 25-hydroxy vitamin D.
The rate of hospitalizations in the first year of dialysis therapy was higher for patients with 25-OH vitamin D concentrations in the lower 2 tertiles compared with patients with 25-OH vitamin D concentrations .>15.5 ng/mL. P value for trend = .0531.
Adjusted for age, gender, race, body mass index, diabetes, treatment modality (hemodialysis vs. peritoneal dialysis), and facility.
Finally, to account for any misclassified events of hospitalization among patients who were not Medicare eligible, we performed a sensitivity analysis excluding these patients (n = 46 [18%]). There was a similar increase in time to first hospitalization (HR 1.93, 95% CI 1.35–2.75) and rate of first hospitalization (incidence rate ratio 1.60, 95% CI 1.06 to 2.42) among the patients in the lower tertile of 25-OH vitamin D.
Discussion
We previously showed nearly ubiquitous vitamin D insufficiency or deficiency in a subsample of the CDS cohort.1 Herein, we show that among patients new to dialysis, those with 25-OH vitamin D concentrations below 10.6 ng/mL, the lowest tertile, were approximately 75% more likely to die and to be hospitalized after adjustment for key confounding factors. The magnitude of the increase in risk for mortality for a patient in the lowest 25-OH vitamin D tertile was equivalent to aging 15 years in our model. Patients in the lower 2 tertiles were also hospitalized at a 65% to 70% higher rate in the first year.
Our findings confirm previous studies demonstrating associations between 25-OH vitamin D deficiency and mortality in the general population,14–16 in patients with nondialysis-requiring chronic kidney disease,17,18 and in patients receiving dialysis.2,9,10,18–20 The prevalence of 25-OH vitamin D deficiency was markedly higher in our cohort compared with studies of the general population, whereas the magnitude of the association with mortality was similar to a previous study of incident patients on hemodialysis2 and higher than for the general population.14,16 A recent meta-analysis pooling patients with nondialysis-requiring chronic kidney disease and on dialysis therapy also confirmed a higher risk for mortality in the group with 25-OH vitamin D deficiency.18
In the study conducted by Wolf and colleauges,2 25-OH vitamin D deficiency had a stronger association with mortality than 1,25-(OH)2 vitamin D deficiency. The association with 25-OH vitamin D deficiency was mitigated completely among patients who subsequently received 1,25-(OH)2 vitamin D derivatives, suggesting that 1,25-(OH)2 vitamin D or its analogues may act as key intermediaries. However, randomized clinical trials evaluating the effects of 1,25-(OH)2 vitamin D sterol therapy have been small, and meta-analyses have suggested no benefit on survival or hospitalization.21 Cardiovascular status may be another potential mediator of the relationship between 25-OH vitamin D and mortality. For example, when Wang and colleagues adjusted for echocardiographic measures of left ventricular hypertrophy in their prospective analysis, the association between 25-OH vitamin D concentrations and mortality in patients on peritoneal dialysis was attenuated toward null.19
Evidence for a direct link between 25-OH vitamin D concentrations and survival is primarily preclinical. Zehnder and colleagues6,7 first hypothesized that 25-OH vitamin D may have an autocrine function, with conversion into its active form occurring locally and leading to downstream antiproliferative, cell-modulatory, or immune-protective effects. In support, these authors6 and others22 have demonstrated the existence of “extrarenal” 1 α-hydroxylase in skin, lymph nodes, vascular endothelial cells, colon, brain, and placenta. One study found that local upregulation of this enzyme generated antimicrobial (anti-tuberculosis) peptides by macrophages in response to in-flammation.23 It is plausible that if local production of 1,25-(OH)2 vitamin D at extrarenal sites facilitates cell differentiation or immune regulation effects of vitamin D, and if this in turn requires sufficient circulating 25-OH vitamin D, then 25-OH vitamin D deficiency may be directly linked to survival, independent of 1,25-(OH)2 vitamin D status and/or therapy.
Because adequate nutrition and/or sun exposure is required to maintain sufficient 25-OH vitamin D concentrations, it is possible (arguably probable) that deficiency of 25-OH vitamin D is a marker of a patient in ill health. Although we adjusted for several potential confounders, significant residual confounding may still exist. Ideally, we would have obtained repeat measurements of our key exposure, 25-OH vitamin D, to create a seasonally adjusted average for each patient. Our small sample size limited adjustment for state of residence, season, nutritional markers, or a larger number of comorbidities. Season and latitude are critical to cutaneous synthesis of 25-OH vitamin D, particularly relevant to patients on dialysis—1 small study suggested that relative to persons without kidney disease, patients on dialysis may require higher “doses” of ultraviolet light to simulate 25-OH vitamin D production.24 We did not collect data on markers of mineral metabolism.
