Abstract
Whether consciousness is an all-or-none or graded phenomenon is an area of inquiry that has received considerable interest in neuroscience and is as of yet, still debated. In this magnetoencephalography (MEG) study we used a single stimulus paradigm with sub-threshold, threshold and supra-threshold duration inputs to assess whether stimulus perception is continuous with or abruptly differentiated from unconscious stimulus processing in the brain. By grouping epochs according to stimulus identification accuracy and exposure duration, we were able to investigate whether a high-amplitude perception-related cortical event was (1) only evoked for conditions where perception was most probable (2) had invariant amplitude once evoked and (3) was largely absent for conditions where perception was least probable (criteria satisfying an all-on-none hypothesis). We found that averaged evoked responses showed a gradual increase in amplitude with increasing perceptual strength. However, single trial analyses demonstrated that stimulus perception was correlated with an all-or-none response, the temporal precision of which increased systematically as perception transitioned from ambiguous to robust states. Due to poor signal-to-noise resolution of single trial data, whether perception-related responses, whenever present, were invariant in amplitude could not be unambiguously demonstrated. However, our findings strongly suggest that visual perception of simple stimuli is associated with an all-or-none cortical evoked response the temporal precision of which varies as a function of perceptual strength.
Keywords: Magnetoencephalography, all-or-none, temporal precision
Are the neuronal events that support consciousness simply an extension of those that do not or rather, does consciousness result from an all-or-none response that is easily dissociated from unconscious processing in the brain? This central issue in neuroscience has been heavily debated and is, as of yet, still poorly understood.
In the case of visual perception, several studies indicate that conscious access results from a gradual increase in cortical activity also associated with unconscious stimulus processing— a view point known as the ‘activation strength hypothesis’ (Kanwisher, 2001). For example, recent fMRI work showed a linear increase in activity localized to the fusiform gyrus with increasing recognition performance for familiar objects. These findings demonstrated a continuous rather than abrupt change in cortical activity as subjects transitioned from a state of ambiguous to more robust stimulus recognition (Bar, et al., 2001). Similar results, using a binocular fusion paradigm, showed identical brain regions to be activated in the same, stimulus-specific manner for objects that were and were not perceived. Again, the presence and absence of perception differed merely by way of activation levels, with trials where perception occurred, correlating with larger blood-oxygen dependant changes (Moutoussis & Zeki, 2002). In addition, visual masking studies have also shown that varying degrees of conscious awareness exist with — in between perceptual states only being adequately captured with the use of continuous awareness response criteria (Overgaard, Rote, Mouridsen, & Ramsoy, 2006; Sergent & Dehaene, 2004).
By contrast, other studies assert that visual perception results from a high-amplitude, all-or-none event in the brain that is largely absent when perception is not supported. For example, using the attentional blink paradigm, Sergent and Dehaene demonstrated that target perception was reported in a bimodal manner (seen and not seen) even when subjects were given a continuous scale with which to report awareness (Sergent & Dehaene, 2004). More recent work using backward-masking in combination with electroencephalography demonstrated that reported awareness and ERP amplitudes increased in a non-linear fashion with increasing target-mask onset asynchrony (Del Cul, Baillet, & Dehaene, 2007). Specifically, a sudden onset of high-amplitude activity recorded in frontal, parietal and temporal cortices was only seen for trials where stimuli were consciously accessed. Additionally, at the single neuron level, recordings from human medial temporal lobe show prolonged firing to consciously perceived visual stimuli with firing rates increasing as a non-linear function of stimulus duration— again indicating an all-or-none response reflecting conscious recognition (Quiroga, Mukamel, Isham, Malach, & Fried, 2008).
To better characterize the nature of visual awareness, we used a single stimulus paradigm combined with magnetoencephalography (MEG) to assess whether stimulus perception was correlated with all-or-none vs. graded changes in evoked brain responses. Specifically, we delivered sub-threshold, threshold and supra-threshold duration stimuli and assessed whether cortical responses were evoked in full when stimuli were perceived and absent when they were not, regardless of stimulus exposure times. We found that while averaged evoked responses gradually increased in amplitude with increasing perceptual strength, single trial cortical responses were evoked in an all-or-none manner with decreasing temporal jitter observed for conditions with increasing perceptual strength.
1. EXPERIMENTAL PROCEDURES
In this study, we tested the hypothesis that evoked cortical responses to identified visual stimuli are all-on-none in nature—i.e. that these responses are either evoked in full when stimuli are recognized or are absent when they are not, regardless of the actual exposure times of the presented inputs. To this effect, we assessed whether elicited cortical responses to (1) threshold duration stimuli that were recognized were similar to supra-threshold duration stimuli that were recognized and (2) threshold duration stimuli that were not recognized were similar to sub-threshold duration stimuli that were not recognized.
In order to ensure that stimulus-evoked neuromagnetic signals were unambiguously related to the target stimuli used, masking was not used to disrupt perception. Instead, we used a single-stimulus paradigm with extremely short exposure durations (milliseconds), made possible by using LED displays for all stimulus presentations (see section 1.2. below). Averaged event related fields (ERFs) as well as single trial data were analyzed to investigate the nature of the cortical responses associated with visual perception (see below).
1.1. Participants
Nineteen healthy volunteer subjects (9 males, 18 right –handed, mean age 28.5 ± 1.1 years) provided written informed consent to participate in this study. Data obtained from one left-handed subject were used after visual inspection confirmed that there were no gross differences in psychophysics or MEG signals when compared with right-handed subjects. All participants had normal or corrected to normal vision. Data from 1 subject were excluded from analysis due to poor attentional performance (<75% identification accuracy for maximal duration stimuli).
1.2. Stimuli
Due to the slow refresh rates of typically available hardware and the long exposure times that result from that, stimuli brief enough to impair conscious perception could not be generated using conventional displays compatible with the magnetically shielded room (MSR) used for brain scanning. Consequently, a stimulation device was designed and assembled that could display visual signals of sub-millisecond duration.
The apparatus consisted of 8 seven-segment LEDs (maximum digit height: 14mm; maximum digit width: 8mm; color: red) arranged in a circle with radius 30 mm. Each seven segment display was equidistant from the center of the circle. The center contained a small, circular LED (5mm; red) that served as a fixation point. The entire circuit was covered with a black fiberglass case so the internal LEDs were invisible to the viewer until turned on.
In this study, only the two horizontally aligned seven-segment units were used. With the subject fixating on the central LED position, stimuli were randomly delivered to either the subject’s left or right visual field during any particular trial at one degree eccentricity from the foveal center. This was done to avoid expectation of stimulus presentation at one single location.
Stimuli consisted of the digits 0–9 and during each trial, one of these were presented at random. During experimentation, the device was placed 1770mm from the subject’s face giving a stimulus visual angle of 0.45 degrees. Stimuli of sufficient duration were clearly visible under these conditions.
All light stimuli durations were oscilloscopically calibrated to be accurate to the nearest 100 microseconds. During experimentation, stimulus exposure times ranged from 1.8 ms to 100 ms. The device had a separate output port used to send a pulse to the MEG acquisition computer 10 ms prior to stimulus onset. This allowed MEG data acquisition and analysis to be precisely aligned with stimulus onset.
Stimulus luminance was measured using an optometer (United Detector Technology; Model Number: 61 CRT; Serial number: 62111). Threshold stimulus durations were averaged across subjects for each number and the resulting values were used to calculate the relevant stimulus durations used in the study (i.e threshold and ½ threshold).
For each stimulus, a total of five luminance measurements were taken for each of the three non sequential stimulus exposure durations used (½ threshold, threshold, and 100 ms). These five samples were averaged to give a more stable luminance measure for each stimulus and exposure duration. Luminance measures ranged between 1.8 cd/m2 (1/2 threshold duration) to 94 cd/m 2 (100ms duration).
Our stimulation apparatus received input via a serial port connected to a PC running the Presentation software package (version 12.2; Neurobehavioral Systems). The presentation program sent output parameters for each trial (stimulus duration, identity and location) to the device. The device controlled the exact timing of stimulation and eliminated extraneous jitter in event timing. Five-hundred ms after stimuli were delivered, the presentation software sent a brief sound pulse (50 ms, 500Hz sound wave) to cue for the subject’s response. All responses were collected by the Presentation program and logged into a separate text file for later MEG data analysis by trial-type.
Auditory stimuli for the response cue were delivered via a speaker system placed outside the MSR adjacent to a pass through that allowed access into the room. Sound volume was adjusted to be at a comfortable level for each subject.
1.3. Task
1.3.1. Dark adaptation and Threshold Determination
Prior to any stimulus exposure, subjects were seated in the MSR with the lights turned off for 30 minutes in order to be adequately dark adapted (Barlow, Kohn, & Walsh, 1947). Since stimuli were short (≤ 100 ms) and small (< 1 deg), they were delivered in the dark to optimize the signal to noise ratio of elicited MEG activity.
Following dark-adaptation, the accelerated stochastic approximation method (Kesten, 1958; Robbins & Monro, 1951) was used to determine the threshold duration (here 50% accurate identification) for each number stimulus (0–9) for each subject. The stimulus sequence was terminated when the step-size remained at 0.1 ms for 20 iterations of each stimulus type. For a given subject, the times recorded for these 20 iterations per number were averaged to yield unique threshold durations for digits 0–9. This ensured that individual thresholds were perceptually matched across both number stimuli and subjects. That is, the threshold for conscious access varied depending on the stimulus (digit) and the subject. Thus, the duration at which each stimulus could be identified correctly 50% of the time by each subject was calculated to normalize across such variation. In this manner, when threshold stimuli were shown, we could ensure that perception/identification occurred 50% of the time regardless of the stimulus type or the subject.
Measured threshold durations were used to calculate relevant stimulus exposure times for each individual during experimentation.
1.3.2. Experimental Paradigm
On each trial, a fixation LED was displayed for 200 ms and then turned off. Subjects were instructed (1) to maintain fixation even after the central LED was turned off and (2) not to saccade towards the stimuli that would appear shortly in the periphery.