The most recent Kidney Disease Improving Global Outcomes guidelines for management of chronic kidney disease mineral bone disorder suggest periodic measurement of 25-OH vitamin D concentrations in patients with chronic kidney disease Stages 3 to 5D.25 The inclusion of patients on dialysis was an update from the 2003 guidelines, based in part on benefit from 25-OH vitamin D supplementation reported in the general population. A meta-analysis of randomized control trials of 25-OH vitamin D supplementation in patients with a range of comorbidities demonstrated a modest risk reduction (0.92) for mortality with mean daily supplementation of 528 IU.26
Small studies on supplementation with cholecalciferol in patients on dialysis have demonstrated some improvement in mineral bone disease markers with supplementation. Armas and colleagues27 reported higher 1,25-(OH)2 vitamin D concentrations among patients randomized to receive weekly doses of 10,000 IU of cholecalciferol; a larger randomized study28 also reported a trend toward lower parathyroid hormone concentrations. In addition, a small study showed a decrease in inflammatory cytokines such as interleukin-6 and tumor necrosis factor after 7 hemodialysis patients received cholecalciferol therapy.29 A placebo-controlled randomized trial of weekly and/or monthly ergocalciferol supplementation, which will examine clinical outcomes including mortality and hospitalization, is currently underway (DIVINE, NCT 00892099).
In addition to residual confounding, our study has several limitations. We did not have data on calcium, phosphorus, intact parathyroid hormone, or 1,25-(OH)2 vitamin D, all of which are potential modifiers of the relationship between vitamin D deficiency and survival. For example, in a cohort of 762 patients receiving dialysis in the Nether-lands, Drechsler and colleagues10 only reported increased cardiovascular mortality and all-cause mortality among patients with 25-OH vitamin D deficiency and serum parathyroid hormone concentrations above the median (452 pg/mL). We also could not ascertain information about whether patients with 25-OH vitamin D deficiency subsequently started cholecalciferol supplementation.
Practical Application
Our study adds to the current observational literature demonstrating that patients with severe 25-OH vitamin D deficiency have poorer survival. It is not yet clear whether measurement of 25-OH vitamin D concentrations and supplementation in deficient patients will improve survival or reduce hospitalization or other adverse health outcomes.
Acknowledgments
Support: This work was funded by contract N01-DK-7-0005 from the National Institute of Diabetes and Digestive and Kidney Diseases. S.A. was supported by F32 DK 084697, G.M.C. was supported by K24 DK 080645, G.A.K. was supported in part by a grant from Dialysis Clinic Inc. G.M.C., K.L.J., B.G., and G.A.K. were also supported by N01-DK012450. M.K.T. was supported in part by the American Society of Nephrology and Association of Subspecialty Professors T. Franklin Williams Award. The data reported here have been supplied by the U.S. Renal Data Service. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the U.S. government.
References
- 1.Anand S, Kaysen GA, Chertow GM, et al. Vitamin D deficiency, self-reported physical activity and health-related quality of life: the Comprehensive Dialysis Study. Nephrol Dial Transplant. 2011;26:3683–3688. doi: 10.1093/ndt/gfr098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf M, Shah A, Gutierrez O, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72:1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 3.Bhan I, Burnett-Bowie SA, Ye J, Tonelli M, Thadhani R. Clinical measures identify vitamin D deficiency in dialysis. Clin J Am Soc Nephrol. 2010;5:460–467. doi: 10.2215/CJN.06440909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer RF. Vitamin D in dialysis: defining deficiency and rationale for supplementation. Semin Dial. 2012;26:40–46. doi: 10.1111/sdi.12010. [DOI] [PubMed] [Google Scholar]
- 5.Jones G. Expanding role for vitamin D in chronic kidney disease: importance of blood 25-OH-D levels and extra-renal 1alpha-hydroxylase in the classical and nonclassical actions of 1alpha,25-dihydroxyvitamin D(3) Semin Dial. 2007;20:316–324. doi: 10.1111/j.1525-139X.2007.00302.x. [DOI] [PubMed] [Google Scholar]