After a delay period (randomly jittered between 1–1.2 secs for each trial) subjects were shown a stimulus (digit 0–9) in one of two locations (left or right visual field) for a particular duration (½ threshold (sub-threshold stimulus), threshold or 100 ms (supra-threshold stimulus)). The delay period between fixation and stimulus onset was chosen to minimize contamination of the recorded pre-stimulus period (500ms before stimulus onset) with fixation-evoked brain responses. Trials belonging to a particular condition (i.e. stimulus duration) were equally divided so half were displayed in the subject’s right visual field and half in the left visual field. Three hundred trials of each type (½ threshold, threshold and 100 ms duration trials) were delivered giving a total of 900 trials. A previous study from our lab, using the same experimental set up, showed no significant differences in stimulus recognition and evoked responses due to the visual field of stimulus presentation. Thus, trials were collapsed across visual fields for data analysis to increase the number of trials belonging to each trial type and thereby increase the signal-to-noise ratio of recorded MEG signals. In order to minimize fatigue, the 900 trials were divided into 5 separate runs (180 trials per run) each taking ~10 minutes. Subjects were given small breaks between runs while they remained in the MSR, and the 5 runs were later concatenated for analysis. All trials were pseudo-randomized (for stimulus type (digits 0–9), duration and visual field of stimulation). See figure 1 for a schematic representation of the experimental protocol and relevant event timings.
Figure 1. Experimental Protocol.
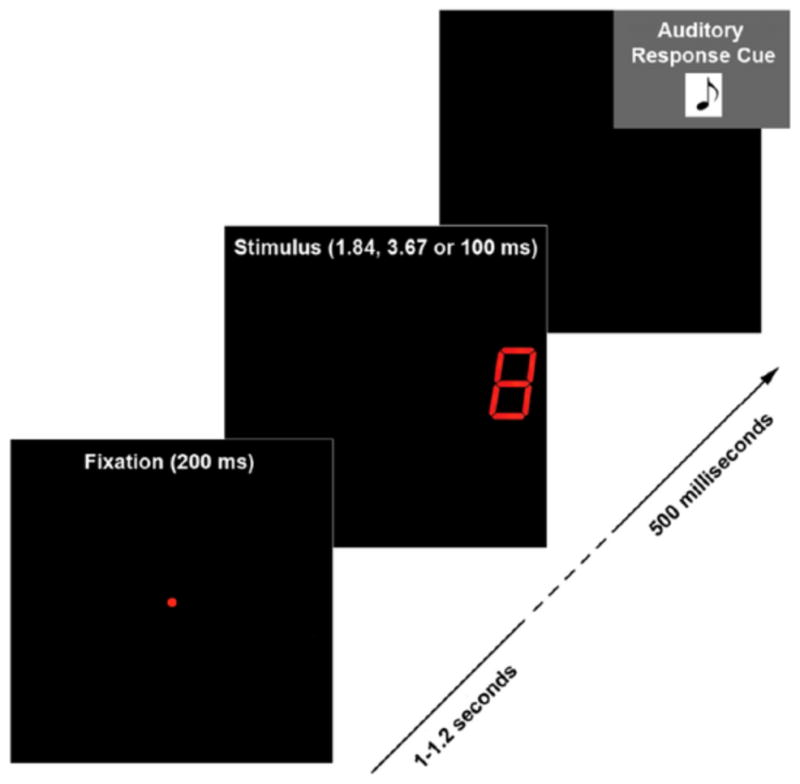
On each trial, a fixation was displayed for 200ms and then turned off. Following a delay period (randomly jittered between 1–1.2 seconds) a stimulus (digit 0–9) was delivered for one of the 3 experimental durations (½ threshold; threshold and 100ms). A response tone was played 500ms after the stimulus was turned off cuing subjects to report the number they saw using a button press. If subjects were unable to recognize the displayed token, they were instructed to press a random button and continue to the next trial.
Subjects reported the identity of the number they saw (via button press) when cued with the auditory beep (500 ms post-stimulus). There were 10 button choices in total– one for each digit 0–9. When the subject saw a given digit he/she would press the button corresponding to that specific stimulus. If the subject was unable to identify a stimulus, he/she was instructed to press any one of the digit buttons (i.e. guess), at random, to move to the next trial.
Behavioral Data were collected using a fiber-optic button press [Lumitouch, Photon Control] that registered all responses to the acquisition computer as input from an additional channel.
1.4. Data Acquisition
MEG data were recorded in 1 second epochs using a 275-channel, synthetic 3rd order gradiometer, whole-head system (VSM Med Tech Ltd.) at a sampling rate of 600Hz. Epochs contained ~500 ms each of pre and post stimulus periods (recording stopped before the response cue was given so MEG data were restricted to stimulus processing, with limited response-related activity). Epochs containing abnormal MEG signals (aberrant deflections in time-series data for any MEG channel exceeding a maximal amplitude of 4 pT) were manually rejected. Data were baseline corrected using the pre-stimulus window and then band pass filtered between 2.5–30 Hz for the analysis of the event-related fields (ERF). This frequency range was selected to minimize low-frequency drifts in the time series data and keep the signal smooth by limiting high frequency content. At the start and end of each run, the subject’s head position, relative to the sensor array, was recorded using digitized coils placed at the nasion and left and right pre-auricular points (~1 cm anterior to the tragus of each ear). This was done to ensure minimal head movement during recordings. Filtered data for all subjects, and for each MEG recording run per subject, were realigned so head positions were matched to that measured for the first recording run for subject 1 using the ft_megrealign function from the open source Matlab FieldTrip toolbox (Fieldtrip Toolbox for MEG/EEG Analysis. Copyright (C) 2003–2007, F.C. Donders Centre, Radboud University Nijmegen, the Netherlands). This subroutine interpolates MEG signals onto standard gradiometer locations by projecting individual, timelocked data towards a coarse source reconstruction. Resulting signals are then recalculated for standard gradiometer locations. In this manner, changes in head position between MEG recordings for each subject would be corrected and activity originating from similar brain regions would be recorded in nearby MEG sensors for all participants.
1.5. Data Analysis
1.5.1. Psychophysics
Recognition performance was measured for each subject during experimentation. Chance level accuracy was 10% (10 number stimuli used). Data were analyzed to yield the total percentage of correct identifications per stimulus exposure time. Statistically significant differences in stimulus identification due to changes in exposure duration were determined using a two-tailed rank sum test. Variance measures were reported as standard errors of the mean.
Recognition performance data recorded for each epoch, belonging to each respective trial type (½ threshold, threshold and 100 ms stimulus exposure durations), were later used to group trials according to the following criteria: ½ threshold incorrect trials; threshold incorrect trials; threshold correct trials; and 100 ms correct trials. MEG data recorded for each of these conditions were compared to assess whether cortical responses associated with stimulus recognition were all-or-none (i.e. (1) whether all trials where stimuli were accurately identified (threshold correct and 100 ms correct) displayed the same, high amplitude brain response and (2) whether all trials where stimuli were not recognized (½ threshold incorrect and threshold incorrect) showed little deviation in MEG activity from baseline).
1.5.2. MEG
The open source MATLAB Fieldtrip tool box (Fieldtrip Toolbox for MEG/EEG Analysis. Copyright (C) 2003–2007) was used to analyze the MEG data. Stimulus-evoked changes in the magnetic field over time were analyzed as (1) averaged event related field (ERF) measurements of epochs grouped by trial type (i.e. average of the time-series data for all single trials of a particular experimental category) and (2) evoked responses in single trial data grouped by trial type (i.e. time series data for each epoch of a particular experimental category). For analysis methods not supported by this tool box, software was written in-house using MATLAB. In addition, functions from the Mathworks signal processing and statistics tool boxes (rank sum function) were also used when necessary.
1.5.2.1 Event-related Field- Timing of evoked responses
For each subject, data collected for all trial types were averaged across 180 trials (1 run) for each MEG sensor. These data were plotted to observe the timing of all salient brain responses (ERFs) evoked by the general paradigm used [see Fig 3 for data from subject 1]. Using these results, relevant time windows of interest were calculated for each salient evoked response observed.
Figure 3. Topographical Plot of the Grand-Averaged Event Related Field Pattern Recorded during the peak of the middle ERF.
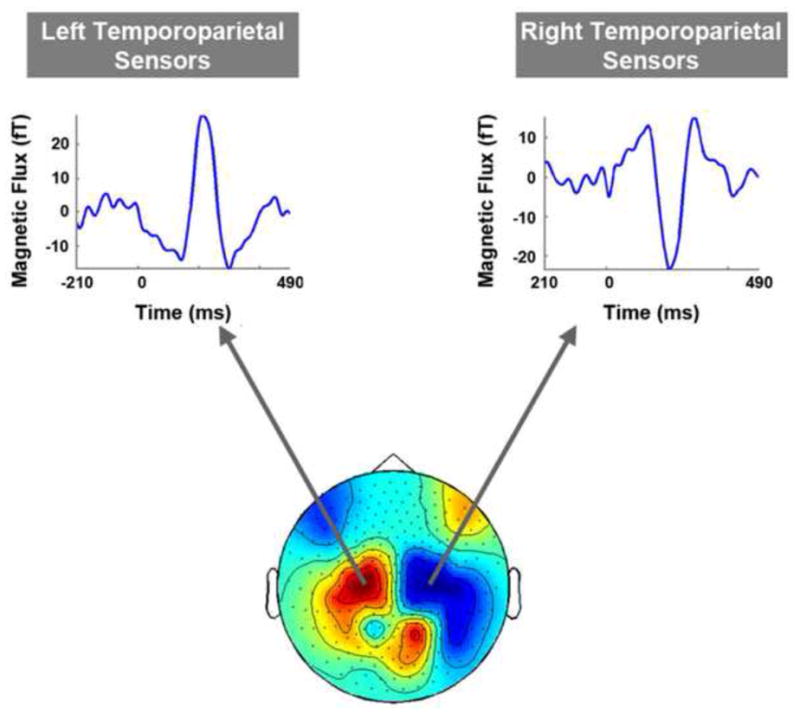
Left and right temporo-parietal sensors were robustly activated during the selected time point. Both sensor groups showed three main event related fields (ERFs) - early, middle and late- with the middle response having the largest peak amplitude. Each time series data trace is from one MEG sensor that is representative of the sensor group form which it was selected. The selected time point approximates the peak latency of the middle ERF recorded in right and left temporo-parietal sensors for data averaged across all subjects.
1.5.2.2. Event-related Field- Global Field Pattern
The time point of peak of the most salient (largest amplitude) ERF was approximated for data averaged across all subjects. In order to visualize the global field pattern of MEG activity at this time point, grand average data were topographically plotted and resulting sensor groups (i.e. regions of gradiometer space) that were maximally activated were identified [see Fig. 4]. Identified sensor groups were used to select channels for further data analysis (see section 1.5.2.3. below).
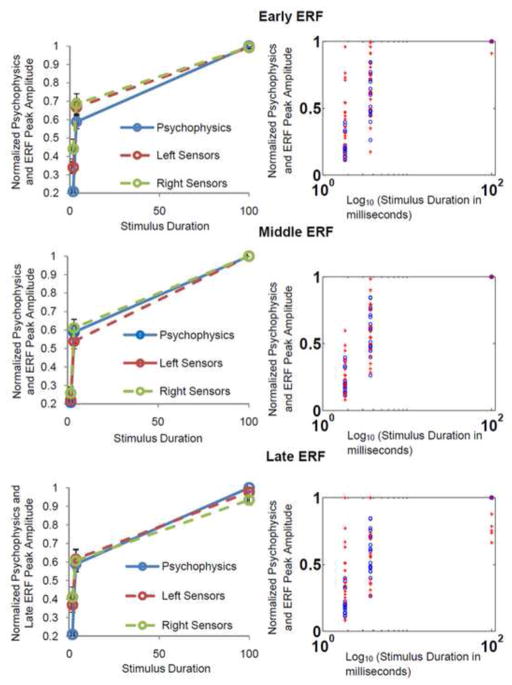
Figure 4. Normalized Percent of Correct Responsese and Peak Response Amplitude For Early, Middle and Late ERFs.
Left column: Normalized mean psychophysics (percentage of correct stimulus identifications) plotted together with normalized mean ERF peak amplitudes for all three ERFs of interest. The blue solid trace represents normalized percent of correct identifications (psychophysics). Red and green dotted traces denote ERF peak amplitude values for left and right temporo-parietal sensors respectively. Open circles represent data points. All three ERFs show a strong correlation with behavioural data for both sensor groups, however, this is strongest for the middle ERF. Error bars represent the standard error of the mean. Right Column- Normalized percentage of correct responses: ‘
 ’ and ERF peak amplitudes: ‘
’ and ERF peak amplitudes: ‘
 ’ for all 18 subjects. All three ERFs show a tight overlap of data points for behavioural measures and brain activity at the individual level, however, this is greatest again for the middle ERF. The stimulus durations were plotted on a log10 rather than a linear scale to more clearly display data points for shorter stimulus exposurs. Individual data are only shown for right temporo-parietal sensors and are representative of results seen for left temporo-parietal sensors.
’ for all 18 subjects. All three ERFs show a tight overlap of data points for behavioural measures and brain activity at the individual level, however, this is greatest again for the middle ERF. The stimulus durations were plotted on a log10 rather than a linear scale to more clearly display data points for shorter stimulus exposurs. Individual data are only shown for right temporo-parietal sensors and are representative of results seen for left temporo-parietal sensors.
1.5.2.3. Event-related Fields- Sensor Selection
Since response maxima varied slightly in sensor location for each subject, the set of sensors used for further ERF analyses were selected on an individual basis with the following criteria: 10 sensors were selected for each of the chosen MEG sensor groups showing strong evoked responses in the topographical plot of grand averaged data (described above). Channels selected for each sensor group had to display large deflections from baseline (for the time window of interest-i.e. corresponding to the peak of the most salient ERF) that were statistically different to the pre-stimulus period (KS-test corrected for multiple comparisons using FDR of 0.05). In addition, the time series data for selected channels had to be representative of that recorded in surrounding sensors.
For each individual, MEG data were later grouped according to the experimental conditions (trial types) used in the study. For the following trial types: ½ threshold, threshold and 100 ms duration stimuli, all 300 trials belonging to each condition were grouped together. For the experimental conditions combining stimulus duration and response accuracy (½ threshold incorrect, threshold incorrect, threshold correct and 100 ms correct), the number of trials per condition were normalized for each individual to ensure that all conditions had equal number of trials and therefore, equal signal-to-noise ratios per subject. The normalized number of trials for each subject corresponded to the minimum number of trials recorded for one of the four experimental conditions (½ threshold incorrect, threshold incorrect, threshold correct and 100 ms correct). For conditions where only a subset of the total number of trials were used (i.e. conditions with more than the normalized number), trials were randomly sorted before the relevant number were chosen for analysis. This was done to ensure that selected epochs were not restricted to the start or end of a recording run and as such, fatigue would not bias results for these conditions.
For each experimental condition (½ threshold, threshold, 100 ms, ½ threshold incorrect, threshold incorrect, threshold correct and 100 ms correct), ERFs (i.e. averaged time-series data for all single-trials corresponding to that condition) were calculated for each MEG sensor. In order to generalize these data across spatially localized sensor areas, ERFs for the 10 selected channels of each identified sensor group were averaged together to yield a region-specific event related field per each sensor group and experimental condition (trial type). Similarly for single trial data analysis, time series data for each epoch of a particular trial type were averaged together for the 10 sensors selected for each sensor group of interest to yield region-specific time-series traces for each epoch per sensor group and trial type.
Grouping of trials in this manner resulted in 14 sets of ERF data per subject (2 sensor groups of interest X 7 trial types including trials grouped by stimulus duration only and trials grouped by stimulus duration and response accuracy) and 8 sets of single trial data (2 sensor groups of interest X 4 trial types including only trials grouped by stimulus duration and response accuracy).
1.5.2.4. Event-related Fields- Amplitude and latency measures for ERFs of trials grouped by stimulus duration only
To evaluate how stimulus-evoked responses were modulated by stimulus duration (½ threshold, threshold and 100 ms), for each subject, the peak amplitude and latency of each salient ERF were measured (see Event-related Fields: Timing of evoked responses) given a particular stimulus duration and sensor-group.
ERF peak amplitudes were measured as the absolute value of the maximal signal deflection from baseline for each identified time window of interest (i.e. corresponding to each salient ERF). Latency measures were taken as the time-points of these maximal deflections. Amplitude and latency were calculated for all identified sensor-groups that were strongly activated (see above)-i.e. amplitude and latency were calculated per condition (stimulus duration) from the ERFs averaged across the 10 channels representing each sensor-group of interest for that condition.
Amplitude differences were first compared for conditions (stimulus durations) within a given sensor group. Pair-wise comparisons of conditions yielded similar trends in significant differences for all identified sensor groups of interest. Hence, peak amplitude measures for each experimental condition were combined for sensors groups and statistical analysis was repeated to yield a more general pattern of significant differences common to all identified sensor groups. Statistical significance was determined using a two-tailed ranksum test, corrected for multiple comparisons using a false discovery rate (FDR) of 0.05 (Genovese, Lazar, & Nichols, 2002). Variance measures were reported as standard errors of the mean.
Average latency was calculated for each ERF of interest (across all stimulus durations and sensor groups) to yield approximations of the time point at which each averaged evoked response peaked.
1.5.2.5. Event-related Fields- Correlation between psychophysics and ERFs of trials grouped by stimulus duration only
To assess whether brain activity was related to the behavioral measurements being collected, response accuracy and ERF peak amplitude values (for each ERF of interest) were normalized and plotted together for the three stimulus durations used (½ threshold, threshold and 100 ms). ERF peak amplitude and psychophysical data were also normalized and plotted at the individual level to assess whether trends seen in the group data were representative of results seen for the majority of subjects. For each ERF of interest, correlation measures were calculated between response accuracy and ERF peak amplitude using Spearman’s method (Spearman, 1904). Significant differences in correlation measures were assessed using a 1-tailed ranksum test, corrected for multiple comparisons using an FDR of 0.05 [since the matlab ranksum function assumes symmetry of results (i.e. the 2-sided p-value is calculated by doubling the most significant 1-sided p-value), the p-value for a 2-sided tail was halved to get the corresponding 1-sided p-value]. Variance measures were reported as standard errors of the mean.
1.5.2.6. Event-related Fields- Amplitude measures for ERFs of trials grouped by stimulus duration and response accuracy: are evoked responses all-or-none?
To assess whether evoked responses reflected stimulus recognition in an all-or-none manner, we compared ERF peak amplitudes calculated for the following trial types: ½ threshold incorrect, threshold incorrect, threshold correct and 100 ms correct. We hypothesized that if ERF peak amplitude reflected an all-or-none recognition-related cortical response, peak amplitudes for ½ threshold incorrect and threshold incorrect conditions would be similarly low while peak amplitudes for threshold correct and 100 ms correct conditions would be similarly high.
ERF Peak amplitudes were calculated in the same manner as described above for trials grouped by stimulus duration only (see paragraph 2 under: section 1.5.2.4.) and the resulting values for each condition were compared to each other. Amplitude differences were first compared for conditions within a given sensor group. Results yielded similar trends in significant differences for all identified sensor groups of interest. Hence, peak amplitude measures for each experimental condition were combined for sensors groups and statistical analysis was repeated to yield a more general pattern of significant differences common to all identified sensor groups. Statistical significance was determined using a two-tailed ranksum test, corrected for multiple comparisons using a false discovery rate (FDR) of 0.05 (Genovese, et al., 2002). Variance measures were reported as standard errors of the mean.
1.5.2.7. Single-trial analysis of trials grouped by stimulus duration and response accuracy: are evoked responses all-or-none?