- 6.Zehnder D, Bland R, Williams MC, et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86:888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 7.Hewison M, Zehnder D, Chakraverty R, Adams JS. Vitamin D and barrier function: a novel role for extra-renal 1 alpha-hydroxylase. Mol Cell Endocrinol. 2004;215:31–38. doi: 10.1016/j.mce.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Melamed ML, Thadhani RI. Vitamin D therapy in chronic kidney disease and end stage renal disease. Clin J Am Soc Nephrol. 2012;7:358–365. doi: 10.2215/CJN.04040411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drechsler C, Pilz S, Obermayer-Pietsch G, et al. Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. Eur Heart J. 2010;31:2253–2261. doi: 10.1093/eurheartj/ehq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drechsler C, Verduijn M, Pilz S, et al. Vitamin D status and clinical outcomes in incident dialysis patients: results from the NECOSAD study. Nephrol Dial Transplant. 2011;26:1024–1032. doi: 10.1093/ndt/gfq606. [DOI] [PubMed] [Google Scholar]
- 11.London GM, Guerin AP, Verbeke FH, et al. Mineral metabolism and arterial functions in end-stage renal disease: potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol. 2007;18:613–620. doi: 10.1681/ASN.2006060573. [DOI] [PubMed] [Google Scholar]
- 12.Matias PJ, Ferreira C, Jorge C, et al. 25-Hydroxyvitamin D3, arterial calcifications and cardiovascular risk markers in haemodialysis patients. Nephrol Dial Transplant. 2009;24:611–618. doi: 10.1093/ndt/gfn502. [DOI] [PubMed] [Google Scholar]
- 13.Kutner NG, Johansen KL, Kaysen GA, et al. The comprehensive dialysis study (CDS): a USRDS special study. Clin J Am Soc Nephrol. 2009;4:645–650. doi: 10.2215/CJN.05721108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melamed ML, Michos ED, Post W, Astor B. 25-Hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ginde AA, Scragg R, Schwartz RS, Camargo CA., Jr Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older U.S. adults. J Am Geriatr Soc. 2009;57:1595–1603. doi: 10.1111/j.1532-5415.2009.02359.x. [DOI] [PubMed] [Google Scholar]
- 16.Levin GP, Robinson-Cohen C, de Boer IH, et al. Genetic variants and associations of 25-hydroxyvitamin D concentrations with major clinical outcomes. JAMA. 2012;308:1898–1905. doi: 10.1001/jama.2012.17304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehrotra R, Kermah DA, Salusky IB, et al. Chronic kidney disease, hypovitaminosis D, and mortality in the United States. Kidney Int. 2009;76:977–983. doi: 10.1038/ki.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilz S, Iodice S, Zitterman A, Grant WB, Gandini S. Vitamin D status and mortality risk in CKD: a meta-analysis of prospective studies. Am J Kidney Dis. 2011;58:374–382. doi: 10.1053/j.ajkd.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Wang AY, Lam CW, Sanderson JE, et al. Serum 25-hydroxyvitamin D status and cardiovascular outcomes in chronic peritoneal dialysis patients: a 3-y prospective cohort study. Am J Clin Nutr. 2008;87:1631–1638. doi: 10.1093/ajcn/87.6.1631. [DOI] [PubMed] [Google Scholar]
- 20.Gracia-Iguacel C, Gallar P, Qureshi AR, et al. Vitamin D deficiency in dialysis patients: effect of dialysis modality and implications on outcome. J Ren Nutr. 2010;20:359–367. doi: 10.1053/j.jrn.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Palmer SC, McGregor DO, Macaskill P, Craig JC, Elder GJ, Strippoli GF. Meta-analysis: vitamin D compounds in chronic kidney disease. Ann Intern Med. 2007;147:840–853. doi: 10.7326/0003-4819-147-12-200712180-00004. [DOI] [PubMed] [Google Scholar]
- 22.Chonchol M, Kendrick J, Targher G. Extra-skeletal effects of vitamin D deficiency in chronic kidney disease. Ann Med. 2011;43:273–282. doi: 10.3109/07853890.2010.543923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 24.Cuppari L, Garcia Lopes MG, Kamimura MA. Vitamin D biology: from the discovery to its significance in chronic kidney disease. J Ren Nutr. 2011;21:113–116. doi: 10.1053/j.jrn.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Uhlig K, Eckardt KU. A decade after the KDOQI CKD guidelines: impact on CKD guidelines. Am J Kidney Dis. 2012;60:705–706. doi: 10.1053/j.ajkd.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 26.Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730–1737. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- 27.Armas LA, Andukuri R, Barger-Lux J, Heaney RP, Lund R. 25-Hydroxyvitamin D response to cholecalciferol supplementation in hemo-dialysis. Clin J Am Soc Nephrol. 2012;7:1428–1434. doi: 10.2215/CJN.12761211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jean G, Terrat JC, Vanel T, et al. Evidence for persistent vitamin D 1-alpha-hydroxylation in hemodialysis patients: evolution of serum 1,25-dihydroxycholecalciferol after 6 months of 25-hydroxycholecalciferol treatment. Nephron Clin Pract. 2008;110:c58–c65. doi: 10.1159/000151534. [DOI] [PubMed] [Google Scholar]
- 29.Stubbs JR, Idiculla A, Slusser J, Menard R, Quarles LD. Cholecalciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. J Am Soc Nephrol. 2010;21:353–361. doi: 10.1681/ASN.2009040451. [DOI] [PMC free article] [PubMed] [Google Scholar]