Trial type specific event related fields are calculated by averaging time series data for all single-trials belonging to a particular experimental condition. Thus, ERF peak amplitudes may be different for a pair of conditions due to two reasons: 1— because single trial evoked response peak amplitudes are different for the two conditions or 2— because the single trial peak amplitudes for the two conditions are the same but the latency of evoked response maxima are different for the two conditions. That is, if one experimental condition is associated with more jitter (i.e. less precision) in the timing of evoked responses than another, then averaged evoked response amplitudes (i.e. ERF amplitudes) may differ even though peak amplitudes of single trial responses are identical for both conditions (Brazier, 1964).
In order to assess whether the temporal jitter associated with single-trial evoked responses were different for each condition, we compared the mean variance of single trial evoked response peak latencies for the experimental conditions of interest (½ threshold incorrect, threshold incorrect, threshold correct and 100 ms correct). Single trial peak latencies for each experimental condition were calculated as the time point of maximal MEG signal deflection recorded during each epoch of a particular trial type for all trials belonging to that category. For each single-trial, peak latency measures were calculated for all salient evoked responses seen in ERF data (i.e. peak latency was measured for each time-window of interest associated with each salient ERF). Variance of single trial latency values for each experimental condition were then calculated (for each evoked response of interest) using latency values measured for all trials belonging to that condition. Single trial peak latency variance was then compared among all four experimental conditions for each evoked response.
Mean single-trial evoked response peak amplitudes for each experimental condition of interest were also calculated and compared to each other. Mean single trial peak amplitudes were calculated as follows: for each ERF time window of interest the absolute value of the maximal signal deflection from baseline for each epoch were measured for all epochs belonging to a particular trial type. This was done for each trial type and the resulting amplitude values were averaged across all single trials belonging to each respective condition for each evoked response. Mean single-trial peak amplitudes calculated for each evoked response were then compared among experimental conditions.
Differences in mean single-trial amplitude and latency variance were first compared for conditions within a given sensor group. Results yielded similar trends in significant differences for all identified sensor groups of interest. Hence, amplitude and latency variance for each experimental condition were combined for sensors groups and statistical analysis was repeated to yield a more general pattern of significant differences common to all identified sensor groups. Statistical significance was determined using a two-tailed t-test, corrected for multiple comparisons using a false discovery rate (FDR) of 0.05 (Genovese, et al., 2002). Variance measures were reported as standard errors of the mean.
Single trial data were also plotted for one representative subject (with high signal to noise ratio) to visualize evoked response peak amplitudes and latency jitter for each experimental condition being compared.
1.5.2.8. Corrected Averages of Single Trial Data- are evoked responses all-or-none?
Latency jitter correction of single trial evoked responses was carried out using Woody’s method (Woody, 1967) which reconstructs averaged waveforms after adjusting for temporal jitter—i.e. for each trial type, new ERFs were calculated by aligning the times of all single-trial evoked responses and then averaging across corrected epochs for each condition. Woody’s method was implemented using the built-in function from the signal detection toolbox for MATLAB. All relevant input parameters were set to their default values.
For each condition, corrected ERF Peak amplitudes were calculated in the same manner as described above for trials grouped by stimulus duration only (see section 1.5.2.4.) and resulting values were compared to each other. Peak amplitude measures for each experimental condition were combined for sensors groups before statistical analysis was applied. Statistical significance was determined using a two-tailed ranksum test, corrected for multiple comparisons using a false discovery rate (FDR) of 0.05 (Genovese, et al., 2002).
1.5.2.9. Localization
Source data were localized onto individual structural MRIs for 6 subjects with clear event related responses. Structural MRIs were acquired as T1- weighted scans without contrast injection using a 1.5 Tesla MRI scanner. To prepare for cortical source localization, MRIs were individually segmented and subsequently, lead-field matrices were calculated using the segmented brain anatomy for each subject.
Using the Linearly Constrained Minimum Variance (LCMV) method (part of the Fieldtrip tool box), event related activity were localized independently for each MEG recording run for each subject (each run consisted of 180 trials collapsed across all trial types).
Source localizations were calculated for all salient ERFs by selecting an appropriate time window for each event related field per subject. Time windows were selected such that (1) each window was roughly (±10 ms) within the bounds used to measure ERF peak amplitude and latency values for each respective ERF of interest and (2) the topographical plot of activated sensors within each window were stable and similar to the activation profile used to select sensors of interest (see event related fields-sensor selection section for details).
Source power was calculated as the decibel increase above noise (noise being the source power calculated for a pre-stimulus time interval equal to the time-window of interest for each respective ERF). This measure was calculated for each ERF and for each recording run, giving a total of 15 estimates per subject (3 ERFs of interest X 5 recording runs).
For each subject, all 15 final source localizations were interpolated onto their own MRI brain anatomy. Subsequently, source data as well as cortical anatomy were averaged across all five MEG recording runs for each ERF of interest to yield a single localized source per ERF. Figure 8 shows source localization results for each ERF of interest for two representative subjects.
Figure 8. Localizations of Early, Middle and Late ERFs for Two Representative Subjects.
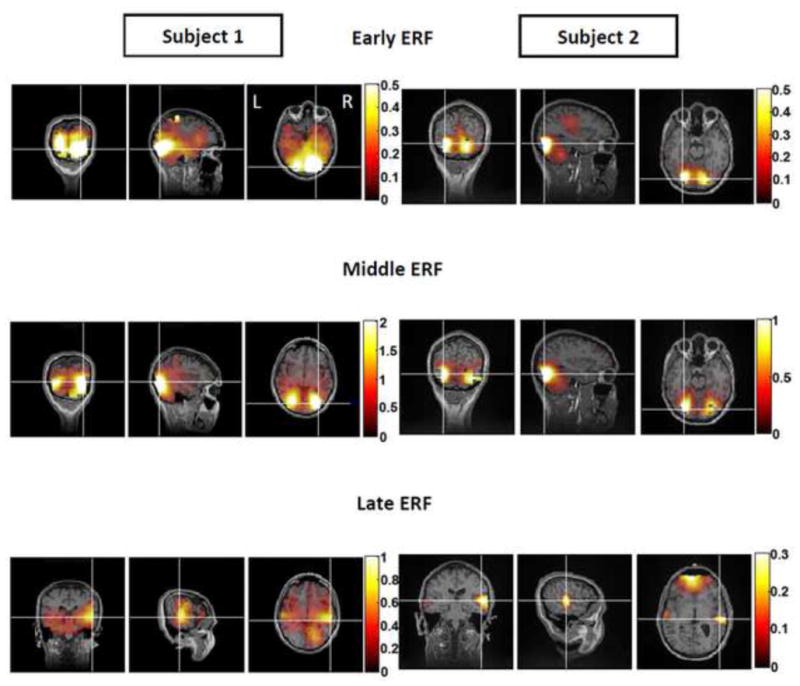
For each ERF, coronal, sagittal and axial views (left to right) of source localizations are shown for two subjects displaying similar areas of activation for early and middle evoked responses. Source activity was calculated as the decibel increase in power above noise (noise = activity recorded during the pre-stimulus period). Cross hairs represent areas of maximal power increase. Left (‘L’) and right (‘R’) cortical hemispheres are depicted in the axial view of the early ERF source localization result for subject 1. The early ERF localized mainly to medial, posterior occipital cortices in both brain hemispheres for three out of six subjects. The remaining three subjects showed more variable results. The middle ERF localized primarily to lateral occipital cortices in both brain hemispheres for the same three subjects with similar results seen for the early response. The remaining three subjects again showed greater variation in areas of activation. The late ERF localized primarily to the right, lateral occipital cortex and also incorporated activation of the anterior regions of inferior occipitotemporal cortex bilaterally for subject 1. For Subject 2, areas of activation were limited to anterior regions of occipitotemporal cortex with additional frontal lobe involvement. The late evoked response was associated with the highest level of individual variations in resulting localizations. The post-stimulus time windows used for localizing the early middle and late ERFs for subjects 1 and 2 were: subject 1— early response: 30–130 ms; subject 2— early response: 60–120 ms; subject 1— middle response: 130–270 ms; subject 2— middle response: 130–200 ms; subject 1— late response: 270–330 ms and subject 2— late response : 230 – 290 ms.
2. RESULTS
2.1. Thresholds
Typical threshold exposure durations ranged from 2.3 ± 0.3 ms to 5.4 ± 1.0 ms depending on the stimulus delivered (average thresholds per digit). Typical threshold exposure durations also varied among subjects and ranged from 1.6 ± 0.1 ms to 12.7 ± 1.3 ms (averaged threshold per subject). The mean threshold duration, averaged across all stimuli and all subjects, was 3.7 ± 0.3 ms (mean of the average thresholds per number, collapsed across individual and stimulus-specific differences).
2.2. Psychophysics
Stimulus identification accuracy was 20.1% (near chance) for subliminal (1/2 threshold) stimuli, sharply increased to 56.9% for threshold duration stimuli and increased again, although less abruptly, to 96.6% for maximal duration stimuli (100 ms). Response accuracy levels were statistically different for all pair-wise comparisons (p = 8.6 e−007 for comparison of ½ threshold and threshold duration stimuli and p = 3.1 e−007 for comparisons of ½ threshold vs. maximal duration as well as threshold vs. maximal duration stimuli). See section 1.5.1 for details regarding statistical methods used.
2.3. MEG
2.3.1. Event-related Fields- Timing of evoked responses
For each subject, recorded MEG data were collapsed across all experimental conditions and plotted to visualize salient evoked responses (ERFs) related to the general paradigm used. Three strong ERFs were seen in resulting time-series traces: an early ERF (peak amplitude between 40–140 ms post stimulus), a middle ERF (peak amplitude between 140–290 ms post-stimulus) and a late ERF (peak amplitude between 190–390 ms post-stimulus) (see Fig 2 for data collected from subject 1). Visualization of resulting signals for all subjects consistently showed the middle ERF to be of higher amplitude than early and late ERFs.
Figure 2. Timing of MEG Evoked Responses.
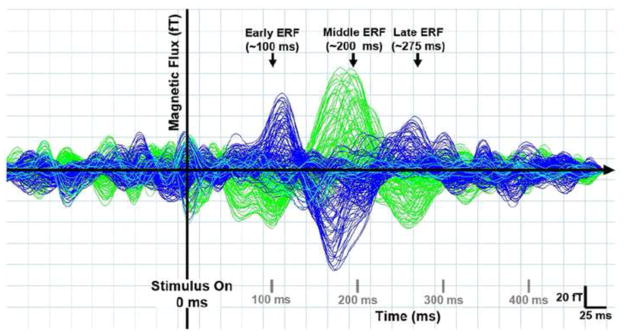
Representative data from one subject showing three main stimulus evoked responses in magnetic signals averaged across all trial types (i.e. after collapsing across all experimental conditions). Traces represent the averaged magnetic response for each MEG sensor as a function of time for one recording run (180 trials). Blue and green traces represent sensors positioned over the right and left cortical hemispheres respectively while aqua traces represent midline sensor positions. The earliest evoked response peaked between 40–140 ms post stimulus (early ERF). A second, higher amplitude response peaked between 140–290 ms post stimulus (middle ERF) and was followed by a third, lower amplitude response that peaked between 190–390 ms post stimulus.
2.3.2. Event-related Fields- Global Field Pattern & Sensor Selection
Since the middle ERF was consistently the strongest (highest amplitude) evoked signal observed, MEG responses (collapsed across all experimental conditions) were averaged across all subjects (grand-averaged ERF data) and the time point of the peak of the middle response was calculated from the resulting data. In order to visualize which MEG sensors were maximally activated at this time point, grand-averaged ERF data were then topographically plotted.
Results showed two main clusters of MEG sensors being robustly activated (i.e. 2 main sensor groups of interest): left and right temporo-parietal sensors (see figure 3, bottom). Although frontal and occipital sensors were also active during this time point, previous work form our lab (using the same stimuli and experimental set up) showed neuromagnetic responses recorded in the temporo-parietal channels to be robustly correlated with stimulus perception. Thus, we restricted sensor-level data analysis to left and right temporo-parietal channels only. The main deflection in ERF waveforms for this time point had opposite directionality in left vs. right temporo-parietal sensors, possibly reflecting activation of inversely-oriented dendritic currents recorded from homologous cortical areas in left vs. right brain hemispheres. Time series data for representative channels from each sensor group also showed strong ERFs during the early (40–140 ms post stimulus) and late (190–390 ms post stimulus) time windows of interest (see figure 3, top left and right traces).
In order to quantify how early, middle and late responses were affected by changes in stimulus exposure duration, trial-type specific ERFs were generated for each identified sensor group of interest per subject. That is, for each subject, ERFs (averaged time series data for all trials of a particular type) were calculated for ½ threshold, threshold and 100 ms stimulus duration trials per sensor. Data were then averaged for 10 sensors representing left temporo-parietal and right temporo-parietal sensor-groups respectively for each subject (see 1.5.2.3 for details). Amplitude and latency measures were calculated from the resulting data and compared to characterize how these parameters were altered as stimulus exposure duration changed from sub-threshold to threshold and supra-threshold durations.
2.3.3. Event-related Fields- Amplitude and latency measures for ERFs of trials grouped by stimulus duration only
ERF Peak amplitudes and latencies were calculated from trial type specific (1/2 threshold, threshold and supra-threshold) and sensor group specific (left and right temporo-parietal) data for the following temporal windows: 40–140 ms post-stimulus (early ERF), 140–290 ms post-stimulus (middle ERF) and 190–390 ms post-stimulus (late ERF). These data were compared to quantify differences in cortical response amplitude and timing resulting from stimulus exposure duration manipulations.
For all 3 ERFs and for both sensor groups of interest, peak amplitudes were low for the half threshold duration condition, sharply increased to intermediate values for the threshold duration condition and increased further (although less abruptly) for the 100 ms duration condition. For all three ERFs of interest, peak amplitudes were statistically different for pair-wise comparisons of the three stimulus durations used (i.e. peak amplitudes differed for ½ threshold vs. threshold conditions, ½ threshold vs. supra-threshold conditions and for threshold vs. supra-threshold conditions; p ≤ 4.2 e−005 for all pair-wise comparisons).
The average peak latency for early, middle and late ERFs was 110 ± 3.3 ms, 234 ± 6.1 ms and 302.9 ± 4.8 respectively (collapsed across subjects, sensor-groups and experimental conditions).
2.3.4. Correlation between psychophysics and ERFs of trials grouped by stimulus duration only
The above results demonstrated that psychophysical data (response accuracy) and ERF peak amplitudes were similar functions of stimulus exposure duration, being low for sub-threshold duration stimuli, sharply increasing to intermediate values for threshold duration stimuli and increasing further, yet less abruptly, for 100 ms duration stimuli. Thus, these two parameters were directly compared to one another to assess whether they were separate measures of a similar brain perceptual response.
Plotting normalized ERF peak amplitudes with psychophysical data demonstrated how similarly these variables changed as a function of stimulus duration. Upon visualization, early ERF average peak amplitudes trended strongly with psychophysical data for ½ threshold, threshold and 100 ms duration stimuli (see figure 4, top left panel). This result was also seen when data were plotted individually for each subject (see figure 4 top right panel). Similarly, for the middle ERF, averaged peak amplitudes trended strongly with psychophysical measurements (figure 4, middle left panel). Visualization of data plotted at the individual level, showed the tightest overlap for psychophysical and evoked response peak amplitudes for the middle ERF (figure 4, middle right panel). Finally, for the late ERF, averaged as well as individual peak amplitudes again trended strongly with psychophysical data (figure 4, bottom left and right panels respectively).
Since ERF peak amplitudes and psychophysical measures trended robustly together on visualization, these parameters were directly correlated with one another to quantify results. Mean correlation values for early, middle and late ERFs and psychophysics were 0.89 ± 0.1, 1 ± 0.0 and 0.92 ± 0.0 respectively. These results demonstrated that for all three ERFs of interest, response amplitudes were strongly correlated with psychophysics. Statistical analysis yielded significantly greater correlation for the middle ERF compared to early and late ERFs (p = 0.01 and 3.6 e−004 respectively). Correlation values for early vs. late ERFs were not significantly different (p = 0.17). Thus, the middle evoked response measured was most strongly correlated with psychophysical measures of perception.
2.3.5. ERF peak amplitudes for trials grouped by stimulus duration and response accuracy: are evoked responses all-or-none?
Since ERF peak amplitudes were strongly correlated with psychophysical measures of stimulus recognition accuracy, we used amplitude data to assess whether stimulus recognition was an all-or-none event. Specifically, for each exposure duration used, trials were regrouped according to correct and incorrect stimulus identifications respectively. Resulting ERF peak amplitudes were then compared. If stimulus recognition occurs as an all-or-none event in the brain, ERF amplitudes for ½ threshold incorrect trials should be similar and low to threshold incorrect trials (no recognition conditions). ERF amplitudes should also be similar and high for threshold correct and supra-threshold correct trials (recognition conditions). See section 1.5.2.6 for methodological details.
ERF peak amplitudes were not an all-or-none function of recognition accuracy. Specifically, results indicated a gradual, step-wise increase in averaged ERF peak amplitudes from low values recorded for ½ threshold incorrect trials up to high values for 100 ms correct trials. This result was seen for all three ERFs of interest (see figure 5 top left for early ERF, middle left for middle ERF and bottom left for late ERF).
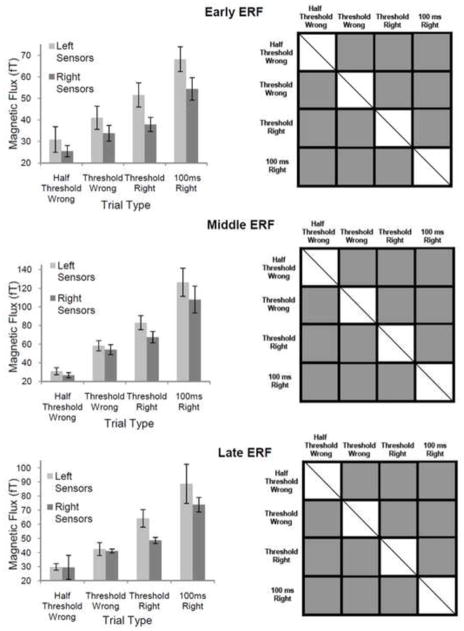
Figure 5. Mean ERF Peak Amplitudes for Correctly and Incorrectly Identified Stimuli of ½ Threshold, Threshold and 100 ms Duration.
Left column— average ERF peak amplitudes for each trial type (error bars represent standard errors of the mean). For all three ERFs (early, middle and late), the mean peak amplitude of evoked responses gradually increased from low values for trials where ½ threshold duration stimuli were incorrectly identified to high values for trials where 100 ms duration stimuli were correctly identified. Right column— Statisticlaly significant differences between the various trial types. Grey squares depict pairwise comparisons that were significantly different. Squares with diagonal lines represent conditions that could not be compared. Statistical analysis was done after combining data recorded from left and right sensor groups for each trial type. All pairwise comparisons yielded significant differences. These data support a graded rathar than all-or-none model of stimulus recognition.
All possible pair-wise comparisons of ERF peak amplitudes for the different trial types (½ threshold incorrect, threshold incorrect, threshold correct and 100 ms correct) were significantly different to one another (p ≤ 0.04 for all comparisons), reinforcing a step-wise or graded increase in response amplitude from ½ threshold incorrect to 100 ms correct conditions (see figure 5 right column for statistically significant comparisons of ERF peak amplitudes for each pair of conditions; top right for early ERF, middle right for middle ERF and bottom right for late ERF).
These results further weaken an all-or-none hypothesis relating ERF peak amplitude to stimulus recognition. According to an all-or-none hypothesis, ERF peak amplitudes should be statistically similar for ½ threshold incorrect and threshold incorrect trials as well as for threshold correct and 100 ms correct trials. Both these expected findings were not corroborated by our results (see figure 5, right column). An all-or-none hypothesis would also imply statistically different ERF peak amplitudes for threshold correct and threshold incorrect trials. This was seen in our results for all three ERFs of interest (see figure 5, right column).
2.3.6. Single-trial analysis of trials grouped by stimulus duration and response accuracy: latency jitter
Since averaged ERFs were not an all-or-none function of stimulus recognition, single trial data for each condition (½ threshold incorrect, threshold incorrect, threshold correct and 100 ms correct) were compared to assess whether these results reflected psychophysical measures of perception in an all-or-none manner. Specifically, single trial peak latency jitter (variance) as well as single trial peak amplitude parameters were compared across experimental conditions (see section 1.5.2.7 for methodological details).
For all three ERFs of interest, single-trial latency variance of evoked response maxima was significantly less for trials where 100 ms duration inputs were correctly identified than for the remaining trial types (½ threshold incorrect, threshold incorrect, and threshold correct) (p ≤ 0.002 for comparisons of 100 ms correct condition with all other conditions for the early ERF, p ≤ 0.003 for comparisons of 100 ms correct condition with all other conditions for the late ERF, p ≥ 0.2 for all other pair-wise comparisons. See figure 6, right column for significant differences among conditions.
Figure 6. Mean Variance of Single Trial Evoked Response Peak Latency for Correctly and Incorrectly Identified Stimuli of ½ Threshold, Threshold and 100 ms Duration.
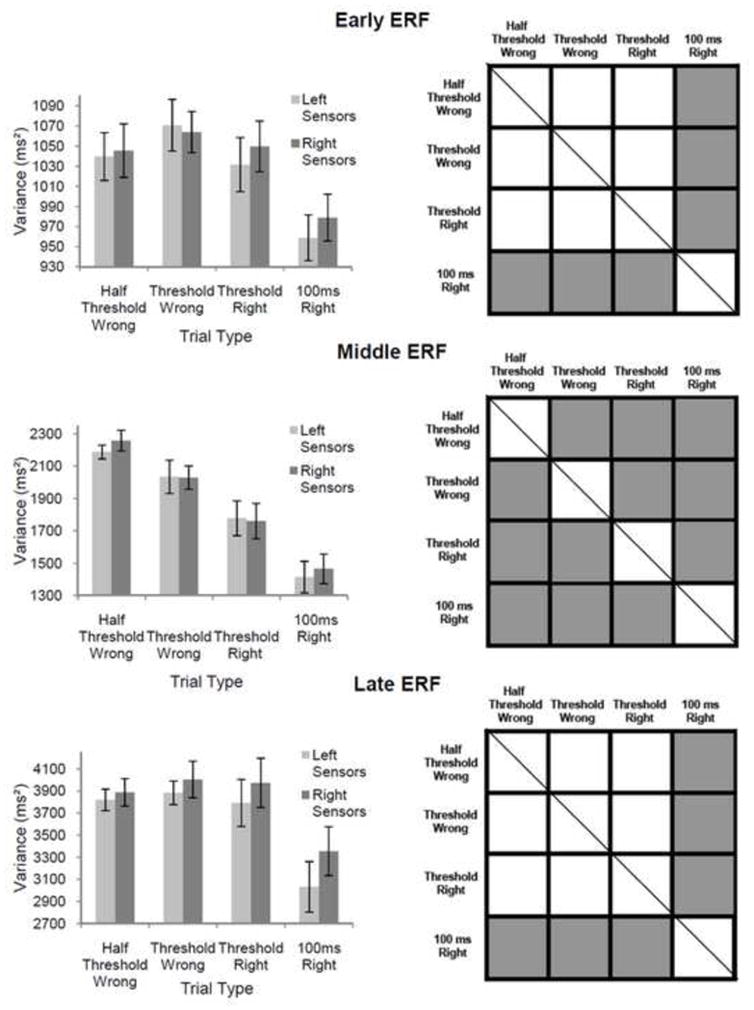
Left column— average variance of evoked response peak latency for single trial data recorded per trial type (error bars represent standard errors of the mean). Right column— Statisticlaly significant differences between conditions (grey squares depict pairwise comparisons that were significantly different and squares with diagonal lines represent conditions that could not be compared). Statistical analysis was done after combining data recorded from left and right sensor groups for each trial type. For early and late ERFs, the variance of single trial evoked response peak latency was significantly lower for 100 ms duration stimuli than for all other trial types. For the middle ERF, mean variance of evoked response peak latency decreased gradually from high values for trials where subthreshold stimuli were incorrectly identified to low values for trials where supra-threshold stimuli were correctly identified (all pairwise comparisons showed significant differences).
For the middle ERF, which was most tightly correlated with stimulus recognition, the latency jitter of single-trial evoked response maxima gradually decreased from high values for ½ threshold incorrect trials to low values for 100 ms correct trials (see figure 6, middle left column). Pair-wise comparisons of these data showed significant differences for all possible conditional pairs (p ≤ 0.01 for all comparisons; see figure 6, middle right column).
This result demonstrated that as stimulus duration and perceptual strength increased, the temporal precision associated with the middle evoked response increased systematically (note: perceptual strength refers to an assumed increase in perceptual clarity as subjects transitioned from no perception (½ threshold incorrect trials), to perception without identification (threshold incorrect trials), to ambiguous perception (threshold correct trials) and finally, to robust perception (100 ms correct trials).
2.3.7. Single-trial analysis of trials grouped by stimulus duration and response accuracy: amplitude
The temporal precision of the middle evoked response increased with inferred perceptual strength. Thus, single trial peak amplitudes were compared among conditions to assess whether stimulus perception was associated with an all-or-none response. That is, if perception occurs as an all-or-none event, the same, high amplitude response would be present for all trials where perception occurred. However, the trial-by-trial timing of this response may be more jittered for less-robust perceptual experience. If this were true, the graded increase in averaged ERF peak amplitudes described above (see 2.3.5) may result from an all-or-none perceptual event that occurs in all single trials associated with stimulus perception but with greater latency jitter for weaker perception conditions (see 1.5.2.7. for methodological details).
Due to the low signal to noise ratios associated with single-trial neuromagnetic responses, there were no significant differences detected in evoked response peak amplitudes for any pair of conditions (data not shown). Hence, it was not possible to determine whether single trial evoked responses generated for ambiguous and robust perception conditions had similar amplitudes and therefore, temporal jitter differences were the sole cause for the observed differences in averaged ERF peak amplitudes for these conditions.
2.3.8. Single-trial analysis of trials grouped by stimulus duration and response accuracy: visualization of responses
Single trial data for each experimental condition were also plotted and visualized to assess whether stimulus perception is all-or-none.
Visual inspection of individual, single-trial data did not show any strong deflections in MEG signals from baseline for the threshold incorrect condition (see figure 7, top left panel). However, threshold incorrect, threshold correct and 100 ms correct conditions all showed similar, high amplitude evoked responses in the single trial data (see figure 7, top right, bottom left and bottom right panels respectively). These responses tended to be strongest for the time window of the middle ERF. Visual inspection also demonstrated a systematic decrease in the temporal jitter of single-trial response maxima from the ½ threshold incorrect condition to the threshold correct condition and finally to the 100 ms correct condition (see Figure 7 top right, bottom left and bottom right panels respectively).
Figure 7. Neuromagnetic Responses Recorded for Single Trials of Each Trial Type.
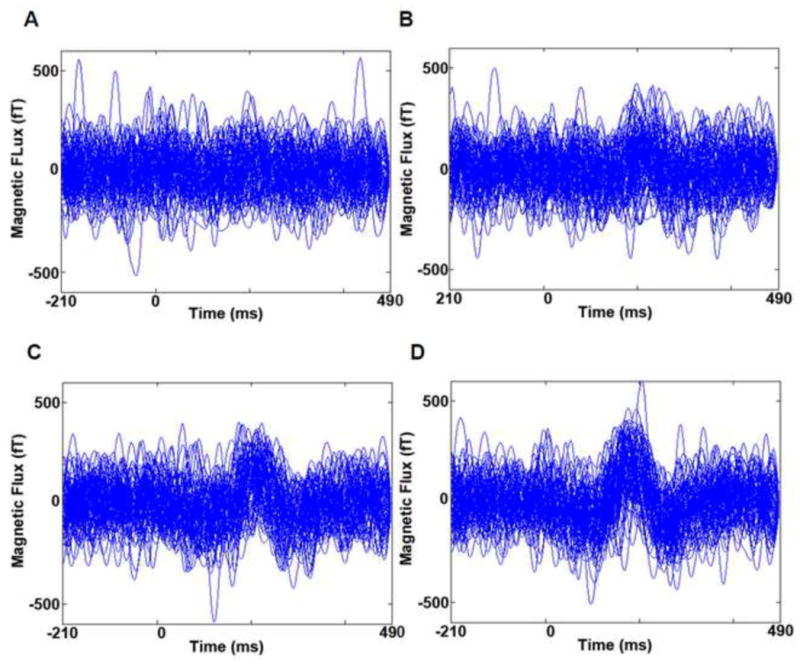
Each trace shows time series data from one single trial (per trial type) recorded in the left temporo-parital sensor group for subject 1. These data are representative of the single trial, neuromagnetic responses seen for right temporo-parietal sensors for Subject 1. A total of 89 single trials are shown per condition. A. Single trial responses for ½ threshold duration stimuli that were incorectly identified. Traces do not show consistant differences from the prestimulus period (i.e. evoked responses are largely absent). B. Single trial responses for threshold duration stimuli that were incorrectly identified. Tracres show the emergence of an evoked response that peaks at ~210 ms post stimulus with considerable intertrial latency jitter. C. Single trial responses for threshold duration stimuli that were correctly identified. Tracres show an evoked response peaking at ~210 ms with an intermediate level of intertrial latency jiter. D. Single trial responses for 100 ms duration stimuli that were correctly identified. Tracres show an evoked response peaking at ~210 ms with very little intertrial latency jitter.
2.3.9. Corrected Averages of Single Trial Data- are evoked responses all-or-none?
Averaged ERFs were recalculated after realigning temporally mismatched single-trial evoked responses for each condition. This was done to see if corrected, averaged ERF peak amplitudes reflected perception in an all-or-none rather than a graded manner (see 1.5.2.8 for methodological details).
Statistical analysis yielded no significant differences between any pair of conditions for recalculated ERF peak amplitude values after correcting for single-trial latency jitter.
2.3.10. Summary of averaged ERF vs. Single-trial Analysis Findings
Averaged ERF data suggested that stimulus perception was graded. However, visualization of the single trial data showed that it was all-or-none: a response with similar amplitude was seen for all conditions where perception was supported (figure 7). Qualitative inspection of single trial data (figure 7) showed that random noise fluctuations (during the pre-stimulus period) were very similar in peak amplitude to the evoked response being characterized. Thus, significance was not reached when single trial peak amplitudes were compared, even between ½ threshold incorrect and 100 ms correct conditions. However qualitative inspection of the single-trial evoked response data for these 2 conditions clearly showed that peak amplitudes differed (figure 7). Hence, the graded increase in amplitude seen in averaged ERF results likely results, at least in part, from the increased temporal jitter seen for the all-or-none single trial response for less robust perception conditions. These results are again evident in figure 7.
2.3.11. Localization
MEG responses recorded during the early, middle and late time windows of interest were localized to identify activated cortical regions (see 1.5.2.9 for methodological details).
Source localization showed robust activation of posterior occipital cortices, lateral occipital cortices and lateral, occipito-temporal areas. For several subjects, parietal and frontal-lobe activation was also seen for some of the ERF time windows analyzed. The degree of activation of each cortical area varied with the timing of each ERF (early, middle and late). Additionally, the degree of individual variations in activated cortical areas increased from early to late evoked responses.
Source localization of the earliest event related field showed maximal activation of posterior regions of the occipital cortex for three out of six subjects. For these individuals, posterior occipital cortices were activated bilaterally for two of the three subjects with activity for the third subject localizing primarily to right posterior occipital areas. For the remaining three individuals, localization results showed more variability. Specifically, for one subject, activity localized primarily to right posterior and lateral occipital areas with some activity seen in the left anterior frontal lobe. For another subject, activity localized to bilateral occipito-temporal cortices close to the temporal poles. For the final subject, activity localized to bilateral parieto-frontal regions.
Source localization of the middle ERF showed bilateral regions of lateral, occipital cortices to be maximally activated for three individuals (note: the same three individuals displayed similar areas of activation pertaining to early and middle ERF activity). For these subjects, activated regions of the occipital cortex were more anterior to those seen for localization of the early ERF (for one of these three individuals, activity localized to bilateral occipito-temporal regions). For the remaining three individuals, areas of activation were more varied. For one subjects, activity localized to right lateral occipital cortex with some involvement of left anterior frontal areas. For another subject, activity localized to bilateral occipito-temporal cortices close to the temporal poles with some additional activity seen in medial frontal cortex. For the final subject, activity localized to medial parietal areas with some activity also seen in right temporo-parietal regions.
Source localization of the late ERF showed the most individual variation in resulting areas of maximal activation. One subject showed activation of right lateral occipital cortex with additional involvement of bilateral occipito-temporal areas. Another subject showed activation of bilateral occipito-temporal regions near the temporal poles and also included anterior frontal regions bilaterally. Two more individuals showed frontal lobe activation with one individual primarily indicating left, anterior, frontal lobe involvement and the other showing medial, anterior, frontal lobe involvement. The remaining two subjects showed activity in the right hemisphere only with right temporal lobe activation (near the temporal pole) seen for one individual and right temporo-parietal activity seen for the other.
In summary, Localization of MEG signals points to the involvement of multiple, ventral visual stream cortices that are activated at various time windows following stimulus presentation.
See figure 8 for representative source localization data for two subjects with similar early and middle ERF localization results.
2.3.12. Limitations of Localizing Evoked Responses
Localization results varied among subjects, obscuring the identification of activated areas common to the majority of participants. This resulted from multiple factors including limited access to structural MRIs (a maximum of 6 could be acquired). In addition, individual variability in signal-to-noise ratios for MEG data likely also contributed to the observed variability in source reconstruction accuracy and as a result, in source localization. Combining all experimental runs for each subject prior to source localization may have helped normalize these differences. However, this was not possible after MEG data had been realigned to standard gradiometer coordinates. Data that was not realigned was not localized to avoid errors resulting from changes in head positions between recording runs. Subtle differences in task performance may have contributed to the variability seen among subjects. Finally, for the majority of selected individuals, activated areas were highly reproducible for two related experiments using the same experimental paradigm— lending validity to these results.
3. DISCUSSION
The dynamics of central nervous system activity are ongoing (Llinás, 2001), yet our conscious, visual experiences can seem strikingly abrupt in nature. Indeed, we either do or do not see an item regardless of the subtle variations that can exist regarding the certainty, clarity and degree of richness with which we experience perceiving it. Thus, our waking visual perceptions, be they ambiguous or robust, seem to exist as all-or-none events generated by the brain in response to stimuli of sufficient strength. Indeed our findings suggest that while averaged cortical responses to visual stimuli reflect recognition in a graded fashion, single trial responses related to perception seem to be all-or-none.
Specifically, we found that a high-amplitude evoked response, peaking at ~230 ms and involving lateral, occipito-temporal cortices, was robustly correlated with stimulus identification. Previous work form our lab has shown this response to also be observed for visual symbols that are perceived and not identified, characterizing it as primarily perceptual in nature (Sekar 2011 – thesis). Peak amplitudes of averaged ERFs for the response in question increased gradually with increasing stimulus duration and identification accuracy. However, single trial analyses of the same response showed that it was evoked in an all-or-none manner— i.e. being present for all conditions where stimulus perception was most probable and largely absent for the condition where perception was least probable. Additionally, the temporal jitter associated with single-trial responses decreased systematically as stimulus duration and identification accuracy increased, contributing at least partly to the graded increase seen for averaged ERF peak amplitudes in relation to these measures. Taken together, these results suggest that (1) averaged ERF measures can be misleading when there is a high degree of inter-trial variance for evoked responses and (2) that perception is in fact correlated with an all-or-none, cortical evoked response, the temporal precision of which increases systematically as stimulus perception transitions from ambiguous to robust states.
3.1. Averaged ERFs Can Incorrectly Represent Single Trial Evoked Responses
Averaged responses serve as clearer estimates of single-trial activity when the signal of interest has stable characteristics for all epochs (K.M. Spencer, 2005). Conversely, signal averaging can also conceal trial-by-trial variability of evoked response characteristics. For example, if evoked response strength is invariant but response latency changes on single trials, then the averaged signal will be distorted and with lower amplitude (Brazier, 1964). Secondly, variations in amplitude between two averaged ERFs, may not reflect differences in single trial response strength but rather, differences in the proportion of trials with a response of constant amplitude (Otten & Rugg, 2005).
Given these caveats, we investigated whether ERF amplitudes for threshold input trials comprised of varied proportions of high and low values corresponding to trials where these inputs were and were not recognized respectively. We found a graded increase in averaged ERF peak amplitude with increasing stimulus duration and response accuracy. However, single trial responses revealed an all-or-none type relationship between evoked response generation and expected perceptual strength.
3.2. Single Trial Evoked Responses suggest that perception is all-or-none
The evoked response correlated with stimulus identification was similar for single trial categories where perception was most probable (threshold stimuli incorrectly identified—perception without identification, threshold stimuli correctly identified—ambiguous perception, and supra-threshold stimuli correctly identified—robust perception) and was largely absent for single trials where perception was least probable (sub-threshold stimuli incorrectly identified no—perception). Though it is possible that ‘threshold-incorrect’ trials were associated with no perception or, conversely, ‘sub-threshold-incorrect’ trials with perception, previous work form our laboratory indicates that this is unlikely. In a preliminary study consisting of 10 participants, we measured identification as well as subjective awareness reports for the same stimuli. Regarding trials where threshold stimuli were incorrectly identified, only 41± 8% were associated with awareness reports of ‘didn’t see (i.e. ‘didn’t see’ indicating that conscious perception had not occurred). However, for trials where sub-threshold duration inputs were incorrectly identified, the majority (81± 6%) of awareness reports were ‘didn’t see’. It is also conceivable that threshold and supra-threshold correct conditions were not associated with perception (i.e. stimulus discrimination can sometimes occur without perception as in the case of blind sight (Ptito & Leh, 2007)), however, this too is unlikely given that so few trials were associated with awareness reports of ‘didn’t see’ for these conditions (23.9 ± 9.4% and & 0.2 ± 0.1% respectively).
3.3. What Does an All-or-None Response Imply?
The all-or-none law, initially described for contraction of heart muscle, states that an inducing shock either does or does not produce contraction depending on its amplitude. For all successful contractions, strength is maximal (Cannon, 1924). In our case, this law can be restated that stimuli either evoked the cortical response of interest or failed to do so depending on whether perception occurred. However, all evoked responses seemed maximal in amplitude. Thus, it is possible that increases in stimulus strength may have resulted in increasing initial numbers of depolarized cortical cells in areas of the brain that are involved in generating perception. However, once a threshold level of excitement was reached, an explosive, self-sustaining response took over, regardless of stimulus strength or the initial degree of excitation. Importantly, such a response implies that the neurons involved must communicate with each other in some way since a proportion of activated cells must be capable of recruiting the remainder necessary for a synchronized, maximal amplitude response. Such implications lend important insights into the complex neuronal interactions that may be required for generating cognition.
3.4. Single Trial Latency Jitter Amplitudes
We also found that as stimulus duration and identification accuracy increased, the temporal jitter associated with single trial responses decreased systematically, contributing at least partly to the graded increase seen in averaged ERF amplitudes for these parameters.
Amplitude measures for single trial data and for averaged signals corrected for temporal jitter showed no differences between any pair of conditions. This may signify that the amplitude of unaveraged responses were unrelated to changes in stimulus duration or identification. In such case, perceptual strength would be correlated solely with variations in the trial-by-trial timing of the signal which was presumably evoked in an all-or-none manner for all trials. This is unlikely however, given that single trials plotted for subjects with high signal-to-noise ratios showed the response in question to be largely absent for the sub-threshold wrong condition and clearly present for all remaining conditions. Thus, for single trial signal strength comparisons, the high amplitude of background fluctuations in unaveraged signals may have obscured differences in peak amplitudes between conditions where perception was and was not supported. In the case of temporally realigned averages, the method used assumed that all trials contained the response of interest. Thus for conditions where the measured response was absent for the majority of epochs (presumably for the sub-threshold incorrect condition), single-trial detection methods may have introduced false components into the recalculated average (K.M. Spencer, 2005). On the other hand, that corrected ERF peak amplitudes were invariant for the remaining conditions (threshold incorrect, threshold correct and supra-threshold correct) strengthens the assertion that the studied response was evoked with equal magnitude for conditions where perception was most probable.
3.5. Perceptual Strength in Relation to the Temporal Jitter of Evoked Responses
As subjects transitioned from absent to ambiguous to robust perception, the trial-by-trial timing of evoked responses became more precise. Previous studies have shown that such differences in ‘latency jitter’ between conditions can result from greater variability in stimulus complexity (and resulting stimulus evaluation times) for one condition vs. another (Kutas, Mccarthy, & Donchin, 1977; K. M. Spencer, Abad, & Donchin, 2000). In our case, the stimuli themselves were identical for all trial types (save durational manipulations). However, it is conceivable that the evoked response latency we measured is indicative of the timing required for cortical processes to form perceptual representations of presented inputs. If so, correctly identified supra-threshold stimuli, would be expected to reliably engage cortical processes related to perception at ceiling levels-regardless of inter-trial variations in subjects’ attention, interest etc. Thus, resulting perceptual representations would be obtained with high temporal precision for this condition. However, as stimulus duration decreases to threshold values, it is possible that trial-by-trial variations in subjects’ attention, interest etc. may play a larger role in how long such stimuli must be processed in order for them to be perceived or whether they are perceived at all. Thus, a broader distribution of latency values required for the formation of a stable percept would result.
Moreover, decreased latency jitter (seen for robust perception conditions) implies that evoked responses are more phase-locked to stimulus presentation—i.e. the phase of resulting signal deflections are temporally aligned for all epochs pertaining to that condition. Similarly, for conditions with high degrees of latency jitter, the phase of neuromagnetic deflections will be out of register for different trials. Increases in such inter-trial coherence (Delorme & Makeig, 2004) or phase locking (Tallon-Baudry, Bertrand, Delpuech, & Pernier, 1996) in the theta frequency band (4.1–8.5 Hz) have recently been shown to correlate strongly with stimulus identification recorded during an attentional blink task (Slagter, Lutz, Greischar, Nieuwenhuis, & Davidson, 2009). Further, several researchers have asserted that evoked potentials are generated by a stimulus-induced partial phase-reset of ongoing neuronal oscillations (Makeig, 2002; Salinas & Sejnowski, 2001; Sayers, Beagley, & Henshall, 1974) and that the pre-stimulus phase of thetha and alpha rhythms may influences whether perception occurs (Busch, Dubois, & VanRullen, 2009). If early visual processing events can reset the phase of ongoing oscillations to align optimally for later, perceptual responses to be evoked, then our data may suggest that this process occurs more reliably for strong, supra-threshold stimuli than for weaker, threshold inputs. Such timing-related changes in perceptual clarity may indicate that supra-threshold inputs (with more optimal response timing) are more effectively bound into the context of a unified percept that is also being generated at the same (optimal) time. Thus, the degree to which inputs are enmeshed into a unified percept with other sensory processing events (i.e. depending on the timing of stimulus-specific evoked brain responses relative to the timing of perceptual brain mechanisms) may dictate how clearly such stimuli are subsequently perceived.
Highlights.
Magnetoencephalography measurements were strongly correlated with visual object perception.
Averaged neuromagnetic responses incorrectly showed perception as a graded event in the brain.
Single trial neuromagnetic responses suggested that perception is an all-or-none event in the brain.
The temporal precision of perception-related brain activity increased systematically as perception transitioned from being ambiguous to robust.
Acknowledgments
We wish to thank Alex Porras for technical assistance in the development and assembly of the stimulation apparatus used in this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Krithiga Sekar, Email: Krithiga.Sekar@med.nyu.edu.
William M. Findley, Email: William.Findley@nyumc.org.
Rodolfo R. Llinás, Email: Rodolfo.Llinas@nyumc.org.
References
- Bar M, Tootell RBH, Schacter DL, Greve DN, Fischl B, Mendola JD, et al. Cortical mechanisms specific to explicit visual object recognition (vol 29, pg 529, 2001) Neuron. 2001;30(1):299–299. doi: 10.1016/s0896-6273(01)00224-0. [DOI] [PubMed] [Google Scholar]
- Barlow HB, Kohn HI, Walsh EG. The Effect of Dark Adaptation and of Light Upon the Electric Threshold of the Human Eye. American Journal of Physiology. 1947;148(2):376–381. doi: 10.1152/ajplegacy.1947.148.2.376. [DOI] [PubMed] [Google Scholar]
- Brazier MA. Evoked Responses Recorded from the Depths of the Human Brain. Ann N Y Acad Sci. 1964;112:33–59. doi: 10.1111/j.1749-6632.1964.tb26741.x. [DOI] [PubMed] [Google Scholar]
- Busch NA, Dubois J, VanRullen R. The Phase of Ongoing EEG Oscillations Predicts Visual Perception. Journal of Neuroscience. 2009;29(24):7869–7876. doi: 10.1523/JNEUROSCI.0113-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WB. Biographical memoir, Henry Pickering Bowditch, 1840–1911. Washington, D.C: U.S. Govt. Print. Off; 1924. (Vol. volume xvii,eighth memoir) [Google Scholar]
- Del Cul A, Baillet S, Dehaene S. Brain dynamics underlying the nonlinear threshold for access to consciousness. PLoS Biol. 2007;5(10):e260. doi: 10.1371/journal.pbio.0050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Kanwisher N. Neural events and perceptual awareness. Cognition. 2001;79(1–2):89–113. doi: 10.1016/s0010-0277(00)00125-6. [DOI] [PubMed] [Google Scholar]
- Kesten H. Accelerated Stochastic-Approximation. Annals of Mathematical Statistics. 1958;29(1):41–59. [Google Scholar]
- Kutas M, Mccarthy G, Donchin E. Augmenting Mental Chronometry - P300 as a Measure of Stimulus Evaluation Time. Science. 1977;197(4305):792–795. doi: 10.1126/science.887923. [DOI] [PubMed] [Google Scholar]
- Llinás R. I of the Vortex: From Neurons to Self. Cambridge: MIT Press; 2001. [Google Scholar]
- Makeig S. Dynamic brain sources of visual evoked responses (vol 295, pg 690, 2002) Science. 2002;295(5559):1466–1466. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- Moutoussis K, Zeki S. The relationship between cortical activation and perception investigated with invisible stimuli. Proc Natl Acad Sci U S A. 2002;99(14):9527–9532. doi: 10.1073/pnas.142305699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD. Interpreting Event-Related Brain Potentials. In: Handy TC, editor. Event-Related Potentials A Methods Handbook. Cambridge: The MIT Press; 2005. pp. 3–16. [Google Scholar]
- Overgaard M, Rote J, Mouridsen K, Ramsoy TZ. Is conscious perception gradual or dichotomous? A comparison of report methodologies during a visual task. Conscious Cogn. 2006;15(4):700–708. doi: 10.1016/j.concog.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Ptito A, Leh SE. Neural substrates of blindsight after hemispherectomy. Neuroscientist. 2007;13(5):506–518. doi: 10.1177/1073858407300598. [DOI] [PubMed] [Google Scholar]
- Quiroga RQ, Mukamel R, Isham EA, Malach R, Fried I. Human single-neuron responses at the threshold of conscious recognition. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(9):3599–3604. doi: 10.1073/pnas.0707043105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins H, Monro S. A Stochastic Approximation Method. Annals of Mathematical Statistics. 1951;22(3):400–407. [Google Scholar]
- Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nature Reviews Neuroscience. 2001;2(8):539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers BMA, Beagley HA, Henshall WR. Mechanism of Auditory Evoked Eeg Responses. Nature. 1974;247(5441):481–483. doi: 10.1038/247481a0. [DOI] [PubMed] [Google Scholar]
- Sergent C, Dehaene S. Is consciousness a gradual phenomenon? Evidence for an all-or-none bifurcation during the attentional blink. Psychol Sci. 2004;15(11):720–728. doi: 10.1111/j.0956-7976.2004.00748.x. [DOI] [PubMed] [Google Scholar]
- Slagter HA, Lutz A, Greischar LL, Nieuwenhuis S, Davidson RJ. Theta phase synchrony and conscious target perception: impact of intensive mental training. J Cogn Neurosci. 2009;21(8):1536–1549. doi: 10.1162/jocn.2009.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearman C. The proof and measurement of association between two things. American Journal of Psychology. 1904;15:72–101. [PubMed] [Google Scholar]
- Spencer KM. Averaging, Detection, and Classification of Single-Trial ERPs. In: Handy TC, editor. Event-Related Potentials A Methods Handbook. Cambridge: The MIT Press; 2005. pp. 209–227. [Google Scholar]
- Spencer KM, Abad EV, Donchin E. On the search for the neurophysiological manifestation of recollective experience. Psychophysiology. 2000;37(4):494–506. [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Delpuech C, Pernier J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J Neurosci. 1996;16(13):4240–4249. doi: 10.1523/JNEUROSCI.16-13-04240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody CD. Characterization of an adaptive filter for the analysis of variable latency neuroelectric signals. Medical and Biological Engineering. 1967;5:539–553. [Google Scholar]